Abstract
Hypertension is one of the most prevalent diseases that leads to end organ damage especially affecting the heart, kidney, brain, and eyes. Numerous studies have evaluated the association between hypertension and impaired sexual health, in both men and women. The detrimental effects of hypertension in men includes erectile dysfunction, decrease in semen volume, sperm count and motility, and abnormal sperm morphology. Similarly, hypertensive females exhibit decreased vaginal lubrication, reduced orgasm, and several complications in pregnancy leading to fetal and maternal morbidity and mortality. The adverse effect of hypertension on male and female fertility is attributed to hormonal imbalance and changes in the gonadal vasculature. However, mechanistic studies investigating the impact of hypertension on gonads in more detail on a molecular basis remain scarce. Hence, the aim of the current review is to address and summarize the effects of hypertension on reproductive health, and highlight the importance of research on the effects of hypertension on gonadal inflammation and lymphatics.
Introduction
Reproductive health is vital for human flourishing and survival of the species, and includes the sexual wellness of both men and women along with maternal and infant health. A male’s ability to produce sperm in the testes coupled with a female’s release of matured eggs from the ovaries are necessary for successful reproduction. Human reproduction is affected by lifestyle, genetics, and environmental factors. It is also affected by physiological abnormalities like obesity, dyslipidemia, insulin resistance, and hypertension [1,2]. Hypertension, present in almost half of the population, is a well-known factor increasing cardiovascular disease risk by altering the macro- and micro-vasculature in target organs like the brain, heart, and kidney. However, hypertension also vastly affects reproductive health in both men and women [3–7]. While the roles that the primary male gonadal hormone testosterone (T) and the primary female gonadal hormone estrogen play in cardiovascular disease have been studied extensively, the mechanisms by which hypertension affects gonads are less known. In this review, we discuss how hypertension impacts fertility with a focus on the effects on male and female gonadal function. How hypertension influences gonadal inflammation and what roles lymphatic vessels might play in these tissues are highlighted to bring attention to novel findings that impact fertility.
Hypertension and reproductive health
The major public health epidemic of hypertension can result in target-organ injury and is associated with inflammation and sexual dysfunction in both men and women [3–7]. For example, the prevalence of erectile dysfunction (ED) is higher in hypertensive men than normotensive men [8,9]. Similarly, hypertensive women exhibit decreased vaginal lubrication, less frequent orgasm, and more frequent genital pain than normotensive women [4,10]. It is known that hypertension affects the reproductive ability of both men and women (Figure 1), but the mechanisms by which hypertension impacts reproductive health and function remain to be fully unraveled.
Figure 1. Effect of hypertension on gonadal function.
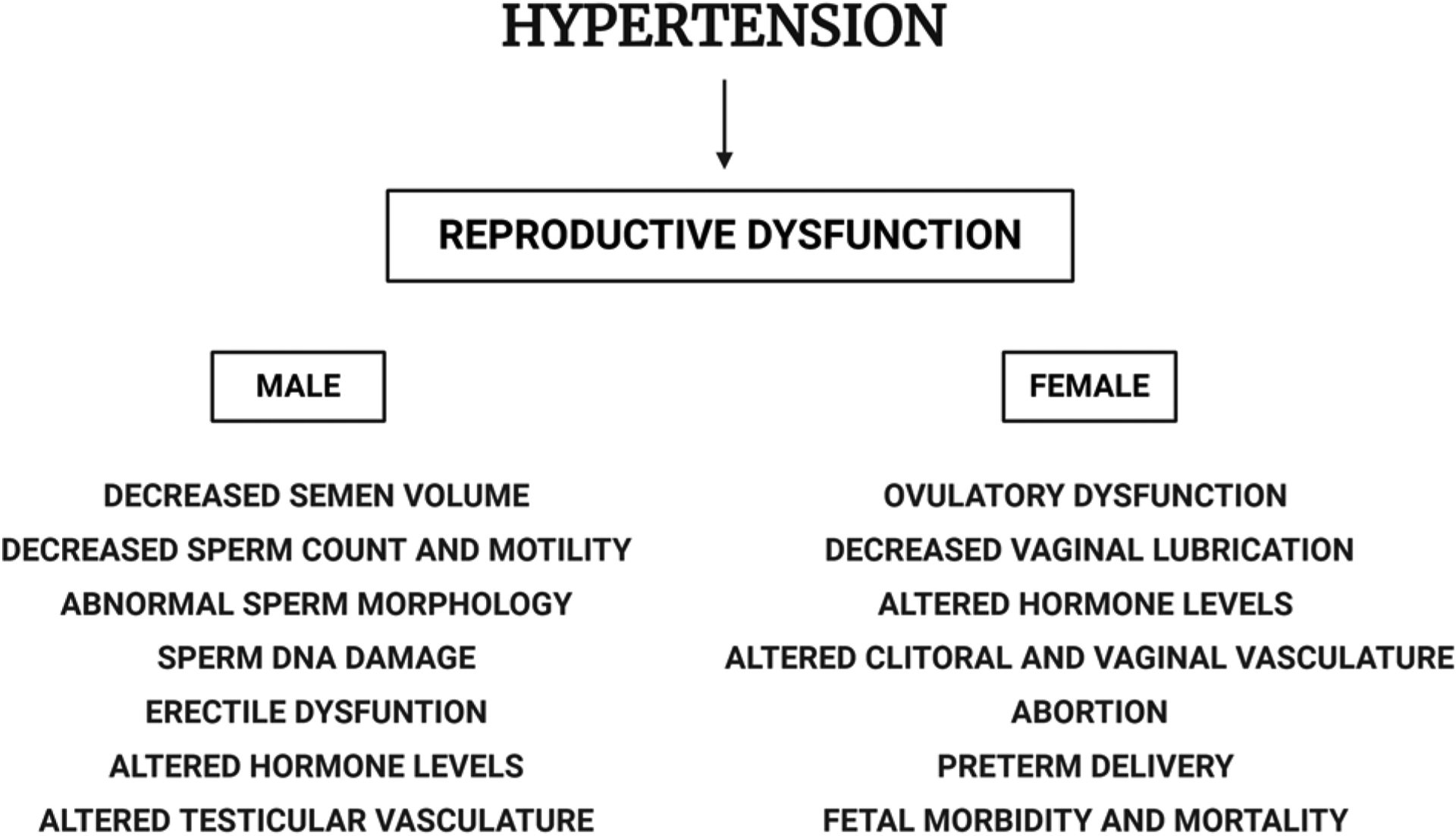
Hypertension affects reproductive health by altering hormone levels and reproductive tissue vasculature in both males and females, thereby disturbing spermatogenesis and oogenesis, respectively. Created with BioRender.com.
Hypertension and male fertility
Hypertension and spermatogenesis
There are several studies on hypertensive men that established an inverse relationship between blood pressure and total serum T [11,12], free T [13–15], and the sex hormone-binding globulin15. Nevertheless, only a few studies are available to date that relate hypertension and male reproductive function. A study involving 110 newly diagnosed, never treated hypertensive men and 110 normotensive men attributed a reduction in sexual activity reported in hypertensive men to a decrease in T levels [12]. Using a rat model exhibiting renovascular hypertension, diminished sexual behavior and impaired spermatogenesis were attributed to attenuated levels of prolactin, T, and follicle stimulating hormone [16]. Another group evaluated the quality of ejaculated spermatozoa in a group of 25 normotensive and 25 hypertensive subjects by measuring (i) the levels of clusterin, a glycoprotein associated with abnormal sperm morphology, and (ii) sperm DNA damage. There was a significant increase in clusterin levels and sperm DNA damage in hypertensive subjects that established a strong relationship between hypertension and poor sperm quality [17]. Guo et al. [18] explored the association between hypertension and semen quality utilizing collected data from the Stanford Reproductive Endocrinology and Infertility Center. The study cohort included male patients who visited the infertility center between 1994 and 2011 who had been diagnosed with hypertension prior to or within one year after semen analysis. Abnormal semen parameters were characterized based on the World Health Organization Manual on Semen Analyses [19]. Hypertensive males were found to have decreased semen volume, sperm motility, total sperm count, and motile sperm count with respect to normotensive males [18,20]. Eisenberg et al. [21] also revealed higher rates of semen abnormalities, including reduced sperm count and motility, in hypertensive men. These studies reinforce a strong association between hypertension and impaired semen and sperm quality. As correlations, however, these studies lack thorough investigation and a direct end-organ effect of hypertension on the testes.
Hypertension and erectile dysfunction
There are several studies that link hypertension with reproductive dysfunction based primarily on hormonal imbalance and/or ED. It is well established that hypertensive men have an increased risk to develop ED [22–24]. ED-associated erectile tissue morphological changes including smooth muscle tissue hypertrophy and stenosis in the corpus cavernosum correlate positively with hypertension [25–27]. One of the plausible mechanisms behind the morphological changes in erectile tissue may be the oxidative stress caused by chronic hypertension, leading to endothelial dysfunction resulting in inefficient dilation of the arteries of the corpus cavernosum [28]. Spontaneously hypertensive rats that are stroke prone (SHRSP) demonstrated a significant reduction in erectile response to ganglionic stimulation. However, this improved after a single intracavernous injection of drug Y-27632 [29]. This drug binds to the ATP-binding site on Rho-kinase, preventing the phosphorylation and subsequent inactivation of myosin light chain (MLC) phosphatase, thereby promoting cavernosal smooth muscle relaxation leading to erection [29–34]. This demonstrates the RhoA/Rho-kinase pathway as a critical regulator controlling the erectile process by regulating the phosphorylation of MLC phosphatase. A similar trend was observed using the mineralocorticoid-salt model of hypertension, where treatment with Y-27632 improved the erectile response [29]. Nitric oxide (NO) induces vasodilation in cavernosal tissues leading to erection by inhibiting RhoA activity [32–34]. Sildenafil, a commonly prescribed ED drug, acts by promoting NO-mediated activation of guanylyl cyclase and eventual accumulation of cGMP leading to relaxation of the corpus cavernosum and subsequent erection [35,36]. This drug slows down the process of apoptosis in corpora cavernosa and ameliorate spermatogenesis, eventually improving the microcirculation in men with ED [36]. Few studies have reported that antihypertensive drugs (e.g. β-blockers and diuretics) themselves are associated with ED [37], while others had controversial reports [38,39]. Despite these sometimes-conflicting reports, evidence suggests a relationship between hypertension and male fertility that warrants further mechanistic investigations.
Hypertension and testes
Despite strong evidence for the association between hypertension, sperm quality, and male fertility [2,9,12,16–18,20,21], the target organ testes have not been studied extensively. Rats made hypertensive using a deoxycorticosterone acetate treatment protocol exhibited reduced testis weight in addition to a reduction in sexual behavior, seminal vesicle weight, and T levels when compared with the uninephrectomized controls [40,41]. SHRs demonstrated testicular hypertrophy and ED [42]. A study in the SHRSP model reported the hypertensive changes in intra-testicular arterioles resulted in the impairment of Sertoli cell (SC) function with subsequent loss of spermatogenic cells [43]. Another study using the same model confirmed the marked alterations in intra-testicular arteries including increased intimal thickness, fibrinoid necrosis, and hyalinization [44]. These alterations in testicular arterial vasculature induce ischemia and a significant reduction in the nutritional supply to the organ [45–47]. This study also identified an impairment in spermatogenesis likely due to atrophic seminiferous tubules with reduced numbers of spermatids in hypertensive rats [44]. Due to the presence of the blood–testis barrier (BTB), germ cells (GCs) depend on SCs for nutrients [48]. Hence, a reduced blood supply to SCs in testes resulted in impaired spermatogenesis. Akagashi et al. [45] measured a significant reduction in the concentration of transferrin in SHRSP rats that coincides with impaired spermatogenesis. Treatment with manidipine (a long-lasting calcium channel blocker, CCB) to improve blood flow led to improvement in spermatogenesis. Atanassova et al. [49], in support of the previous studies, demonstrated deleterious testicular changes with GC depletion in seminiferous tubules of SHRs. Elevations in the local expression of testicular angiotensin-converting enzyme (ACE) in SHRs occurred earlier in the stage of spermiogenesis and correlated with decreased fertility when compared with age-matched normotensive rats. Increased ACE activity is known to be involved in vascular remodeling. Therefore, the compromise in fertility observed in SHR may be attributed in part to the dysfunctional intra-testicular vasculature [49].
In a study with SHRs, enalapril (an ACE inhibitor) treatment restored the morphological changes in the testes and normalized spermatozoid production by preventing vascular remodeling in the testes [50]. A recent study in hypertensive rats identified increased testicular weight and altered testicular morphology resulting from arterial alterations (Figure 2) and impaired testicular vasomotion [51]. These rats had a reduction in sperm concentration and DNA integrity, and increased percentages of sperm with dysfunctional mitochondria, intracellular superoxide anion activity, and abnormal morphology. Histological studies showed perturbed spermatogenesis with immature GCs in the tubular lumen and tubular necrosis. The hypertensive rats also displayed increased arteriolar adventitia in the testicular microvasculature. The authors also reported elevated hypoxia-inducible factor-1α gene expression in the testes, suggesting an inadequate oxygen supply to the tissue in hypertensive animals. Increased levels of vascular endothelial growth factor (VEGF) were also observed in the testes of hypertensive animals due to testicular hypoxia-induced protein expression [51]. In total, these studies help us to have a better understanding of the mechanisms implicated under hypertensive conditions with respect to male fertility.
Figure 2. Hematoxylin and eosin staining of testis from normal and spontaneously hypertensive rats.
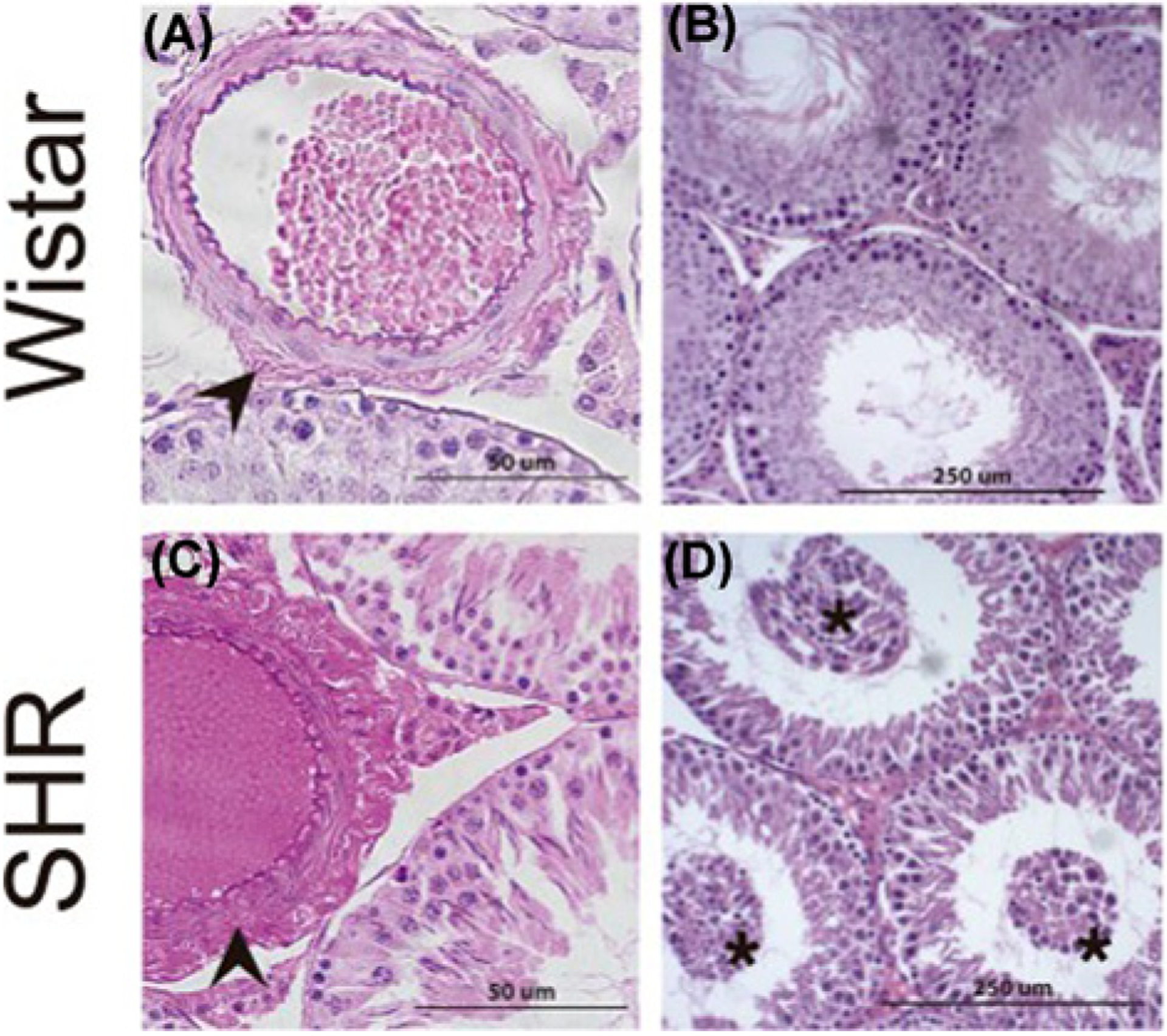
(A) Normal histoarchitecture of testes from 26-week-old Wistar rats. Arrowheads indicate arteriolar adventitia. (B) Testes from 26-week-old Wistar rats showing intact seminiferous tubules and interstitial compartment. (C) A significant increase in adventitial layers in testicular arterioles (arrowheads) independent of their diameter in spontaneously hypertensive rats (SHR). (D) Testes from SHR showing immature germ cells in the tubular lumen (asterisk). The images are reproduced from the reference [51] with permission.
