Abstract
Spermatozoa transport within the male reproductive tract is a highly dynamic and biologically important reproductive event. However, due to the lack of live volumetric imaging technologies and quantitative measurements, there is little information on the dynamic aspect and regulation of this process. Here, we presented ex vivo dynamic volumetric imaging of the mouse testis, efferent duct, epididymis, and vas deferens at a micro-scale spatial resolution with optical coherence tomography (OCT). Micro computed tomography imaging is presented as a reference for the proposed OCT imaging. Application of functional OCT analysis allowed for 3D mapping of the cilia beat frequency in the efferent duct, which volumetrically visualized the spatial distribution of the ciliated cells and corresponding ciliary activities. Potentially these analyses could be expanded to in vivo settings through intravital approach. In summary, this study demonstrated that OCT has a great potential to investigate the microstructure and dynamics, such as cilia beating, muscle contractions, and sperm transport, within the male reproductive tract.
1. Introduction
In mammals, the male reproductive tract consists of a series of organs including the testes, efferent ducts, epididymis, vas deferens, and accessory sex glands. The testis is responsible for the development of germ cells and production of spermatozoa, which is termed spermatogenesis. The spermatogenesis takes place inside the long, convoluted tube called seminiferous tubule, which is tightly packed within the testis. The time required to produce sperm is approximately 30 to 40 days in most mammals [1]. A previous study demonstrated that human daily sperm production is approximately 45 million per day per testis, which means ∼1000 sperm are produced per second [2]. This fact suggests that testes highly specialize in producing sperm, however, the testicular sperm are not functionally mature. Sperm released from the testes are transported into the epididymis which is the site of sperm maturation and storage. The epididymis is a duct-like organ that connects efferent ducts of the testes to vas deferens and is largely divided into three regions: caput, corpus, and cauda. Sperm undergo a number of physiological and biochemical modifications by interacting with the epithelium and luminal environment of the epididymal ducts, which allows sperm to acquire potential motility and fertilizing ability [3]. In both, humans and mice, after 1 to 2 weeks of transit through the epididymis, mature sperm are stored in the distal part of the cauda epididymis until ejaculation. The vas deferens is a tubular structure with a narrow lumen surrounded by thick muscle layers. This smooth muscle coat consists of a circular layer, and inner and outer longitudinal layers. The major role of the vas deferens is to transport sperm from the cauda epididymis to the urethra. During ejaculation, the muscular contractions of the vas deferens propel sperm toward the urethra [4].
Sperm transport, which is crucial for mammalian reproduction, begins in the seminiferous tubules inside the testes, followed by the transit through the efferent ducts, epididymis, and vas deferens. Although sperm acquire the motility in the epididymis, they keep a quiescent state in the male reproductive tract and turn into an active state after ejaculation by the interactions with female reproductive tract. Therefore, the contractions of male reproductive tract make huge contributions to the sperm transport in testes [5], efferent ducts [6], epididymis [7,8], and vas deferens [4]. Interestingly, the efferent duct harbors ciliated cells which are also involved in sperm transport through the tubules [7]. The efferent duct is a tiny tubule that connects the testis to the epididymis and is the only region of the male reproductive tract where ciliated cells exist on the epithelium [8]. Several previous studies showed that the defective or lack of cilia in the efferent ducts can lead to male infertility, suggesting that the ciliary activity of the efferent ducts plays an important role in sperm transport and male fertility [8,9]. Taken together, the dynamics of male reproductive tract is key for successful sperm transport. However, due to the lack of volumetric imaging technologies to capture these dynamics in humans of mammalian animal models, there is little information on the dynamic aspect and regulation of sperm transport in the male reproductive tract.
Toward this gap, current study investigates the use of the optical coherence tomography (OCT) toward volumetric dynamic imaging of mouse male reproductive tract with a potential for in vivo application. OCT is a noninvasive, label-free, and depth-resolved imaging modality that provides micro-scale spatial resolution with an imaging depth of 1 to 2 mm [10]. There are very few previous studies exploring the use of OCT to investigation of the male reproductive tract in different animal models; all these studies reported static two-dimensional imaging. Trottmann et al. showed that OCT is a promising imaging tool for investigating the microstructure of the male reproductive organs in a bovine model [11]. Cilip et al. performed OCT imaging of the vas deferens in a canine model [12]. Ramasamy et al. demonstrated that OCT has a potential to evaluate spermatogenesis within seminiferous tubules in rat testes [13]. However, there were no studies attempting volumetric imaging of the male reproductive organs using OCT. Additionally, to our knowledge, OCT imaging of mouse male reproductive system was never reported even though mouse is a superior animal model in many fields of biomedical research, including reproductive biology, due to the availability of genetic manipulation tools and mutants modeling human disorders. Toward investigation of mouse reproductive processes, our group recently established a set of structural and functional methods with OCT for live volumetric dynamic visualization of the female reproductive tract in mice, providing insights into the oocyte/embryo transport and the process of sperm migration in the native state [14,15]. We also established an OCT based functional approach for in vivo mapping of cilia beat frequency in the mouse fallopian tube [16]. Those studies revealed great potential for functional OCT in exploration of numerous aspects of reproduction in mouse models and motivated the expansion of OCT use to unveil the dynamics of reproduction not only in females, but also within the male reproductive tract.
