Abstract
Background:
Oleuropein, the main constituent of olive fruit and leaves, has been reported to protect against insulin resistance and diabetes. While many experimental investigations have examined the mechanisms by which oleuropein improves insulin resistance and diabetes, much of these investigations have been carried out in either muscle cell lines or in vivo models two scenarios with many drawbacks. Accordingly, to simplify identification of mechanisms by which oleuropein regulates specific cellular processes, we resort, in the present study, to isolated muscle preparation which enables better metabolic milieu control and permit more detailed analyses.
Methods:
For this purpose, soleus muscles were incubated for 12 h without or with palmitate (1.5 mM) in the presence or absence of oleuropein (1.5 mM), and compound C. Insulin-stimulated glucose transport, glucose transporter type 4 (GLUT4) translocation, Akt substrate of 160 kDa (AS160) phosphorylation and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation were examined.
Results:
Palmitate treatment reduced insulin-stimulated glucose transport, GLUT4 translocation and AS160 phosphorylation, but AMPK phosphorylation was not changed. Oleuropein administration (12 h) fully rescued insulin-stimulated glucose transport, but partially restored GLUT4 translocation. However, it fully restored AS160 phosphorylation, raising the possibility that oleuropein may also have contributed to the restoration of glucose transport by increased GLUT4 intrinsic activity. Inhibition of AMPK phosphorylation with compound C (50 µM) prevented oleuropein -induced improvements in insulin-stimulated glucose transport, GLUT4 translocation, and AS160 phosphorylation.
Conclusion:
Our results clearly indicate that oleuropein alleviates palmitate-induced insulin resistance appears to occur via an AMPK-dependent mechanism involving improvements in the functionality of the AS160-GLUT4 signaling system.
Key Words: AMPK, GLUT4, Muscle, Insulin resistance, Oleuropein
Introduction
Nowadays, several medications are available to help manage Type 2 diabetes. However, most of these medications have several side effects, and their compliance is less than optimal (1). Therefore, the search for novel antidiabetic bioactive compounds with less, if any, side effects for maintaining glucose homeostasis and improving insulin sensitivity has been receiving increasing scientific attention.
In this context, oleuropein (OLE), a phenolic compound found in abundance in olive fruit and leaves of the olive tree (2,3), has attracted scientific attention in recent years because of a variety of reported therapeutic and pharmacological properties (3–8), including its ability to protect against insulin resistance and diabetes. It is very important to be noted that these studies, to a large extent, have been carried out either in muscle cell lines (9) or in vivo (8,10). While such studies are important, they are unable to isolate the precise mechanisms by which OLE manage insulin resistance. In the in vivo model a myriad of biochemical and molecular analyses can take place that creates disturbance in the substrate-endocrine milieu that can further exacerbate insulin resistance (i.e., increased glucose and insulin concentrations). On the other hand, muscle cells line such as L6 and C2C12 are not representative of mature mammalian muscles. It is also well known that metabolic rates in cell lines are low, and they respond less well than mammalian muscle to hormones such as insulin. Accordingly, using these models, with these concomitant problems would complicate identification of specific mechanisms via which OLE ameliorates insulin resistance and diabetes. To overcome these challenges and to better understand OLE’S protective mechanism of action, we resort, in the present study, to isolated muscle preparation (11). This approach permits control of the substrate-endocrine milieu and is therefore amenable to identifying more clearly the mechanisms via which OLE rescue insulin resistance (11,12).
The central anomaly in Type 2 diabetes, a worldwide metabolic disease, is the chronically elevated concentration of blood glucose (i.e hyperglycemia). This anomaly has a toxic effect on many tissues. Therefore, uncontrolled, or poorly controlled hyperglycemia contributes to a wide variety of other metabolic diseases (13). In Type 2 diabetes, hyperglycemia is due in large part to the insulin resistance in skeletal muscles (14,15) which is now widely considered a key tissue that accounts for whole body insulin resistance. This resistance has been linked to the impairments in the insulin-activated signaling machinery in muscle (16,17) and possibly in a reduced activity of cell surface glucose transporter type 4 (GLUT-4).