Hypertension and female fertility
Hypertension and sexual health
The prevalence of hypertension in women of reproductive age is relatively less compared with age-matched males [52,53], but should not be ignored in women as it is one of the most prevalent risk factors for cardiovascular disease and can also greatly complicate pregnancy and offspring health. Women with hypertension may show sexual dysfunction due to alterations in clitoral and vaginal vasculature, reduced blood flow in the pelvic region, and thinning of the vaginal wall and clitoral smooth muscles resulting in vaginal dryness [54,55]. Subsequent complications include pain during sexual intercourse, lack of orgasm, adverse effects of the arousal phase, and sexual reluctance in the woman have also been reported [54]. Hypertension can lead to fibrosis of the clitoris and the vaginal wall due to lower NO levels and reduced blood flow in the pelvic region [56,57]. A reduction in blood flow with inefficient vasocongestion during sexual arousal results in decreased lubrication and finally dyspareunia in hypertensive women [58,59].
Hypertension and pregnancy
It is reported that out of the estimated 4 million pregnancies in the United States each year, around 5% of women face complications due to hypertension [60], leading to maternal and fetal mortality [60–62]. Women with chronic hypertension are highly susceptible to preeclampsia [63–65]. In hypertensive women, the chances of placental abruption [63–65], maternal stroke [66–68], renal failure, pulmonary edema, and death [67,68] are tremendously increased. The adverse effects of chronic hypertension on fetal growth can lead to perinatal and maternal morbidity and mortality through premature delivery; intrauterine growth restriction [64,65]; hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome; perinatal death; and maternal convulsion or eclampsia [64,67,69–71]. A retrospective study conducted using medical records at Mettu Karl Referral Hospital, Mettu, Ethopia, for the period January 1, 2010 to December 1, 2013 reported increased rates of fetal death, low birth weight, low APGAR score, abortion, preterm delivery, and HELLP syndrome in pregnant women with hypertensive disorders [72]. Khosravi et al. [73] reported a higher prevalence of hypertensive disorders among pregnant women who were admitted to a tertiary center in Tehran for delivery. Zhou et al. [74] reported higher risk of pregnancy loss in hypertensive women among 2940 women attempting pregnancy in Anhui, China. Several clinical studies have associated both preconception and early pregnancy blood pressures with risk of pregnancy loss [71,72,74,75]. While clearly linked, the actual underlying mechanisms of sexual dysfunction and pregnancy loss in hypertensive women remains obscure.
Hypertension and ovaries
Possibly due to the lower prevalence of hypertension in young reproductive women than their male counterparts, studies on the effects of hypertension on the ovarian vasculature and folliculogenesis have been neglected. To date, there are only a few studies that have specifically focused on the mechanisms by which hypertension affects female fertility. Studies on female SHRs demonstrated an association between hypertension and sexual dysfunction with reduced ovulation and morphological changes in the clitoris [76]. In female rats with renovascular hypertension, there was a delay in re-establishing estrous cyclicity. Resumed hypertensive rats demonstrated a significant decrease in lordosis quotient (a measure of sexual posturing) and oocyte number, demonstrating a reduction in sexual behavior and ovulation, respectively [77]. Although many studies have identified a higher prevalence of sexual dysfunction in hypertensive women when compared to normotensive women [78–80], further studies are needed to identify the exact pathophysiological mechanisms underlying the adverse effects on reproduction. With the profound effects that hypertension has on the male testis vasculature, it seems likely that the ovaries may demonstrate similar changes, but this area of research remains unexplored.
Antihypertensive drugs and reproduction
The antihypertensive drugs are mainly classified into five categories namely β-blockers, CCBs, ACE inhibitors, angiotensin II receptor blockers, and diuretics [18]. The effect of antihypertensive drugs on sexual function has always remained a topic of debate. CCBs have been reported to attenuate hormone levels viz., T, luteinizing hormone, and follicular stimulating hormone, leading to impaired spermatogenesis and sperm parameters [81,82]. Many in vitro studies have demonstrated the negative impact of CCBs on reproductive function by hampering spermatozoa–oocyte interaction and the fertilizing capability of sperm [83–86]. These effects are obvious since it is well known that Ca2+ is crucial for spermatogenesis, sperm motility, capacitation, acrosome reaction, and fertilization [83–92]. Clonidine, a selective agonist of α2-adrenoceptors, induced desensitization of functional α2-adrenoceptors and elicited contractions of the rat testicular capsule affecting the proper transport of spermatozoa out of the testes [93]. It was reported that rats treated with lisinopril, an ACE inhibitor, exhibit decreased sperm density and motility along with a decrease in sperm acrosomal reaction [94]. β-Blockers hamper the relaxation of smooth muscles in the corpora cavernosum by blocking the β2 adrenergic receptors and cause a reduction in plasma T levels [38,95–100]. In contrast with these findings, a few studies elucidated beneficial aspects of these drugs on sperm parameters and sexual activity [50,101,102].
ACE inhibitors or angiotensin II receptor blockers are teratogenic and have been shown to increase the likelihood of congenital anomalies; hence they are generally not recommended to women who are planning to get pregnant [103–106]. Methyldopa has been considered safe even in the early trimester, with no potential harm to the growth and development of the fetus [107,108]. β-Blockers have been implicated in intrauterine growth restriction and premature birth [109]. CCBs such as nifedipine have been reported to reduce blood pressure in pregnant women without compromising fetal health and prevent premature labor [110]. Further extensive research is required in this field to choose the appropriate treatment and improve the impact of hypertension treatment on the patient’s reproductive health. Since it is difficult to delineate the effect of hypertension and antihypertensive drugs on reproductive function, a better understanding of the direct effects of hypertension on reproductive organs can pave the way for alternative treatments.
It is well known that hypertension promotes inflammation, immune cell trafficking, and inflammation-associated lymphangiogenesis in several organs like the skin, intestine, heart, kidney, and airway tract [111–113], but the impact of hypertension on gonadal lymphatics remains obscure. In the following sections, we will focus on the development of gonadal lymphatic vasculature and inflammation-associated lymphangiogenesis in gonads under several pathological conditions to emphasize the need for extensive research in this area.
Gonads and lymphatics
The lymphatic system plays a crucial role in the maintenance of tissue fluid homeostasis by recirculating the interstitial fluid, formed by blood vessel extravasation, and in the transportation of antigen-presenting cells and lymphocytes from tissues to lymph nodes [114–116]. These roles are particularly important during inflammation, when blood vascular permeability is enhanced and immune cell numbers and activation increase in the tissue.
The lymphatic network in testes originates 9.5 days post coitum in the mouse and its distribution varies across mammalian species [117–119]. The lymphatic network arises on the surface of the testes spreading across the tunica albuginea in both large animals and rodents [118–121]. Using immunolabeling with lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) antibodies or Prox-1-EGFP transgenic lymphatic reporter mice to identify lymphatics, it is reported that testicular lymphatics are found only on the surface and do not penetrate into the interstitium (Figures 3 and 4) [118,119,122]. Similarly, lymphatic vessels were also absent in the interstitium in rat testes [123]. In contrast, lymphatic capillaries were detectable within the testicular interstitium of large animals; these vessels drain into the tunica albuginea via fibrous septa and finally into larger collecting lymphatic vessels in the mediastinum testis [117,120,121,124]. In large animals, these large lymphatics carrying testicular lymph drain into the latero- or para-aortic lymph node groups (Figure 5) [122,125,126]. In rats, collecting lymphatics from three regions (superior, middle, inferior) combine to form a large testicular lymphatic trunk [127]. The testicular lymphatics in rats may drain to either the renal or lumbar lymph nodes, but in certain cases bypass lymph nodes and lead directly to the thoracic duct [128]. Interestingly, there are distinct routes of lymphatic drainage of testes from those of the scrotum (that drains to the inguinal lymph node) (Figure 5) [129]. It was concluded in studies using rams that testicular lymphatics do not play an important role in the return of T into the blood due to both the lower concentration of the hormone in lymph and lower flow rate of lymph when compared to venous blood [130]. Nevertheless, studies on pigs and horses have demonstrated a comparatively higher concentration of conjugated steroids like estrone sulfate and dehydroepiandrosterone sulfate in testicular lymph than in the testicular venous blood accounting for the contribution in returning these to the circulation [131,132]. Hence, the role of testicular lymphatics in hormone transport and distribution may be species- and molecule-dependent [122].
Figure 3. Distribution of lymphatic vasculature in the prenatal testes of Prox1-EGFP reporter mice.
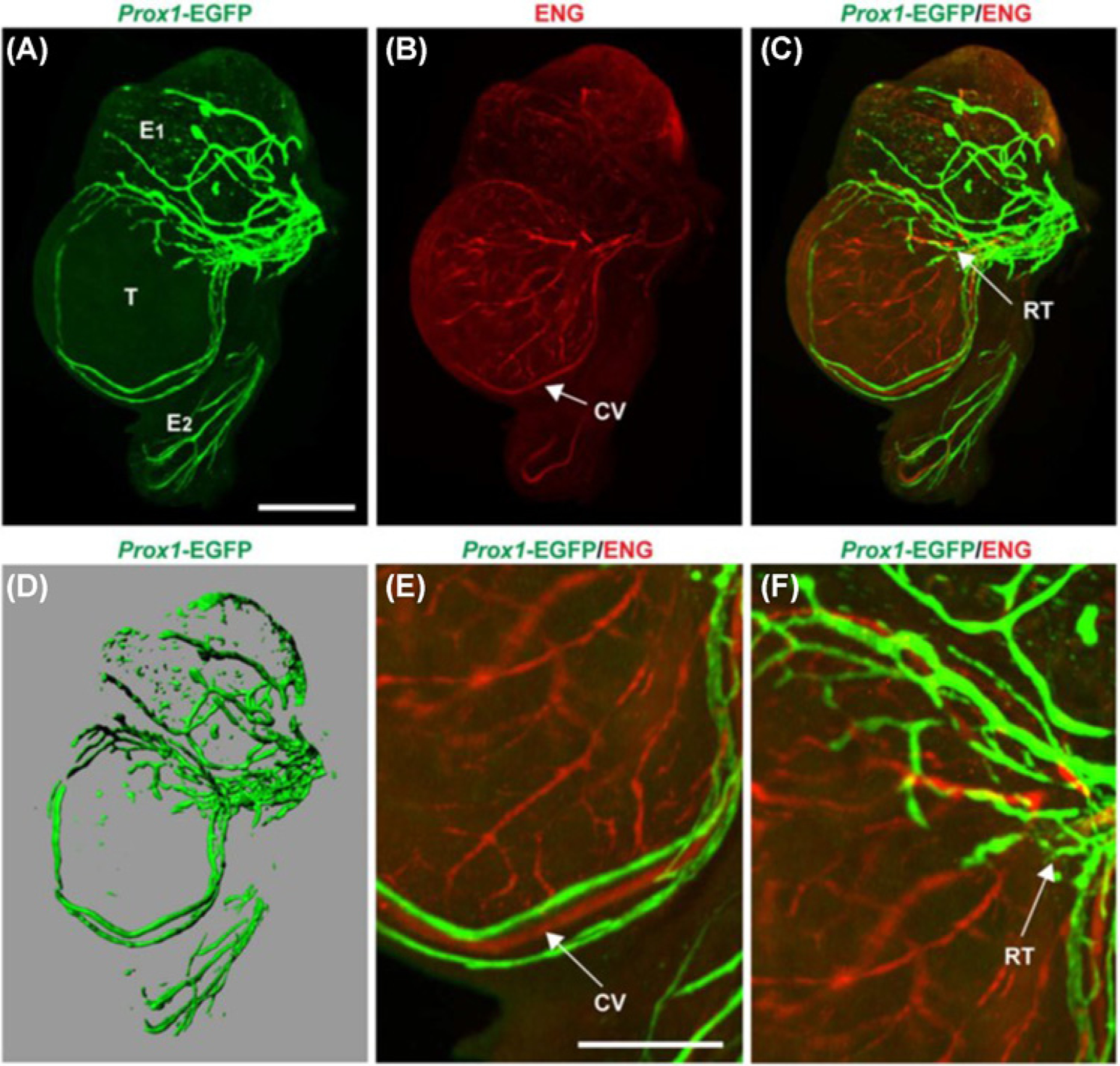
(A) During embryonic development (E17.5), EGFP-positive lymphatic vessels begin to grow from the spermatic cord across the surface of the testis (T) but does not cross the testis cap. Lymphatics are also established on the epididymal head (E1) and tail (E2). (B) Endoglin (ENG) staining was done to view the distribution of blood vessels in fetal testes. (C) The lymphatics (Prox 1-EGFP-positive) and blood vessels (ENG labelled) did not colocalize. Regions in yellow represent overlapping of both green and red signals from different planes. (D) A 3-D representative image of the lymphatics in fetal testes. (E) Magnified image of fetal testes illustrating the lymphatic vessels running alongside the coelomic vessel (CV). (F) Magnified view of the rete testes (RT). Scale bar for panels (A–D) = 500 μm; (E and F) = 250 μm. The images are reproduced from the reference [119] with permission.
Figure 4. Distribution of the lymphatic vasculature in the adult testes of Prox1-EGFP reporter mice.