In this study, we present first volumetric visualizations of different regions within the mouse male reproductive tract, revealing structural details of the organs. We report visualization of tubular dynamics, suggesting a potential for investigation of sperm transport. We also demonstrate volumetric mapping of cilia and cilia beat frequency in the efferent ducts, which, to the best of our knowledge, is the first volumetric and quantitative measurement of the ciliary activity in the efferent ducts. The presented results set a platform for variety of future studies investigating normal and abnormal male reproductive physiology in mouse models of human defects, leading to a more complete understanding and improved management of male reproductive disorders.
2. Materials and methods
2.1. Tissue preparation
The wild-type CD1 male mice aged 7 to 14 weeks were used in this study. All animal procedures have been approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, and all experiments were conducted following the approved guidelines and protocols. After the animals were euthanized, we dissected the male reproductive tract including testes, efferent ducts, epididymis, and vas deferens. The freshly extracted reproductive organs were used for ex vivo OCT imaging or processed for micro computed tomography (Fig. 1(A)). For OCT analysis, the extracted tissues were placed in a 35-mm-diameter petri dish containing pre-heated 37°C phosphate buffered saline (PBS) (Sigma-Aldrich). All samples were imaged within 1 hour after the dissection on a pre-heated stage maintained at 37°C.
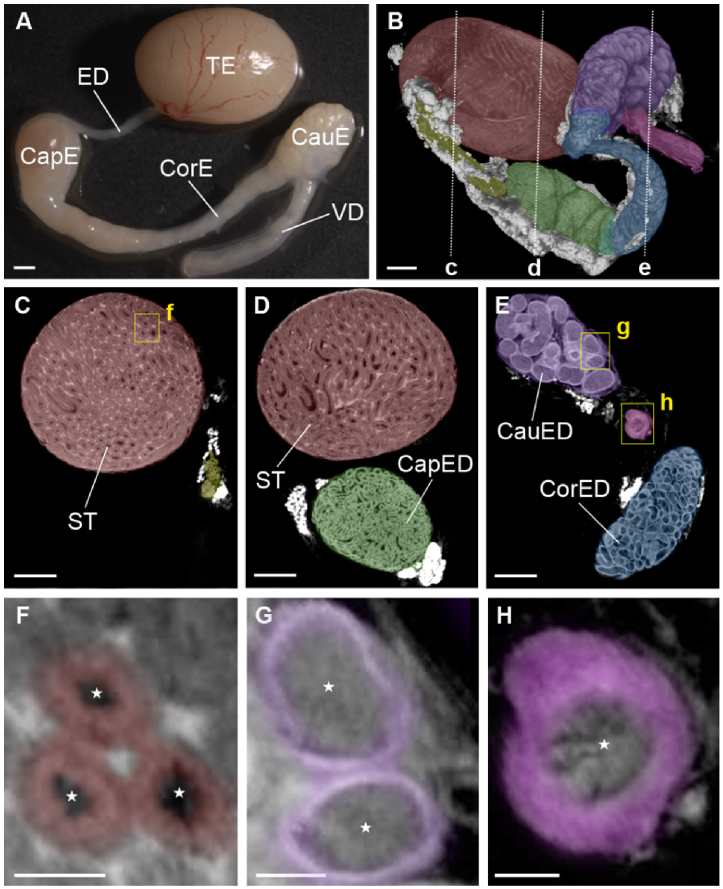
Fig. 1.
Overall structure of the mouse male reproductive tract. (A) Bright-field microscopy image of the male reproductive tract, including the testis (TE), efferent ducts (ED), caput epididymis (CapE), corpus epididymis (CorE), cauda epididymis (CauE), and vas deferens (VD). (B) Micro CT imaging of the male reproductive tract. The dashed line represents the location for the corresponding cross-section shown in panels C-E. Different organs and distinct regions of the epididymis are color-coded: testis (red), efferent ducts (yellow), caput (green), corpus (blue), cauda (purple), and vas deferens (magenta). (C-E) Corresponding depth-resolved micro CT images of the male reproductive tract revealing microstructural details such as seminiferous tubules (ST), caput epididymal ducts (CapED), corpus epididymal ducts (CorED), and cauda epididymal ducts (CauED). The yellow rectangles represent the location for the corresponding magnified images shown in panels F-H. Corresponding magnified images of seminiferous tubules (F), cauda epididymal ducts (G), and the duct of vas deferens (H). The color coding correspond to germ cells (red), epididymal epithelium (purple), and muscle layers of vas deferens (magenta). The stars in (F-H) indicate the lumens of the seminiferous tubules, epididymal ducts, or vas deferens, respectively. Scale bars in (A,B) correspond to 1000 µm, in (C-E) to 500 µm, and in (F-H) to 200 µm.
2.2. Micro computed tomography imaging
To set a reference for structural analysis of the reproductive system with OCT, we performed micro computed tomography (µCT) imaging on the extracted male reproductive tract including testes, efferent ducts, epididymis, and vas deferens. After the dissection, the samples were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) in PBS at 4°C for at least 24 hours. The samples were transferred to a 1.5 ml tube containing 1 ml of X-CLARITY Hydrogel Solution (Logos Biosystems) with 0.25% X-CLARITY Polymerization Initiator (Logos Biosystems) and incubated at 4°C overnight for the polymer to diffuse through the samples. We then incubated the samples at 37°C to initiate the crosslinking reaction for 3 hours for the acrylamide-paraformaldehyde to crosslink with the tissue and create a hydrogel-tissue mixed structure. After crosslinking, we carefully removed the external hydrogels from the specimens and stored them in PBS with 0.1% sodium azide (Sigma-Aldrich) at 4°C overnight. The samples were then immersed in 5 ml of 0.1N iodine solution (Fisher Chemical) at 4°C until µCT imaging for at least 2 days. We acquired the images on SKYSCAN 1272 (Bruker) at 4 µm/pixel over 16.1 mm × 10.8 mm; 1921 images per data set; the X-ray source was set at 70 kV and 142 µA with 0.5 mm aluminum filter. The acquired µCT data was reconstructed with NRecon software (Bruker) and then visualized with Imaris software (Bitplane).