Insulin facilitates the transport of glucose into muscle cells via the GLUT4 in the following manner: briefly, after insulin binds to its receptor a series of signaling molecules is activated (insulin receptor substrate 1 (IRS-1)→ phosphatidylinositol 3-kinase (PI3 kinase)→ Akt substrate of 160 kDa (AS160)), which induce the translocation of GLUT4 to the cell surface (18). Another distinct mechanism responsible for glucose transport is AMPK activation. AMPK activation leads to GLUT4 translocation from the cytosol to the cell surface (19). Activation of AMPK has been proposed to explain the anti-diabetic potential of metformin, commonly used medication for the treatment of Type 2 diabetes (20), as well as several other natural bioactive compounds, including thujone (21), eugenol (22), and resveratrol (23). Given these points, in addition to the fact that AMPK signaling system is not disturbed in insulin-resistance states (24), it seems reasonable to assume that activation of AMPK could be an attractive therapeutic option to protect against insulin resistance and diabetes.
Here, the primary objective was to determine how OLE clearly facilitates recovery from insulin resistance using an isolated muscle preparation. To examine this question, we rapidly.
induced insulin resistance with a high concentration of palmitate, as previously reported (12). Subsequently, we attempted to restore insulin sensitivity with OLE.
Materials and Methods
All institutional and national guidelines for the care and use of laboratory animals were followed in this study.
Materials
Oleuropein was obtained from Sigma-Aldrich (St. Louis, MO) (purity> 98%). Total and phosphorylated proteins were determined with commercially available antibodies from the following sources: anti-Glut4 from Chemicon International (Temecula, CA); anti-AMPK and antiphospho-AMPK Thr 172 from Upstate (NY, USA); goat-anti-rabbit secondary antibodies from Chemicon International; donkey-anti-rabbit secondary antibody from Amersham Biosciences (Oakville, Ontario, Canada). All other reagents and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
All experiments were approved by Yarmouk University Animal Care and Use Committees. Male Sprague-Dawley rats (55–75 g) were used in these studies. The animals consumed normal laboratory chow and water ad libitum.
Muscle incubation
On the experimental day, the intact soleus muscles were isolated gently, cleaned free of adipose, connective tissues and blood, and immediately incubated free floating for 12 hours in 20 ml vials in a shaking water bath at 30 °C. Each vial contained 10.0 ml of warmed (30 °C) pre-gassed (95% O2-5% CO2) Medium 199 (M199) containing 5 mM glucose and supplemented with 4% Bovine Serum Albumin (BSA), penicillin (100 IU/ml), streptomycin (0.1 mg/ml), with (1.5 mM) or without (control) palmitate. During this 12 h of incubation, some muscles were treated with OLE (1.5 mM) or with OLE (1.5 mM)+compound C (50 µM), an AMPK inhibitor. We previously (25) demonstrated that 1.5 mM OLE induced maximum glucose uptake after 12 h incubation. Therefore, this concentration of OLE, along with this incubation time, was used in all subsequent experiments. Additionally, for all experiments a concentration of 1.5 mM palmitate was selected, this high concentration of palmitate in the present study is consistent with approaches in the literature (26,27).
Glucose transport
To determine the rate of glucose transport, our previously described method was used (12,21,28). Briefly, at the end of the incubation period (12 h), isolated soleus muscles that had been treated for the various experiments as described above were incubated (30 °C, 30 min, 95% O2-5% CO2) in two ml of palmitate-free Krebs-Henseleit buffer [8 mM glucose, 32 mM mannitol, and 0.1% BSA with (20 mU/ml) or without insulin]. Subsequently, muscles were washed twice [10 min, 30 °C, glucose-free Krebs-Henseleit buffer, 40 mM mannitol, 0.1% BSA, with (20 mU/ ml] or without insulin). Glucose transport was then determined in palmitate-free Krebs-Henseleit buffer (2 ml) supplemented with 0.5 µCi [3H]- 3-O-methyl glucose (3-O-MG), 1.0 µCi [14C]-mannitol, 32 mM 3-O-MG, 4 mM mannitol,4 mM pyruvate, and 0.1% BSA, in the presence (20 mU/ml) or absence of insulin for 20 min, as previously reported (21,28).
Thereafter, muscles were blotted, weighed, and solubilized followed by scintillation counting of muscle extracts. It is noteworthy to mention that soleus muscles were stimulated with insulin for 70 minutes because during this time interval the supraphysiological concentration of insulin (20 mU/ml) has been shown to evoke a significant increase in muscle insulin signaling (21,28).
