Abstract
Previous human postmortem brain tissue research has implicated abnormalities of 5-HT receptor availability in depression and suicide. Although altered abundance of 5-HT 1A, 5-HT 2A, and 5-HT 2C receptors (5-HT1A, 5-HT2A, and 5-HT2C) has been reported, the causes remain obscure. This study evaluated the availability of these three receptor subtypes in postmortem brain tissue specimens from persons with a history of major depression (MDD) and normal controls and tested the relationships to protein kinases A and C (PKA, PKC). Samples were obtained from postmortem brain tissue (Brodmann area 10) from 20 persons with a history of MDD and 20 matched controls as determined by a retrospective diagnostic evaluation obtained from family members. Levels of 5-HT1A, 5-HT2A, and 5-HT2C receptor were quantitated via Western blot analyses. Basal and stimulated PKA and PKC activity were also determined. The depressed samples showed significantly increased 5-HT2A receptor abundance relative to controls, but no differences in 5-HT1A or 5-HT2C receptors. Basal and cyclic AMP-stimulated PKA activity was also reduced in the depressed sample; PKC activity was not different between groups. 5-HT2A receptor availability was significantly inversely correlated with PKC activity in controls, but with PKA activity in the depressed sample. Increased 5-HT2A receptor abundance and decreased PKA activity in the depressed sample are consistent with prior reports. The correlation of 5-HT2A receptor levels with PKA activity in the depressed group suggests that abnormalities of 5-HT2A receptor abundance may depend on receptor uncoupling and heterologous regulation by PKA.
Keywords: 5-HT receptors, protein kinases, protein kinase A, protein kinase C, depression, postmortem
Major depression (MDD) is a serious, potentially disabling, and even life threatening disorder. Although the underlying pathophysiology is undoubtedly multifactorial, a huge literature implicates the 5-HT system as a possible causal factor in depression in general and suicidal behavior in particular (Stockmeier, 2003; Pandey et al., 2003b; Mann et al., 2001; Jans et al., 2007; Arango et al., 2003). 5-HT serves a modulatory role with regard to mood, and a variety of factors may lead to dysfunction of the 5-HT system, leading to abnormal mood states. A variety of mechanisms regulate 5-HT response in brain, including enzymes involved in the synthetic pathway (e.g. tryptophan hydroxylase, indoleamine 2,3-dioxygenase), synaptic regulation (e.g. the 5-HT transporter protein) and a variety of 5-HT receptors.
5-HT 2A receptors (5HT2A) have been shown to be elevated in frontal cortex of depressed persons and suicide victims (Turecki et al., 1999; Stanley and Mann; Pandey et al., 2002; Hrdina and Du, 2001; Hrdina et al., 1993; Arranz et al., 1994; Arango et al., 1990, 1997), particularly involving pyramidal cells of cortical layer V (Pandey et al., 2002). 5-HT2A receptors have been shown to have a significant role in the modulation of mood state, consistent with their widespread distribution in brain regions known to modulate mood responses, including cortex, hippocampus, and amygdala (Weisstaub et al., 2006). Activation of 5-HT2A has been shown to enhance anxious responding in animal and human studies (Mora et al., 1997; Graeff et al., 1996), whereas selective blockade (Kleven et al., 1997; Griebel et al., 1997), antisense inhibition (Sibille et alt., 1997), or disruption (Weisstaub et al., 2006) has been shown to reduce anxiety and learned helplessness behavior. Furthermore, learned helplessness in rats related to chronic inescapable shock, a putative model for human depression, is associated with significant up-regulation of 5-HT2A mRNA and protein expression in frontal cortex (Dwivedi et al., 2005).
