ABSTRACT
Esophageal squamous cell carcinoma (ESCC) is a deadly malignant tumor that threatens human health. Long noncoding RNA (lncRNA) is widely expressed in eukaryotes and is closely associated with human disease progression. However, its role in ESCC remains incompletely understood. In this study, we analyzed the results of three gene expression omnibus (GEO) databases containing lncRNA expression data of ESCC and normal tissues. The results showed that HCP5 was significantly overexpressed in ESCC tissues, which was further verified in our collected ESCC samples. The functional study suggested that HCP5 knockdown inhibited ESCC cell proliferation and invasion. Regarding the mechanism, HCP5 was able to directly interact with YTHDF1, a N6-methyladenosine (m6A) reader, enhancing the binding of YTHDF1 to m6A-modified HK2 mRNA, leading to increasing HK2 stability, thereby promoting the Warburg effect (aerobic glycolysis) of ESCC cells. The nude mice model showed that the knockdown of HCP5 in vivo remarkably reduced tumor size. Clinically, high HCP5 was positively correlated with larger tumor volume, higher TNM stage and lymph node metastasis. Moreover, ESCC patients with high HCP5 exerted shorter survival time than patients with low HCP5. These findings uncover the importance of HCP5 in human ESCC progression; the turbulence of HCP5/YTHDF1/HK2 axis may be responsible for ESCC carcinogenicity.
KEYWORDS: ESCC, HCP5, warburg effect, m6A, biomarker
Graphical abstract
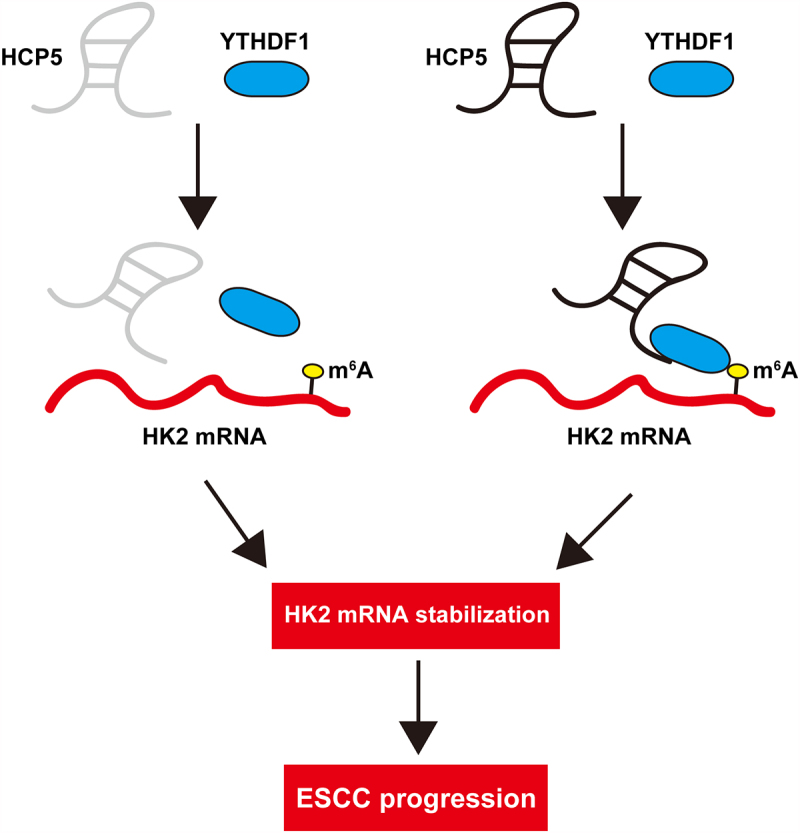
Introduction
Esophageal squamous cell carcinoma (ESCC) is one of the most invasive and fatal cancers in digestive tract tumors, ranks seventh in terms of incidence (604,000 new cases) and sixth in mortality overall (544,000 deaths) [1]. Eastern Asia has the highest incidence, mainly due to the high burden in China [2]. Although some advances have been made in ESCC treatment in the recent years, the 5-year overall survival in ESCC patients at stage III is only 10–15%, and the median survival in patients at stage IV is less than 1 year [3]. This is mainly due to the late onset of symptoms, the cost or inapplicability of endoscopy, insensitivity and non-specificity of biomarkers [4]. Therefore, there is an urgent need to find new sensitive and cost-effective biomarkers for diagnosis, treatment and prognosis of ESCC.
