Abstract
The origin of second harmonic generation (SHG) signal in otoconia was investigated. SHG signal intensity from otoconia was compared to pure calcite crystals, given calcite is the primary component of otoconia and is known to emit surface SHG. The SHG intensity from calcite was found to be ∼41× weaker than the SHG intensity from otoconia signifying that the SHG signal from otoconia is likely generated from the organic matrix. Furthermore, the SHG intensity from otoconia increased when treated with a chelating agent known to dissolve calcite which confirms that calcite is not the source of SHG. Additionally, polarization-resolved SHG microscopy imaging revealed that the arrangement of the SHG emitters is radial and can form highly ordered domains.
1. Introduction
Otoconia are composite mineral and biological crystals, and part of the vestibular system within the inner ear, responsible for gravity and linear acceleration perception in most vertebrates [1–3]. As a result, otoconia crystals are important for maintaining spatial orientation and balance. Otoconia crystals are formed in the embryonic state and mature shortly after birth. Demineralization and alterations to both the composition and the crystalline structure of human otoconia occur due to aging, infection, trauma, and consumption of ototoxic substances such as certain antibiotics [4]. Consequently, otoconia develop a hollow structure and break into fragments often causing the displacement of otoconia in the inner ear, resulting in sensations of vertigo and loss of balance [4]. To develop methods to prevent degeneration, and possibly promote repair and regeneration, it is important to study the crystalline structure of otoconia to understand how these biological crystals are developed.
Mammalian otoconia are primarily composed of calcium carbonate crystals in the polymorphic form of calcite [5] where a large portion of their mass is calcite (>90 weight %) and a small portion of their mass is an organic matrix (<5 weight %) [3,6–9]. The organic matrix embedded within otoconia has a fibrillar structure of glycoproteins and glycosaminoglycans that continues to extend outwards from their surface as fibrils, and helps to join neighboring otoconia together [7,10–14]. The most abundant glycoprotein within otoconia is otoconin-90 [9]. Otoconin-90 participates in collecting and assembling the necessary components for the development of the remaining organic matrix [4]. This in turn helps direct the morphology of otoconia [4,15]. Another essential glycoprotein in otoconia development is otolin which is structurally and chemically similar to collagen and makes up the part of the fibril that helps anchor and join otoconia together [16].
Otoconia have a 3-fold rotation axis and a center of symmetry with a symmetry point group close to -3m. However, recent research suggests that the overall symmetry of these crystals may not be as close to -3m as previously thought due to uneven size observations of their rhombohedral faces [17]. Despite recent advancements, there is still limited and conflicting information regarding the internal structural properties of otoconia including the crystal and protein arrangement.
Nonlinear optical microscopy is quickly becoming a standard analytical tool for structural investigations of biological samples. Nonlinear optical microscopy allows convenient sample investigation without pretreatment or the addition of a dye, permitting a quick measurement of samples in their native state, and thus diminishing the probability of artifacts. Therefore, nonlinear optical microscopy lends itself a convenient research tool for the structural analysis of otoconia. In this paper, we use nonlinear optical microscopy to investigate the origin of second harmonic generation (SHG) signal from otoconia and to perform an initial polarization-resolved SHG study.
