Introduction
The Coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) quickly spread worldwide on an unprecedented scale after emerging in late 2019. Neurological complications of the central, peripheral, and autonomic nervous systems are being reported with increasing frequency. Patients with autonomic disorders, including postural orthostatic tachycardia syndrome (POTS), experienced significant disability for several months after resolving acute infection [1, 2]. After the emergent authorization of COVID-19 vaccines to save lives, various neurological adverse events, ranging from a mild headache to cerebral venous thrombosis, have been reported [3]. We report a case of a 40-year-old man who was diagnosed with POTS after the messenger RNA COVID-19 vaccination.
Case report
A 40-year-old man presented with a 8-week history of intermittent headache, palpitation, fatigue, and dyspnea, which developed one week after receiving the first dose of Moderna COVID-19 vaccine. The palpitation was especially aggravated in orthostatic and postprandial status. He had no significant past medical history, including adverse side effects to any prior vaccines. Before being referred to the neurologic clinic, he underwent several studies, including echocardiography, pulmonary function test, computed tomography of chest and abdomen, and laboratory studies such as complete blood count, routine chemistry, liver enzymes, and thyroid function test. However, these studies revealed no apparent abnormal findings. 24-h Holter monitoring showed episodes of sinus tachycardia.
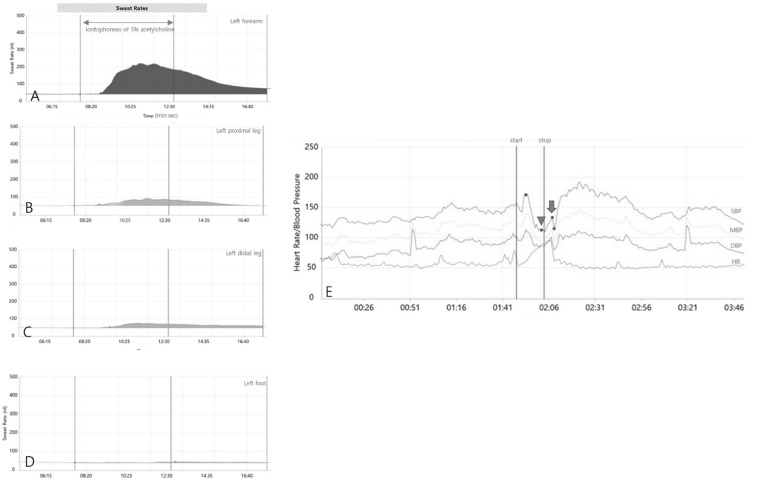
Neurological examination was normal. Autonomic function tests were performed using the technique of Low et al. [4] and in the following order: sudomotor, cardiovagal, adrenergic, and head-up tilt. The quantitative sudomotor axon reflex test (QSART) was performed using Q-Sweat (Quantitative Sweat Measurement System, WR Electronics, Rochester, MN, USA). QSART revealed reduced reflex sweating in the proximal and distal leg and in the proximal foot (Fig. 1A–D). The heart rate response to deep breathing and Valsalva ratio was normal. In the systolic blood pressure response (SBP) to a Valsalva maneuver, SBP decreased up to 33 mmHg compared with baseline during early phase II and did not return to baseline during late phase II (Fig. 1E). Head-up tilt test revealed orthostatic tachycardia with an increment of heart rate from 72 to 110 beats per minute without significant decrement of blood pressure. Additional serologic tests, including autoimmune disease, thiamine, vitamin B12, and porphobilinogen, showed no remarkable findings. With a diagnosis of POTS and mild autonomic dysfunction, he received propranolol (20 mg, three times daily) for 2 months, resulting in improved symptoms. Five months after onset, symptoms were nearly resolved without medication.
Fig. 1.
Quantitative sudomotor axon reflex test revealed normal response in the forearm (A) and reduced response in the proximal leg (B), distal leg (C), and foot (D). During Valsalva maneuver, systolic blood pressure decreased up to 33 mmHg in early phase II (arrowhead) and did not return to baseline in late phase II (arrow) (E). SBP systolic blood pressure, MBP mean blood pressure, DBP diastolic blood pressure, HR heart rate
Discussion
POTS is defined as a sustained heart rate increment of more than 30 beats per minute within 10 min of active standing or head-up tilt without orthostatic hypotension. It has a wide range of clinical manifestations, such as palpitation, dizziness, presyncope, fatigue, dyspnea, mental clouding, blurry vision, and orthostatic intolerance. Diagnosing POTS can be challenging due to a lack of awareness of the syndrome and heterogeneity of symptoms, so patients often report a significant delay in diagnosis, more than 5 years between the onset of symptoms and diagnosis [5]. Although the underlying pathophysiology is not fully understood, three hypotheses were suggested, including a partial autonomic neuropathy, a persistent predilection for hypovolemia, and a central hyperadrenergic state [6]. However, POTS patients will sometimes manifest features from more than one of the three mechanisms, resulting in the clinical heterogeneity seen in this syndrome [5].
POTS can be triggered by infection, surgery, pregnancy, or concussion, with post-infectious disease being the most common mode of onset. Although the pathophysiological mechanism of post-COVID-19 autonomic disorders remains speculative, autoimmunity is one of the major mechanisms in the pathophysiology of post-COVID-19 POTS [1]. Several reports have demonstrated that the SARS-CoV-2-generated antibodies cross react with components of the autonomic ganglia, autonomic nerve fibers, G-protein-coupled receptors, or other neuronal or cardiovascular receptors, which can lead to dysfunction of the autonomic nervous system [1].
There have been case reports of autonomic disorders including POTS after human papillomavirus vaccine (HPV), and autoantibodies were present in a subset of these patients [7]. Through further investigation to determine the possible causation between autonomic disorders and HPV vaccines, vaccine-triggered, immune-mediated autonomic dysfunction was proposed as a pathogenesis. Although a firm conclusion cannot be drawn from few case reports [8], COVID-19 vaccine can cause autonomic dysfunctions, such as sympathetic adrenergic, postganglionic sympathetic sudomotor dysfunction, and POTS.
Since a substantial population worldwide received a COVID-19 vaccine, many patients suffering from autonomic dysfunction, which can significantly impair quality of life, could be underdiagnosed. Clinicians need a high index of suspicion for various autonomic disorders to enable appropriate diagnostic studies and treatment.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The case report been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 Dysautonomia. Front Neurol. 2021;12:624968. doi: 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2022;43:3–40. doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Mar PL, Raj SR. Postural orthostatic tachycardia syndrome: mechanisms and new therapies. Annu Rev Med. 2020;71:235–248. doi: 10.1146/annurev-med-041818-011630. [DOI] [PubMed] [Google Scholar]
- 6.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 7.Blitshteyn S, Brinth L, Hendrickson JE, Martinez-Lavin M. Autonomic dysfunction and HPV immunization: an overview. Immunol Res. 2018;66:744–754. doi: 10.1007/s12026-018-9036-1. [DOI] [PubMed] [Google Scholar]
- 8.Reddy S, Reddy S, Arora M. A case of postural orthostatic tachycardia syndrome secondary to the messenger RNA COVID-19 vaccine. Cureus. 2021;13:e14837. doi: 10.7759/cureus.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]