Long noncoding RNA (lncRNA) refers to a class of RNA with a length of more than 200 nucleotides, which is mainly transcribed by RNA polymerase II, III or V in eukaryotes [5]. Mature lncRNA can interact with a variety of molecules to form supramolecular structures, such as RNA/RNA, RNA/DNA, RNA/protein, DNA/RNA /protein or DNA/RNA/RNA complex [6]. Although a large number of studies have shown that lncRNAs play a role at the transcriptional, post-transcriptional and translation levels, the function of most of the lncRNAs remains unknown [7,8]. LncRNA is differentially expressed in some pathological processes, such as inflammation, anoxia and especially in cancer [9,10]. Emerging evidence suggests that the proliferation, invasion, differentiation, senescence and death of cancer cells are tightly controlled by lncRNA [11]. For instance, SPRY4-IT1 activated by NF-κB was a pro-metastatic lncRNA that enhanced TCEB1 mRNA decay via binding to STAU1 [12]. Linc00284 was significantly upregulated in human colorectal cancer, and promoted c-Met expression by sponging miR-27a [13]. Besides, LINC00857 was recently shown to be a tumor promoter in colorectal cancer by regulating the miR-150-5p /HMGB3 axis [14]. DHRS4-AS1 was a novel suppressor that inhibited hepatocellular carcinoma cell proliferation and induced apoptosis [15].
HLA complex P5 (HCP5), a hybrid HLA Class I endogenous retroviral gene that codes for a lncRNA, was involved in human disease progression [16]. Nevertheless, its role in ESCC is still unclear. In the present study, we aimed to explore the expression and clinical implication of HCP5 in human ESCC, further, we revealed the function and mechanism of HCP5 via conducting a series of experiments in vitro and in vivo. Our study provides new important clues for the pathogenesis of ESCC.
Materials and methods
ESCC sample and cell culture
We collected a total of 86 fresh frozen ESCC and corresponding nontumor normal tissues from The Second People’s Hospital of Huai’an, the clinicopathological data were statistically analyzed for the correlation with HCP5. We obtained informed consent from each patient, and this study was approved by the Medical Ethics Committee of The Second People’s Hospital of Huai’an (No. 20,190,301). The normal HET-1A cells and ESCC cells (EC109 and TE10) were purchased from National Collection of Authenticated Cell Cultures (Shanghai, China), and were cultured in Dulbecco’s Modified Eagle Medium (D-MEM) (GIBCO, USA) added with 10% fetal bovine serum (FBS) (GIBCO).
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted by Trizol solution (Invitrogen, USA) as per the manufacturer’s instructions. Then, cDNA was synthesized using SuperScript IV Reverse Transcription Reagent (Thermo Fisher Scientific, USA). ABsolute qPCR SYBR Green Mixes (Thermo Fisher Scientific) were used to amplify and quantify cDNA. Gene expression was calculated by using 2^-ddCt formula. GAPDH was used as reference control. The primer sequences are shown below:
HCP5: Forward: 5`-GCATTCTCAAGACTGCCACA-3`, Reverse: 5`-CCCTCTACTGGTGTCCCAAA-3`;
HK2: Forward: 5`-AACGCCTGCTACATGGAAGA-3`, Reverse: 5`-ACTCAGTGCGAATGTCGTTG-3`;
GAPDH: Forward: 5`-GTCAGTGGTGGACCTGACCT-3`, Reverse: 5`-ACCTGGTGCTCAGTGTAGCC-3`;
Detection of HCP5 location
RNA was isolated from the nucleus and cytoplasm using Cytoplasmic & Nuclear RNA Purification Kit (Norgen Biotek, Canada). GAPDH and U6 were used as cytoplasmic and nuclear references, respectively. Besides, HCP5 probe was designed (RiboBio, China) and Fluorescent In Situ Hybridization (FISH) Kit (RiboBio) was used to analyze the subcellular localization of HCP5, the results were observed by confocal microscopy.
Lentiviral vector
Three short hairpin (shRNA) sequences targeting HCP5 were synthesized and inserted into psi-LVRU6GP lentiviral vector (GeneCopoeia, USA). ESCC cells were infected with the lentivirus. The medium was replaced with fresh medium 24 h after infection. 1.8 μg/mL puromycin was used to screen stable HCP5-silenced cell clones, and the efficiency was detected by qRT-PCR.
Colony formation assay
Monolayer cultured ESCC cells in logarithmic growth phase were digested with 0.25% trypsin and beaten into single cells. 500 cells were plated onto 6-well plate, followed by two weeks of cultivation. Cells were washed by PBS and stained by crystal violet dye.
Cell invasion assay
Transwell chamber was used to test cell invasion assay as described previously [17], the upper chamber was coated with 50 mg/L Matrigel at a dilution of 1:8. The lower chamber was added with DMEM with 10% FBS. 5 × 105 ESCC cell suspension was added into the upper chamber, and cultured for 24 h. The invasive cells were stained with crystal violet dye.
Cell viability detection
1000 cells were plated onto 96-well plate, followed by incubation with CCK-8 reagent (Dojindo, Japan) for 1 h. The absorption value at 450 nm was tested using an automatic microplate spectrophotometer (SpectraMax, USA).
Glucose uptake, lactate production, ATP level and extracellular acidification rate (ECAR)
The glucose uptake rate, lactate production, ATP and ECAR levels were quantified using glucose assay kit (Sigma, USA), Lactate Assay kit (BioVision, USA), CellTiter-Glo Luminescent Cell Viability Assay (Promega, USA) and Seahorse XF 96 Extracellular Flux Analyzer, respectively, based on the supplier’s instructions.