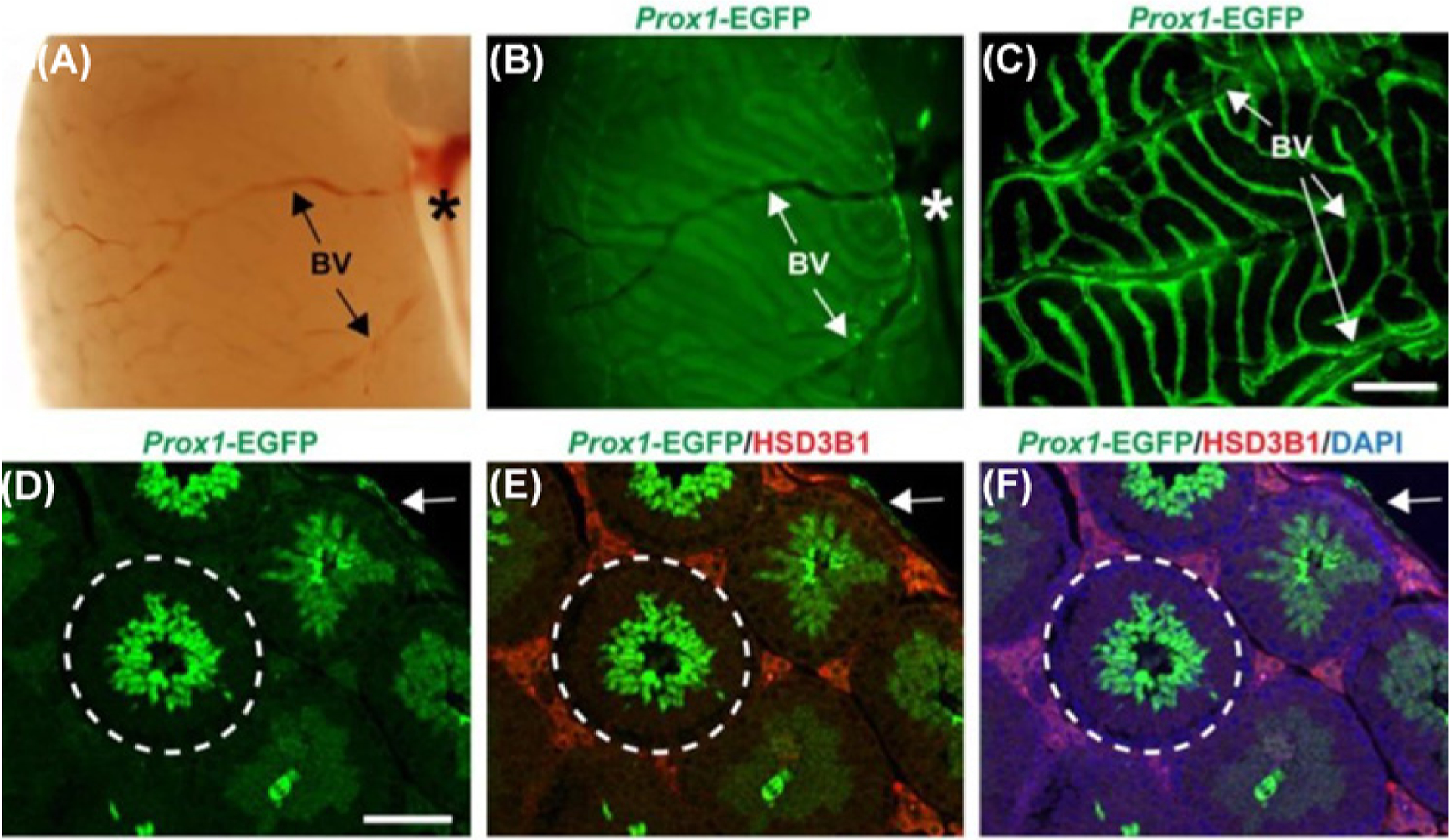
(A) A brightfield image of the surface of adult mouse testes exhibiting blood vessels (BV) and the spermatic cord (asterisk). (B) Prox 1-EGFP-positive signals were observed from the same surface view. (C) A confocal image showing the Prox1-EGFP-positive lymphatic network across the tunica albuginea originating from the spermatic cord. (D–F) Prox 1-EGFP positive lymphatics were confined within the tunica albuginea (arrow) but was not found inside the seminiferous tubule (encircled) when co-stained with the Leydig cell marker HSD3B1 and counterstained with DAPI. Within the seminiferous tubules, EGFP expression was observed in spermatids that are in close proximity to the lumen. Scale bar for panel (C) = 600 mm, (D) = 100 mm. The images are reproduced from the reference [119] with permission.
Figure 5. Lymphatic drainage of the testes and scrotum.
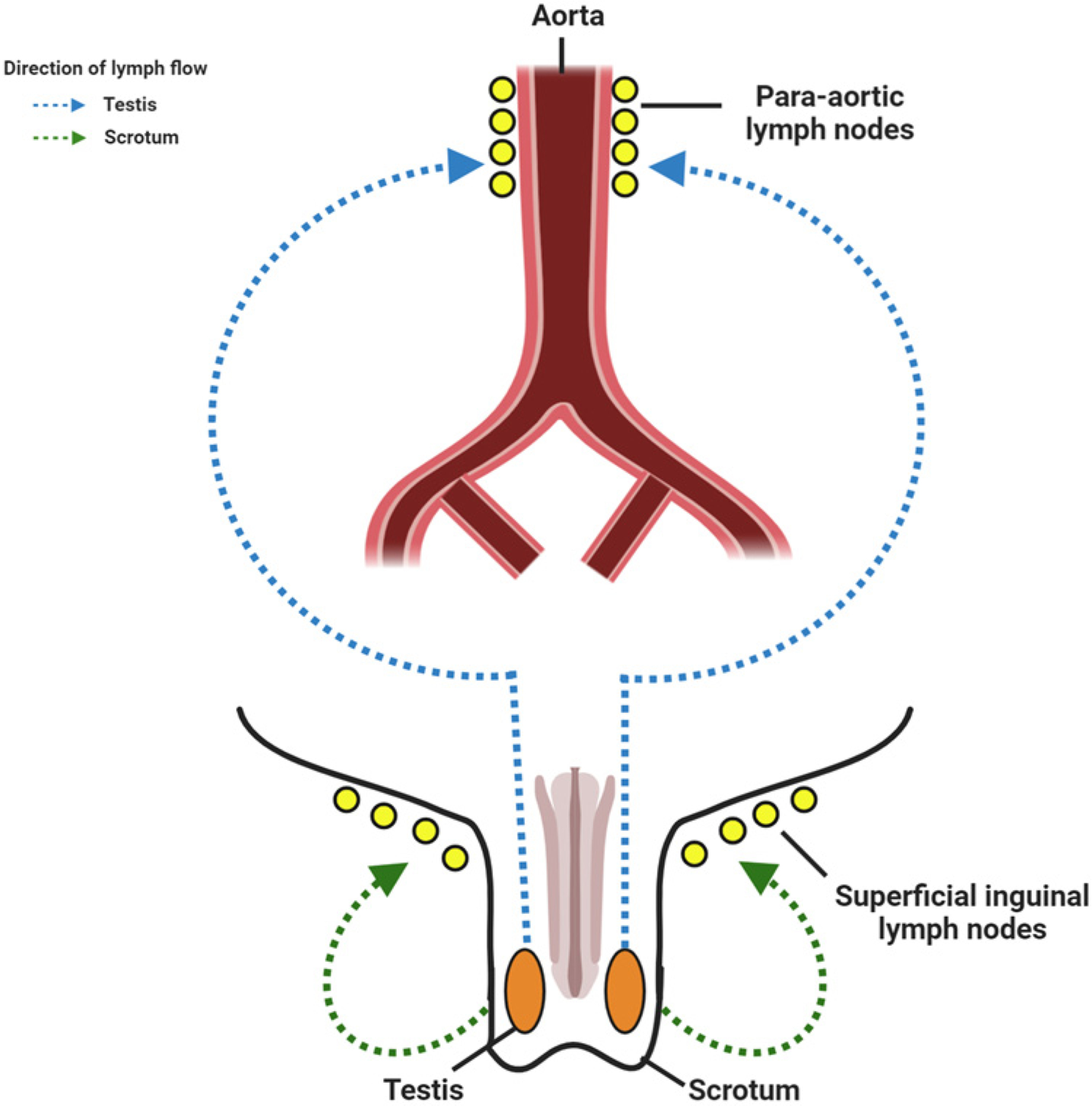
Lymphatics from the testes drain into the para-aortic lymph nodes, whereas the lymphatics from the scrotum drain into the superficial inguinal lymph nodes. Created with BioRender.com.
Ovaries secrete hormones and produce ova for the maintenance of female fertility. Unlike testes, ovaries possess a dense network of lymphatics (Figure 6) and the distribution of lymphatic networks are consistent among many mammalian species where the lymphatic vessels reside in the thecal layer surrounding the developing follicles and the periphery of the corpus luteum [119,121,133–136]. The density and size of the lymphatic network depends on the different stages of the estrous cycle in pigs and sheep [121]. The formation and degeneration of the lymphatic network corresponds to the follicular development and regression of the corpus luteum, respectively [137–139]. The first appearance of ovarian lymphatics in mice occurs after birth around post-natal day 10 and starts expanding along the length of the uterine horn [119,136]. These lymphatics undergo remodeling corresponding to folliculogenesis, attributed to the increased expression of VEGF-C, VEGF-D, and vascular endothelial growth factor receptor-3 (VEGFR-3) genes in adult mice [136]. The ovarian lymphatic network combines at the hilum into a dense plexus and eventually into 4–6 larger lymphatic vessels anastomosing with other lymphatics from the uterus and Fallopian tube and finally drains into the lumbo-aortic nodes [125,140–142]. A recent study suggested three lymphatic drainage paths occurring in the human ovary (Figure 7) [143]. The first major route arises from the cranial side of the ovary, accompanying the ovarian artery in the infundibulopelvic ligament towards the para-aortic and paracaval nodes [143]. The second arises from the caudal side of the ovary that travels along the ovarian artery that anastomoses to the uterine artery in the ovarian ligament, ultimately draining to internal iliac lymph nodes [143]. The third route appears to be minor and involves sparse lymphatic vessels following the round ligament to the inguinal nodes [122,143].
Figure 6. Distribution of the lymphatic vasculature in the adult ovary of Prox1-EGFP reporter mice.
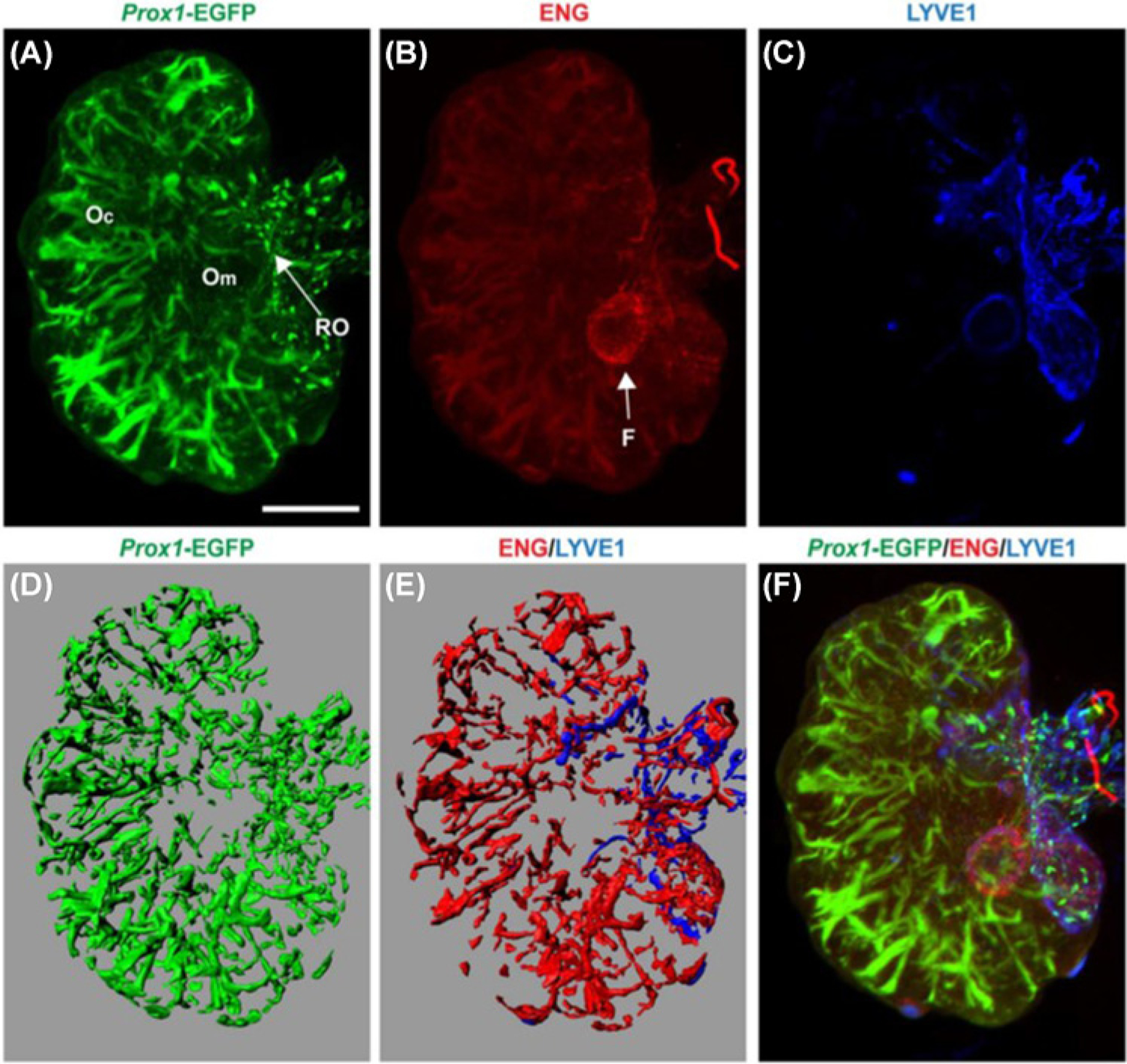
The adult ovary possesses a rich lymphatic network largely overlapping with the blood vasculature. (A) Prox 1-EGFP-positive lymphatics were observed throughout the ovary, originating from the rete ovarii (RO) (arrow), and extending into the ovarian medulla (Om) and ovarian cortex (Oc). (B) Endoglin (ENG) labeling showed an extensive network of blood vessels in the ovary with the follicle (F) indicated by an arrow. (C) Lyve-1 positive lymphatic vessels were confined to the ovarian and extraovarian rete. (D) A 3-D representative image showing Prox1-EGFP-positive lymphatics. (E) Lyve1- positive lymphatic vessels and ENG-positive blood vessels. (F) Combined image showed some colocalization of Prox1, Lyve1, and ENG and a distinct pattern of the blood and lymphatic network in the adult ovary; scale bar = 1 mm. The images are reproduced from the reference [119] with permission.
Figure 7. Lymphatic drainage of the ovary and uterus.
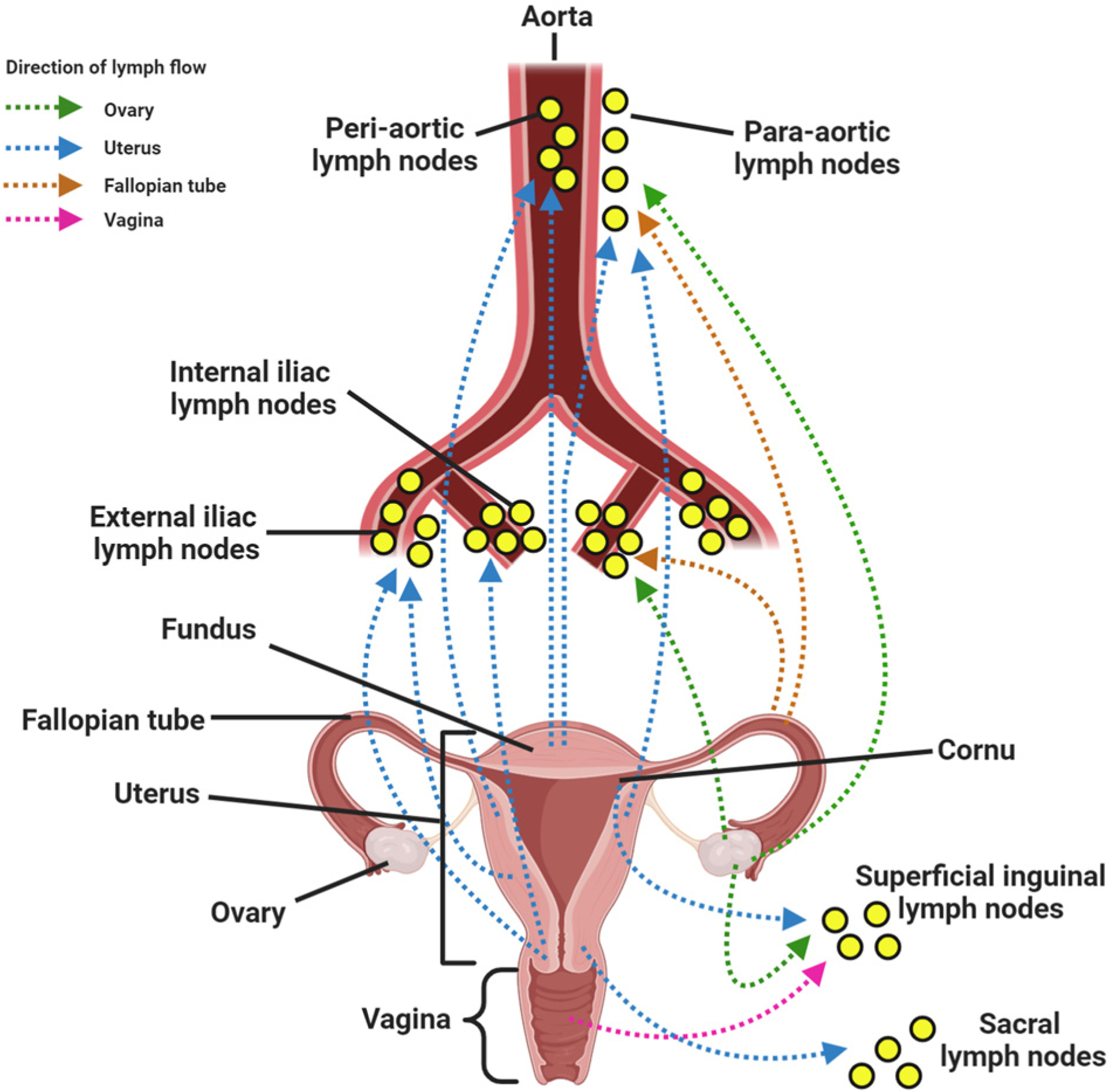
(A) The lymphatic vessels from ovaries drain into (i) the para-aortic lymph nodes, (ii) internal iliac lymph nodes, and (iii) inguinal lymph nodes. (B) Lymphatics from the fallopian tube drain into para-aortic and internal iliac lymph nodes. (C) Lymphatic vessels from the (i) fundus and superior uterine body drain into pre-aortic and para-aortic lymph nodes, cornu into the superficial inguinal lymph nodes, (ii) middle uterine body into the external iliac nodes, and (iii) the lower portion (cervix) into the internal iliac, external iliac, and sacral lymph nodes. (D) Lymphatics from the vagina drain into the superficial inguinal lymph nodes. Created with BioRender.com.