Elevated availability of 5-HT2A receptors in brains of depressed and suicide victims has been attributed to decreased 5-HT leading to receptor upregulation (Jans et al., 2007). However, the regulation of 5-HT2A receptors is complex and, under certain conditions, paradoxical (Van Oekelen et al., 2003). For example, both agonists and antagonists induce down-regulation in 5-HT2A receptor availability (Van Oekelen et al., 2003). 5-HT2A receptors are also susceptible to both homologous and heterologous receptor-mediated down-regulation via protein kinases (Saucier et al., 1998; Saucier and Albert). Although homologous desensitization can occur via protein kinase C (PKC)–dependent phosphorylation by activation of the 5-HT2A–Gq/11–phospholipase C–diacylglycerol cascade, heterologous desensitization of 5-HT2A receptors by other enzymes including protein kinase A (PKA) or calcium-calmodulin kinase (CaMK) also occurs (Van Oekelen et al., 2003). Phosphorylation-dependent internalization to endosomes results in either dephosphorylation leading to resensitization or degradation (Van Oekelen et al., 2003). Therefore, regulation of 5-HT2A receptors via phosphorylation may significantly affect availability.
Our research group and others have demonstrated decreased activity and protein availability for PKA (Shelton et al., 1996, 1999; Perez et al., 1995, 1999, 2001; Pandey et al., 2007; Manier et al., 1996, 2000; Dwivedi et al., 2002, 2004b; Akin et al., 2004, 2005) and PKC (Pandey et al., 1997, 1998; Coull et al., 2000; Akin et al., 2005) in a significant subset of patients with MDD using both peripheral tissue models and postmortem brain tissue. This has also been tested functionally by demonstrating decreased phosphorylation of target proteins such as cyclic AMP response element binding protein (CREB) (Pandey et al., 2007; Manier et al., 2001) and myristoylated alanine-rich C kinase substrate (MARCKS) (Pandey et al., 2003a). However, to our knowledge, the relationship between reduced PKA and PKC and 5-HT receptor availability has not been previously tested.
Other 5-HT receptors have been implicated in the regulation of mood. For example, presynaptic 5-HT1A receptors inhibit release of 5-HT and down-regulation is required for the antidepressant response to 5-HT selective reuptake inhibitors (SSRIs) (Lemonde et al., 2003; Blier et al., 2001). Post-synaptic 5-HT1A receptors also appear to mediate some of the antidepressant actions of SSRIs and related drugs. For example, activation of 5-HT1A receptors enhances the activity of both norepinephrine and dopamine neurons (Szabo and Blier, 2001; Ichikawa and Meltzer, 1999; Ichikawa et al., 2001), which is likely to be involved in antidepressant effects (Szabo and Blier, 2001; Stahl and Shayegan; Simon and Nemeroff; Haddjeri et al., 1997). 5-HT1A also has been studied in depressed and suicide samples using both brain imaging and postmortem brain tissue methods (Tochigi et al., 2008; Szewczyk et al., 2008; Stockmeier et al., 1997, 1998; Matsubara et al., 1991; Lemonde et al., 2003; Hsiung et al., 2003; Drevets et al., 1999, 2007; Arranz et al., 1994; Arango et al., 2001), with variable results (for a review, see Stockmeier, 2003). A number of studies have demonstrated reduced binding, availability, or activity of 5-HT1A receptors, but this appears to vary depending on both the brain region analyzed and the clinical subtype tested (Stockmeier, 2003; Drevets et al., 2007). For example, Drevets et al. conducted two different positron emission tomography (PET) studies of 5-HT1A binding using carbonyl-[11C]WAY-100635, a relatively selective 5-HT1A ligand in depressed and control samples. They showed decreased 5-HT1A binding in medial temporal cortex and raphe nuclei, but not in other brain regions, a finding that appeared to be specific for recurrent, familial depression (Drevets et al., 1999, 2007). Stockmeier et al. (1997) did not find any differences [3H]8-hydroxy-2-(di-n-propyl)-aminotetralin ([3H]8-OH-DPAT) binding to 5-HT1A receptors in right anterior prefrontal cortex (PFC) (Brodmann area [BA] 10) from depressed suicide victims in comparison to controls, although in a related study, 5-HT1A protein abundance was found to be reduced in PFC samples from depressed females (Szewczyk et al., 2008).