Western blot
The protocol of western blot was shown before [18]. Briefly, total protein was extracted by RIPA buffer, and centrifuged at 16,000 × g for 20 min, the supernatant was taken for protein quantification. Then, 30 μg protein was used for sample loading, and transferred onto PVDF membrane, followed by incubation with HK2 antibody (ab104836, Abcam), YTHDF1 (ab220162, Abcam), METTL3 (ab195352, Abcam) and GAPDH (ab2735, Abcam) overnight at 4°C. The second day, the membrane was incubated with HRP-conjugated IgG at 37°C for 1 h. After washing with TBST, the blot was developed using ready-to-use ECL solution (Invitrogen).
Detection of mRNA half-life
ESCC cells were treated with 5 µg/ml actinomycin D (Sigma-Aldrich, MO, USA) for the indicated time (0 h, 2 h, 4 h and 8 h). Then, the total RNA was collected by Trizol reagent. The remaining mRNA level of HK2 was tested by qRT-PCR.
RNA pull-down and RNA immunoprecipitation (RIP) assays
The biotin-conjugated HCP5 probe was synthesized and incubated with ESCC cell lysates at 4°C for 5 h, then the streptavidin-labeled magnetic beads (Invitrogen) were added into the above lysates and incubated for 1 h. The enriched proteins were analyzed by western blot assay. Besides, RIP assay was carried out using Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, Germany) as per the standard protocol, followed by qRT-PCR analysis.
Methylated RNA immune-precipitation PCR (MeRIP-PCR)
The Magna MeRIP™ m6A Kit (Millipore, Germany) was used to assess m6A enrichment. In brief, the total RNA was extracted and fragmented, followed by incubation with protein A/G magnetic beads in IP buffer, containing anti-m6A antibody overnight at 4°C. The enrichment of HK2 mRNA was tested by qRT-PCR analysis.
Xenograft tumor model
Six-week-old male nude BALB/C mice were randomly divided into two groups, NC and sh-HCP5#1 groups. 2 × 106 EC109 cells were subcutaneously injected into nude mice and grown for 5 weeks. Then, the mice were sacrificed and tumor tissues were collected for qRT-PCR and western blot assays. This animal protocol was in line with the Animal Protection Ethics Committee of The Second People’s Hospital of Huai’an.
Statistical analysis
Comparison of continuous variables was performed by Student’s t-test. The correlations between HCP5 and clinical features of ESCC patients were tested by Chi-square test. All statistical results were two-side, P < 0.05 was considered as statistical significance.
Results
In this study, we first determined the overexpression of HCP5 in ESCC. Further, we tested the correlations between HCP5 and clinical features of ESCC patients, aiming to reveal its clinical value. Lastly, we conducted a series of functional assays and established in vivo model, uncovering the key oncogenic role of HCP5. HCP5 promoted ESCC cell Warburg effect by regulation of HK2 mRNA stability via binding m6A reader YTHDF1. Dysregulation of HCP5/HK2.YTHDF1 axis may be responsible for ESCC tumorigenesis and progression.
HCP5 is overexpressed in human ESCC
We first analyzed the expression of HCP5 in GEO database, as shown in Figure 1(a). HCP5 was significantly increased in ESCC tissues as compared to normal tissues. The qRT-PCR results in our cohort also exerted the upregulation of HCP5 in ESCC tissues (Figure 1(b)). High HCP5 was closely correlated with tumor size, TNM stage and lymph node metastasis (Table 1), but not with gender, age, differentiation smoking and drinking status (Table 1). Importantly, ESCC patients with high HCP5 had shorter survival time than patients with low HCP5 (Figure 1(c)), and this was also verified in KM database (http://kmplot.com/analysis) (Figure 1(d)). Cox regression model showed that HCP5 was an independent prognostic factor for ESCC overall survival (Table 2). Next, we tested the location of HCP5 using qRT-PCR and FISH assays, and found that HCP5 was mainly located in the cytoplasm in normal HET-1A and two ESCC cell lines (Figure 1(e, F)).
Figure 1.