2. Materials and methods
2.1. Otoconia dissection
The common house mouse (Mus musculus) model species, was chosen to extract otoconia due to their structural and compositional similarities to humans along with analogous mechanisms of otoconia formation [4]. Female mice of the strain B6.Cg-Tg(Thy1-YFP)16Jrs/J (The Jackson Laboratory) bred and raised at the Dalhousie University animal care facilities were used. To avoid age-related structural irregularities, young adult mice between 70-108 days old were used [18]. All animal housing and care, euthanasia procedures described and tissue collection were approved by The University Committee on Laboratory Animals, Dalhousie University, Halifax via Protocol #I20-04. The following procedure for otoconia isolation was modified from that used by Ou et al. [19]. Mice were euthanized with an overdose of the anesthetic sodium pentobarbitol (250 mg/kg), and transcardially perfused and exsanguinated with ice-cold phosphate buffered saline (pH 7.4). Following decapitation and removal of surrounding skin and muscle, the skulls were bisected along the mid-sagittal plane and brain tissue discarded. The temporal bones (housing the middle and inner ear structures) were isolated by carefully trimming away surrounding areas of the skull and placed onto a petri dish containing 0.1 M sodium cacodylate buffer (pH 7.4). Under a dissecting microscope the anterior semicircular canal on the inner surface of the temporal bone was used as a landmark to find the utricle. Perpendicular to the anterior semicircular canal lies the petrous ridge and a small area of translucent bone just above the ridge was carefully removed using a finely sharpened tool. The utricle located under this bone was removed using fine tipped forceps. The membrane around the utricle was torn by pulling apart with two pairs of #55 forceps to expose the numerous otoconia. Otoconia suspended in sodium cacodylate buffer were sandwiched between a microscope slide and a coverslip for SHG imaging.
2.2. Calcite preparation and calcite dissolution
Calcite was acquired from Dr. Jacob Hanley (Department of Geology, Saint Mary’s University). Using a porcelain mortar and pestle, the mm sized calcite was ground down until a fine powder resulted with a confirmed size below 500 nm. Larger calcite crystals were also grown. A supersaturated solution of pure calcite powder (Sigma-Aldrich) and deionized water was placed onto a separate petri dish with a paperclip to be used as a nucleation site for crystal formation. The petri dishes were then left for 48 hours until crystallization was complete. Approximately 0.2 g of the calcite powder was placed into an Eppendorf tube containing 1 mL of 0.1 M sodium cacodylate buffer (pH 7.4). The crystalline samples suspended in buffer were sandwiched between a microscope slide and a coverslip for SHG imaging. The grown calcite was confirmed to have a size range of 0.5–5 µm.
A chelating agent known to dissolve calcite, ethylenediaminetetraacetic acid (EDTA), was used for otoconia. Otoconia and calcite powder immersed in 0.1 M sodium cacodylate buffer were initially imaged on separate slides. Then, a syringe was used to flush the samples with a solution of 0.5 M EDTA. A timer was started and SHG images of the same samples initially imaged were taken every 10 minutes for a total of ∼80 minutes.
2.3. SHG imaging
SHG imaging was performed using a femtosecond duration pulsed laser (FemtoLux 3, Ekspla) operating at 1030 nm, 5 MHz repetition rate and ∼250 fs pulse duration. The laser was coupled to a custom-built nonlinear optical microscope. The laser beam was raster scanned across the sample using a pair of galvanometric scan mirrors (ScannerMAX, Pangolin Laser Systems Inc.) with a pixel dwell time of 6 µs to create images with 100×100 pixels with a size of 18×18 µm or 36×36 µm. The laser was focused onto the sample using a 0.8 numerical aperture (NA) air immersion objective lens (Plan-Apochromat 20×, Carl Zeiss AG). The average laser power at the sample was 1–4 mW. A custom built 0.85 NA collection objective lens (Omex Technologies) was used to collect SHG signal in transmission mode. SHG signal was separated from the laser light using an interference filter centered at 525 nm with 25 nm bandwidth (87-789, Edmund Optics Inc.) and a Schott colour glass filter (48-637, Edmund Optics Inc.). SHG signals were measured using a single-photon-counting photomultiplier detector (H10682-210, Hamamatsu Photonics K.K.).