By contrast, 5-HT2C receptors have received less attention, in spite of their apparent involvement in mood regulation. Activation of 5-HT2C receptors attenuates PFC norepinephrine and dopamine release in rodent models (Pozzi e al., 2002; Li et al., 2005; Gobert et al., 2000), and blockade of 5-HT2C receptors by atypical antipsychotics has been hypothesized to underlie their antidepressant properties (Shelton and Papakostas). There have been limited postmortem studies of 5-HT2C receptors; studies (Schmauss, 2003; Niswender et al., 2001; Gurevich et al., 2002) have shown an increase in an edited form of 5-HT2C receptor (isoleucine–asparagine–isoleucine to valine–glycine–valine editing at positions 156, 158, and 160) that is associated with decreased coupling of the receptor to G-proteins in samples from persons with MDD. One study of 5-HT2C receptors in various human postmortem brain regions contrasted samples from suicide victims and controls (Pandey et al., 2006). 5-HT2C receptors were found to be widely distributed, with greater abundance in choroid plexus, hypothalamus, and nucleus accumbens, and lesser availability in PFC and cerebellum. However, only PFC (BA8/9) showed decreased abundance of 5-HT2C receptors in the depressed sample relative to controls.
The primary purpose of the current study was to contrast the abundance of 5-HT1A, 5-HT2A, and 5-HT2C receptors and to test the association of these receptors with PKA and PKC levels in postmortem orbitofrontal cortex tissue (BA10) from depressed and control samples. BA10 is involved in a variety of functions of significance to depression, including executive function (Rogers et al., 1999; Okuda et al., 2007; Leung et al., 2005; Konishi et al., 2000) and reward behavior (Rogers et al., 1999). BA10 is also relatively selectively activated with administration of cocaine (Kufahl e al., 2005) and amphetamine (Devous et al., 2001), which is consistent with the rich innervations of this region by norepinephrine and dopamine containing neurons (Volkow et al., 2000). We hypothesized that there would be alterations in the availability of these receptor subtypes in depressed subjects versus controls, consistent with previous observations in postmortem samples. We also hypothesized that there would be reduced PKA and PKC activity and that the activity of these enzymes would be correlated with the abundance of 5-HT receptors, particularly 5-HT2A. A final goal of this study was to test whether altered 5-HT receptors and kinase activity are specific to depressed patients who died by suicide.
EXPERIMENTAL PROCEDURES
Brain specimens were obtained from the Brain Tissue Donation Program at the University of Pittsburgh Medical Center and were acquired during autopsies after consent was given by the next of kin. Samples of PFC (BA10) were collected from 20 persons with a history of MDD and 20 age, sex, and postmortem interval (PMI) matched controls (Table 1). All procedures were conducted in accordance with the Declaration of Helsinki and were approved by the University of Pittsburgh’s Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research and the Vanderbilt University Health Sciences Institutional Review Board. An independent diagnostic conference was conducted with a committee of experienced research clinicians who assigned DSM-IV diagnoses by consensus for each subject on the basis of all information obtained from a standardized psychological autopsy which incorporated structured interviews (e.g. the Structured Clinical Interview for DSM-IV; First et al., 1996), conducted by trained and experienced clinicians with family members of the index case (patients and controls), to assess diagnosis, psychopathology, medical, developmental, social and family history, medication history, history of alcohol, tobacco and other substance use, and handedness. The use of multiple informants, including physicians and other health care workers, provided both an extensive range of detailed information and the opportunity to corroborate critical elements of the history. Ancillary data from clinical records, toxicology and neuropathology examinations, and the Medical Examiner’s investigation were also reviewed. Written informed consent was obtained from all participants. Based on the consensus findings, the deceased were given primary (e.g. MDD) and subtype (e.g. melancholia) diagnoses. All samples came from persons free from all known psychotropic agents based on toxicology and recent substance abuse. The right hemisphere of each brain was blocked coronally, immediately frozen and stored at −80 °C.
Table 1.