Plasma membrane preparation
To determine the content of the plasmalemmal GLUT4 protein, muscle samples were transferred into fresh pre-gassed M199 supplemented with (20 mU/ml) or without insulin in the last 70 min of the 12 h incubation for the various experiments as described above. Following the 70min incubation with or without insulin, giant vesicles were prepared in which plasma membrane content of GLUT4 was measured, as we have previously reported (21,28). Briefly, soleus muscles were cut into thin layers (1–3 mm thick) with a scalpel. The scored muscles were then incubated for 75 min at 34 °C in 140 mM KCl-10 mM MOPS (pH 7.4), collagenase (150 U/ml), and aprotinin (1 mg/ml). Thereafter, the incubating medium was collected, and the remaining muscle debris as washed with 10 mM EDTA in KCl/MOPS until 7 ml had been collected. Percoll (final concentration 16%) and aprotinin (1 mg/ml) were added to the collected medium. The resulting mixture was placed at the bottom of a density gradient consisting of a 3-ml middle layer of 4% Nycodenz (wt/vol) and a 1-ml KCl-MOPS upper layer. The samples were spun at 60 g for 45 min at room temperature. After centrifugation, the vesicles were harvested from the interface of the two upper solutions and centrifuged at 12,000 g for 5 min. The supernatant fraction was aspirated, and the resulting pellet was re-suspended in KCl/MOPS. Vesicles were stored at -80 °C for subsequent analysis.
Muscle protein extraction and Western blot analysis
The expression of selected proteins was determined in soleus muscle incubated in M 199 for 12 h under the various experiments as described above. Thereafter, muscles were rapidly blotted, frozen in liquid nitrogen, and stored at -80 °C until analyzed. To measure the phosphorylation of AS160, muscles were incubated for the various experiments, as described above, followed by incubation with insulin (20 mU/ml) for 10 min, the time in which maximal phosphorylation was observed (12).
For whole muscle protein determination, frozen soleus muscles were homogenized in 2 ml of buffer. Muscle homogenate and plasma membrane protein concentrations were determined using the bicinchoninic acid assay. As we have previously reported (25,28). Proteins were separated using SDS-polyacrylamide gel electrophoresis and were detected using Western blotting.
Statistical analyses
Data were analyzed using analyses of variance. Fisher’s least squares difference test was used as the post-hoc test to determine statistical significance at selected points. All data are reported as mean ± SEM.
Results
Effects of OLE on glucose transport in palmitate-induced insulin resistant muscle
After 12 h of incubating without palmitate, the insulin-stimulated glucose uptake was increased compared to the control muscles. Palmitate incubation for 12 h lowered the insulin-stimulated glucose uptake to the rates observed in the control muscles. In the absence of palmitate, and in the presence of insulin, no further increase in glucose uptake by OLE was observed. In comparison to the markedly reduced insulin stimulated glucose uptake in muscles incubated with palmitate for 12 h. OLE, despite the presence of palmitate, was found to be sufficient to enhance the insulin-stimulated glucose uptake to levels that are similar to that seen in the muscles treated with insulin alone (p< 0.05, Fig. 1).
Fig. 1.
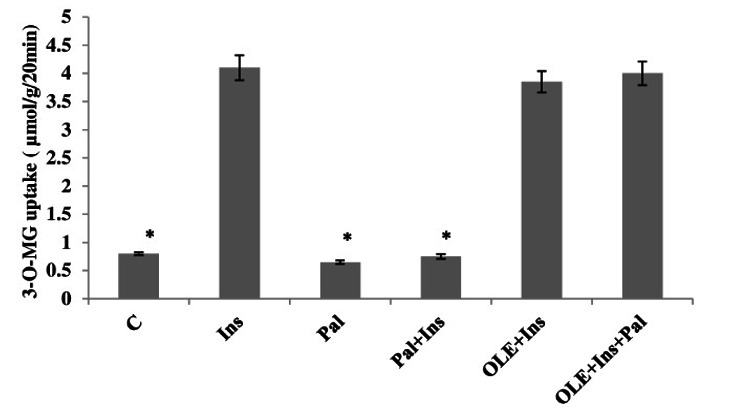
Effect of OLE treatment (12 h) on insulin (20 mU/ml) stimulated 3-O-MG transport in isolated soleus muscle incubated for 12 h with a high concentration of palmitate (2 mM). Data are presented as means±SEM, n= 6 muscles per data point. * p< 0.05 vs all other conditions.