A higher concentration of ovarian steroid hormones is detected in the draining lymph from ovaries compared with peripheral blood, but these concentrations are comparatively lower than in sampled ovarian venous blood [144,145]. These ovarian hormones are transported back to the ovarian arteries through retrograde transfer, revealing the potential role of lymphatics in the feedback mechanism in hormonal regulation and thereby female reproductive health [146,147].
Testicular inflammation and lymphangiogenesis
The predominant process underlying male infertility is the disrupted or disturbed spermatogenesis. A few studies have correlated impaired spermatogenesis with inflammatory and/or immunological factors by demonstrating immunoglobulin and complement reactivity on the thickened basement membrane of seminiferous tubules in testis biopsy specimens from infertile men [148–152]. The testis is an immunologically privileged organ that protects the auto-immunogenic spermatids from the male’s immune system through the BTB between SCs [153]. However, testes are still vulnerable to immune activation leading to inflammatory reactions. Testicular inflammation, or orchitis, can occur due to bacterial, viral, or other pathogenic infections as well as exposure to drugs and heavy metals [154]. Autoimmune orchitis can lead to male infertility [153,155–157]. This condition is characterized by an increased infiltration of immune cells (macrophages, dendritic cells, and subsets of T cells) that results in elevated proinflammatory cytokines levels [158–160], leading to degeneration and apoptosis of GCs. It is known that resident macrophages and mast cells, as well as SCs, can produce an array of cytokines, including both pro- and anti-inflammatory molecules such as interleukin −1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), members of the transforming growth factor-β (TGF-β) family, and IL-10 [161–164]. Increased levels of mRNAs of proinflammatory cytokines, such as IL-1β, TNF, and IFN-γ were linked to disturbed spermatogenesis and inflammatory lesions in human testicular biopsies showing GC neoplasia [165]. Stimulation with TNF-α and IL-1α resulted in upregulation of IL-6 in cultured SCs [166]. In an experimental autoimmune orchitis (EAO) model, peritubular and intratubular immune cell infiltration was reported that was attributed to chemotactic gradients established in the testes due to up-regulation of cell adhesion molecules (CD31, CD44, CD106), chemokines [monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory proteins (MIP) 1α and 1β] and chemokine receptors (CCR2, CCR5) [161,167]. There was an increase in the expression of CCR7 in dendritic cells isolated from EAO rat testes [168]. TNF-α has also be shown to upregulate MCP-1, IL-6, and cyclooxygenase-2 in cultured human testicular peritubular cells [169].
Spiess et al. [170] reported an increased mRNA expression of high-affinity IgE receptor and the mast cell-related fractalkine receptor in a cross-sectional microarray analysis study involving testicular biopsies with spermatogenic failure. A similar study reported an increase in transcript levels associated with inflammatory activity in human testicular biopsies [171]. This is in accordance with earlier studies that pointed out increased numbers of mast cells in testicular biopsies from infertile men with impaired spermatogenesis [165,172,173]. Mast cells, in addition to secreting proinflammatory cytokines like TNF and IL-6, also produce serine protease tryptase that enhances the synthesis of collagen and subsequently tubular fibrosis by exerting its mitogenic effect on fibroblasts and peritubular cells [174–177]. Mast cell tryptase activates proteinase-activated receptor-2 on isolated peritubular cells in vitro leading to up-regulation of inflammatory molecules such as MCP-1, cyclooxygenase-2, and TGF-β2 [177]. Translocation of high-mobility group box protein-1 from the nuclei in EAO rat testes has been shown to regulate inflammatory responses. A similar result was found in testes of infertile men with impaired spermatogenesis along with lymphocytic infiltrates [178]. Galectin-1, activins, and inhibin have also been reported to play a crucial role in the development of testicular immunopathology [179–182].
Immune cell infiltration has been associated with disruption of the BTB in seminiferous tubules [183,184]. IL-6 has proved to be an essential factor in the development of testicular inflammatory responses, which is found to disrupt the integrity of the BTB in rats, thereby attacking the immunological barrier in testes [185]. IL-6 inhibits protein degradation and activates phosphorylated ERK in SCs [186]. IL-6 also interferes with GC differentiation or degeneration by acting through the transcription factor Zfp637 on spermatogonia. TNF/TNFR1, Fas/FasL, and Bax/Bcl-2 systems have all been implicated along with IL-6 and its receptor in GC apoptosis in the rat EAO model [164,168,187]. Oh et al. [188] reported attenuated mRNA expression of SC tight junction proteins such as Claudin-11 (Cldn11), Occludin, and Zona occludens-1 in varicocele testes when compared with normal testes. Immunolocalization showed that Cldn11 was found in the cytoplasm rather than in the periphery of the seminiferous tubule suggesting impaired subcellular localization. Increased mRNA levels of proinflammatory cytokines (Tnfa, Il1a, and Il6), leukocyte marker (Cd45), and T-cell markers (Cd3g and Cd3d) were observed in varicocele testes, indicating immune cell infiltration. This might have deregulated Cldn11 expression in SCs in varicocele testes, thereby attenuating the permeability of the BTB and finally resulting in impaired spermatogenesis [188]. Another study using rats with EAO reported an increase in IL-6 along with a decrease in Occludin, and delocalization of Cldn 11 and Zona occludens-1 leading to a disrupted BTB and membrane permeability [185]. Hence, it is very clear from the available literature that impaired spermatogenesis is associated with testicular inflammation and immune cell infiltration (Table 1).
Table 1.
Inflammatory markers altered in testis during inflammation
| S.No. | Inflammation model | Findings of the study | References |
|---|---|---|---|
| 1. | Experimental autoimmune orchitis model | Increased infiltration of macrophages, dendritic cells and subsets of T cells, elevated pro-inflammatory cytokines levels IL-6, TNF- α, up-regulation of cell adhesion molecules (CD31, CD44, CD106), chemokines (MCP-1, MIP 1 α and 1 β) and chemokine receptors (CCR2, CCR5 and CCR7) Increased levels of IL-6 disrupted the integrity of the BTB by down-regulating the expression of Occludin, and delocalization of Claudin 11 and Zona occludens-1 in the testes, IL-6 also induces GC apoptosis |
[153,158–162,164,167,168,185,187] |
| 2. | Testicular cancer biopsies | Increased levels of mRNAs of pro-inflammatory cytokines, such as IL-1β, TNF-α, and IFN-γ | [165] |
| 3. | Testicular biopsies from patients with spermatogenic failure | Increased mRNA expression of high-affinity IgE receptor and the mast cell-related fractalkine receptor revealing increased inflammatory activity in the testis | [170,171] |
| 4. | Lipopolysaccharide-induced inflammation | Up-regulation of IL-1β and IL-6 in the testis | [163] |
| 5. | Varicocele testes model | Increased mRNA levels of proinflammatory cytokines (Tnfa, //1a, and //6), leukocyte marker (Cd45), and T-cell markers (Cd3g and Cd3d), attenuated mRNA expression of SC tight junction proteins such as Claudin-11, Occludin and Zona occludens-1 in varicocele testes | [188] |
Expansion of the local lymphatic vasculature, lymphangiogenesis, is common under inflammatory conditions and generally considered to be a beneficial process in restoring tissue homeostasis [111–113,189–191]. Several tissue cells and infiltrating immune cells secrete the predominant lymphangiogenic proteins VEGF-C and VEGF-D during inflammation. It was also reported that TNF-α, VEGF-A, VEGF-C, and VEGF-D are produced by macrophages and induced angiogenesis and lymphangiogenesis [192–198]. Few studies have directly examined lymphangiogenesis in orchitis. It is reported that seminiferous tubules are immersed in lymph and the tissue fluid is drained through lymphatic capillaries extended beneath the tunica albuginea but not within the testicular interstitium [118]. Naito et al. [199] demonstrated an expanded interstitium area in the EAO model that was restored under post-inflammatory condition. The increased lymphatics observed around the inflammatory lesions point out the importance of interstitial fluid and infiltrated immune cell clearance. Hirai and colleagues [200] identified a significant increase in VEGF-D in the testes of EAO mice. Quantifying inflammation-associated lymphangiogenesis is complicated in some tissues because LYVE-1, a commonly used marker to identify lymphatic endothelial cells, is expressed on a subset of macrophages under inflammatory conditions [196,201,202]. LYVE-1+ cells were identified in the interstitium proper of inflamed testes; however, all of these cells were F4/80+ and CD31- indicating that these LYVE-1+ cells were mature macrophages and not true lymphatic endothelial cells. An incorporation of LYVE-1+ macrophages into the wall of lymphatic capillaries under the tunica albuginea was also identified in this model. This indicates that F4/80+ macrophages expressing LYVE-1 may play a role in promoting testicular lymphangiogenesis during inflammation [200]. Whether gonadal lymphangiogenesis occurs in hypertension has not yet been described.
Ovarian inflammation, endomteriosis, and lymphangiogenesis
Autoimmune ovarian disease (AOD), a chronic inflammatory disease, is linked to lymphocytic infiltration in the ovarian follicle in women with premature ovarian failure [158]. Researchers have established a rodent model of AOD by immunization of animals with zona pellucida (ZP) antigens [203–206]. ZP is an extracellular glycoprotein found around the oocytes and aids follicular development, spermatozoa–oocyte interaction, and fertilization [206]. Immunization with ZP led to the release of anti-ZP autoantibodies and activation of autoreactive T cells eventually resulting in autoimmune oophoritis, a condition identified by inflammation in the ovarian interstitium and organized monocytic granulomata [203–207]. Adoptive transfer of ZP peptide-specific T cells into näıve mice caused granulomatous oophoritis and up-regulation of proinflammatory markers including IL-1, TNF-α, and IFN-γ, but did not alter ovarian function or fertility [207]. Ovarian granulosa cells had ectopic expression of major histocompatibility complex (MHC) II in the site of inflammation, induced by IFNγ [208]. Similar results were observed with cynomolgus macaques where pZP3 (zona pellucida 3 peptide) immunization showed co-localization of T cell clusters with MHC-II+ macrophages in the ovarian interstitium [205]. Increased level of chemokines such as MIP-1α, IL8, eotaxin-1, and interferon inducible protein-10, as well as the pro-lymphangiogenic factor VEGF-D, was reported to be associated with an early stage of premature ovarian insufficiency [209]. Beyond the ovary, an increase in the production of chemokines and macrophage recruitment have been identified in the uterine tissues of women with endometriosis [210–212]. Increased production of intercellular adhesion molecule-1, insulin-like growth factor-I, IL-1, IL-6, IL-8, IL-12, MCP-1 (CCL2), MIP-1α (CCL3), RANTES (CCL5), eotaxin (CCL11), VEGF, and TNFα were associated with endometriosis [211,213]. It is to be noted that inflammation in the ovaries and uterus are associated with reproductive dysfunction in females (Table 2).
Table 2.
Inflammatory markers altered in ovaries and uterus during inflammation
| S.No. | Inflammation Model | Findings of the study | References |
|---|---|---|---|
| 1. | Autoimmune ovarian disease model | Up-regulation of proinflammatory markers such as IL-1, TNF-α, and IFN-γ | [207] |
| 2. | Ovarian biopsies from women with premature autoimmune ovarian failure. | Ovarian granulosa cells had ectopic expression of MHC-II in the site of inflammation. | [208] |
| 3. | Autoimmune ovarian disease model (Cynomolgus macaques) | Co-localization of T cell clusters with MHC-II+ macrophages in the ovarian interstitium. | [205] |
| 4. | Females with premature ovarian insufficiency | Increased level of chemokines such as MIP-1α, IL-8, eotaxin-1 and interferon inducible protein-10 as well as lymphangiogenic factor VEGF-D in follicular fluid. | [209] |
| 5. | Endometriosis | Elevated levels of intercellular adhesion molecule-1, insulin-like growth factor-I, IL-1, IL-6, IL-8, IL-12, MCP-1, MIP-1α, RANTES, eotaxin, VEGF and TNFα were associated with endometriosis. | (Explained in detail in the cited reviews) [211,213] |
Lymphangiogenesis occurs in the ovaries and uterus under normal physiological conditions with respect to reproductive cycles and pregnancy [139,214]. While studied somewhat extensively in ovarian cancer due to lymphatics serving as a route of metastases, lymphangiogenesis in the ovary during inflammation has been understudied. Studies have reported that proinflammatory cytokines like IL-6 and TNF-α might be involved in pathologic lymphangiogenesis in ovarian cancer-related inflammation [215,216]. Reichelt et al. [217] demonstrated lymphangiogenesis in peritoneal endometriosis with up-regulation of VEGF-C and VEGF-D expression. The pathological lymphangio-genesis observed may be ascribed to the chronic inflammatory responses during endometriosis induced by VEGF-C and VEGF-D expressing macrophages [217]. Recently, it was reported that VEGFR1 signaling is responsible for the induction of lymphangiogenesis in endometrial tissues and contributes to the pathogenesis of endometriosis in a mouse model [218]. Whether hypertension causes inflammation-associated lymphangiogenesis in female reproductive organs and whether this is beneficial or detrimental remains an area to be explored.
Future perspectives and conclusion
To conclude, studies conducted so far demonstrate that hypertension has a deleterious effect on male and female fertility. It is also now appreciated that hypertension induces inflammation, immune cell trafficking, and inflammation-associated lymphangiogenesis in various organs, but it remains largely unknown whether hypertension induces inflammation and inflammation-associated lymphangiogenesis in the gonads (Figure 8). Moreover, while limited in reports thus far, inflammation-associated lymphangiogenesis occurs in gonads in certain other pathological conditions (Figure 8). Hence, identifying how and to what extent inflammation and lymphangiogenesis occurs as a result of hypertension in reproductive tissues is a question of keen interest to our group. Preliminary results from our lab demonstrate up-regulation of lymphatic vessel markers, pro-lymphangiogenic growth factors, their respective receptors, and proinflammatory cytokines and chemokines in the testes of hypertensive mice (Figure 9). Studies are underway to examine how hypertension may induce inflammation-associated lymphangiogenesis in the gonads of hypertensive male and female mice. Whether enhancing lymphatic density in gonads may help in combating the inflammatory response and its consequences on reproductive function are key questions to consider. Further studies along these lines are required to support our hypothesis that may enlighten the mechanisms behind the deleterious effect of hypertension on fertility.