Demographic and postmortem data
| Depressed | Controls | |
|---|---|---|
| N | 20 | 20 |
| Age (S.D.) | 45.5 (14.2) years | 46.9 (13.4) years |
| PMI (S.D.) | 18.2 (6.4) hours | 17.9 (5.6) hours |
| Brain pH (S.D.) | 6.65 (0.23) | 6.74 (0.27) |
| Sex (%) | 3 Females (15%) | 3 Females (15%) |
| Race (%) | 20 White (100%) | 20 White (100%) |
Western blot analysis
Tissues were homogenized by sonication in 10 volumes of ice-cold buffer containing 20 mM tris(hydroxymethyl)aminomethane (Tris)–HCl, (pH 7.4 at 25 °C), 2 mM EDTA, 25 mM 2-mercaptoethanol, 0.5 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride, plus 0.5% Triton X-100, 2 μg/ml leupeptin, 3 μg/ml aprotinin, and 0.2 mg/ml soybean trypsin inhibitor. The homogenate was centrifuged at 12,000×g for 10 min at 4 °C. Equal volumes of supernatant (20 μl containing 30 μg of protein) and gel loading solution (50 mM Tris–HCl, pH 6.8, 4% β-mercaptoethanol, 1% sodium dodecyl sulfate [SDS], 40% glycerol, and a trace amount of Bromphenol Blue) were mixed, then boiled for 3 min and kept on ice for 10 min. Samples were loaded onto 10% (w/v) SDS–polyacrylamide gel using the Mini Protein II gel apparatus (Bio-Rad, Hercules, CA, USA). The gels were run using 25 mM Tris-base, 192 mM glycine, and 0.1% (w/v) SDS at 200 V. The proteins were transferred electrophoretically to an enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham) using the Mini TransBlot transfer unit (Bio-Rad) at 0.25 amp constant current. Membranes were washed with phosphate-buffered saline containing 0.05% Tween 20 for 10 min. The blots were blocked by incubation with 3% (w/v) powdered nonfat milk in phosphate-buffered saline. They were incubated overnight at 4 °C with primary antibody (anti-5-HT1A, 5-HT2A, 5-HT2C, [all 5-HT receptor antibodies were from Santa Cruz Biotechnologies, Santa Cruz, CA, USA] or phosphorylated cyclic AMP response element binding protein (CREB-P) [Upstate Cell Signaling Solutions, Charlottesville, VA, USA]) at a dilution of 1:1000–1:3000 depending on the antibody used. The membranes were washed with phosphate-buffered saline and incubated with horseradish peroxidase–linked secondary antibody (anti-rabbit immunoglobulin G; 1:3000) for 1.5 h at room temperature. The membranes were washed with water followed by phosphate-buffered saline containing 0.05% Tween 20 and exposed to ECL film, then standardized using 10–100 μg of protein. The optical density of the bands varies linearly with a concentration of up to 100 μg of protein. The band optical density was quantified using Un-Scan-It gel digitizing software (Orem, UT, USA); the optical density is corrected by the total protein in the sample, determined by the methods of Lowry et al. (1990). All samples were done in triplicate. For representative blots, see Fig. 1.
Fig. 1.
Representative Western blots for 5-HT1A, 5-HT2A, and 5-HT2C receptors (triplicates, controls left, depressed right).
PKA and PKC activity
Brain specimens were homogenized using a glass-Teflon homogenizer (1000 rpm, five strokes) in 50 vol buffer containing 30% sucrose). PKA activity was determined in the soluble fraction (±100 μM cAMP), as previously described (Shelton et al., 1996; Manier et al., 1996). PKA activity was defined as the transfer of PO4 from ATP (100 μM; 32P tracer, 300 counts per minute/pmol) to the heptapeptide Kemptide (leucine–arginine–arginine–alanine–leucine–glycine; 50 μM) and normalized per units protein and time.
The PKC assay was based on the phosphorylation of CREB in tissue homogenates after treatment by phorbol 12-myristate 13-acetate (PMA) (100 μM, 10 min at 37 °C). Stimulated and endogenous phosphorylation of CREB was determined. CREB-P was quantitated by Western blotting, using methods described above.