Effects of OLE on plasmalemmal GLUT4 in palmitate-induced insulin resistant muscle
The plasma membrane GLUT4 contents were increased by insulin stimulation in muscles that had not been incubated with palmitate. These increments were similar to the muscles that were treated with OLE in the presence of insulin, but in the absence of palmitate. Inclusion of palmitate in the incubation medium for 12 h, inhibited the appearance of GLUT4 at the plasma membrane when muscles were stimulated with insulin, at this point, the control and insulin-stimulated plasmalemmal GLUT4 are similar. However, despite the presence of palmitate, inclusion of OLE to the incubating medium for 12 h restored of plasmalemmal GLUT4, when muscles were stimulated with insulin, although not quite to the levels observed in the muscles treated with insulin alone. On the other hand. The expression of GLUT4 was not altered with any of the experimental treatments (p> 0.05; Fig. 2).
Fig. 2.
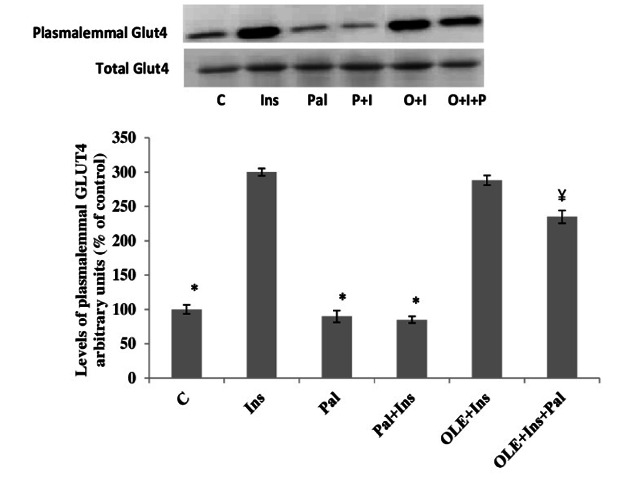
Effect of OLE treatment (12 h) on insulin (20 mU/ml) stimulated plasma membrane GLUT4 content in muscle incubated with 2 mM palmitate for 12 h. Data are presented as means±SEM, n= 6 muscles per data point. *p< 0.05 vs all other conditions; ¥< 0.05 vs Insulin (Ins) or OLE+Ins treated groups.
Effect of OLE on AS160 phosphorylation in palmitate-induced insulin resistant muscle
Neither palmitate, nor insulin, nor OLE altered the expression of AS160. Alternatively, in muscles incubated without palmitate for 12 h the magnitude of insulin stimulated AS160 phosphorylation was increased compared to the control muscles. The insulin-stimulated increased AS160 phosphorylation in the OLE -treated muscles was found to be similar to the muscles treated with insulin alone. Incubation with palmitate for 12 h reduced the magnitude of insulin stimulated AS160 phosphorylation. In contrast, treatment with OLE fully restored insulin stimulated AS160 phosphorylation to normal levels despite the continued presence of palmitate (p< 0.05, Fig. 3).
Fig. 3.
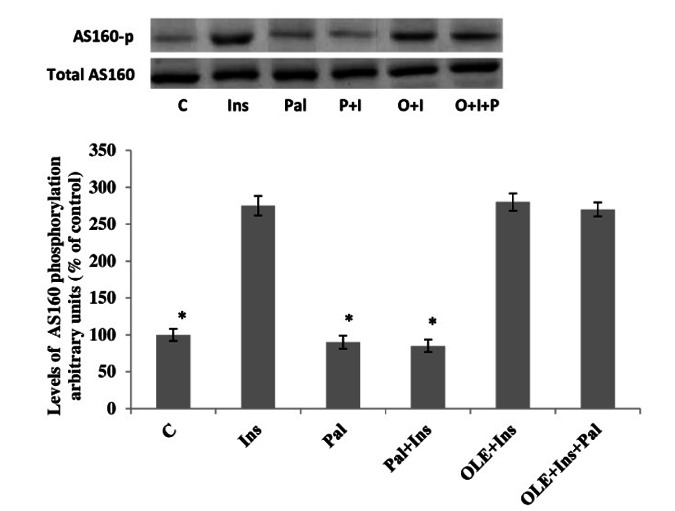
Effect of OLE treatment (12 h) on AS160 phosphorylation in soleus muscle incubated with 2mM palmitate for 12 h. Data are presented as means±SEM, n= 6 muscles per data point. * p< 0.05 vs all other conditions.