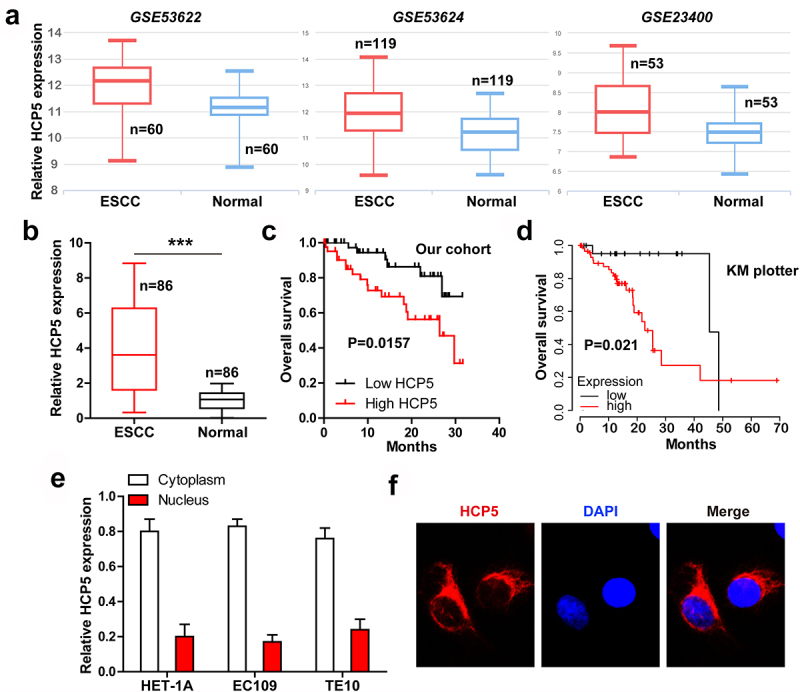
HCP5 is significantly upregulated in ESCC linking to poor prognosis. A. The expression of HCP5 in three GEO databases. B. qRT-PCR analysis of HCP5 level in 86 pairs of ESCC and normal tissues. C, D. The survival curve of ESCC patients based on HCP5 expression in our cohort and KM plotter database. E, F. qRT-PCR and FISH testing the location of HCP5 in normal and ESCC cells. ***P < 0.001.
Table 1.
Correlation between HCP5 expression and clinicopathological features in ESCC patients (n = 86)
| Parameters | All cases | HCP5 expressiona |
P value | |
|---|---|---|---|---|
| Low (n = 43) | High (n = 43) | |||
| Gender | ||||
| Male | 50 | 23 | 27 | 0.382 |
| Female | 36 | 20 | 16 | |
| Age (years) | ||||
| ≤ 65 | 44 | 25 | 19 | 0.196 |
| > 65 | 42 | 18 | 24 | |
| Tumor size | ||||
| ≤ 3 | 34 | 23 | 11 | 0.008 |
| > 3 | 52 | 20 | 32 | |
| Differentiation | ||||
| Well/moderate | 48 | 28 | 20 | 0.082 |
| Poor | 38 | 15 | 23 | |
| TNM stage | ||||
| I–II | 56 | 34 | 22 | 0.007 |
| III–IV | 30 | 9 | 21 | |
| Lymph node metastasis | ||||
| No | 53 | 35 | 18 | <0.001 |
| Yes | 33 | 8 | 25 | |
| Smoking | ||||
| No | 35 | 15 | 20 | 0.272 |
| Yes | 51 | 28 | 23 | |
| Drinking | ||||
| No | 47 | 25 | 22 | 0.516 |
| Yes | 39 | 18 | 21 | |
aThe median HCP level was used as the cuf-off value.
Table 2.
Uni- and multivariate analysis of prognostic predictors for overall survival in ESCC patients (n = 86)
| Variable |
Univariate analysis |
|
Multivariate analysis |
|
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gender | 1.325(0.416–2.325) | 0.527 | ||
| Age | 1.051(0.785–1.638) | 0.657 | ||
| Tumor size | 2.177 (0.865–2.985) | 0.065 | ||
| Differentiation | 1.687 (0.662–5.427) | 0.178 | ||
| TNM stage | 3.574(1.838–7.965) | 0.027 | 2.178 (1.275–6.074) | 0.048 |
| Smoking | 1.008 (0.375–1.736) | 0.578 | ||
| Drinking | 1.374 (0.837–2.813) | 0.758 | ||
| HCP5 expression | 3.863 (2.008–9.275) | <0.001 | 4.331 (1.896–9.774) | 0.006 |
Silencing of HCP5 represses ESCC cell malignant behavior
We designed three shRNA sequences against HCP5, and found that only HCP5#1 could effectively block HCP5 expression in both EC109 and TE10 cells (Figure 2(a)). Knockdown of HCP5 significantly reduced the number of ESCC cell clones (Figure 2(b)). Similarly, HCP5-silenced EC109 and TE10 cells were less aggressive than control cells (Figure 2(c)). Then, we conducted CCK-8 assay, and found that HCP5 knockdown resulted in a significant decrease in cell viability (Figure 2(d)).
Figure 2.