Statistical analysis
Demographic and postmortem (e.g. PMI) variables were compared via independent samples t-test or chi square analysis as appropriate. Western blot data and kinase activity were compared via independent samples t-test. Within-groups analyses (e.g. suicide versus non-suicide) were done with independent samples t-tests. Missing data were handled pairwise. The primary outcome contrast determined a priori was a comparison of 5-HT receptors in samples from persons with MDD against matched controls, with 5-HT2A being the first entered. The relationships between PKA and PKC activity versus 5-HT receptor availability was tested via the Pearson product moment correlation coefficient. The a priori hypotheses were as follows: 1. The availability of 5-HT1A receptors would be reduced in depressed versus control samples; 2. 5-HT2A receptors would be increased relative to controls; 3. 5-HT2C receptors would be decreased in depressed versus controls; 4. PKA and PKC activity would both be reduced in depressed relative to control samples; 5. Both PKA and PKC activity would be significantly correlated with 5-HT receptor abundance; 6. The contrast of suicide versus non-suicide depressed samples would not show any significant differences in 5-HT receptors or kinase activity. All hypotheses were tested independently. The effects of age, PMI, and brain pH on protein levels and kinase activity were tested by Pearson product moment correlation analysis. Data were analyzed using SPSS 15.0 (SPSS, Chicago, IL, USA).
RESULTS
Demographic characteristics and tissue sample descriptions are shown in Table 1 and the characteristics of the individual samples are shown in Table 2. There were no significant differences in age (t=−317, df=38, P=0.75), PMI (t=1.47, df=38, P=0.88), pH of the samples (t=−1.184, df=38, P=0.024), or sex (both groups were 15% female). All samples were from Caucasians.
Table 2.
Sample characteristicsa
| Subject number | Group | Pair | Sex | Race | Age | PMI (hours) | pH | Cause of death | Manner of Death |
|---|---|---|---|---|---|---|---|---|---|
| 511 | MDD | 1 | M | W | 43 | 17.9 | 6.91 | Arteriosclerotic cardiovascular disease | Natural |
| 596 | MDD | 2 | M | W | 68 | 20.5 | 6.88 | Gunshot wound | Suicide |
| 600 | MDD | 3 | M | W | 63 | 9.9 | 6.72 | Hanging | Suicide |
| 613 | MDD | 4 | M | W | 59 | 15.6 | 6.95 | Gunshot wound | Suicide |
| 614 | MDD | 5 | M | W | 39 | 19.5 | 6.67 | CO poisoning | Suicide |
| 628 | MDD | 6 | M | W | 26 | 21.6 | 6.73 | CO poisoning | Suicide |
| 668 | MDD | 7 | M | W | 34 | 24.3 | 7.00 | Hanging | Suicide |
| 699 | MDD | 8 | M | W | 65 | 5.5 | 6.71 | Gunshot wound | Suicide |
| 735 | MDD | 9 | F | W | 40 | 14.0 | 6.84 | Pulmonary embolism | Accidental |
| 927 | MDD | 10 | M | W | 58 | 24.9 | 6.11 | Arteriosclerotic cardiovascular disease | Natural |
| 949 | MDD | 11 | M | W | 38 | 25.0 | 6.23 | Cardiac arrhythmia | Natural |
| 1013 | MDD | 12 | M | W | 46 | 16.1 | 6.27 | Nail-gun wound | Suicide |
| 1017 | MDD | 13 | M | W | 27 | 18.8 | 5.69 | Diabetic ketoacidosis | Natural |
| 1028 | MDD | 14 | M | W | 39 | 14.5 | 6.18 | Gunshot wound | Suicide |
| 1053 | MDD | 15 | M | W | 47 | 24.0 | 6.57 | Arteriosclerotic cardiovascular disease | Natural |