Effects of OLE on AMPK phosphorylation
Western blot analysis revealed that the phosphorylation of AMPK was considerably increased by OLE treatment, indicating an elevation in AMPK activity. Similar increase was also observed with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) (2 mM), a well-known activator of AMPK, which served as a positive control. Although palmitate treatment did not affect the phosphorylation of AMPK, the presence of OLE was observed to enhance the phosphorylation of AMPK despite the presence of palmitate. Of further note, AMPK total expression was not altered by any of the experimental treatments (Fig. 4; p> 0.05).
Fig. 4.
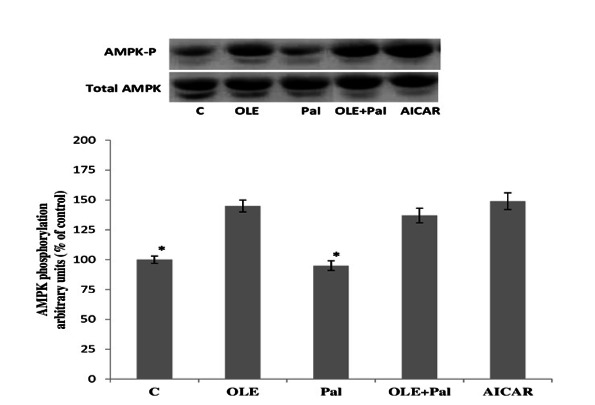
Effect of OLE treatment (12 h) on AMPK phosphorylation in soleus muscle incubated with 2mM palmitate for 12 h. Data are presented as means±SEM, n= 6 muscles per data point. * p< 0.05 vs all other conditions.
To elucidate the involvement of AMPK in OLE promoted glucose uptake, some soleus muscles were treated with 50 μM of compound C. Treatment of muscles with compound C in the presence of OLE completely blocked OLE-stimulated glucose uptake (Fig. 5A; p< 0.05). Similarly, compound C blocked OLE-induced GLUT4 translocation (Fig. 5B; p< 0.05) and AS160 phosphorylation (Fig. 5C; p< 0.05), indicating that OLE was effective in ameliorating palmitate-induced insulin resistance by AMPK-dependent pathway.
Fig. 5.
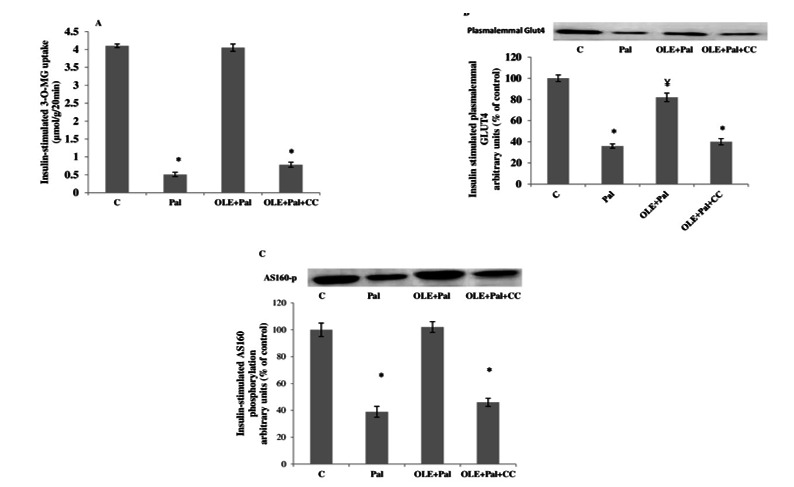
A. Effect of compound C (CC) on insulin-stimulated 3-O-MG transport in muscles that were treated with OLE (12 h) and without and with (2mM) palmitate. Data are presented as means±SEM, n= 6 muscles per data point. * p< 0.05 vs all other conditions. B. Effect of compound C (CC) on insulin stimulated plasma membrane GLUT4 content in muscles that were treated with OLE (12 h) and without and with (2 mM) palmitate. Data are presented as means±SEM, n= 6 muscles per data point. *p< 0.05 vs all other conditions; ¥< 0.05 vs C group. C. Effect of compound C (CC) on insulin stimulated AS160 phosphorylation in muscles that were treated with OLE (12 h) and without and with (2 mM) palmitate. Data are presented as means±SEM, n= 6 muscles per data point. *p< 0.05 vs all other conditions.