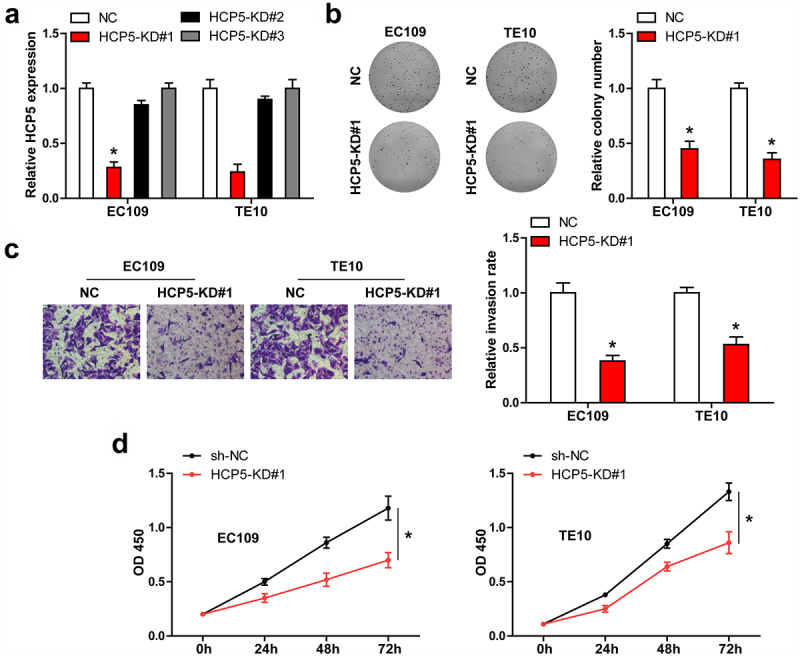
HCP5 is a promoter of ESCC. A. qRT-PCR verifying the knockdown of HCP5 by these three shRNAs. B-D. Colony formation, cell invasion and CCK-8 assays in EC109 and TE10 cells after HCP5 silencing. *P < 0.05.
HCP5 promotes Warburg effect of ESCC cells
Through analyzing the KEGG pathways of GSE53624 data, we found that HCP5 may be closely associated with metabolic pathways (Figure 3(a)). As expected, glucose uptake, lactate production and ATP level in EC109 and TE10 cells were significantly decreased after knockdown of HCP5 (Figure 3(b-d)). Likewise, ECAR assay showed that HCP5 silencing markedly promoted the glycolytic capacity of ESCC cells (Figure 3(e)).
Figure 3.
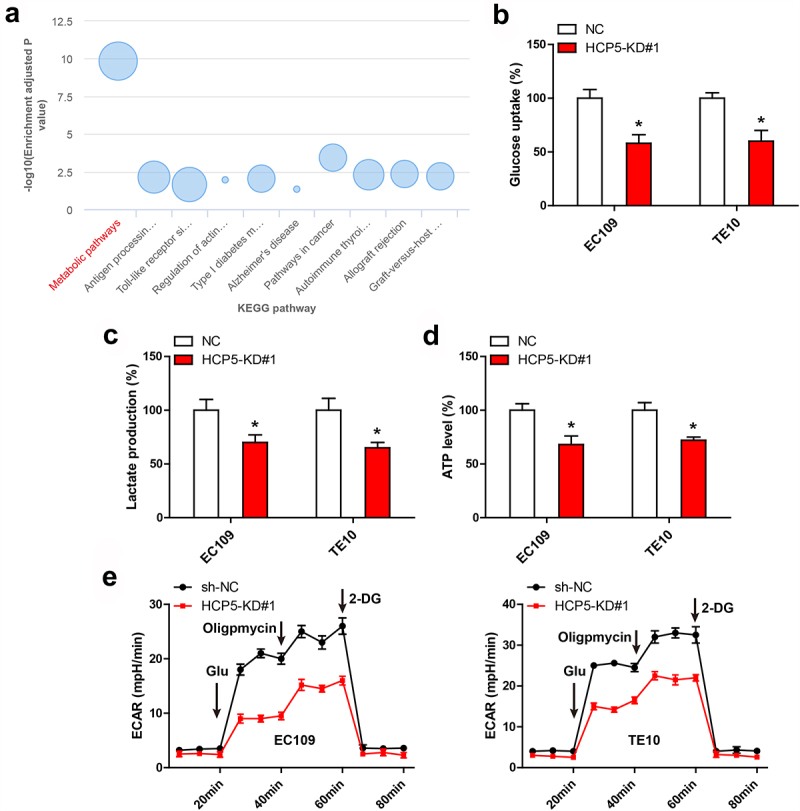
HCP5 affects aerobic glycolysis. A. KEGG pathway enrichment analysis based on HCP5 expression in GSE53624. B-E. Glucose uptake, lactate production, ATP level and ECAR analysis in EC109 and TE10 cells after HCP5 silencing. *P < 0.05.
HCP5 increases HK2 mRNA stability via YTHDF1
Next, we detected some key enzymes responsible for Warburg effect. As shown in Figure 4(a), only HK2 expression was notably altered in both EC109 and TE10 cells, and western blot assay confirmed the decrease of HK2 protein levels in HCP5-silenced cells (Figure 4(b)). To reveal how HCP5 affected HK2 mRNA level, we first analyzed the promoter activity of HK2, the luciferase reporter assay showed that HK2 promoter activity did not change after HCP5 knockdown (Figure 4(c)). But the half-time of HK2 mRNA was markedly shortened in HCP5-silenced cells (Figure 4(d)). A recent study suggests that HK2 mRNA stability is affected by m6A modification via METTL3/YTHDF1 axis [19], we then overexpressed METTL3 in ESCC cells, and found that the reduced HK2 level caused by HCP5 knockdown was entirely rescued by METTL3 (Figure 4(e)). Further, RNA pull-down assay showed that HCP5 could directly bind to YTHDF1 protein, but not METTL3 protein (Figure (4f)), which was verified by RIP assay (Figure 4(g)). Moreover, knockdown of HCP5 reduced the binding of YTHDF1 to HK2 mRNA (Figure 4(h)), accompanied by a decrease in m6A level (Figure 4(i)).