2.3. OCT system and ex vivo 3D imaging
We used a house-built spectroscopic OCT system. The system employs the supercontinuum laser (NKT Photonics) relying on a central wavelength of ∼800 nm and a bandwidth of ∼100 nm. The system uses a fiber-based Michaelson interferometer; the interference of light from the reference and sample arms is directed to a spectrometer based on a 250 kHz e2V OctoPlus camera (Teledyne Technologies Inc.). Fast Fourier transform (FFT) is used to obtain the OCT intensity A-line from equally k-spaced interference fringes. The system provides an A-line rate of up to 250 kHz, and has an axial and transverse resolutions of ∼4 µm. To perform three-dimensional (3D) transverse scanning, we used a set of galvanometer mirrors (GVS012, Thorlabs Inc.), which provides a high flexibility of adjusting the number of pixels and the scanning distance. A variety of settings were employed in the experiments to accommodate the size and structural differences in reproductive organ regions. The volumetric rendering and dynamic visualizations were conducted using Imaris software (Bitplane).
2.4. CBF analysis
The cilia beat frequency (CBF) analysis based on the OCT intensity fluctuations in pixels corresponding to cilia locations, as previously reported in [16]. Briefly, while the resolution of the system did not allow for the direct visualization of cilia movement, the periodic beats of cilia created speckle variations of the OCT intensity over time with a dominant frequency corresponding to the cilia beat frequency at the corresponding pixels. First, structural imaging was used to create a mask excluding pixels, which do not correspond to tissue based on an absolute OCT intensity. For all remaining spatial pixels, OCT structural time lapses were analyzed through FFT to obtain the amplitude spectra of intensity fluctuations for each pixel. To distinguish pixels corresponding to cilia, the cut off threshold was set as an average amplitude plus three standard deviations within high frequency window (30-35 Hz) corresponding to noise. The dominant frequency peaks above the threshold at the window of 2-15 Hz were counted as the CBF signal; the corresponding pixels indicated the cilia location. The datasets were acquired as 10,000 frames over 0.54 X 0.35 mm, which resulted in a spatial step of 35 nm between frames. The sampling frame rate of the OCT B-scanning was 120 Hz. The FFT analysis was performed over 256 frames as a sliding window over the whole volume with a step of 10 frames. The color-coded volumetric CBF maps were overlaid with the structural data sets and rendered using Imaris software (Bitplane).
3. Results
3.1. Microstructure of male reproductive tract
The male reproductive tract includes testis, efferent ducts, epididymis, and vas deferens (Fig. 1(A)). To get a general understanding of the male reproductive system morphology and organization and to set a reference for evaluation of the OCT imaging capabilities, we first performed a µCT imaging of the male reproductive organ microarchitectures. The representative 3D reconstruction of µCT images of the whole male reproductive tract is shown in Fig. 1(B) and Visualization 1 (12.2MB, mp4) . The seminiferous tubules and epididymal ducts inside the organs were clearly identified by µCT imaging. Figure 1(C)-(E) shows the cross-sectional images of each region of the reproductive tract. The germ cells and lumen inside the seminiferous tubules, the epithelium and lumen inside the epididymal duct, and the muscle layers and lumen inside the vas deferens were all visualized (Fig. 1(F)-(H)). As µCT scanners become more common in biomedical research institutions, µCT is gaining popularity as a tool for structural morphological analysis of different organs and tissues. These results demonstrated that µCT imaging allows for micro-scale visualization of the whole male reproductive tract including the inner luminal environment at high level of structural detail. While by itself, this result suggests strong potential for µCT in morphological phenotyping of male reproductive organs. However, µCT requires for tissue fixation and sample preparation over multiple days, which sets the motivation for the development of OCT imaging, which does not require tissue processing and could potentially be done in vivo.
3.2. 3D OCT imaging of testis
We performed ex vivo OCT imaging of the testes extracted from the adult male mice. Figure 2(A) shows a typical 3D OCT reconstruction of the mouse testis. Figure 2(B) and Visualization 2 (3.9MB, mp4) are the top-view cross-sectional image showing the coiled structure of seminiferous tubules tightly packed within the testis. Figure 2(C) is the representative three-dimensional OCT image showing the blood vessels running through the surface of the testis as indicated by the arrow heads. The cross-sectional images of seminiferous tubules are shown in Fig. 2(D)-(E) and Visualization 3 (6.5MB, mp4) . The lumen, germ cells, released sperm, and the outer membrane of the testis were visualized (Fig. 2(F)). These structural features captured by 3D OCT imaging were clearly recognizable based on the µCT images as shown in red in Fig. 1(B)-(F).
Fig. 2.