Discussion
This study was undertaken to determine whether OLE, a polyphenolic compound enriched in olive oil and leaves, was able to rescue palmitate (1.5 mM)-induced insulin resistance in isolated mammalian soleus muscle. There are several novel observations, which are as follows: i) In insulin-resistant muscle, OLE treatment for 12 h completely restored insulin-stimulated glucose uptake a process that was inhibited by palmitate. Importantly, ii) this marked improvement in insulin action on glucose uptake was closely associated with complete restoration of AS160 phosphorylation, but iii) there was only a partial restoration of insulin stimulated GLUT4 translocation to the plasma membrane. The data further suggest iv) that increasing AMPK phosphorylation appears to be one of the mechanisms by which OLE ameliorates palmitate-induced insulin resistance. In control muscles, alterations in either insulin stimulated glucose transport or in the post-receptor insulin signaling pathway, after 12 h of incubation with palmitate, parallel the observations in our previous studies (29,30). Additionally, treatment with OLE for 12 h provides no further stimulation beyond that provided by insulin. Therefore, the alleviation of palmitate-induced insulin resistance, after OLE treatment, is a consequence of an additive effect. Therefore, OLE treatment appears to induce corrections in the mechanisms by which palmitate induced insulin resistance, and thereby make the soleus muscles more responsive to insulin action.
It has been observed that OLE treatment reduced serum glucose, in various diabetic models in rodents. It has been shown that OLE improved serum glucose levels in diabetic rats induced by alloxan (10) or streptozotocin (8). OLE has also shown that treating C2C12 cells with OLE normalized palmitate-induced insulin resistance (31). The end results of these studies are that OLE improves insulin mediated peripheral glucose disposal. However, the precise action mechanism contributing to OLE-ameliorated insulin resistance has not been fully elucidated. It has been demonstrated that defect in the GLUT4 recruitment from the intracellular storage sites to cell surfaces plays a pivotal role in the induction of insulin resistance (30,32,33).
A major finding of the present study is that 12 h of OLE treatment ameliorated the inhibitory effect of palmitate on insulin stimulated GLUT4 translocation to the plasma membrane. This effect of OLE on GLUT4 translocation is also supported by other studies (31). The restorative effects of OLE on insulin-stimulated glucose transport and GLUT4 translocation did not completely parallel each other; suggesting that regulation of GLUT4 translocation may not be the only mechanism involved in glucose-lowering effect of OLE. However, OLE treatment might exert an insulin-sensitizing effect in muscle by other mechanism(s), possibly an improved intrinsic activity of GLUT4.
Several previous studies (12,29,34) have suggested that phosphorylation/activation of AS160 is critical and prerequisite step for full GLUT4 translocation to cell surfaces by insulin and/or AMPK signaling systems. This possibility was examined in the present study when insulin-resistant muscles were treated with OLE. Our results reveal that OLE treatment for 12 h fully restored insulin-stimulated AS160 phosphorylation in the palmitate-treated muscles, this restoration was also associated with full restoration in insulin-stimulated plasma membrane GLUT4 content. Collectively, this entails that other signal may also be needed to fully restore GLUT4 translocation.
AMPK is an interesting mechanism that has been widely thought that its activation in skeletal muscles contributes to insulin signaling through AS160-GLUT4 signaling molecules (35,36), where AMPK activation promotes the activation of AS160 which in turns stimulates the translocation of GLUT4 to the muscle cells membrane, thus it results involved in the peripheral glucose uptake, contributing to controlling the blood glucose levels. In the present study, the role of AMPK activation on OLE ameliorating palmitate induced insulin resistance has been questioned. When AMPK phosphorylation was inhibited by compound C, OLE failed to exert its insulin-sensitizing effects. This observation, as well as others (9), suggests that OLE contributes to insulin signaling in an AMPK-dependent manner to sensitize the insulin signaling proteins to insulin. Such effects may account, in part, to the beneficial effects of OLE on insulin stimulated glucose uptake in insulin resistant muscle.
Collectively, these results indicate that normalizing insulin sensitivity in palmitate-treated muscle by OLE administration is largely accounted for by the improvement in insulin-stimulated GLUT4 translocation, which may be related to improvements in the AS160 functionality. This restoration in the AS160 functionality and GLUT4 translocation could easily be attributed to the activation of AMPK, as inhibition of AMPK phosphorylation provoked a complete inhibition in insulin-stimulated glucose uptake, GLUT4 translocation and AS160 phosphorylation.