Figure 4.
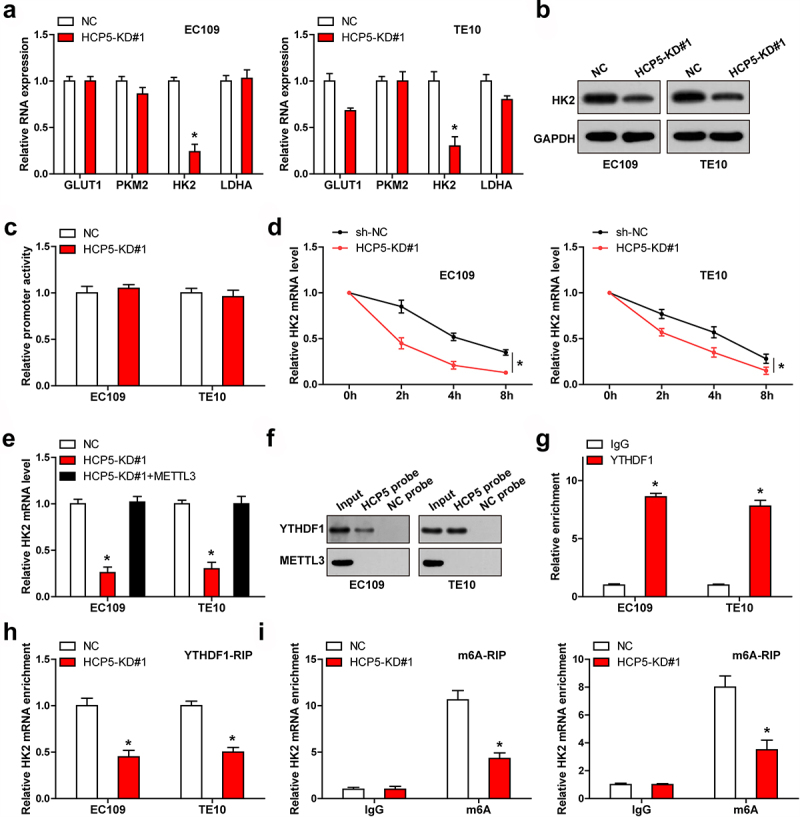
HCP5 regulates HK2 mRNA stability via m6A. A, B. qRT-PCR and western blot analyzing the indicated RNA and protein levels in ESCC cells with HCP5 knockdown. C. Luciferase reporter assay testing the effect of HCP5 knockdown on HK2 promoter. D. qRT-PCR testing the half-life of HK2 mRNA after HCP5 knockdown. E. qRT-PCR testing HK2 mRNA level in HCP5-silenced ESCC cells overexpressed METTL3. F. RNA pull-down assay testing the binding of HCP5 to METTL3 and YTHDF1. G, H. RIP assay testing the binding of YTHDF1 to HCP5 and HK2 mRNA. I. MeRIP assay testing the m6A level on HK2 mRNA. *P < 0.05.
Identification of HCP5/HK2 axis in vivo
Last, we subcutaneously injected EC109 cells with HCP5 knockdown into nude mice. The results showed that tumor volume and weight were significantly less in HCP5-depleted group than those in control group (Figure 5(a-c)). Consistently, HK2 mRNA and protein levels were both dramatically reduced in HCP5-depleted tissues in comparison to control tissues (Figure 5(d, e)).
Figure 5.