Figure 8. Graphical overview of the effects of hypertension on gonadal function and the plausible mechanisms behind these effects.
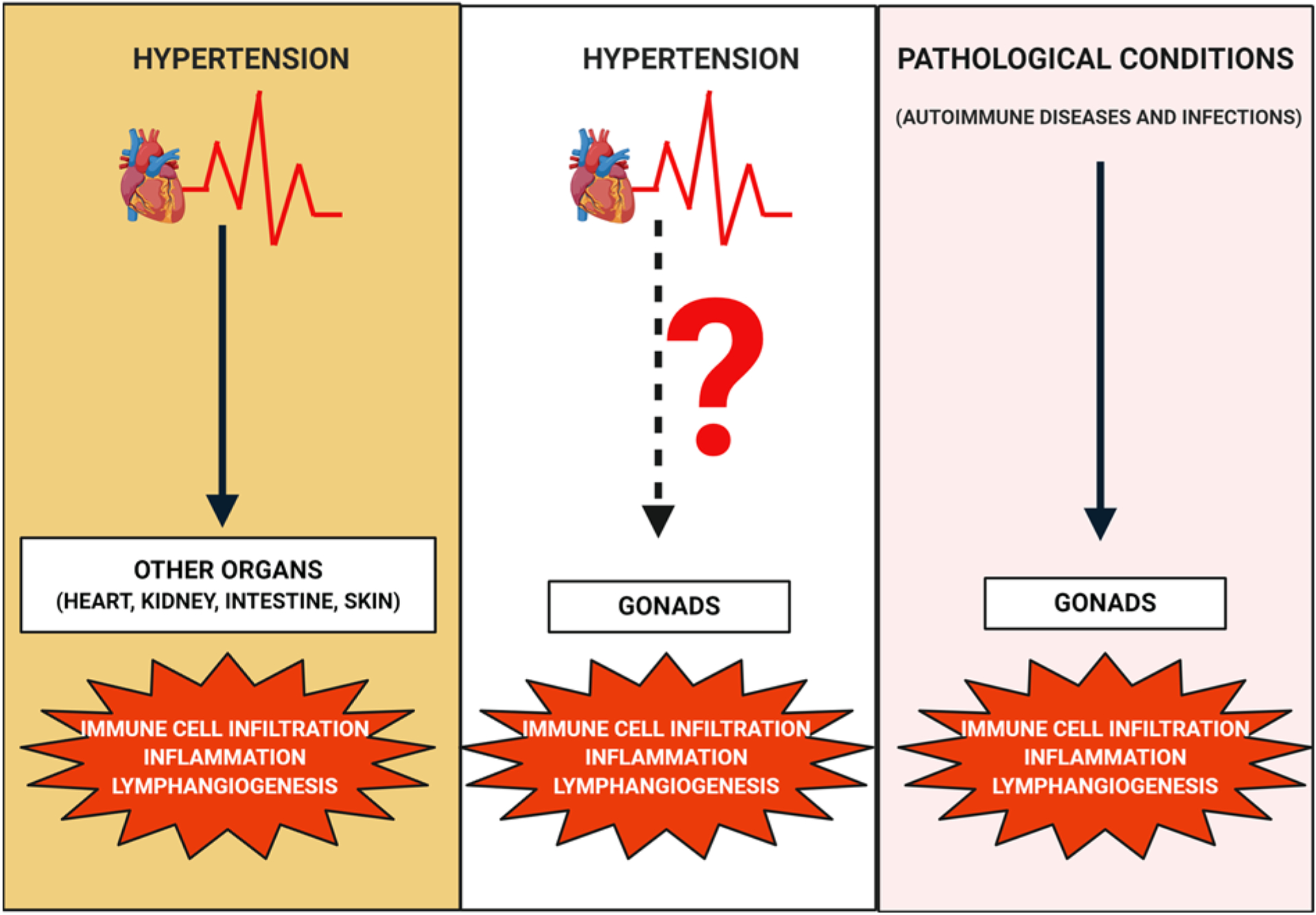
Hypertension is known to promote immune cell infiltration, inflammation, and inflammation-associated lymphangiogenesis in several organs like the heart, kidneys, intestine, and skin. Similarly, immune cell infiltration, inflammation, and lymphangiogenesis have been observed in gonads under certain pathological conditions. However, the exact molecular mechanisms by which hypertension affects gonads are still unclear. Hence, one of the plausible mechanisms might be immune cell infiltration and inflammation in gonads thereby leading to reproductive dysfunction. Inflammation-associated lymphangiogenesis may occur in a compensatory manner and its role in gonadal function in hypertension is unknown. Created with BioRender.com.
Figure 9. Testes from L-NAME induced hypertensive mice had a significant increase in gene expression of lymphatic markers, proinflammatory cytokines, chemokines, and adhesion molecules.
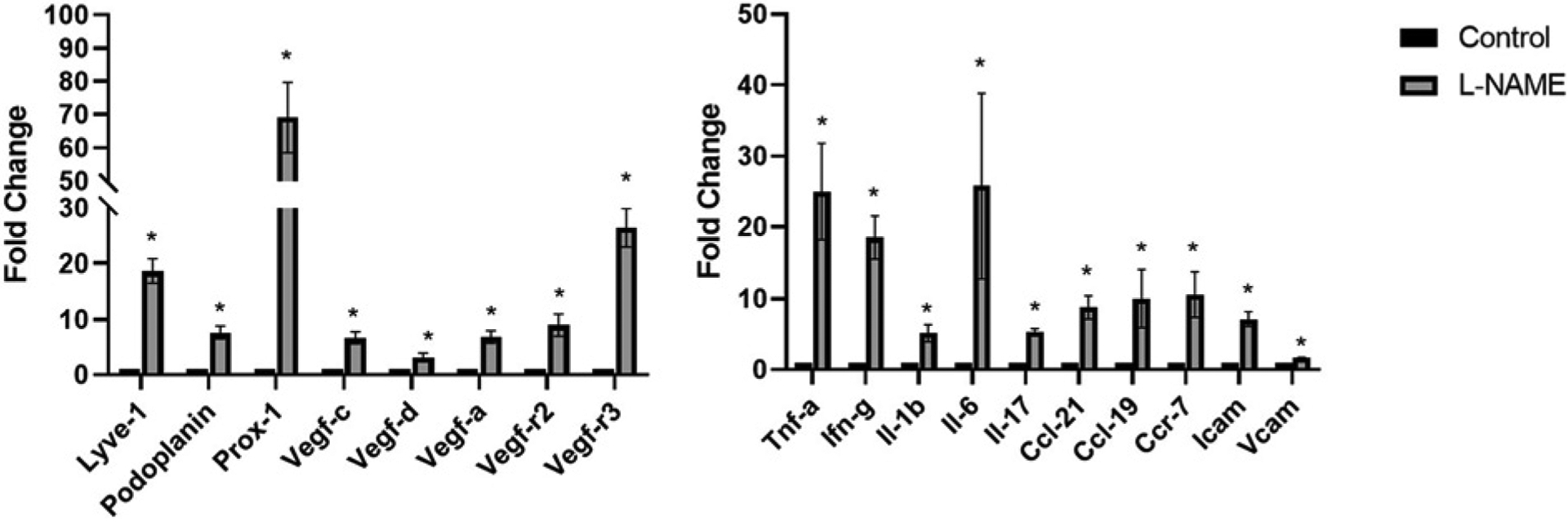
Results are expressed as mean ±SEM (n=4 per group), and statistical analyses were performed with a Student’s t test. *P<0.05 vs. Control mice.
Acknowledgements
All authors have read the journal’s authorship statement and agree that this article is original and has not been presented in any form previously or is under consideration elsewhere.
Funding
This work was supported by the National Institutes of Health [grant number DK120493 (to B.M.M.)].
Abbreviations
- AOD
autoimmune ovarian disease
- BTB
blood–testis barrier
- CCL
C-C motif chemokine ligand
- Cldn11
Claudin-11
- EAO
experimental autoimmune orchitis
- ED
erectile dysfunction
- GC
germ cell
- IFN-γ
interferon-γ
- IL
interleukin
- L-NAME
L-arginine methyl ester hydrochloride
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor-1
- MCP-1
monocyte chemoattractant protein-1
- MHC-II
major histocompatibility complex-II
- MIP-1α
macrophage inflammatory protein-1α
- MLC
myosin light chain
- NO
nitric oxide
- SC
Sertoli cell
- SHRSP
spontaneously hypertensive rats that are stroke prone
- SHR
spontaneously hypertensive rat
- T
testosterone
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- ZP
Zona pellucida
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Awlaqi AA and Alkhayat K (2016) Hammadeh ME Metabolic Syndrome and Infertility in Women. IJWHR 4, 89–95, 10.15296/ijwhr.2016.23 [DOI] [Google Scholar]
- 2.Martins AD and Majzoub A (2019) Agawal A Metabolic Syndrome and Male Fertility. World J. Mens Health 37, 113–127, 10.5534/wjmh.180055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis C, Duncan LE and Balance DI (1998) Pearson TA Is sexual dysfunction in hypertensive women uncommon or understudied? Am. J. Hypertens 11, 733–735 [PubMed] [Google Scholar]
- 4.Duncan LE, Lewis C, Jenkins P and Pearson TA (2000) Does hypertension and its pharmacotherapy affect the quality of sexual function in women? Am. J. Hypertens 13, 640–647, 10.1016/S0895-7061(99)00288-5 [DOI] [PubMed] [Google Scholar]
- 5.Fogari R, Preti P, Zoppi A, Corradi L, Pasotti C, Rinaldi A et al. (2004) Effect of valsartan and atenolol on sexual behavior in hypertensive postmenopausal women. Am. J. Hypertens 14, 77–81, 10.1016/j.amjhyper.2003.08.016 [DOI] [PubMed] [Google Scholar]
- 6.Kearney PM, Whelton M, Raynolds K, Muntner P, Whelton PK and He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365, 217–223, 10.1016/S0140-6736(05)17741-1 [DOI] [PubMed] [Google Scholar]
- 7.Viigimaa M, Doumas M, Vlachopoulos C, Anyfanti P, Wolf J, Narkiewicz K et al. (2011) Hypertension and sexual dysfunction: time to act. J. Hypertens 29, 403–407, 10.1097/HJH.0b013e328342c659 [DOI] [PubMed] [Google Scholar]
- 8.Aranda P, Ruilope LM, Calvo C, Luque M, Coca A and Gil de Miguel A (2004) Erectile dysfunction in essential arterial hypertension and effects of sildenafil: results of a Spanish national study. Am. J. Hypertens 17, 139–145, 10.1016/j.amjhyper.2003.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Foy CG, Newman JC, Berlowitz DR, Russell LP, Kimmel PL, Wadley VG et al. (2019) Blood Pressure, Sexual Activity, and Erectile Function in Hypertensive Men: Baseline Findings from the Systolic Blood Pressure Intervention Trial (SPRINT). J. Sex Med 16, 235–247, 10.1016/j.jsxm.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doumas M, Tsiodras S, Tsakiris A, Douma S, Chounta A, Papadopoulos A et al. (2006) Female sexual dysfunction in essential hypertension: a common problem being uncovered. J. Hypertens 24, 2387–2392, 10.1097/01.hjh.0000251898.40002.5b [DOI] [PubMed] [Google Scholar]
- 11.Khaw KT and Barrett-Connor E (1988) Blood pressure and endogenous testosterone in men: an inverse relationship. J. Hypertens 6, 329–332, 10.1097/00004872-198804000-00010 [DOI] [PubMed] [Google Scholar]
- 12.Fogari R, Zoppi A, Preti P, Rinaldi A, Marasi G, Vanasia A et al. (2002) Sexual activity and plasma testosterone levels in hypertensive males. Am. J. Hypertens 15, 217–221, 10.1016/S0895-7061(01)02280-4 [DOI] [PubMed] [Google Scholar]
- 13.Hughes GS, Mathur RS and Margolius HS (1989) Sex steroid hormones are altered in essential hypertension. J. Hypertens 7, 181–187, 10.1097/00004872-198903000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Phillips GB, Jing TY, Resnick LM, Barbagallo M, Laragh JH and Sealey JE (1993) Sex hormones and hemostatic risk factors for coronary heart disease in men with hypertension. J. Hypertens 11, 699–702, 10.1097/00004872-199307000-00003 [DOI] [PubMed] [Google Scholar]
- 15.Svartberg J, von Muhlen D, Schirmer H, Barrett-Connor E, Sundfjord J and Jorde R (2004) Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur. J. Endocrinol 150, 65–71, 10.1530/eje.0.1500065 [DOI] [PubMed] [Google Scholar]
- 16.Breigeiron MK, Lucion AB and Sanvitto GL (2007) Effects of renovascular hypertension on reproductive function in male rats. Life Sci. 80, 1627–1634, 10.1016/j.lfs.2007.01.030 [DOI] [PubMed] [Google Scholar]
- 17.Muciaccia B, Pensini S, Culasso F, Padula F, Paoli D, Gandini L et al. (2012) Higher clusterin immunolabeling and sperm DNA damage levels in hypertensive men compared with controls. Hum. Reprod 27, 2267–2276, 10.1093/humrep/des173 [DOI] [PubMed] [Google Scholar]
- 18.Guo D, Li S, Behr B and Eisenberg ML (2017) Hypertension and male fertility. World J. Mens Health 35, 59–64, 10.5534/wjmh.2017.35.2.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM et al. (2010) World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 16, 231–245, 10.1093/humupd/dmp048 [DOI] [PubMed] [Google Scholar]
- 20.Guo D, Li S, Behr B and Eisenberg M (2015) PD52–12 The impact of hypertension and antihypertensives on semen quality. J. Urol 193, e1117, 10.1016/j.juro.2015.02.1786 [DOI] [Google Scholar]
- 21.Eisenberg ML, Li S, Behr B, Pera RR and Cullen MR (2015) Relationship between semen production and medical comorbidity. Fertil. Steril 103, 66–71, 10.1016/j.fertnstert.2014.10.017 [DOI] [PubMed] [Google Scholar]
- 22.Burchardt M, Burchardt T, Baer L, Kiss AJ, Pawar RV, Shabsigh A et al. (2000) Hypertension is associated with severe erectile dysfunction. J. Urol 164, 1188–1191, 10.1016/S0022-5347(05)67138-8 [DOI] [PubMed] [Google Scholar]
- 23.Ponholzer A, Temml C, Mock K, Marszalek M, Obermayr R and Madersbacher S (2005) Prevalence and risk factors for erectile dysfunction in 2869 men using a validated questionnaire. Eur. Urol 47, 80–85, 10.1016/j.eururo.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM and Coltman CA (2005) Erectile dysfunction and subsequent cardiovascular disease. JAMA 294, 2996–3002, 10.1001/jama.294.23.2996 [DOI] [PubMed] [Google Scholar]
- 25.Toblli JE, Stella I, Inserra F, Ferder L, Zeller F and Mazza ON (2000) Morphological changes in cavernous tissue in spontaneously hypertensive rats. Am. J. Hypertens 13, 686–692, 10.1016/S0895-7061(99)00268-X [DOI] [PubMed] [Google Scholar]
- 26.Ushiyama M, Morita T, Kuramochi T, Yagi S and Katayama S (2004) Erectile dysfunction in hypertensive rats results from impairment of the relaxation evoked by neurogenic carbon monoxide and nitric oxide. Hypertens. Res 27, 253–261, 10.1291/hypres.27.253 [DOI] [PubMed] [Google Scholar]
- 27.Viigimaa M, Vlachopoulos C, Lazaridis A and Doumas M (2014) Management of erectile dysfunction in hypertension: Tips and tricks. World J. Cardiol 6, 908–915, 10.4330/wjc.v6.i9.908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kloner R (2006) Erectile dysfunction and hypertension. Int. J. Impot. Res 19, 296–302, 10.1038/sj.ijir.3901527 [DOI] [PubMed] [Google Scholar]
- 29.Chitaley K, Webb RC, Dorrance AM and Mills TM (2001) Decreased penile erection in DOCA-salt and stroke prone-spontaneously hypertensive rats. Int. J. Impotence Res 13, S16–S20, 10.1038/sj.ijir.3900773 [DOI] [PubMed] [Google Scholar]
- 30.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M et al. (2000) Pharmacological properties of Y-27632, a specific inhibitor of Rho-associated kinases. Mol. Pharmacol 57, 976–983 [PubMed] [Google Scholar]
- 31.Narumiya S, Ishizaki T and Uehata M (2000) Use and properties of ROCK-specific inhibitor Y-27632. Methods Enzymol. 25, 273–284, 10.1016/S0076-6879(00)25449-9 [DOI] [PubMed] [Google Scholar]
- 32.Chitaley K, Webb RC and Mills TM (2001) RhoA/Rho-kinase: a novel player in the regulation of penile erection. Int. J. Impotence Res 13, 67–72, 10.1038/sj.ijir.3900647 [DOI] [PubMed] [Google Scholar]
- 33.Mills TM, Chitaley K, Lewis RW and Webb RC (2002) Nitric oxide inhibits RhoA/Rho-kinase signaling to cause erection. Eur. J. Pharmacol 439, 173–174, 10.1016/S0014-2999(02)01408-5 [DOI] [PubMed] [Google Scholar]
- 34.Mills TM, Lewis RW, Wingard CJ, Chitaley K and Webb RC (2002) Inhibition of tonic contraction-a novel way to approach erectile dysfunction. J. Androl 23, S5–S9 [PubMed] [Google Scholar]
- 35.Jeremy JY, Ballard SA, Naylor AM, Miller MA and Angelini GD (1997) Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br. J. Urol 79, 958–963, 10.1046/j.1464-410X.1997.00206.x [DOI] [PubMed] [Google Scholar]
- 36.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD and Wicker PA (1998) Oral sildenafil in the treatment of erectile dysfunction. Sildenafil study group. N. Engl. J. Med 338, 1397–1404, 10.1056/NEJM199805143382001 [DOI] [PubMed] [Google Scholar]
- 37.La Torre A, Guipponi G, Duffy D, Conca A and Ctanzariti D (2015) Sexual dysfunction related to drugs: a critical review. Part IV: cardivascular drugs. Pharmacopsychiatry 48, 1–6 [DOI] [PubMed] [Google Scholar]
- 38.Grimm RH, Grandits GA, Prineas RJ, McDonald RH, Lewis CE, Flack JM et al. (1997) Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women: Treatment of Mild Hypertension Study (TOMHS). Hypertension 29, 8–14, 10.1161/01.HYP.29.1.8 [DOI] [PubMed] [Google Scholar]
- 39.Erdmann E (2010) Safety and tolerability of beta-blockers: prejudices and reality. Indian Heart J. 62, 132–135 [PubMed] [Google Scholar]
- 40.Mrotek JJ, Barrow RY, Evans SL and Clark JT (1988) Deoxycorticosterone (DOC) has differential effects depending on age. Seventh Annual Symposium on Geriatrics and Gerontology: Endocrine Function and Aging, St. Louis, MO, (Abstract) [Google Scholar]
- 41.Mrotek JJ, Barrow R, Evans S and Clark JT (1989) Deoxycorticosterone (DOC)-reproductive and adrenal effects. Annual Meeting of the Federation of American Societies for Experimental Biology, New Orleans, LA, (Abstract) [Google Scholar]
- 42.Clark JT, Sahu A, Mrotek JJ and Kalra SP (1991) Sexual function and neuropeptide Y levels in selected brain regions in male spontaneously hypertensive rat. Am. J. Physiol 261, R1234–R1241 [DOI] [PubMed] [Google Scholar]
- 43.Itoh N, Akagashi K, Kumamoto Y, Suzuki T and Ohta Y (1995) Influence of hypertensive vascular changes in intratesticular arteries on spermatogenesis in SHRSP. Clin. Exp. Pharmacol. Physiol. Suppl 22, S134–S135, 10.1111/j.1440-1681.1995.tb02852.x [DOI] [PubMed] [Google Scholar]
- 44.Akagashi K, Itoh N, Kumamoto Y, Tsukamoto T, Suzuki T and Ohta Y (1996) Hypertensive changes in intratesticular arteries impair spermatogenesis of stroke-prone spontaneously hypertensive rat. J. Androl 17, 367–374 [PubMed] [Google Scholar]
- 45.Akagashi K, Kumamoto Y, Itoh N, Tsukamoto T, Suzuki T and Ohta Y (1997) Manidipine improves spermatogenesis in the stroke-prone spontaneously hypertensive rat. J. Androl 18, 210–216 [PubMed] [Google Scholar]
- 46.Azu OO (2015) Testicular morphology in spontaneously hypertensive rat model: oxidant status and stereological implications. Andrologia 47, 123–137, 10.1111/and.12233 [DOI] [PubMed] [Google Scholar]
- 47.Sasanka KB, Bagchi P, Sharma D, Rajeev TP and Dhekial PP (2015) Segmental Testicular Infarction: A Clinical Dilemma. J. Genit Syst. Disor 4, 2 [Google Scholar]