Our work results have identified OLE as a compound that possesses potent anti-diabetic potential, where it facilitates and normalizes insulin sensitivity in insulin resistance muscle via activation of the GLUT4-AMPK signaling pathway. Therefore, OLE could represent a promising therapeutic agent to prevent and treat Type 2 diabetes.
Acknowledgements
We sincerely thank Dr Christopher Perry from York University, Canada, for his help with the language editing of this manuscript.
References
- 1.Howlett HC, Bailey CJ. A risk-benefit assessment of metformin in type 2 diabetes mellitus. . Drug Saf. . 1999;20(6):489–503. doi: 10.2165/00002018-199920060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rigacci S, Stefani M. Nutraceutical properties of olive oil polyphenols. an itinerary from cultured cells through animal models to humans. . Int J Mol Sci. . 2016;17(6):843. doi: 10.3390/ijms17060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marino S, Festa C, Zollo F, Nini A, Antenucci L, Raimo G, et al. Antioxidant activity and chemical components as potential anticancer agents in the olive leaf (olea europaea l. Cv leccino.) Decoction. . Anticancer Agents Med Chem. . 2014;14(10):1376–85. doi: 10.2174/1871520614666140804153936. [DOI] [PubMed] [Google Scholar]
- 4.Bulotta S, Celano M, Lepore SM, Montalcini T, Pujia A, Russo D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. . J Transl Med. . 2014;12:219. doi: 10.1186/s12967-014-0219-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro B, Toietta G, Maggio R, Arciello M, Tarocchi M, Galli A, et al. Effects of the olive-derived polyphenol oleuropein on human health. Int J Mol Sci. . 2014;15(10):18508–18524. doi: 10.3390/ijms151018508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murotomi K, Umeno A, Yasunaga M, Shichiri M, Ishida N, Koike T, et al. Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. J Agric Food Chem. 2015;63(30):6715–22. doi: 10.1021/acs.jafc.5b00556. [DOI] [PubMed] [Google Scholar]
- 7.Nekooeian A, Khalili A, Khosravi M. Oleuropein offers cardioprotection in rats with simultaneous Type 2 diabetes and renal hypertension. . Indian J Pharmacol. . 2014;46(4):398–403. doi: 10.4103/0253-7613.135951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sangi SA, Sulaiman M, El-wahab MA, Ahmedani E, Ali S. Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. . Pharmacogn Mag. 2015;11(Suppl 2):S251–7. doi: 10.4103/0973-1296.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadrich F, Garcia M, Maalej A, Moldes M, Isoda H, Feve B, et al. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. . Life Sci. . 2016;151:167–173. doi: 10.1016/j.lfs.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Jemai H, Feki AEL, Sayadi S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J Agric Food Chem. . 2009;57(19):8798–804. doi: 10.1021/jf901280r. [DOI] [PubMed] [Google Scholar]
- 11.Alkhateeb H, Chabowski A, Bonen A. Viability of the isolated soleus muscle during long-term incubation. . Appl Physiol Nutr Metab. 2006;31(4):467–76. doi: 10.1139/h06-022. [DOI] [PubMed] [Google Scholar]
- 12.Alkhateeb H, Chabowski A, Glatz JFC, Gurd B, Luiken JJFP, Bonen A . Restoring AS160 phosphorylation rescues skeletal muscle insulin resistance and fatty acid oxidation while not reducing intramuscular lipids. . Am J Physiol Endocrinol Metab. 2009;297(5):E1056–66. doi: 10.1152/ajpendo.90908.2008. [DOI] [PubMed] [Google Scholar]
- 13.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. . Annu Rev Nutr. . 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 14.Solinas G, Summermatter S, Mainieri D, Gubler M, Pirola L, Wymann MP, et al. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. . FEBS Letters. 2004;577(3):539–44. doi: 10.1016/j.febslet.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes. 2004;5(4):219–26. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 16.Watson RT, Pessin JE. Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, GLUT4. . Exp Cell Res. . 2001;271(1):75–83. doi: 10.1006/excr.2001.5375. [DOI] [PubMed] [Google Scholar]