| 1131 | MDD | 16 | M | W | 29 | 26.6 | 6.92 | Gunshot wound | Suicide |
| 1186 | MDD | 17 | M | W | 45 | 6.6 | 6.25 | Traumatic asphyxiation | Accidental |
| 1215 | MDD | 18 | M | W | 44 | 11.0 | 6.54 | Arteriosclerotic cardiovascular disease | Natural |
| 1221 | MDD | 19 | F | W | 28 | 24.8 | 6.61 | Pulmonary embolism | Natural |
| 10028 | MDD | 20 | F | W | 72 | 23.1 | 6.66 | Gunshot wound | Suicide |
| 739 | Control | 1 | M | W | 40 | 15.8 | 6.52 | Arteriosclerotic cardiovascular disease | Natural |
| 841 | Control | 2 | M | W | 70 | 21.2 | 7.18 | Hypertrophic cardiomyopathy | Natural |
| 510 | Control | 3 | M | W | 63 | 12.4 | 6.51 | GI hemorrhage | Natural |
| 685 | Control | 4 | M | W | 56 | 14.5 | 7.06 | Hypoplastic coronary artery | Natural |
| 604 | Control | 5 | M | W | 39 | 19.3 | 7.08 | Hypoplastic coronary artery | Natural |
| 585 | Control | 6 | M | W | 26 | 16.0 | 6.67 | Trauma | Accidental |
| 694 | Control | 7 | M | W | 38 | 20.7 | 6.73 | Subarachnoid hemorrhage | Natural |
| 615 | Control | 8 | M | W | 62 | 7.2 | 6.39 | Ruptured aortic aneurysm | Natural |
| 567 | Control | 9 | F | W | 46 | 15.0 | 6.77 | Mitral valve prolapse | Natural |
| 902 | Control | 10 | M | W | 60 | 23.6 | 6.74 | Arteriosclerotic cardiovascular disease | Natural |
| 700 | Control | 11 | M | W | 42 | 26.1 | 6.95 | Arteriosclerotic cardiovascular disease | Natural |
| 10003 | Control | 12 | M | W | 49 | 21.2 | 6.54 | Trauma | Accidental |
| 871 | Control | 13 | M | W | 28 | 16.5 | 6.33 | Trauma | Accidental |
| 1047 | Control | 14 | M | W | 43 | 13.8 | 6.63 | Arteriosclerotic cardiovascular disease | Natural |
| 643 | Control | 15 | M | W | 50 | 24.0 | 6.23 | Arteriosclerotic cardiovascular disease | Natural |
| 789 | Control | 16 | M | W | 22 | 20.0 | 7.04 | Asphyxiation | Accidental |
| 1067 | Control | 17 | M | W | 49 | 6.5 | 6.55 | Hypertensive heart disease | Natural |
| 857 | Control | 18 | M | W | 48 | 16.6 | 6.54 | Arteriosclerotic cardiovascular disease | Natural |
| 1282 | Control | 19 | F | W | 39 | 24.5 | 6.84 | Cardiac arrhythmia | Natural |
| 818 | Control | 20 | F | W | 67 | 24.0 | 7.06 | Anaphylaxis | Accidental |
Abbreviations: M, male; F, female; PMI, postmortem interval; ASCVD, arteriosclerotic cardiovascular disease.
Immunolabeling of 5-HT receptors
There were no statistically significant differences between depressed and control samples in 5-HT1A receptors (t=0.358, df=38, P=NS) or 5-HT2C receptors (t=0.043, df=38, P=NS) (Table 3). Alternatively, 5-HT2A receptors were dramatically and significantly increased in depressed versus controls (t=2.767, df=38, P=0.009); the depressed samples were increased to 217.30% of matched controls.
Table 3.
5-HT1A, 5-HT2A, and 5-HT2C Western blot results for depressed and controls (means and standard deviation), and depressed percent matched controls (means and standard deviations)
| Protein | Controls | Depressed | Percent matched controls |
|||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| 5-HT1A | 326.40 | 233.99 | 306.15 | 234.00 | 115.25 | 69.52 |
| 5-HT2A | 406.75 | 247.52 | 636.85* | 277.62 | 217.30 | 153.40 |
| 5-HT2C | 326.25 | 127.77 | 328.05 | 134.57 | 110.65 | 55.18 |
Values expressed as relative density.
P=0.009.