2.4. Polarization-sensitive SHG image analysis
Polarization measurements of the SHG signal from otoconia were performed using the polarization-in, polarization-out (PIPO) technique [20]. Briefly, a linear polarizer followed by a rotating half-wave plate was placed before the excitation objective lens for rotation of the incident laser linear polarization. Polarization measurements of the SHG signal were performed via rotating a linear polarizer after the collection objective lens. A PIPO SHG measurement consisted of recording an SHG image at 64 combinations of 8 emission polarization angles for each of 8 half-wave plate angles, followed by a scan at the initial condition to check for photodamage or sample movement. Analysis of PIPO SHG data was performed assuming the SHG active domains are structures with cylindrical symmetry, which seems reasonable for otoconial fibrils, allowing the use of Eq. (1). This equation describes the SHG intensity as a function of the laser electric field polarization orientation ( ), the orientation of the analyzer (φ), the in-plane angle between the crystal axis and the laboratory z-axis ( ), and the second-order nonlinear optical susceptibility tensor ratio value assuming C6v and Kleinman symmetry:
| (1) |
The primed second-order nonlinear optical susceptibility tensor ratio denotes the molecular susceptibility projected onto the image plane. This parameter is determined by the molecular orientations of the SHG emitters, and has been linked to the helical tilt of the SHG emitters in collagen, muscle and starch [21] as well as the orientation of structured water [22]. R is also a function of disorder of SHG emitters in the focal plane [23]. Additionally, the laboratory and molecular R parameters are a function of their angle to the image plane (α) by the following equation:
| (2) |
This equation shows that R represents the laboratory frame molecular component of the second-order nonlinear optical susceptibility tensor ratio when the SHG emitter is in the image plane ( ).
3. Results
Otoconia immersed in buffer were observed to give rise to intense SHG signal while crushed calcite powder immersed in buffer did not emit SHG signal at similar or even slightly higher laser powers. To ensure that the size of the pure calcite powder was not too small and comparable to the size of otoconia, larger pure calcite crystals were grown. SHG signal from the larger calcite crystals was detected however, the SHG signal was found to be ∼41× lower than the SHG signal observed with otoconia.
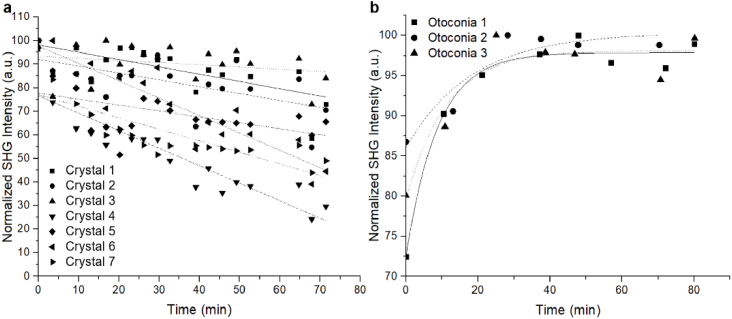
Next, otoconia and calcite were exposed to 0.5 M EDTA and imaged over a period of ∼80 minutes. Small regions were selected in 7 calcite crystals and 3 otoconia for analysis, and the corresponding SHG intensities were plotted against time (Fig. 1). The SHG intensities of calcite crystals were observed to decrease as little as 13% and as much as 58% over the course of 71 minutes. On the other hand, the SHG intensity increased for otoconia exposed to 0.5 M EDTA, and began to plateau after 29-53 minutes of exposure.
Fig. 1.
Graphs of the SHG intensity of (a) calcite and (b) otoconia with increasing exposure time to 0.5 M EDTA.
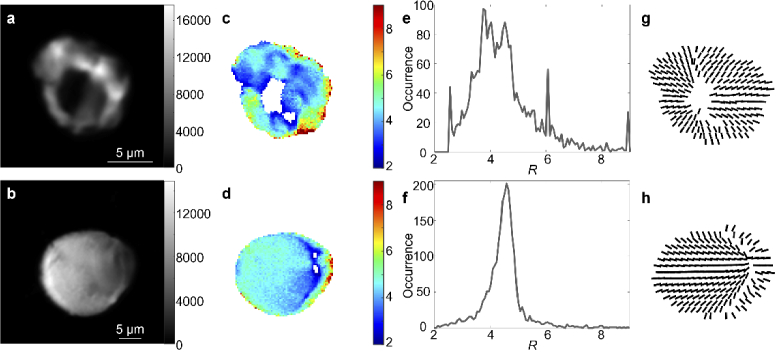
The ultrastructural organization of otoconia was also investigated with PIPO SHG microscopy. Representative findings are demonstrated in Fig. 2, where SHG intensity images visualize the otoconia (Fig. 2(a), 2(b)). Fitted R values for each pixel in the SHG images are shown by color (Fig. 2(c), 2(d)) and summarized with occurrence histograms (Fig. 2(e), 2(f)). The mean R values for two typical otoconia shown in Fig. 2(c) and 2(d) were 4.15 ± 0.06 and 4.53 ± 0.02, respectively. Corresponding plots of the fitted parameters are displayed by vector images in Fig. 2(g) and 2(h), and indicate SHG emitting structures in otoconia are radially arranged with respect to the nucleation regions.