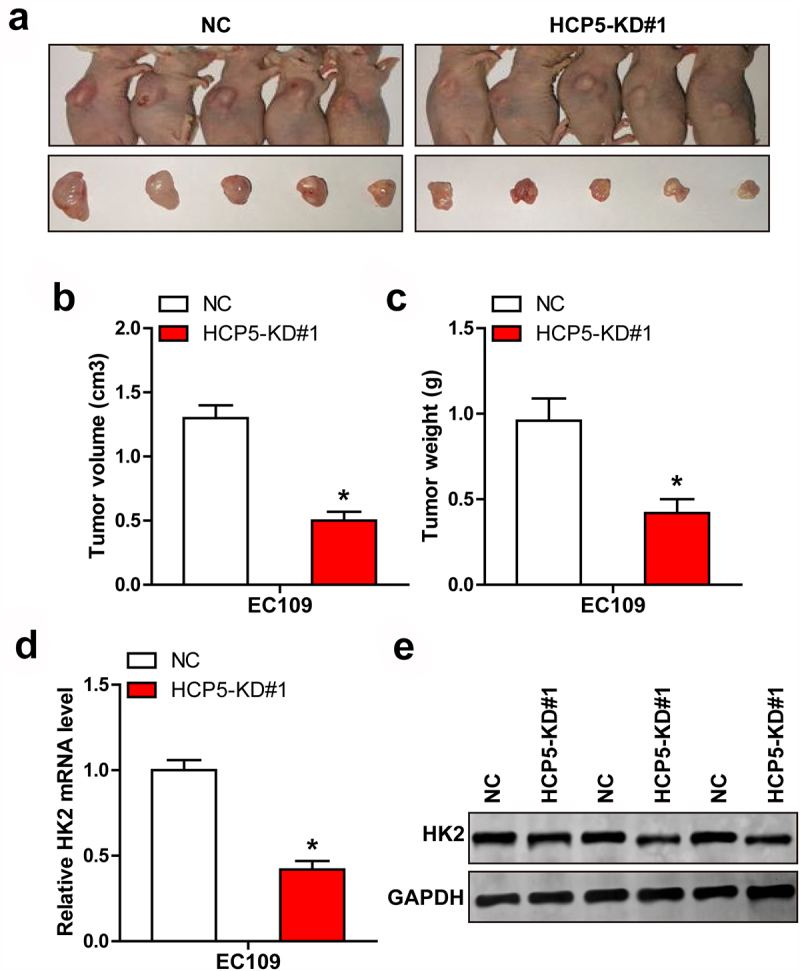
The effect of HCP5 in vivo. A-C. Tumor image, volume and weight in HCP5-silenced group. D, E. qRT-PCR and western blot testing HK2 mRNA and protein levels in control and HCP5-silenced tissues. *P < 0.05.
Discussion
In this study, we characterized the role of HCP5 in human ESCC, and found that it was significantly upregulated in ESCC, linking to aggressive behaviors and clinical features. The stable HCP5-silenced cell lines were established and found that HCP5 knockdown notably inhibited ESCC cell proliferation and invasion. Further investigation revealed that HCP5 promoted aerobic glycolysis via regulating HK2, in detail, HCP5 could bind to YTHDF1 and increase the interaction between YTHDF1 and m6A-modified HK2 mRNA, resulting in strengthening HK2 mRNA stability. Moreover, the nude mice model showed that obstruct of HCP5 delayed tumor growth by reducing HK2 level. Therefore, our study highlights the importance of HCP5 in ESCC and provides new evidence that lncRNA and protein cross-linking patterns are functional in human cells.
The role of lncRNA depends on its subcellular localization, in which cytoplasmic lncRNA plays an important role in a variety of molecular mechanisms, including mRNA stability and translation regulation, protein modification, as a precursor of miRNA or as a competitive endogenous RNA sponging miRNA [20]. The interaction mode of lncRNA and protein is critical for cancer initiation, development and progression [21]. For example, AC073352.1 was reported as a binding partner of YBX1, and increased YBX1 protein stability, promoting breast cancer metastasis and angiogenesis [22]. BGL3 was shown to be a stabilizer of PTEN protein via binding to PTEN and inhibited papillary thyroid carcinoma growth and metastasis [23]. ZFAS1 could bind to the SRSF3 protein directly and prevent degrading, thereby promoting osteosarcoma cell malignant behaviors [24]. Herein, by qRT-PCR and FISH assays, we found that HCP5 was mainly located in the cytoplasm. And further exploration revealed that HCP5 could physically interact with YTHDF1, a m6A ‘reader’, and enhance the recognition of YTHDF1 to m6A-modified HK2 mRNA caused by METTL3, a m6A ‘writer’. m6A is a widely present methylation modification in mRNA, which is dynamic and reversible, involving in m6A methyltransferase, demethylase and recognition protein [25]. m6A participates in the regulation of mRNA nuclear export, splicing, stability, translation and degradation [26]. YTHDF1, belongs to YT521-B homology (YTH) domain family, is reported as an mRNA or translation regulator, some targets such as Myc [27], HINT2 [28], EIF3C [29] and TRAF6 [30]. Recently, HK2, the first and rate-limiting enzyme of the glycolysis pathway, is a well-known driver of aerobic glycolysis in human cancer [31]. It has been shown as target of YTHDF1 [19], YTHDF1 recognized the m6A site on HK2 mRNA 3’-UTR, and prevented it from decay. In this study, we found that HCP5 functioned as a scaffold for YTHDF1 binding to HK2 mRNA 3’-UTR, knockdown of HCP5 significantly reduced their interaction, resulting in increased HK2 mRNA stability. Hence, our results reveal a previously unappreciated lncRNA linking m6A modification and mRNA fate; further research is needed to clarify the specific binding regions or sites between them.
Conclusion
In sum, our findings for the first time suggest that HCP5 is an oncogenic lncRNA in human ESCC, implying a promising prognostic indicator and druggable target of ESCC.
Supplementary Material
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Highlights
HCP5 is overexpressed in human ESCC, which is correlated with poor prognosis.
Depletion of HCP5 inhibits ESCC cell malignant behavior.
HCP5 promotes Warburg effect of ESCC cells.
HCP5 increases HK2 mRNA stability via m6A reader YTHDF1.