3D OCT imaging of mouse testis. (A) Three-dimensional OCT rendering of the mouse testis showing the external morphology. The dashed rectangle represents the location for the cross-sectional plane shown in panel B. (B) Corresponding depth-resolved OCT cross-sectional image of seminiferous tubules (ST) inside the testis. (C) Three-dimensional OCT image of the testis and blood vessels (arrow heads) running through the surface of the testis. The dashed line represents the location for the cross-section shown in panels D-E. (D-E) Corresponding depth-resolved cross-sectional images of the seminiferous tubules within the mouse testis. (F) Different structural features inside the testis were color-coded: lumen and released sperm (green), germ cells (red), and outer membrane of the testis (yellow). Scale bars in (A-E) correspond to 500 µm, and scale bar in (F) correspond to 200 µm.
3.3. 3D OCT imaging of epididymis
Ex vivo OCT imaging was performed on three different regions of the mouse epididymis. The 3D OCT reconstruction of the whole epididymis is shown in Fig. 3(A) and Visualization 4 (2.6MB, mp4) . The overall structure of the epididymis was comparable among all different imaging approaches; bright-field microscopy imaging, µCT imaging, and OCT imaging (Fig. 1(A)-(B)). Figure 3(B) is the top-view cross-sectional image showing that the epididymal ducts are tightly packed inside the epididymis. The depth-resolved cross-sectional images of epididymal ducts in the caput, corpus, and cauda epididymis are shown in Fig. 3(C), Fig. 3(D), and Fig. 3(E), respectively. The lumen and walls of each duct were clearly identified (Fig. 3(C)’-E’). These cross-sectional OCT images also demonstrated that the duct diameter and lumen diameter dramatically increased along the epididymal duct from the caput to the cauda, which is consistent with the µCT imaging results (Fig. 1(D)-(E)) and the previous paper [17]. Dynamic imaging revealed contractions of the epididymal ducts (Visualization 5 (3.2MB, mp4) ), which suggests a potential for this technology to study dynamics of sperm transport and transfer disorders in mouse models.
Fig. 3.
Ex vivo OCT imaging of mouse epididymis. (A) Three-dimensional OCT reconstruction of the whole epididymis including caput, corpus, and cauda regions. CapE: caput epididymis, CorE: corpus epididymis, CauE: cauda epididymis. (B) OCT cross-sectional image of the epididymis showing the epididymal ducts tightly packed inside the organ. (C-E) Depth-resolved cross-sectional OCT images of the epididymal ducts within the caput (C), corpus (D), and cauda epididymis (E). The lumen and the walls of each epididymal duct are shown in green and blue, respectively. Scale bars in (A) and (B) correspond to 1 mm, and scale bars in (C-E) correspond to 200 µm.
3.4. 3D OCT imaging of vas deferens
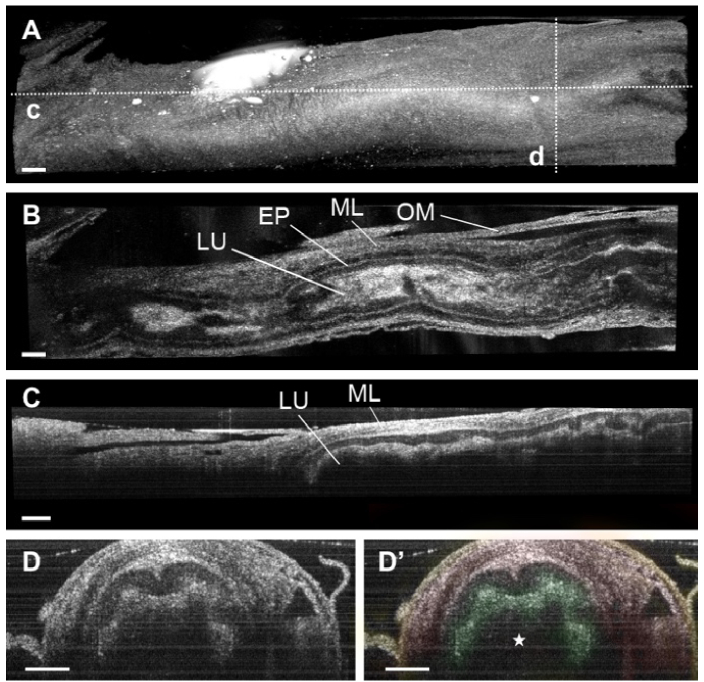
The representative 3D OCT reconstruction of the mouse vas deferens is shown in Fig. 4(A) and Visualization 6 (5.9MB, mp4) . Figure 4(B)-(C) is the longitudinal OCT images showing the single duct with inner lumen surrounded by thick muscle layers. The cross-sectional images of the duct are shown in Fig. 4(B)-(D). The lumen, epithelium, smooth muscle layers, and the outer membrane of the vas deferens were clearly visualized with the OCT imaging approach. These structural features were comparable with those in the µCT images of the vas deferens as shown in magenta in Fig. 1(B) and (E).
Fig. 4.
3D reconstructions of mouse vas deferens. (A) Three-dimensional OCT image of the vas deferens showing the external morphology. The dashed lines represent the location for the cross-section shown in panels C-D. (B) OCT cross-sectional image of the vas deferens showing the lumen (LU), epithelium (EP), smooth muscle layers (ML), and the outer membrane (OM) of the duct. (C) Corresponding longitudinal OCT image of the vas deferens. (D) Corresponding depth-resolved cross-sectional images of the mouse vas deferens. Different structural features were color-coded: epithelium (green), muscle layers (red), and outer membrane (yellow). The star indicates the inner lumen of the duct. All scale bars correspond to 200 µm.