Fig. 2.
PIPO SHG analysis of otoconia. SHG intensity images of two otoconia obtained by summing all images in a PIPO SHG image stack (a, b), color-coded maps of the fitted R values (c, d), the corresponding occurrence histograms of the fitted R values (e, f) in the color-coded maps (c, d, respectively) and the fitted orientation of the crystal axis ( ) for each 6th pixel (g, h).
4. Discussion
The results indicate that the SHG intensity of otoconia did not decrease with EDTA induced loss of calcite (Fig. 1(b)) as would be expected if SHG signal was emitted by calcite. This observation indicates that calcite is likely not the source of SHG signal and instead, we attribute otoconial fibrils as the SHG emitters in otoconia. Further, a significant increase in SHG intensity was observed with EDTA treatment, with several possible explanations. The calcite crystals within otoconia have been previously reported to have diameters as low as 50-100 nm [2,3], hence they are within our laser focal volume (0.5 µm lateral and 2 µm axial), and also within our laser coherence length. Therefore, it is reasonable that in untreated otoconia, the crystals hold otoconial fibrils apart in a radial fashion reducing the SHG intensity, which is maximized when fibrils are parallel. Upon calcite dissolution and diffusion out of the otoconium, it is therefore sensible that the otoconial fibrils would adapt an increasingly parallel structure in domains, explaining the increasing SHG intensity. However, if the dissolved calcite does not diffuse out of the otoconia, then the increased SHG intensity may be due to decreased laser scattering. In untreated otoconia, the many surfaces of the small calcite crystals likely induce a significant amount of laser scattering, which reduces the laser intensity at the fibrils. If the calcite crystals are partially or totally dissolved and do not diffuse out of the otoconium, the resulting liquid may act as index matching fluid reducing the laser scattering and hence, increasing the SHG intensity. One interesting, although possibly difficult future step to prove the assertion that otoconial fibrils are the SHG emitters is to dissolve the organic matrix while leaving the calcite intact.
The ultrastructural organization of otoconia was additionally investigated with PIPO SHG microscopy, and the results for two typical otoconia are shown in Fig. 2. Good fits to the data were obtained with the C6v model indicating that the SHG emitters are likely cylindrical structures. The fitted orientation of the cylindrical axis for each 6th pixel at the approximate equator of the otoconia is shown in Fig. 2(g) and 2(h), and it demonstrates a radial arrangement of SHG emitters within the otoconia. This indicates that the SHG emitters in otoconia, which are likely the otoconial fibrils, are radially arranged. This result is in line with previous electron microscopy observations of the organic matrix having a radial fibrillar organization [13].
The fitted R parameter for each pixel of the image (Fig. 2(c), 2(d)) appears to decrease near the supposed otoconia nucleation regions, which can be observed by regions of divergence in the vector diagrams. A similar reduction in the R parameter was previously observed for PIPO SHG measurements of starch granules [22,24,25], which are known to have a radial arrangement, and they have similar radial vector image profiles to otoconia (see for example the fourth column in Fig. 6 in Ref. [22]). The R value of the otoconia near the supposed nucleation region is lower (∼3), as compared with regions closer to the periphery (∼7). A reduced R parameter was also observed near the nucleation regions in starch granules, and was explained by the radial starch ultrastructure, where the laser focal volume encompasses SHG emitters with increasingly larger variation in orientation as it gets closer to the nucleation region. The R parameter is known to tend towards 3 as disorder increases [23], hence, the radial structure induces an artificial disorder at and near nucleation regions. It is likely that the same effect occurs in otoconia, resulting in the observed reduction in R around nucleation regions. A transmission electron microscope study on mouse otoconia indicated that they contain an abundance of otoconin-90 around their core with altered structure as compared to the outer core which could also explain the variation in the R parameter [26]. An interesting future experiment to reveal how the radial otoconia ultrastructure affects the R parameter is to image individual otoconia at different heights, where the R parameter is expected to reduce to 3 as the out of plane angle increases, similar to an earlier experiment performed on starch granules [27].