Knockdown of HCP5 retards tumor growth in vivo.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
References
- [1].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer. J Clin. 2021. May;71(3):209–249 . [DOI] [PubMed] [Google Scholar]
- [2].Abnet CC, Arnold M, Wei WQ.. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Domper AM, Ferrandez AA, Lanas AA. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang C, Wang J, Chen Z, et al. Immunohistochemical prognostic markers of esophageal squamous cell carcinoma: a systematic review. Chin J Cancer. 2017;36(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bohmdorfer G, Sethuraman S, Rowley MJ, et al. Long non-coding RNA produced by RNA polymerase V determines boundaries of heterochromatin. Elife. 2016;5. DOI: 10.7554/eLife.19092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell. 2018;172(3):393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ali T, Grote P. Beyond the RNA-dependent function of LncRNA genes. Elife. 2020;9. DOI: 10.7554/eLife.60583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Statello L, Guo C-J, Chen -L-L, et al. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fang Y, Fullwood MJ. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genomics Proteomics Bioinformatics. 2016;14(1):42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang C, Ren X, Zhang W, et al. Prognostic and clinical significance of long non-coding RNA SNHG12 expression in various cancers. Bioengineered. 2020;11(1):1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhao L, Jiang L, Zhang M, et al. NF-kappaB-activated SPRY4-IT1 promotes cancer cell metastasis by downregulating TCEB1 mRNA via Staufen1-mediated mRNA decay. Oncogene. 2021;40(30):4919–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].You J, Li J, Ke C, et al. Oncogenic long intervening noncoding RNA Linc00284 promotes c-Met expression by sponging miR-27a in colorectal cancer. Oncogene. 2021;40(24):4151–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhou D, He S, Zhang D, et al. LINC00857 promotes colorectal cancer progression by sponging miR-150-5p and upregulating HMGB3 (high mobility group box 3) expression. Bioengineered. 2021;12(2):12107–12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhou Y, Li K, Zou X, et al. LncRNA DHRS4-AS1 ameliorates hepatocellular carcinoma by suppressing proliferation and promoting apoptosis via miR-522-3p/SOCS5 axis. Bioengineered. 2021;12(2):10862–10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kulski JK. Long noncoding RNA HCP5, a Hybrid HLA class I endogenous retroviral gene: structure, expression, and disease associations. Cells-Basel. 2019;8(5):480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang Q, Wang Z, Cheng X, et al. lncRNA DANCR promotes the migration an invasion and of trophoblast cells through microRNA-214-5p in preeclampsia. Bioengineered. 2021;12(2):9424–9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huang L, Dai G. Long non-coding RNA DCST1-AS1/hsa-miR-582-5p/HMGB1 axis regulates colorectal cancer progression. Bioengineered. 2022;13(1):12–26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [19].Wang Q, Guo X, Li L, et al. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020;11(10):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Noh JH, Kim KM, McClusky WG, et al. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip Rev RNA. 2018;9(3):e1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Philip M, Chen T, Tyagi S. A survey of current resources to study lncRNA-Protein Interactions. Noncoding RNA. 2021;7(2). DOI: 10.3390/ncrna7020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kong X, Li J, Li Y, et al. A novel long non-coding RNA AC073352.1 promotes metastasis and angiogenesis via interacting with YBX1 in breast cancer. Cell Death Dis. 2021;12(7):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhao M, Yang F, Sang C, et al. BGL3 inhibits papillary thyroid carcinoma progression via regulating PTEN stability. J Endocrinol Invest. 2021;44(10):2165–2174. [DOI] [PubMed] [Google Scholar]
- [24].Zhang Y, Xu W, Wang Y, et al. Oncogenic lncRNA ZNFX1 antisense RNA 1 promotes osteosarcoma cells proliferation and metastasis by stabilizing serine and argininerich splicing factor 3. Bioengineered. 2022;13(3):5962–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lan Q, Liu PY, Bell JL, et al. The emerging roles of RNA m(6)A methylation and demethylation as critical regulators of tumorigenesis, drug sensitivity, and resistance. Cancer Res. 2021;81(13):3431–3440. [DOI] [PubMed] [Google Scholar]
- [26].Zhao Y, Chen Y, Jin M, et al. The crosstalk between m(6)A RNA methylation and other epigenetic regulators: a novel perspective in epigenetic remodeling. Theranostics. 2021;11(9):4549–4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nishizawa Y, Konno M, Asai A, et al. Oncogene c-Myc promotes epitranscriptome m(6)A reader YTHDF1 expression in colorectal cancer. Oncotarget. 2018;9(7):7476–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jia R, Chai P, Wang S, et al. m(6)A modification suppresses ocular melanoma through modulating HINT2 mRNA translation. Mol Cancer. 2019;18(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu T, Wei Q, Jin J, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48(7):3816–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zong X, Xiao X, Shen B, et al. The N6-methyladenosine RNA-binding protein YTHDF1 modulates the translation of TRAF6 to mediate the intestinal immune response. Nucleic Acids Res. 2021;49(10):5537–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ciscato F, Ferrone L, Masgras I, et al. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int J Mol Sci. 2021;22(9):4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