3.5. 3D OCT imaging of efferent ducts
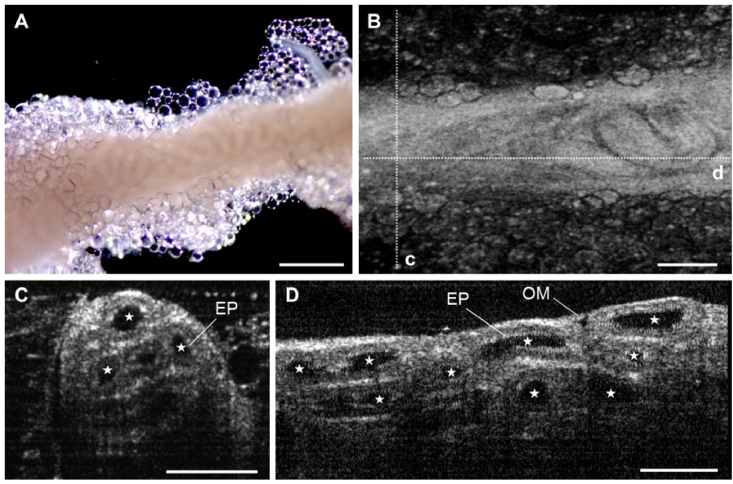
Figure 5(A)-(B) and Visualization 7 (5MB, mp4) show the comparison between 3D OCT imaging of the mouse efferent ducts and bright-field microscopy imaging of corresponding tissues. The structural features, such as the thin, transparent outer membrane and tightly packed coiled ducts, were comparable between these two imaging approaches. Figure 5(C)-(D) shows an in-depth cross-section of the reconstruction along the dashed lines shown in Fig. 5(B). The lumen, epithelium, walls of each duct, and the outer membrane were identifiable with the OCT imaging.
Fig. 5.
3D OCT imaging of mouse efferent ducts. (A) Bright-field microscopy imaging of the mouse efferent ducts. Scale bar corresponds to 500 µm. (B) Three-dimensional OCT reconstruction of the efferent ducts showing the external morphology. The dashed lines represent the location for the cross-section shown in panels C and D. (C-E) Corresponding depth-resolved OCT cross-sectional image of the efferent ducts showing the inner lumen (marked by stars), epithelium (EP), and outer membrane (OM) of the ducts. Scale bars in (C) and (D) correspond to 200 µm.
3.6. 3D mapping of ciliary activity in the efferent ducts
The inner lumen of the efferent ducts is covered with ciliated epithelial cells. Motile cilia are known to maintain their activity in freshly extracted tissues. To visualize the cilia location and analyze the ciliary activities of the efferent ducts, we focused on the analysis of OCT intensity speckle fluctuations over time for volumetric mapping of the CBF in the efferent duct and adapted an approach for the CBF analysis through tissue layers which we previously established for cilia mapping in the mouse fallopian tube [16]. The quantitative measurements of CBF provided by this method have been previously validated. The resulting 3D OCT structural image of the efferent ducts with mapping of the CBF is shown in Fig. 6(A) and Visualization 8 (9.2MB, mp4) . As expected, we detected the spatial location of the ciliated cells specifically on the inner lumen of the efferent ducts and volumetrically visualized the corresponding CBF. Figure 6(B) (B’) and Fig. 6(C) (C’) are the cross-sectional images with OCT mapping of the CBF showing that the ciliated cells were equally distributed across the luminal epithelium. The CBF mostly ranged from 5 to 14 Hz across the duct, and the average CBF of the efferent duct was 9.8 ± 4.8 Hz. Currently, there is no alternative method for mapping cilia location and measuring cilia beat frequency within unstained intact efferent ducts. To the best of our knowledge, this is the first demonstration of such measurement, providing potential opportunities for investigation of the role of motile cilia in sperm transfer and male reproductive physiology.
Fig. 6.
Volumetric cilia and CBF mapping of the mouse efferent ducts. (A) Representative three-dimensional OCT structural image of the efferent ducts with OCT mapping of the CBF showing the location of the cilia distributed on the inner lumen. The CBF values were color-coded according to the presented pattern. The dashed rectangles represent the location for the cross-section shown in panels B-C. (B-C) Corresponding 3D structural images and cross-sectional images (B’-C’) with OCT mapping of the CBF. All scale bars correspond to 100 µm.
4. Discussion and conclusion
In this study, we present a new structural and functional imaging approach that allows for label-free, depth-resolved, three-dimensional high-resolution visualization of the male reproductive tract using OCT. The structural features revealed by the OCT imaging were easily recognizable by comparison to the corresponding µCT images, validating the OCT imaging approach. We demonstrated that the spatial resolution of the OCT system, approximately 4 µm in tissue, is sufficient for visualizing detailed structural features of the testis, efferent ducts, epididymis, and vas deferens in mice.