The R parameter appears heterogeneous throughout the structure of the otoconia (Fig. 2(c), 2(d)), and appears to be formed into domains. In general, variations in R occur either because: (i) the intrinsic R of the SHG emitters is variable and hence there are different species of SHG emitters, or (ii) there are variations in disorder of SHG emitters in different domains, or (iii) the SHG emitters in domains are oriented at different angles off the image plane, see Eq. (2). In typical otoconia we observe that domains further from the nucleation region, as observed by divergence of the vector diagram, have a significantly higher R parameter, near the limit of the sensitivity of the technique (∼R = 10). Assuming that SHG emitters have a uniform intrinsic architecture across any otoconia, we conclude that the high R parameters observed in the otoconia indicate that the intrinsic R parameter of SHG emitters in otoconia is at least 8. One likely conclusion is that domains with intermediate R values indicate the presence of emitters that are tilted at different angles from the image plane , with the higher angles reducing the measured R according to Eq. (1). Additionally, the presence of domains with varying amounts of disorder due to a higher otoconin-90 concentration in the inner core may also reduce R.
5. Conclusions
In order to determine whether the SHG signal from otoconia was generated from its calcite composition, the SHG signal intensity from otoconia was compared to pure calcite crystals. The SHG signal intensity from calcite was found to be ∼41× weaker than the SHG intensity from otoconia. Furthermore, when otoconia was treated with EDTA to promote calcite dissolution, the SHG signal intensity increased. These observations indicate that the SHG signal from otoconia is not generated from calcite and instead, is likely generated from the organic matrix in otoconia. PIPO SHG microscopy imaging was then used to study the ultrastructure of otoconia revealing good fits to the C6v model, indicating emitters likely have a cylindrical ultrastructure in line with what would be expected for fibrils. Further, PIPO SHG measurements revealed that the arrangement of the SHG emitters is radial inside otoconia. These observations suggest that SHG emitters are likely otoconial fibril structures, which appear to be oriented in columns parallel to carbonate crystallites [26]. However, more work must be performed to reveal the exact molecular origin of the noncentrosymmetry within these fibrils needed for SHG. Further analysis revealed the presence of heterogeneity in the ordering parameter inside otoconia (see Fig. 2(c), 2(d)), showing highly ordered domains sometimes near the otoconia periphery. Future research and modelling are required to ascertain the origin of the variations, as they could occur due to variations in the tilt of SHG emitters away from the image plane, disorder of the SHG emitters within the focal volume, or intrinsic disorder of SHG emitters.
Acknowledgments
The authors would like to thank Dr. Mitchell Kerr and Dr. Jacob Hanley (Saint Mary’s University) for providing the calcite samples. The authors would also like to thank Dr. Gabriella Sekerka (Feinberg School of Medicine, Northwestern University) for advice on otoconia dissection as well as Dr. Virginijus Barzda (University of Toronto) for inspiring this project.
Funding
Natural Sciences and Engineering Research Council of Canada10.13039/501100000038 (RGPIN-2018-05444); Canada Foundation for Innovation10.13039/501100000196 (John R. Evans Leaders Fund #37749); Research Nova Scotia10.13039/501100020170 (1868); Canada’s Research Support Fund; Saint Mary’s University10.13039/100012976.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
Data Availability
Data underlying the results presented in this paper are available in Supplement 1 (3.2MB, pdf) .