- 48.França LR, Hess RA, Dufour JM, Hofmann MC and Griswold MD (2016) The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology 4, 189–212, 10.1111/andr.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atanassova N, Lakova E, Bratchkova Y, Krasteva G and Donchev M (2009) Expression of testicular angiotensin-converting enzyme in adult spontaneously hypertensive rats. Folia. Histochem. Cytobiol 47, 117–122, 10.2478/v10042-009-0002-6 [DOI] [PubMed] [Google Scholar]
- 50.Bechara GR, de Souza DB, Simoes M, Felix-Patrício B, Medeiros JL Jr, Costa WS et al. (2015) Testicular Morphology and Spermatozoid Parameters in Spontaneously Hypertensive Rats Treated with Enalapril. J. Urol 194, 1498–1503, 10.1016/j.juro.2015.06.073 [DOI] [PubMed] [Google Scholar]
- 51.Colli LG, Belardin LB, Echem C, Akamine EH, Antoniassi MP, Andretta RR et al. (2019) Systemic arterial hypertension leads to decreased semen quality and alterations in the testicular microcirculation in rats. Sci. Rep 9, 11047, 10.1038/s41598-019-47157-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoder SR, Thornburg LL and Bisognano JD (2009) Hypertension in pregnancy and women of childbearing age. Am. J. Med 122, 890–895, 10.1016/j.amjmed.2009.03.036 [DOI] [PubMed] [Google Scholar]
- 53.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/ NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, e13–e115 [DOI] [PubMed] [Google Scholar]
- 54.Anastasiadis AG, Davis AR, Ghafar MA, Burchardt M and Shabsigh R (2002) The epidemiology and definition of female sexual disorders. WorldJ. Urol 20, 74–78, 10.1007/s00345-002-0272-5 [DOI] [PubMed] [Google Scholar]
- 55.Kütmeç C and Yurtsever S (2011) Effects of sexual function of essential hypertensions in women. Eur. J. Cardiovasc. Nurs 10, 56–63, 10.1016/j.ejcnurse.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 56.Berman JR, Adhikari SP and Goldstein I (2000) Anatomy and physiology of female sexual function and dysfunction: classification, evaluation and treatment options. Eur. Urol 38, 20–29, 10.1159/000020247 [DOI] [PubMed] [Google Scholar]
- 57.Munarriz R, Kim SW, Kim NN, Traish A and Goldstein I (2003) A review of the physiology and pharmacology of peripheral (vaginal and clitoral) female genital arousal in the animal model. J. Urol 170, S40–S44, 10.1097/01.ju.0000075352.03144.15 [DOI] [PubMed] [Google Scholar]
- 58.Berman JR (2005) Physiology of female sexual function and dysfunction. Int. J. Impot. Res 17, S44–S51, 10.1038/sj.ijir.3901428 [DOI] [PubMed] [Google Scholar]
- 59.Latif RA, Muhamad R, Ann AYH, Sidi H, Jaafar NRN, Midin M et al. (2014) Duration of hypertension and antihypertensive agents in correlation with the phases of female sexual response cycle. Compr. Psychiatr 55, S7–S12, 10.1016/j.comppsych.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 60.American College of Obstetricians and Gynecologists (2001) ACOG Practice Bulletin. Chronic hypertension in pregnancy. ACOG Committee on Practice Bulletins. Obstet. Gynecol 98, 177–185, 10.1097/00006250-200107000-00032 [DOI] [PubMed] [Google Scholar]
- 61.Sibai BM (2002) Chronic hypertension in pregnancy. Obstet. Gynecol 100, 369–377, 10.1097/00006250-200208000-00029 [DOI] [PubMed] [Google Scholar]
- 62.Gillon TE, Pels A, von Dadelszen P, MacDonell K and Magee LA (2014) Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS ONE 9, e113715–e113715, 10.1371/journal.pone.0113715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sibai BM, Abdella TN and Anderson GD (1983) Pregnancy outcome in 211 patients with mild chronic hypertension. Obstet. Gynecol 61, 571–576 [PubMed] [Google Scholar]
- 64.Rey E and Couturier A (1994) The prognosis of pregnancy in women with chronic hypertension. Am. J. Obstet. Gynecol 171, 410–416, 10.1016/0002-9378(94)90276-3 [DOI] [PubMed] [Google Scholar]
- 65.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M et al. (1998) Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N. Engl. J. Med 339, 667–671, 10.1056/NEJM199809033391004 [DOI] [PubMed] [Google Scholar]
- 66.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL et al. (2006) Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology 67, 424–429, 10.1212/01.wnl.0000228277.84760.a2 [DOI] [PubMed] [Google Scholar]
- 67.Gilbert WM, Young AL and Danielsen B (2007) Pregnancy outcomes in women with chronic hypertension: a population-based study. J. Reprod. Med 52, 1046–1051 [PubMed] [Google Scholar]
- 68.Kuklina EV, Ayala C and Callaghan WM (2009) Hypertensive disorders and severe obstetric morbidity in the United States. Obstet. Gynecol 113, 1299–1306, 10.1097/AOG.0b013e3181a45b25 [DOI] [PubMed] [Google Scholar]
- 69.Ananth CV, Savitz DA and Bowes WA (1995) Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet. Gynecol. Scand 74, 788–793, 10.3109/00016349509021198 [DOI] [PubMed] [Google Scholar]
- 70.Jain L (1997) Effect of pregnancy-induced and chronic hypertension on pregnancy outcome. J. Perinatol 17, 425–427 [PubMed] [Google Scholar]
- 71.Bramham K, Parnell B, Nelson-Piercy C, Seed PT, Poston L and Chappell LC (2014) Chronic hypertension and pregnancy outcomes: systematic review and meta-analysis. BMJ 348, g2301–g2301, 10.1136/bmj.g2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seyom E, Abera M, Tesfaye M and Fentahun N (2015) Maternal and fetal outcome of pregnancy related hypertension in Mettu Karl Referral Hospital, Ethiopia. J. Ovarian Res 8, 10, 10.1186/s13048-015-0135-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khosravi S, Dabiran S, Lotfi M and Asnavandy M (2014) Study of the prevalence of hypertension and complications of hypertensive disorders in pregnancy. Open J. Prev. Med 4, 860–867, 10.4236/ojpm.2014.411097 [DOI] [Google Scholar]
- 74.Zhou H, Liu Y, Liu L, Zhang M, Chen X and Qi Y (2016) Maternal pre-pregnancy risk factors for miscarriage from a prevention perspective: a cohort study in China. Eur. J. Obstet. Gynecol. Reprod. Biol 206, 57–63, 10.1016/j.ejogrb.2016.07.514 [DOI] [PubMed] [Google Scholar]
- 75.Nobles CJ, Mendola P, Mumford SL, Naimi AI, Yeung EH, Kim K et al. (2018) Preconception Blood Pressure Levels and Reproductive Outcomes in a Prospective Cohort of Women Attempting Pregnancy. Hypertension 71, 904–910, 10.1161/HYPERTENSIONAHA.117.10705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashimoto R and Kimura F (1989) Puberty and ovulatory release of gonadotropins in spontaneously hypertensive rats. Endocrinol. Jap 36, 675–680, 10.1507/endocrj1954.36.675 [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro RA1, Raineki C, Gonçalves O, Franci CR, Lucion AB and Sanvitto GL (2013) Reproductive dysfunction in female rats with renovascular hypertension. Am. J. Hypertens 26, 104–110, 10.1093/ajh/hps026 [DOI] [PubMed] [Google Scholar]
- 78.Lunelli RP, Irigoyen MC and Goldmeier S (2018) Hypertension as a risk factor for female sexual dysfunction: cross-sectional study. Rev. Bras. Enferm 71, 2477–2482, 10.1590/0034-7167-2017-0259 [DOI] [PubMed] [Google Scholar]
- 79.Choy CL, Sidi H, Koon CS, Ming OS, Mohamed IN, Guan NC et al. (2019) Systematic Review and Meta-Analysis for Sexual Dysfunction in Women with Hypertension. J. Sex Med 16, 1029–1048, 10.1016/j.jsxm.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 80.Santana LM, Perin L, Lunelli R, Inácio JFS, Rodrigues CG, Eibel B et al. (2019) Sexual Dysfunction in Women with Hypertension: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep 21, 25, 10.1007/s11906-019-0925-z [DOI] [PubMed] [Google Scholar]
- 81.Almeida SA, Teófilo JM, Anselmo Franci JA, Brentegani LG and Lamano-Carvalho TL (2000) Antireproductive effect of the calcium channel blocker amlodipine in male rats. Exp. Toxicol. Pathol 52, 353–356 [DOI] [PubMed] [Google Scholar]
- 82.Latif R, Aslam M and Mehmood T (2009) Spermatogenesis following discontinuation of calcium channel blocker amlodipine in rats. J. Ayub Med. Coll. Abbottabad 21, 25–27 [PubMed] [Google Scholar]
- 83.Kanwar U, Anand RJ and Sanyal SN (1993) The effect of nifedipine, a calcium channel blocker, on human spermatozoal functions. Contraception 48, 453–470, 10.1016/0010-7824(93)90135-T [DOI] [PubMed] [Google Scholar]
- 84.Benoff S, Cooper GW, Hurley I, Mandel FS, Rosenfeld DL, Scholl GM et al. (1994) The effect of calcium ion channel blockers on sperm fertilization potential. Fertil. Steril 62, 606–617, 10.1016/S0015-0282(16)56953-2 [DOI] [PubMed] [Google Scholar]
- 85.Schlosser J, Nakib I, Carré-Pigeon F and Staerman F (2007) Male infertility: definition and pathophysiology. Annales d’urologie 41, 127–133, 10.1016/j.anuro.2007.02.004 [DOI] [PubMed] [Google Scholar]
- 86.Brezina PR, Yunus FN and Zhao Y (2012) Effects of pharmaceutical medications on male fertility. J. Reprod. Infertil 13, 3–11 [PMC free article] [PubMed] [Google Scholar]
- 87.Morton B, Harrigan-Lum J, Albagli L and Jooss T (1974) The activation of motility in quiescent hamster sperm from the epididymis by calcium and cyclic nucleotides. Biochem. Biophys. Res. Commun 56, 372–379, 10.1016/0006-291X(74)90852-3 [DOI] [PubMed] [Google Scholar]
- 88.Morton BE, Sagadraca R and Fraser C (1978) Sperm motility within the mammalian epididymis: species variation and correlation with free calcium levels in epididymal plasma. Fertil. Steril 29, 695–698, 10.1016/S0015-0282(16)43348-0 [DOI] [PubMed] [Google Scholar]
- 89.Davis BK (1978) Effect of calcium on motility and fertilization by rat spermatozoa in vitro. Proc. Soc. Exp. Biol. Med 157, 54–56, 10.3181/00379727-157-39989 [DOI] [PubMed] [Google Scholar]
- 90.Darszon A, Nishigaki T, Beltran C and Treviño CL (2011) Ca channels in the development, maturation, and function of spermatozoa. Physiol. Rev 91, 1305–1355, 10.1152/physrev.00028.2010 [DOI] [PubMed] [Google Scholar]
- 91.Valsa J, Skandhan KP, Khan PS, Avni KP, Amith S and Gondalia M (2015) Ca and magnesium in male reproductive system and in its secretion.I.Level in normal human semen, seminal plasma and spermatozoa. Urologia 82, 174–178, 10.5301/urologia.5000039 [DOI] [PubMed] [Google Scholar]
- 92.Laganà AS, Vitale SG, Iaconianni P, Gatti S and Padula F (2016) Male infertility during antihypertensive therapy: are we addressing correctly the problem? Int. J. Fertil. Steril 10, 267–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dantas da Silva Júnior E, Palmieri de Souza B, Rodrigues JQD, Caricati-Neto A, Jurkiewicz A and Jurkiewicz NH (2014) Effects of clonidine in the isolated rat testicular capsule. Eur. J. Pharmacol 726, 16–26, 10.1016/j.ejphar.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 94.Saha L, Garg SK, Bhargava VK and Mazumdar S (2000) Role of angiotensin-converting enzyme inhibitor, lisinopril, on spermatozoal functions in rats. Methods Find. Exp. Clin. Pharmacol 22, 159–162 [PubMed] [Google Scholar]
- 95.Fogari R, Zoppi A, Corradi L, Mugellini A and Poletti LP (1998) Sexual function in hypertensive males treated with lisinopril or atenolol: a cross-over study. Am. J. Hypertens 11, 1244–1247, 10.1016/S0895-7061(98)00139-3 [DOI] [PubMed] [Google Scholar]
- 96.Fogari R, Preti P, Derosa G, Marasi G, Zoppi A, Rinaldi A et al. (2002) Effect of antihypertensive treatment with valsartan or atenolol on sexual activity and plasma testosterone in hypertensive men. Eur. J. Clin. Pharmaco 58, 177–180, 10.1007/s00228-002-0456-3 [DOI] [PubMed] [Google Scholar]
- 97.Ferrario CM and Levy P (2002) Sexual dysfunction in patients with hypertension: implications for therapy. J. Clin. Hypertens 4, 424–432, 10.1111/j.1524-6175.2002.00862.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ko DT, Hebert PR, Coffey CS, Sedrakyan A, Curtis JP and Krumholz HM (2002) Beta-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA 288, 351–357, 10.1001/jama.288.3.351 [DOI] [PubMed] [Google Scholar]
- 99.Nudell DM, Monoski MM and Lipshultz LI (2002) Common medications and drugs: how they affect male fertility. Urol. Clin. North America 29, 965–973, 10.1016/S0094-0143(02)00079-4 [DOI] [PubMed] [Google Scholar]
- 100.Boydak B, Nalbantgil S, Fici F, Nalbantgil I, Zoghi M, Ozerkan F et al. (2005) A randomised comparison of the effects of nebivolol and atenolol with and without chlorthalidone on the sexual function of hypertensive men. Clin. Drug Invest 25, 409–416, 10.2165/00044011-200525060-00006 [DOI] [PubMed] [Google Scholar]
- 101.Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A and Corradi L (2001) Sexual activity in hypertensive men treated with valsartan or carvedilol: a crossover study. Am. J. Hypertens 14, 27–31, 10.1016/S0895-7061(00)01214-0 [DOI] [PubMed] [Google Scholar]
- 102.Llisteri JL, Vidal JVL, Aznar Vincente JA, Roca MA, Bravo CP, Zamorano MAS et al. (2001) Sexual dysfunction in hypertensive patients treated with losartan. Am. J. Med. Sci 321, 336–341 [DOI] [PubMed] [Google Scholar]
- 103.Buttar HS (1997) An overview of the influence of ACE inhibitors on fetal-placental circulation and perinatal development. Mol. Cell. Biochem 176,61–71, 10.1023/A:1006822911586 [DOI] [PubMed] [Google Scholar]
- 104.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS et al. (2006) Major congenital malformations after first-trimester exposure to ACE inhibitors. N. Engl. J. Med 354, 2443–2451, 10.1056/NEJMoa055202 [DOI] [PubMed] [Google Scholar]