The presented method has several advantages compared with generally used methods for structural analysis of the reproductive organs, specifically histology and confocal florescence microscopy. These advantages include the deeper imaging depth, a large field of view, no requirement for exogenous contrast agents, and the ability of ex vivo imaging and potential of in vivo imaging. Such features allow for dynamic investigations of the male reproductive organs with minimized alterations of their natural environments, which would facilitate advanced studies in mammalian reproduction. Additionally, OCT imaging is widely used in ophthalmology and cardiology clinics [18], indicating very little influence of the imaging light on the reproductive tissues. Together, our ex vivo 3D OCT imaging approach sets a platform for future investigations on the reproductive process from a dynamic perspective. In contrast, the fluorescence microscopy has the advantage of providing cell type and subcellular labeling specificity. The OCT imaging did not allow us to identify the different developmental stages of germ cells in the testes due to the lower spatial resolution compared with confocal microscopy (Fig. 2(D)-(E)). This limitation of limited resolution can be potentially partially resolved by using the higher-resolution optical coherence microscopy [19]. The multimodal combination of dynamic analysis with OCT and molecular labeling with fluorescence microscopy might be useful to address technical limitations and unexplored questions in mammalian reproduction.
There are a few previous studies exploring the use of OCT to investigation of the male reproductive tract in different animal models including cattle [11], canine [12], and rat [13], which reported static two-dimensional images. Volumetric imaging of the male reproductive organs using OCT was not attempted before to the best of our knowledge. This report presents the first use of OCT for volumetric dynamic visualization of the testis, epididymis, efferent ducts, and vas deferens. This is also the first OCT study of reproductive organs in male mice, which is the only mammalian organism with the variety of mutants and genetic manipulation methods available to model human defects. Therefore, the presented approach sets a platform for a variety of studies investigating normal and pathological mechanisms of sperm maturation and transport in the male reproductive tract relevant to human physiology.
All experiments presented in this study were conducted ex vivo; however, in vivo OCT imaging of the male reproductive organs is highly desirable, as it would open a range of opportunities for reproductive scientists. For example, there is a strong interest in reproductive research community to investigate the mechanism of seminiferous tubule contractions [5] and the behavior of spermatogenic stem cells [20]. In vivo imaging study with OCT would be highly advantageous in this exploration. We previously optimized an intravital OCT imaging protocol through an implantable window in the body wall for in vivo prolonged and longitudinal assessment of reproductive processes within the mouse fallopian tube [21]. Through these in vivo methods, we have been able to uncover fallopian tube contraction patterns, investigate individual sperm trajectories at the site of fertilization, and visualize oocyte/embryo transport in the native state [14,15]. These intravital in vivo imaging methods could be potentially optimized and adapted toward investigations on the reproductive process in the male reproductive tract to significantly enhance potential in studying dynamic reproductive events within the native environment of the male reproductive tract. Due to its millimeter-scale imaging depth, the in vivo application of the presented approach is primarily limited to mouse model. However, since the human testes and epididymis are located outside of the body cavity, further developments in OCT technology might potentially allow for minimally invasive in vivo imaging of the male reproductive organs and help in diagnosis of reproductive disorders in humans.
Sperm transport through the male reproductive tract is a crucial reproductive event. The disruption of the appropriate regulation of sperm transport can lead to incomplete sperm maturation and male infertility. For example, both the acceleration and delay of sperm transit time in the epididymis affect the sperm quality [22,23]. In humans, the failure of sperm transport is a major cause of infertility in male spina bifida patients [24]. Since sperm are immotile before ejaculation, the dynamics of male reproductive tract such as muscle contractions and cilia beating contribute to the process of sperm transport [6]. The presented 4D (3D + time) OCT imaging showed that the ducts in the mouse epididymis exhibited contractions (Visualization 5 (3.2MB, mp4) ), which suggests a potential for imaging dynamics of the sperm transport to investigate the physiological mechanism of sperm transport and factors contributing to associated male infertilities in mouse models of human defects.
The demonstrated ability to volumetrically map cilia and capture ciliary activities is a major milestone. Investigation of ciliary dynamics through OCT imaging is a developing field. The cilia mapping and CBF analysis presented in this study is based on the OCT intensity fluctuations in pixels corresponding to cilia location, which we successfully previously applied to investigation of ciliary beating in mouse fallopian tube in vivo [16,21,25]. Alternative methods for ciliary analysis are being developed by multiple groups taking advantage of phase/Doppler shifts in OCT data created by cilia [26], modified dimension reduction methods of principle component analysis [27], or investigating the microstructural dynamic changes at higher resolution optical coherence microscopy [28]. As new methods are being developed, eventually there might be more optimal cilia and CBF imaging approach for deeply located ciliated structures as those in the male reproductive tract. However, the presented study demonstrates the capability of OCT analysis of ciliary location and dynamics in the male reproductive system.