Supplemental document
See Supplement 1 (3.2MB, pdf) for supporting content.
References
- 1.Lundberg Y. X. W., Zhao X., Yamoah E. N., “Assembly of the otoconia complex to the macular sensory epithelium of the vestibule,” Brain Res. 1091(1), 47–57 (2006). 10.1016/j.brainres.2006.02.083 [DOI] [PubMed] [Google Scholar]
- 2.Mann S., Parker S. B., Ross M. D., Skarnulis A. J., Williams R. J. P., “The ultrastructure of the calcium-carbonate balance organs of the inner-ear - an ultrahigh resolution electron-microscopy study,” Proc. R. Soc. B: Biol. Sci. 218(1213), 415 (1983). [DOI] [PubMed] [Google Scholar]
- 3.Ross M. D., Pote K. G., “Some Properties of Otoconia,” Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 304(1121), 445–452 (1984). [DOI] [PubMed] [Google Scholar]
- 4.Hughes I., Thalmann I., Thalmann R., Ornitz D. M., “Mixing model systems: Using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development,” Brain Res. 1091(1), 58–74 (2006). 10.1016/j.brainres.2006.01.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim D. J., “Formation and fate of the otoconia: Scanning and transmission electron microscopy,” Ann. Otol., Rhinol., Laryngol. 82(1), 23–35 (1973). 10.1177/000348947308200109 [DOI] [PubMed] [Google Scholar]
- 6.Carlström D., Engström H., “The ultrastructure of statoconia,” Acta Oto-Laryngol. 45(1), 14–18 (1955). 10.3109/00016485509118139 [DOI] [PubMed] [Google Scholar]
- 7.Huang Y. X., Buder J., Cardoso-Gil R., Prots Y., Carrillo-Cabrera W., Simon P., Kniep R., “Shape development and structure of a complex (otoconia-like?) calcite-gelatine composite,” Angew. Chem., Int. Ed. 47(43), 8280–8284 (2008). 10.1002/anie.200800968 [DOI] [PubMed] [Google Scholar]
- 8.Simon P., Carrillo-Cabrera W., Huang Y. X., Buder J., Borrmann H., Cardoso-Gil R., Rosseeva E., Yarin Y., Zahnert T., Kniep R., “Structural relationship between calcite-gelatine composites and biogenic (Human) otoconia,” Eur. J. Inorg. Chem. 2011(35), 5370–5377 (2011). 10.1002/ejic.201100756 [DOI] [Google Scholar]
- 9.Walther L. E., Blödow A., Buder J., Kniep R., “Principles of calcite dissolution in human and artificial otoconia,” PLoS ONE 9(7), e102516 (2014). 10.1371/journal.pone.0102516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim D. J., “Otoconia in Health and Disease. A Review,” Annals of Otology, Rhinology and Laryngology, Supplement 112, 17–24 (1984). [DOI] [PubMed] [Google Scholar]
- 11.Pote K. G., Ross M. D., “Ultrastructural Morphology and Protein Content of the Internal Organic Material of Rat Otoconia,” J. Ultrastruct. Mol. Struct. Res. 95(1-3), 61–70 (1986). 10.1016/0889-1605(86)90029-7 [DOI] [PubMed] [Google Scholar]
- 12.Fermin C. D., “High resolution and image processing of otoconia matrix,” Microscopy Research Technique 25(4), 297–303 (1993). 10.1002/jemt.1070250406 [DOI] [PubMed] [Google Scholar]
- 13.Steyger P. S., Wiederhold M. L., “Visualization of newt aragonitic otoconial matrices using transmission electron microscopy,” Hear. Res. 92(1-2), 184–191 (1995). 10.1016/0378-5955(95)00221-9 [DOI] [PubMed] [Google Scholar]
- 14.Zhao X., Yang H., Yamoah E. N., Lundberg Y. W., “Gene targeting reveals the role of Oc90 as the essential organizer of the otoconial organic matrix,” Dev. Biol. 304(2), 508–524 (2007). 10.1016/j.ydbio.2007.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu W., Zhou D., Freeman J. J., Thalmann I., Ornitz D. M., Thalmann R., “In vitro effects of recombinant Otoconin 90 upon calcite crystal growth. Significance of tertiary structure,” Hear. Res. 268(1-2), 172–183 (2010). 10.1016/j.heares.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murayama E., Herbomel P., Kawakami A., Takeda H., Nagasawa H., “Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae,” Mech. Dev. 122(6), 791–803 (2005). 10.1016/j.mod.2005.03.002 [DOI] [PubMed] [Google Scholar]