- 105.Sekine T, Miura K, Takahashi K and Igarashi T (2009) Children’s toxicology from bench to bed-Drug-induced renal injury (1): the toxic effects ofARB/ACEI on fetal kidney development. J. Toxicol. Sci 34, SP245–SP250 [DOI] [PubMed] [Google Scholar]
- 106.American College of Obstetricians and Gynecologists (2013) Task force on hypertension in pregnancy. Hypertension in pregnancy. Report of theAmerican College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol 122, 1122–1131 [DOI] [PubMed] [Google Scholar]
- 107.Cockburn JMV, Ounsted M and Redan CW (1982) Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and deveopment of the children. Lancet 1, 647–649, 10.1016/S0140-6736(82)92202-4 [DOI] [PubMed] [Google Scholar]
- 108.Hoeltzenbein M, Beck E, Fietz AK, Wernicke J, Zinke S, Kayser A et al. (2017) Pregnancy outcome after first trimester use of methyldopa a prospective cohort study. Hypertension 70, 201–208, 10.1161/HYPERTENSIONAHA.117.09110 [DOI] [PubMed] [Google Scholar]
- 109.Meidahl Petersen K, Jimenez-Solem E, Andersen JT, Petersen M, Brødbæk K, Køber L et al. (2012) Beta-blocker treatment during pregnancy and adverse pregnancy outcomes: a nationwide population-based cohort study. BMJ Open 2, e001185, 10.1136/bmjopen-2012-001185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Webster LM, Myers JE, Nelson-Piercy C, Harding K, Cruickshank JK, Watt-Coote I et al. (2017) Labetalol versus nifedipine as antihypertensive treatment for chronic hypertension in pregnancy: a randomized controlled trial. Hypertension 70, 915–922, 10.1161/HYPERTENSIONAHA.117.09972 [DOI] [PubMed] [Google Scholar]
- 111.Kim H, Kataru RP and Koh GY (2014) Inflammation-associated lymphangiogenesis: a double-edged sword? J. Clin. Invest 124, 936–942, 10.1172/JCI71607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abouelkheir GR, Upchurch BD and Rutkowski JM (2017) Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation’s fire? Exp. Biol. Med 242, 884–895, 10.1177/1535370217697385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balasubbramanian D, Lopez Gelston CA, Rutkowski JM and Mitchell BM (2019) Immune cell trafficking, lymphatics and hypertension. Br. J.Pharmacol 176, 1978–1988, 10.1111/bph.14370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aebischer D, Iolyeva M and Halin C (2014) The inflammatory response of lymphatic endothelium. Angiogenesis 17, 383–393, 10.1007/s10456-013-9404-3 [DOI] [PubMed] [Google Scholar]
- 115.Aspelund A, Robciuc MR, Karaman S, Makinen T and Alitalo K (2016) Lymphatic system in cardiovascular medicine. Circ. Res 118, 515–530, 10.1161/CIRCRESAHA.115.306544 [DOI] [PubMed] [Google Scholar]
- 116.Betterman KL and Harvey NL (2016) The lymphatic vasculature: development and role in shaping immunity. Immunol. Rev 271, 276–292, 10.1111/imr.12413 [DOI] [PubMed] [Google Scholar]
- 117.Fawcett DW, Neaves WB and Flores MN (1973) Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol. Reprod 9, 500–532, 10.1093/biolreprod/9.5.500 [DOI] [PubMed] [Google Scholar]
- 118.Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M et al. (2012) The origin of lymphatic capillaries in murine testes. J. Androl 33,745–751, 10.2164/jandrol.111.015156 [DOI] [PubMed] [Google Scholar]
- 119.Svingen T, Francois M, Wilhelm D and Koopman P (2012) Three-dimensional imaging of Prox1-EGFP transgenic mouse gonads reveals divergent modes of lymphangiogenesis in the testis and ovary. PLoS ONE 7, e52620, 10.1371/journal.pone.0052620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rouvière H (1932) L’anatomie des lymphatiques de l’homme, Masson, Paris [Google Scholar]
- 121.Yoffey JM and Courtice FC (1970) Lmphatics, Lymph and the Lymphomyeloid Complex, Academic Press, London [Google Scholar]
- 122.Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP and Murfee WL (2018) Lymphatic Vessel Network Structure and Physiology. Compr.Physiol 9, 207–299, 10.1002/cphy.c180015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clark RV (1976) Three-dimensional organization of testicular interstitial tissue and lymphatic space in the rat. Anat. Rec 184, 203–225, 10.1002/ar.1091840207 [DOI] [PubMed] [Google Scholar]
- 124.Fawcett DW, Heidger PM and Leak LV (1969) Lymph vascular system of the interstitial tissue of the testis as revealed by electron microscopy. J.Reprod. Fertil 19, 109–119, 10.1530/jrf.0.0190109 [DOI] [PubMed] [Google Scholar]
- 125.Poirier P, Cuneo B and Delamere G (1903) The Lymphatics: General Anatomy of the Lymphatics, Archibald Constable and Co, London [Google Scholar]
- 126.Jamieson JK and Dobson JF (1910) The lymphatics of the testicle. Lancet 175, 493–495, 10.1016/S0140-6736(01)74774-5 [DOI] [Google Scholar]
- 127.Perez-Clavier R and Harrison RG (1978) The pattern of lymphatic drainage of the rat testis. J. Anat 127, 93–100 [PMC free article] [PubMed] [Google Scholar]
- 128.Engeset A (1959) The route of peripheral lymph to the blood stream; an x-ray study of the barrier theory. J. Anat 93, 96–100 [PMC free article] [PubMed] [Google Scholar]
- 129.Paul M (1950) The blood and lymph pathways in the spermatic cord.. Ann. R. Coll. Surg. Engl 7, 128–150 [PMC free article] [PubMed] [Google Scholar]
- 130.Lindner HR (1963) Partition of androgen between the lymph and venous blood of the testis in the ram. J. Endocrinol 25, 483–494, 10.1677/joe.0.0250483 [DOI] [Google Scholar]
- 131.Setchell BP and Cox JE (1982) Secretion of free and conjugated steroids by the horse testis into lymph and venous blood. J. Reprod. Fertil. Suppl 32, 123–127 [PubMed] [Google Scholar]
- 132.Setchell BP, Laurie MS, Flint AP and Heap RB (1983) Transport of free and conjugated steroids from the boar testis in lymph, venous blood and rete testis fluid. J. Endocrinol 96, 127–136, 10.1677/joe.0.0960127 [DOI] [PubMed] [Google Scholar]
- 133.Ichikawa S, Uchino S and Hirata Y (1987) Lymphatic and blood vasculature of the forming corpus luteum. Lymphology 20, 73–83 [PubMed] [Google Scholar]
- 134.Otsuki Y, Magari S and Sugimoto O (1987) Fine structure and morphometric analysis of lymphatic capillaries in the developing corpus luteum of the rabbit. Lymphology 20, 64–72 [PubMed] [Google Scholar]
- 135.Xu F and Stouffer RL (2009) Existence of the lymphatic system in the primate corpus luteum. Lymphat. Res. Biol 7, 159–168, 10.1089/lrb.2009.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brown HM, Robker RL and Russell DL (2010) Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology 151,5446–5455, 10.1210/en.2010-0629 [DOI] [PubMed] [Google Scholar]
- 137.Anderson DH (1926) Lymphatics and blood-vessels of the ovary of the sow. Contributions to embryology, vol. 17, pp. 107–123, Carnegie Institution of Washington publication, Washington DC [Google Scholar]
- 138.Morris B and Sass MB (1966) The formation of lymph in the ovary. Proc. R. Soc. Lond. B Biol. Sci 164, S77–S91 [Google Scholar]
- 139.Rutkowski JM, Ihm JE, Lee ST, Kilarski WW, Greenwood VI, Pasquier MC et al. (2013) VEGFR-3 neutralization inhibits ovarian lymphangiogenesis, follicle maturation, and murine pregnancy. Am. J. Pathol 183, 1596–1607, 10.1016/j.ajpath.2013.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Poirier P and Charpy A (1901) Lymphatiques de l’ovaire. Traité d’anatomie humaine, pp. 1199–1200, Masson Et Cie, Paris [Google Scholar]
- 141.Polano O (1903) XIV. Beiträge zur Anatomie der Lymphbahnen im menschlichen Eierstock. Monatsschrift für Geburtshilfe und Gynäkologie 17,281–295 [Google Scholar]
- 142.Bartels P (1909) Das Lymphgefäßsystem. Bardeleben, Handbuch der Anatomie des Menschen, pp. 172–185, Fischer Gustav, Jena [Google Scholar]
- 143.Kleppe M, Kraima AC, Kruitwagen RF, Van Gorp T, Smit NN, van Munsteren JC et al. (2015) Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int. J. Gynecol. Cancer 25, 1405–1414, 10.1097/IGC.0000000000000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daniel PM, Gale MM and Pratt OE (1963) Hormones and related substances in the lymph leaving four endocrine glands-the testis, ovary, adrenal, and thyroid. Lancet 1, 1232–1234, 10.1016/S0140-6736(63)91863-4 [DOI] [PubMed] [Google Scholar]
- 145.Lindner HR, Sass MB and Morris B (1964) Steroids in the Ovarian Lymph and Blood of Conscious Ewes. J. Endocrinol 30, 361–376, 10.1677/joe.0.0300361 [DOI] [PubMed] [Google Scholar]
- 146.Stefanczyk-Krzymowska S and Krzymowski T (2002) Local adjustment of blood and lymph circulation in the hormonal regulation of reproduction in female pigs-facts, conclusions and suggestions for future research. Reprod. Biol 2, 115–132 [PubMed] [Google Scholar]
- 147.Stefanczyk-Krzymowska S, Krzymowski T, Wasowska B and Chlopek J (2002) Retrograde transfer of ovarian steroid hormones to the ovary in the porcine periovarian vascular complex. Exp. Physiol 87, 361–371, 10.1113/eph8702338 [DOI] [PubMed] [Google Scholar]
- 148.De-Casseye MJ, De-Bled G, Gepts W and Schoysman R (1980) An immunohistochemical study for testicular biopsies in cases of male infertility. Andrologia 12, 122–129, 10.1111/j.1439-0272.1980.tb00594.x [DOI] [PubMed] [Google Scholar]
- 149.Salomon F, Saremaslani P, Jakob M and Hedinger CE (1982) Immune complex orchitis in infertile men. Immunoelectron microscopy of abnormal basement membrane structures. Lab. Invest 47, 555–567 [PubMed] [Google Scholar]
- 150.Lehmann D, Temminck B, Da Rugna D, Leibundgut B, Sulmoni A and Müller H (1987) Role of immunological factors in male infertility.Immunohistochemical and serological evidence. Lab. Invest 57, 21–28 [PubMed] [Google Scholar]
- 151.Lehmann D and Emmons LR (1989) Immunological phenomena observed in the testis and their possible role in infertility. Am. J. Reprod. Immunol 19, 43–52, 10.1111/j.1600-0897.1989.tb00547.x [DOI] [PubMed] [Google Scholar]
- 152.Ooba T, Ishikawa T, Yamaguchi K, Kondo Y, Sakamoto Y and Fujisawa M (2008) Expression and distribution of laminin chains in the testis for patients with azoospermia. J. Androl 29, 147–152, 10.2164/jandrol.107.003210 [DOI] [PubMed] [Google Scholar]
- 153.Naito M and Itoh M (2008) Patterns of infiltration of lymphocytes into the testis under normal and pathological conditions in mice. Am. J. Reprod.Immunol 59, 55–61, 10.1111/j.1600-0897.2007.00556.x [DOI] [PubMed] [Google Scholar]
- 154.Fijak M, Pilatz A, Hedger MP, Nicolas N, Bhushan S, Michel V et al. (2018) Infectious, inflammatory and ‘autoimmune’ male factor infertility: how do rodent models inform clinical practice? Hum. Reprod. Update 24, 416–441, 10.1093/humupd/dmy009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sato K, Hirokawa K and Hatakeyama S (1981) Experimental allergic orchitis in mice. Histopathological and immunological studies. Virchows Arch.A Pathol. Anat. Histol 392, 147–158, 10.1007/BF00430817 [DOI] [PubMed] [Google Scholar]
- 156.Kohno S, Munoz JA, Williams TM, Teuscher C, Bernard CC and Tung KS (1983) Immunopathology of murine experimental allergic orchitis. J.Immunol 130, 2675–2682 [PubMed] [Google Scholar]
- 157.Itoh M, Hiramine C and Hojo K (1991) A new murine model of autoimmune orchitis induced by immunization with viable syngeneic testicular germ cells alone. I. Immunological and histological studies. Clin. Exp. Immunol 83, 137–142, 10.1111/j.1365-2249.1991.tb05603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tung KS (1995) Elucidation of autoimmune disease mechanism based on testicular and ovarian autoimmune disease models. Horm. Metab. Res 27, 539–543, 10.1055/s-2007-980021 [DOI] [PubMed] [Google Scholar]
- 159.Guazzone VA, Jacobo P, Theas MS and Lustig L (2009) Cytokines and chemokines in testicular inflammation: A brief review. Microsc. Res.Techniq 72, 620–628, 10.1002/jemt.20704 [DOI] [PubMed] [Google Scholar]
- 160.Jacobo P, Guazzone VA, Theas MS and Lustig L (2011) Testicular autoimmunity. Autoimmun. Rev 10, 201–204, 10.1016/j.autrev.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 161.Guazzone VA, Rival C, Denduchis B and Lustig L (2003) Monocyte chemoattractant protein-1 (MCP-1/CCL2) in experimental autoimmune orchitis. J. Reprod. Immunol 60, 143–157, 10.1016/j.jri.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 162.Suescun MO, Rival C, Theas MS, Calandra RS and Lustig L (2003) Involvement of tumor necrosis factor-alpha in the pathogenesis of autoimmune orchitis in rats. Biol. Reprod 68, 2114–2121, 10.1095/biolreprod.102.011189 [DOI] [PubMed] [Google Scholar]
- 163.O’Bryan MK, Gerdprasert O, Nikolic-Paterson DJ, Meinhardt A, Muir JA, Foulds LM et al. (2005) Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol 288, R1744–R1755, 10.1152/ajpregu.00651.2004 [DOI] [PubMed] [Google Scholar]
- 164.Rival C, Lustig L, Iosub R, Guazzone VA, Schneider E, Meinhardt A et al. (2006) Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 324, 311–318, 10.1007/s00441-005-0129-5 [DOI] [PubMed] [Google Scholar]
- 165.Klein B, Haggeney T, Fietz D, Indumathy S, Loveland KL, Hedger M et al. (2016) Specific immune cell and cytokine characteristics of human testicular germ cell neoplasia. Hum. Reprod 31, 2192–2202, 10.1093/humrep/dew211 [DOI] [PubMed] [Google Scholar]