- 17.Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 18.Smith AG, Muscat GEO. Skeletal muscle and nuclear hormone receptors: Implications for cardiovascular and metabolic disease. . Int J Biochem Cell Biol. . 2005;37(10):2047–63. doi: 10.1016/j.biocel.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. . Genes Dev. 2011;25(18):1895–908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. . J Clin Invest. . 2001;108(8):1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhateeb H, Bonen A. Thujone, a component of medicinal herbs, rescues palmitate-induced insulin resistance in skeletal muscle. . Am J Physiol Regul Integr Comp Physiol. . 2010;299(3):R804–12. doi: 10.1152/ajpregu.00216.2010. [DOI] [PubMed] [Google Scholar]
- 22.Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. . Life Sci. 2019;216:183–188. doi: 10.1016/j.lfs.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YJ, Zhao H, Dong L, Zhen YF, Xing HY, Ma HJ, et al. Resveratrol ameliorates high-fat diet-induced insulin resistance and fatty acid oxidation via ATM-AMPK axis in skeletal muscle. Eur Rev Med Pharmacol Sci. . 2019;23(20):9117–9125. doi: 10.26355/eurrev_201910_19315. [DOI] [PubMed] [Google Scholar]
- 24.Højlund K, Mustard KJ, Staehr P, Hardie DG, Beck-Nielsen H, Richter EA, et al. AMPK activity and isoform protein expression are similar in muscle of obese subjects with and without type 2 diabetes. . Am J Physiol Endocrinol Metab. . 2004;286(2):E239–44. doi: 10.1152/ajpendo.00326.2003. [DOI] [PubMed] [Google Scholar]
- 25.Alkhateeb H, Al-Duais M, Qnais E. Beneficial effects of oleuropein on glucose uptake and on parameters relevant to the normal homeostatic mechanisms of glucose regulation in rat skeletal muscle. . Phyther Res. 2018;32(4):651–656. doi: 10.1002/ptr.6012. [DOI] [PubMed] [Google Scholar]
- 26.Olsen GS, Hansen BF. AMP kinase activation ameliorates insulin resistance induced by free fatty acids in rat skeletal muscle. . Am J Physiol Endocrinol Metab. . 2002;283(5):E965-70. ;283(5):E965–70. doi: 10.1152/ajpendo.00118.2002. [DOI] [PubMed] [Google Scholar]
- 27.Thompson AL, Lim-Fraser MY, Kraegen EW, Cooney GJ. Effects of individual fatty acids on glucose uptake and glycogen synthesis in soleus muscle in vitro. . Am J Physiol Endocrinol Metab. 2000;279(3):E577–84. doi: 10.1152/ajpendo.2000.279.3.E577. [DOI] [PubMed] [Google Scholar]
- 28.Alkhateeb H, Qnais E. Preventive effect of oleate on palmitate-induced insulin resistance in skeletal muscle and its mechanism of action. . J Physiol Biochem. . 2017 ;73(4):605–612. doi: 10.1007/s13105-017-0594-9. [DOI] [PubMed] [Google Scholar]
- 29.Alkhateeb H, Bonen A. Thujone, a component of medicinal herbs, rescues palmitate-induced insulin resistance in skeletal muscle. . Am J Physiol Regul Integr Comp Physio. . 2010;299(3):R804–12. doi: 10.1152/ajpregu.00216.2010. [DOI] [PubMed] [Google Scholar]
- 30.Alkhateeb H, Qnais E. Preventive effect of oleate on palmitate-induced insulin resistance in skeletal muscle and its mechanism of action. . J Physiol Biochem. . 2017;73(4):605–612. doi: 10.1007/s13105-017-0594-9. [DOI] [PubMed] [Google Scholar]
- 31.Fujiwara Y, Tsukahara C, Ikeda N, Sone Y, Ishikawa T, Ichi I, et al. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. . J Clin Biochem Nutr. . 2017;61(3):196–202. doi: 10.3164/jcbn.16-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu PT, Song Z, Zhang WC, Jiao B, Yu ZB, Richter EA. Impaired translocation of GLUT4 results in insulin resistance of atrophic soleus muscle. . Biomed Res Int. . 2015;2015:291987. doi: 10.1155/2015/291987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. . 2007 ;5(4):237–52. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Maria Z, Campolo AR, Lacombe VA. Diabetes alters the expression and translocation of the insulin-sensitive glucose transporters 4 and 8 in the atria. . PLoS One. . 2015;10(12):e0146033. doi: 10.1371/journal.pone.0146033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thong FSL, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. . Diabetes. 2007;56(2):414–23. doi: 10.2337/db06-0900. [DOI] [PubMed] [Google Scholar]
- 36.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. . Diabetes. 2005;54(1):41–50. doi: 10.2337/diabetes.54.1.41. [DOI] [PubMed] [Google Scholar]