- 17.Kniep R., Zahn D., Wulfes J., Walther L. E., “The sense of balance in humans: Structural features of otoconia and their response to linear acceleration,” PLoS ONE 12(4), e0175769 (2017). 10.1371/journal.pone.0175769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang Y. S., Hwang C. H., Shin J. Y., Bae W. Y., Kim L. S., “Age-related changes on the morphology of the otoconia,” Laryngoscope 116(6), 996–1001 (2006). 10.1097/01.mlg.0000217238.84401.03 [DOI] [PubMed] [Google Scholar]
- 19.Ou H. C., Lin V., Rubel E. W., ““In-bone” utricle cultures - A simplified, atraumatic technique for in situ cultures of the adult mouse (Mus musculus) utricle,” Otology & Neurotology 34(2), 353–359 (2013). 10.1097/MAO.0b013e31827ca330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tuer A. E., Krouglov S., Prent N., Cisek R., Sandkuijl D., Yasufuku K., Wilson B. C., Barzda V., “Nonlinear optical properties of type I collagen fibers studied by polarization dependent second harmonic generation microscopy,” J. Phys. Chem. B 115(44), 12759–12769 (2011). 10.1021/jp206308k [DOI] [PubMed] [Google Scholar]
- 21.Tiaho F., Recher G., Rouède D., “Estimation of helical angles of myosin and collagen by second harmonic generation imaging microscopy,” Opt. Express 15(19), 12286–12295 (2007). 10.1364/OE.15.012286 [DOI] [PubMed] [Google Scholar]
- 22.Cisek R., Tokarz D., Krouglov S., Steup M., Emes M. J. M. J., Tetlow I. J. I. J., Barzda V., “Second harmonic generation mediated by aligned water in starch granules,” J. Phys. Chem. B 118(51), 141216070413005 (2014). 10.1021/jp508751s [DOI] [PubMed] [Google Scholar]
- 23.Simpson G. J., Rowlen K. L., “An SHG magic angle: Dependence of second harmonic generation orientation measurements on the width of the orientation distribution,” J. Am. Chem. Soc. 121(11), 2635–2636 (1999). 10.1021/ja983683f [DOI] [Google Scholar]
- 24.Cisek R., Tokarz D., Steup M., Tetlow I. J., Emes M. J., Hebelstrup K. H., Blennow A., Barzda V., “Second harmonic generation microscopy investigation of the crystalline ultrastructure of three barley starch lines affected by hydration,” Biomed. Opt. Express 6(10), 3694–3700 (2015). 10.1364/BOE.6.003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cisek R., Tokarz D., Kontenis L., Barzda V., Steup M., “Polarimetric second harmonic generation microscopy: An analytical tool for starch bioengineering,” Starch/Staerke 70(1–2), 141216070413005 (2014). 10.1002/star.201700031 [DOI] [Google Scholar]
- 26.Andrade L. R., Lins U., Farina M., Kachar B., Thalmann R., “Immunogold TEM of otoconin 90 and otolin e relevance to mineralization of otoconia, and pathogenesis of benign positional vertigo,” Hear. Res. 292(1-2), 14–25 (2012). 10.1016/j.heares.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psilodimitrakopoulos S., Amat-Roldan I., Loza-Alvarez P., Artigas D., “Effect of molecular organization on the image histograms of polarization SHG microscopy,” Biomed. Opt. Express 3(10), 2681–2693 (2012). 10.1364/BOE.3.002681 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results presented in this paper are available in Supplement 1 (3.2MB, pdf) .