- 166.Syed V, Stephan JP, Gerard N, Legrand A, Parvinen M, Bardin CW et al. (1995) Residual bodies activate Sertoli cell interleukin-1a (IL-1a) release, which triggers IL-6 production by an autocrine mechanism, through the lipoxygenase pathway. Endocrinology 136, 3070, 10.1210/endo.136.7.7789334 [DOI] [PubMed] [Google Scholar]
- 167.Guazzone VA, Jacobo P, Denduchis B and Lustig L (2012) Expression of cell adhesion molecules, chemokines and chemokine receptors involved in leukocyte traffic in rats undergoing autoimmune orchitis. Reproduction 143, 651–662, 10.1530/REP-11-0079 [DOI] [PubMed] [Google Scholar]
- 168.Rival C, Guazzone VA, von Wulffen W, Hackstein H, Schneider E, Lustig L et al. (2007) Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol. Hum. Reprod 13, 853–861, 10.1093/molehr/gam067 [DOI] [PubMed] [Google Scholar]
- 169.Schell C, Albrecht M, Mayer C, Schwarzer JU, Frungieri MB and Mayerhofer A (2008) Exploring human testicular peritubular cells: identification of secretory products and regulation by tumor necrosis factor-alpha. Endocrinology 149, 1678–1686, 10.1210/en.2007-1064 [DOI] [PubMed] [Google Scholar]
- 170.Spiess AN, Feig C, Schulze W, Chalmel F, Cappallo-Obermann H, Primig M et al. (2007) Cross-platform gene expression signature of human spermatogenic failure reveals inflammatory-like response. Hum. Reprod 22, 2936–2946, 10.1093/humrep/dem292 [DOI] [PubMed] [Google Scholar]
- 171.Ellis PJ, Furlong RA, Conner SJ, Kirkman-Brown J, Afnan M, Barratt C et al. (2007) Coordinated transcriptional regulation patterns associated with infertility phenotypes in men. J. Med. Genet 44, 498–508, 10.1136/jmg.2007.049650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Meineke V, Frungieri MB, Jessberger B, Vogt H and Mayerhofer A (2000) Human testicular mast cells contain tryptase: increased mast cell number and altered distribution in the testes of infertile men. Fertil. Steril 74, 239–2344, 10.1016/S0015-0282(00)00626-9 [DOI] [PubMed] [Google Scholar]
- 173.Welter H, Köhn FM and Mayerhofer A (2011) Mast cells in human testicular biopsies from patients with mixed atrophy: increased numbers, heterogeneity and expression of cyclooxygenase 2 and prostaglandin D2 synthase. Fertil. Steril 96, 309–313, 10.1016/j.fertnstert.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 174.Frungieri MB, Weidinger S, Meineke V, Kohn FM and Mayerhofer A (2002) Proliferative action of mast-cell tryp-tase is mediated by PAR2, COX2, prostaglandins, and PPARgamma: possible relevance to human fibrotic disorders. Proc. Natl. Acad. Sci. U.S.A 99, 15072–15077, 10.1073/pnas.232422999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Albrecht M, Rämsch R, Köhn FM, Schwarzer JU and Mayerhofer A (2006) Isolation and cultivation of human testicular peritubular cells: a new model for the investigation of fibrotic processes in the human testis and male infertility. J. Clin. Endocrinol. Metab 91, 1956–1960, 10.1210/jc.2005-2169 [DOI] [PubMed] [Google Scholar]
- 176.Mayerhofer A (2013) Human testicular peritubular cells: more than meets the eye. Reproduction 145, R107–R116, 10.1530/REP-12-0497 [DOI] [PubMed] [Google Scholar]
- 177.Iosub R, Klug J, Fijak M, Schneider E, Frohlich S, Blumbach K et al. (2006) Development of testicular inflammation in the rat involves activation of proteinase-activated receptor-2. J. Pathol 208, 686–698, 10.1002/path.1938 [DOI] [PubMed] [Google Scholar]
- 178.Aslani F, Schuppe HC, Guazzone VA, Bhushan S, Wahle E, Lochnit G et al. (2015) Targeting high mobility group box protein 1 ameliorates testicular inflammation in experimental autoimmune orchitis. Hum. Reprod 30, 417–431, 10.1093/humrep/deu320 [DOI] [PubMed] [Google Scholar]
- 179.Pérez CV, Gómez LG, Gualdoni GS, Lustig L, Rabinovich GA and Guazzone VA (2015) Dual roles of endogenous and exogenous galectin-1 in the control of testicular immunopathology. Sci. Rep 5, 12259, 10.1038/srep12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gao J, Wang X, Wang Y, Han F, Cai W, Zhao B et al. (2016) Murine Sertoli cells promote the development of tolerogenic dendritic cells: a pivotal role of galectin-1. Immunology 148, 253–265, 10.1111/imm.12598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nicolas N, Michel V, Bhushan S, Wahle E, Hayward S, Ludlow H et al. (2017) Testicular activin and follistatin levels are elevated during the course of experimental autoimmune epididymo-orchitis in mice. Sci. Rep 7, 42391, 10.1038/srep42391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nicolas N, Muir JA, Hayward S, Chen JL, Stanton PG, Gregorevic P et al. (2017) Induction of EAO in mice: responses to elevated circulating levels of the follistatin. Reproduction 154, 193–205, 10.1530/REP-17-0010 [DOI] [PubMed] [Google Scholar]
- 183.Fijak M and Meinhardt A (2006) The testis in immune privilege. Immunol. Rev 213, 66–81, 10.1111/j.1600-065X.2006.00438.x [DOI] [PubMed] [Google Scholar]
- 184.Pérez CV, Theas MS, Jacobo PV, Jarazo-Dietrich S, Guazzone VA and Lustig L (2013) Dual role of immune cells in the testis - protective or pathogenic for germ cells? Spermatogenesis 3, e23870–e23871, 10.4161/spmg.23870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pérez CV, Sobarzo CM, Jacobo PV, Pellizzari EH, Cigorraga SB, Denduchis B et al. (2012) Loss of occludin expression and impairment of blood-testis barrier permeability in rats with autoimmune orchitis: effect of interleukin 6 on Sertoli cell tight junctions. Biol. Reprod 87, 122, 10.1095/biolreprod.112.101709 [DOI] [PubMed] [Google Scholar]
- 186.Zhang H, Yin Y, Wang G, Liu Z, Liu L and Sun F (2014) Interleukin-6 disrupts blood-testis barrier through inhibiting protein degradation or activating phosphorylated ERK in Sertoli cells. Sci. Rep 4, 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jacobo PV, Fass M, Pérez CV, Jarazo-Dietrich S, Lustig L and Theas MS (2012) Involvement of soluble Fas Ligand in germ cell apoptosis in testis of rats undergoing autoimmune orchitis. Cytokine 60, 385–392, 10.1016/j.cyto.2012.07.020 [DOI] [PubMed] [Google Scholar]
- 188.Oh YS, Jo NH, Park JK and Gye MC (2016) Changes in inflammatory cytokines accompany deregulation of Claudin-11, resulting in inter-Sertoli tight junctions in varicocele rat testes. J. Urol 196, 1303–1312, 10.1016/j.juro.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 189.Alitalo K, Tammela T and Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 428, 946–953, 10.1038/nature04480 [DOI] [PubMed] [Google Scholar]
- 190.Oliver G and Alitalo K (2005) The lymphatic vasculature: recent progress and paradigms. Annu. Rev. Cell Dev. Biol 21, 457–483, 10.1146/annurev.cellbio.21.012704.132338 [DOI] [PubMed] [Google Scholar]
- 191.Ji RC (2006) Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 25, 677–694, 10.1007/s10555-006-9026-y [DOI] [PubMed] [Google Scholar]
- 192.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C et al. (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol 161, 947–956, 10.1016/S0002-9440(10)64255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C et al. (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Invest 113, 1040–1050, 10.1172/JCI20465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ et al. (2005) Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest 115, 247–257, 10.1172/JCI200522037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kerjaschki D (2005) The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest 115, 2316–2319, 10.1172/JCI26354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M et al. (2005) Inflammation induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest 115, 2363–2372, 10.1172/JCI23874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K et al. (2008) Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 15, 68. [DOI] [PubMed] [Google Scholar]
- 198.Rui-Cheng J (2011) Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell. Mol. Life Sci 69,897–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Naito M, Hirai S, Terayama H, Qu N, Kuerban M, Musha M et al. (2012) Postinflammation stage of autoimmune orchitis induced by immunization with syngeneic testicular germ cells alone in mice. Med. Mol. Morphol 45, 35–44, 10.1007/s00795-011-0539-2 [DOI] [PubMed] [Google Scholar]
- 200.Hirai S, Naito M, Terayama H, Qu N, Kuerban M, Musha M et al. (2013) Lymphangiogenesis in chronic inflammation in the testis. Andrology 1,147–154, 10.1111/j.2047-2927.2012.00015.x [DOI] [PubMed] [Google Scholar]
- 201.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R Demory A et al. (2006) Lymphatic endotheliumspecific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80 +, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J. Pathol 209, 67–77, 10.1002/path.1942 [DOI] [PubMed] [Google Scholar]
- 202.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE et al. (2009) Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 113, 5650–5659, 10.1182/blood-2008-09-176776 [DOI] [PubMed] [Google Scholar]
- 203.Rhim SH, Millar SE, Robey F, Luo AM, Lou YH, Yule T et al. (1992) Autoimmune disease of the ovary induced by a ZP3 peptide from the mouse zona pellucida. J. Clin. Invest 89, 28–35, 10.1172/JCI115572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tung KS, Agersborg SS, Alard P, Garza KM and Lou YH (2001) Regulatory T-cell, endogenous antigen and neonatal environment in the prevention and induction of autoimmune disease. Immunol. Rev 182, 135–148, 10.1034/j.1600-065X.2001.1820111.x [DOI] [PubMed] [Google Scholar]
- 205.Bagavant H, Sharp C, Kurth B and Tung KS (2002) Induction and immunohistology of autoimmune ovarian disease in cynomolgus macaques(Macaca fascicularis). Am. J. Pathol 160, 141–149, 10.1016/S0002-9440(10)64358-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lahoti A, Prasannan L and Speiser PW (2016) Premature ovarian failure. In Abnormal Female Puberty (Appelbaum HL, ed.), pp. 67–85, Springer,New York [Google Scholar]
- 207.Bagavant H, Adams SE, Terranova P, Chang A, Kramer FW, Lou YH et al. (1999) Autoimmune ovarian inflammation triggered by proinflammatory (Th1) T cells is compatible with normal ovarian function. Biol. Reprod 61, 635–642, 10.1095/biolreprod61.3.635 [DOI] [PubMed] [Google Scholar]
- 208.Hill JA, Welch WR, Faris HMP and Anderson DJ (1990) Induction of class II histocompatibility complex antigen expression on human granulosa cells by interferon gamma: a potential mechanism contributing to autoimmune failure. Am. J. Obstet. Gynecol 162, 534–540, 10.1016/0002-9378(90)90425-7 [DOI] [PubMed] [Google Scholar]
- 209.Liu P, Zhang X, Hu J, Cui L, Zhao S, Jiao X et al. (2020) Dysregulated cytokine profile associated with biochemical premature ovarian insufficiency. Am. J. Reprod. Immunol 00, e13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lessey B, Lebovic D and Taylor R (2013) Eutopic endometrium in women with ndometriosis: ground zero for the study of implantation defects. Semin. Reprod. Med 31, 109–124, 10.1055/s-0032-1333476 [DOI] [PubMed] [Google Scholar]
- 211.Reis FM, Petraglia F and Taylor RN (2013) Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum.Reprod. Update 19, 406–418, 10.1093/humupd/dmt010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Takebayashi A, Kimura F, Kishi Y, Ishida M, Takahashi A, Yamanaka A et al. (2015) Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol 73, 221–231, 10.1111/aji.12331 [DOI] [PubMed] [Google Scholar]
- 213.Anastasiu CV, Moga MA, Neculau AE, Balan A, Scarneciu I, Dragomir RM et al. (2020) Biomarkers for the Noninvasive Diagnosis ofEndometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci 21, 1750, 10.3390/ijms21051750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Red-Horse K (2008) Lymphatic vessel dynamics in the uterine wall. Placenta 29, S55–S59, 10.1016/j.placenta.2007.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nilsson MB, Langley RR and Fidler IJ (2005) Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 65, 10794–10800, 10.1158/0008-5472.CAN-05-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Szlosarek PW, Grimshaw MJ, Kulbe H, Wilson JL, Wilbanks GD, Burke F et al. (2006) Expression and regulation of tumor necrosis factor α in normal and malignant ovarian epithelium. Mol. Cancer Ther 5, 382–390, 10.1158/1535-7163.MCT-05-0303 [DOI] [PubMed] [Google Scholar]
- 217.Reichelt U, Keichel S, Barcena de Arellano ML, Chiantera V, Schneider A and Mechsner S (2012) High lymph vessel density and expression of lymphatic growth factors in peritoneal endometriosis. Reprod. Sci 19, 876–882, 10.1177/1933719112438440 [DOI] [PubMed] [Google Scholar]
- 218.Hattori K, Ito Y, Honda M, Sekiguchi K, Hosono K, Shibuya M et al. (2020) Lymphangiogenesis induced by vascular endothelial growth factor receptor 1 signaling contributes to the progression of endometriosis in mice. J. Pharmacol. Sci S1347–S8613, 30047–30055 [DOI] [PubMed] [Google Scholar]