The efferent duct is a very unique region of the male reproductive tract that harbors the ciliated cells, which is crucial for male infertility [8,9]. A previous study demonstrated that the ciliary activities of the efferent duct play an essential role in the sperm passage through the reproductive tract, and loss of the cilia beating can cause oligozoospermia in mice [7]. Another paper suggested that loss of efferent duct motile cilia leads to sperm aggregation, sperm granulomas, and ultimately male infertility [6]. However, despite the biological importance of the cilia dynamics of efferent ducts, there were no volumetric and quantitative measurements of ciliary activity in the efferent ducts due to the lack of useful imaging tools. Toward this gap, we integrated the volumetric OCT imaging with the spectral analysis of the speckle variation, which allows us to capture the cilia dynamics of mouse efferent ducts. Taking advantage of this unique method, we volumetrically visualized the spatial distribution of the ciliated cells in the efferent ducts and quantified the corresponding cilia beat frequency. Our 3D CBF mapping showed that the ciliated cells were equally distributed across the luminal epithelium of the efferent ducts, supporting the idea that the cilia play an important role in the reproductive events occurred inside the efferent duct such as the regulation of sperm transport and prevention of sperm aggregation. We also demonstrated that the average CBF of the efferent ducts was 9.8 ± 4.8 Hz, which was in the range of previously reported values acquired through exposure of ciliated layer. Our CBF value was slightly higher than reported by Yuan et al. [6] and slightly lower than reported by Aprea et al. [7]. These subtle differences in CBF of the efferent duct may result from different analysis procedures and experimental conditions. Ex vivo observations showed that the CBF of the fallopian tube varied from 8 to 12 Hz depending on the experimental temperature [29]. Therefore, not only ex vivo investigations but also in vivo studies are necessary to capture the physiological ciliary activity in the native environment of efferent ducts. Finally, because the cilia beating are essential for various biological processes in the mammalian body [30], this presented approach has potentially broad applications in cilia-related biological studies and clinical diagnosis.
In summary, we present ex vivo dynamic volumetric imaging of the male mouse reproductive tract at a micro-scale spatial resolution by structural and functional OCT. The presented method has several technical advantages including the deeper imaging depth, a large field of view, no requirement for exogenous contrast agents, and the potential of in vivo imaging. These unique features allowed for dynamic investigations of the mouse testis, efferent ducts, epididymis, and vas deferens, suggesting that OCT has a great potential to study dynamic reproductive events, such as sperm transport and periodic contractions, within the male reproductive tract. We also introduced the 3D CBF mapping method in the mouse efferent duct, and volumetrically visualized the spatial distribution of the ciliated cells and corresponding ciliary activities in the efferent ducts. These new approaches would provide a platform for a variety of advanced studies in reproductive biology; the integration of the volumetric OCT imaging with genetic mouse models or pharmacological manipulations might contribute to further understanding of the determinants of sperm quality and male fertility in mammals.
Acknowledgments
This study was supported by the Optical Imaging and Vital Microscopy (OIVM) Core at Baylor College of Medicine.
Funding
National Institutes of Health10.13039/100000002 (R01 EB027099).
Disclosures
The authors declare no conflicts of interest.
Data availability
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.
References
- 1.Griswold M. D., “Spermatogenesis: the commitment to meiosis,” Physiol. Rev. 96(1), 1–17 (2016). 10.1152/physrev.00013.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson L., Petty C. S., Neaves W. B., “A comparative study of daily sperm production and testicular composition in humans and rats,” Biol. Reprod. 22(5), 1233–1243 (1980). 10.1093/biolreprod/22.5.1233 [DOI] [PubMed] [Google Scholar]
- 3.James E. R., Carrell D. T., Aston K. I., Jenkins T. G., Yeste M., Salas-Huetos A., “The role of the epididymis and the contribution of epididymosomes to mammalian reproduction,” Int. J. Mol. Sci. 21(15), 5377 (2020). 10.3390/ijms21155377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koslov D. S., Andersson K.-E., “Physiological and pharmacological aspects of the vas deferens-an update,” Front. Pharmacol. 4, 101 (2013). 10.3389/fphar.2013.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleck D., Kenzler L., Mundt N., Strauch M., Uesaka N., Moosmann R., Bruentgens F., Missel A., Mayerhofer A., Merhof D., Spehr J., Spehr M., “ATP activation of peritubular cells drives testicular sperm transport,” eLife 10, (2021). 10.7554/eLife.62885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan S., Liu Y., Peng H., Tang C., Hennig G. W., Wang Z., Wang L., Yu T., Klukovich R., Zhang Y., Zheng H., Xu C., Wu J., Hess R. A., Yan W., “Motile cilia of the male reproductive system require miR-34/miR-449 for development and function to generate luminal turbulence,” Proc. Natl. Acad. Sci. U. S. A. 116(9), 3584–3593 (2019). 10.1073/pnas.1817018116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aprea I., Nöthe-Menchen T., Dougherty G. W., Raidt J., Loges N. T., Kaiser T., Wallmeier J., Olbrich H., Strünker T., Kliesch S., Pennekamp P., Omran H., “Motility of efferent duct cilia aids passage of sperm cells through the male reproductive system,” Mol. Hum. Reprod. 27(3), (2021). 10.1093/molehr/gaab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoque M., Kim E. N., Chen D., Li F.-Q., Takemaru K.-I., “Essential roles of efferent duct multicilia in male fertility,” Cells 11(3), 341 (2022). 10.3390/cells11030341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terré B., Lewis M., Gil-Gómez G., Han Z., Lu H., Aguilera M., Prats N., Roy S., Zhao H., Stracker T. H., “Defects in efferent duct multiciliogenesis underlie male infertility in GEMC1-, MCIDAS- or CCNO-deficient mice,” Development 146(8), 433 (2019). 10.1242/dev.162628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D., Swanson E. A., Lin C. P., Schuman J. S., Stinson W. G., Chang W., Hee M. R., Flotte T., Gregory K., Puliafito C. A., “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trottmann M., Kölle S., Leeb R., Doering D., Reese S., Stief C. G., Dulohery K., Leavy M., Kuznetsova J., Homann C., Sroka R., “Ex vivo investigations on the potential of optical coherence tomography (OCT) as a diagnostic tool for reproductive medicine in a bovine model,” J. Biophotonics 9(1-2), 129–137 (2016). 10.1002/jbio.201500009 [DOI] [PubMed] [Google Scholar]
- 12.Cilip C. M., Allaf M. E., Fried N. M., “Application of optical coherence tomography and high-frequency ultrasound imaging during noninvasive laser vasectomy,” J. Biomed. Opt. 17(4), 046006 (2012). 10.1117/1.JBO.17.4.046006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramasamy R., Sterling J., Manzoor M., Salamoon B., Jain M., Fisher E., Li P. S., Schlegel P. N., Mukherjee S., “Full field optical coherence tomography can identify spermatogenesis in a rodent sertoli-cell only model,” Journal of Pathology Informatics 3(1), 4 (2012). 10.4103/2153-3539.93401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S., Larina I. V., “In vivo three-dimensional tracking of sperm behaviors in the mouse oviduct,” Development 145(6), 649 (2018). 10.1242/dev.157685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S., Larina I. V., “In vivo dynamic 3D imaging of oocytes and embryos in the mouse oviduct,” Cell Rep. 36(2), 109382 (2021). 10.1016/j.celrep.2021.109382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S., Burton J. C., Behringer R. R., Larina I. V., “In vivo micro-scale tomography of ciliary behavior in the mammalian oviduct,” Sci. Rep. 5(1), 13216 (2015). 10.1038/srep13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakata H., Iseki S., “Three-dimensional structure of efferent and epididymal ducts in mice,” J. Anat. 235(2), 271–280 (2019). 10.1111/joa.13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zysk A. M., Nguyen F. T., Oldenburg A. L., Marks D. L., Boppart S. A., “Optical coherence tomography: a review of clinical development from bench to bedside,” J. Biomed. Opt. 12(5), 051403 (2007). 10.1117/1.2793736 [DOI] [PubMed] [Google Scholar]
- 19.Karnowski K., Ajduk A., Wieloch B., Tamborski S., Krawiec K., Wojtkowski M., Szkulmowski M., “Optical coherence microscopy as a novel, non-invasive method for the 4D live imaging of early mammalian embryos,” Sci. Rep. 7(1), 4165 (2017). 10.1038/s41598-017-04220-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hara K., Nakagawa T., Enomoto H., Suzuki M., Yamamoto M., Simons B. D., Yoshida S., “Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states,” Cell Stem Cell 14(5), 658–672 (2014). 10.1016/j.stem.2014.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S., Syed R., Grishina O. A., Larina I. V., “Prolonged in vivo functional assessment of the mouse oviduct using optical coherence tomography through a dorsal imaging window,” J. Biophotonics 11(5), e201700316 (2018). 10.1002/jbio.201700316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meistrich M. L., Hughes T. H., Bruce W. R., “Alteration of epididymal sperm transport and maturation in mice by oestrogen and testosterone,” Nature 258(5531), 145–147 (1975). 10.1038/258145a0 [DOI] [PubMed] [Google Scholar]
- 23.Fernandez C. D. B., Porto E. M., Arena A. C., Kempinas W. D. G., “Effects of altered epididymal sperm transit time on sperm quality,” Int. J. Androl. 31(4), 427–437 (2008). 10.1111/j.1365-2605.2007.00788.x [DOI] [PubMed] [Google Scholar]
- 24.Deng N., Thirumavalavan N., Beilan J. A., Tatem A. J., Hockenberry M. S., Pastuszak A. W., Lipshultz L. I., “Sexual dysfunction and infertility in the male spina bifida patient,” Transl. Androl. Urol 7(6), 941–949 (2018). 10.21037/tau.2018.10.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S., Larina I. V., “In vivo imaging of the mouse reproductive organs, embryo transfer, and oviduct cilia dynamics using optical coherence tomography,” Methods Mol. Biol. 1752, 53–62 (2018). 10.1007/978-1-4939-7714-7_5 [DOI] [PubMed] [Google Scholar]
- 26.Jing J. C., Chen J. J., Chou L., Wong B. J. F., Chen Z., “Visualization and Detection of Ciliary Beating Pattern and Frequency in the Upper Airway using Phase Resolved Doppler Optical Coherence Tomography,” Sci. Rep. 7(1), 8522 (2017). 10.1038/s41598-017-08968-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLean J. P., Ling Y., Hendon C. P., “Frequency-constrained robust principal component analysis: a sparse representation approach to segmentation of dynamic features in optical coherence tomography imaging,” Opt. Express 25(21), 25819–25830 (2017). 10.1364/OE.25.025819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L., Chu K. K., Houser G. H., Diephuis B. J., Li Y., Wilsterman E. J., Shastry S., Dierksen G., Birket S. E., Mazur M., Byan-Parker S., Grizzle W. E., Sorscher E. J., Rowe S. M., Tearney G. J., “Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography,” PLoS One 8(1), e54473 (2013). 10.1371/journal.pone.0054473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y., Qu Y., Jing J. C., Chen Z., “Characterization of oviduct ciliary beat frequency using real time phase resolved Doppler spectrally encoded interferometric microscopy,” Biomed. Opt. Express 10(11), 5650–5659 (2019). 10.1364/BOE.10.005650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibañez-Tallon I., Heintz N., Omran H., “To beat or not to beat: roles of cilia in development and disease,” Hum. Mol. Genet. 12(90001), 27R–35 (2003). 10.1093/hmg/ddg061 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results presented in this paper are not publicly available at this time but may be obtained from the authors upon reasonable request.