PKA and PKC activity
Basal and cyclic AMP–stimulated activity of PKA was compared between depressed and control samples. Depressed patients showed a statistically significant reduction of basal PKA activity (depressed = 1023 [S.D. =290.54], controls= 1273 [S.D.=302.97] pmol/min/mg protein [t=−2.664, df=38, P=0.011]), and a trend toward reduction of cyclic AMP–stimulated PKA activity (depressed=2953.90 [S.D.=768.23], controls=3218.65 [S.D.=759.65] pmol/min/mg protein [t=−1.510, df=38, P=0.139]).
Basal- and PMA-stimulated PKC activity was also compared between depressed and control groups (n=16, each group). No significant differences were found between groups on basal (depressed=3379.00 [S.D.=2356.62], controls=4439.75 [S.D.=3977.23]) or PMA activated (depressed=3475.56 [S.D.=2975.02], controls=4874 [S.D.=3787.50]) PKC activity.
Correlations between PKA and PKC and 5-HT receptors
The relationships between abundance of 5-HT receptors and both PKA and PKC were tested by Pearson product moment correlation. There were no statistically significant correlations between 5-HT1A or 5-HT2C receptors and either basal or activated PKA or PKC in the total sample, depressed, or control groups (Table 3). There were strong trends for a significant positive correlations between 5-HT2C receptor versus PKA in the depressed sample; basal PKA (r=0.432, P=0.057), activated PKA (r=0.437, P=0.054). However, when one depressed outlier sample with an extremely low 5-HT2C receptor value was removed, the association disappeared (PKA basal, r=0.118, P=0.473; PKA activated, r=0.235, P=0.150).
Alternatively, the 5-HT2A receptor showed significant, inverse correlations with PKA activity in the total sample (basal PKA, r=−0.365, P=0.021); activated PKA, r=−0.348, P=0.028). However, when examined separately, only the depressed group showed significant associations, including a strong trend for basal PKA (r=−0.421, P=0.065) and a significant correlation for activated PKA (r=−0.560, P=0.010) (Fig. 2A). The control sample did not show significant correlations between PKA and 5-HT2A receptor (basal PKA, r=0.051, P=0.831; activated PKA, r=0.031, P=0.896) (Fig. 2B).
Fig. 2.
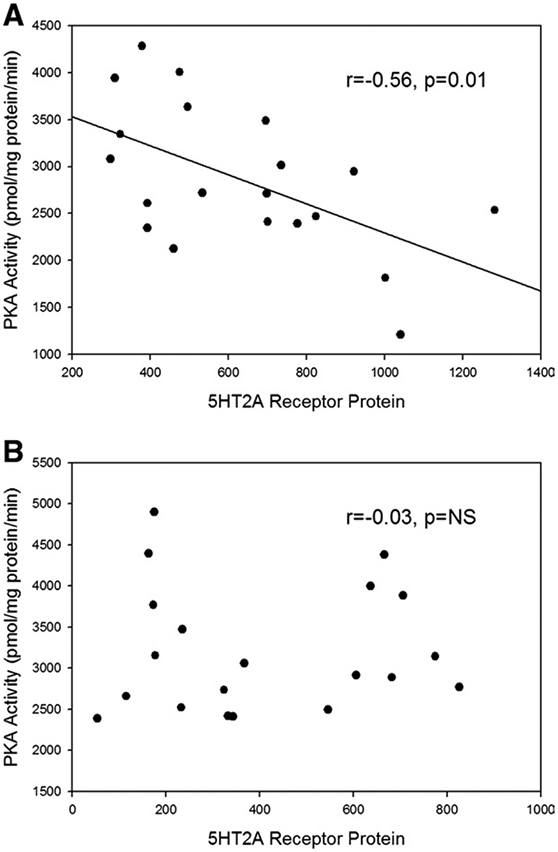
Correlations, PKA activity versus 5-HT2A receptor protein. A=Depressed, B=Controls.
There were no significant correlations between PKC activity and any 5-HT receptor in the total sample or depressed subgroup (Table 4). There were no significant correlations between PKC activity and either 5-HT1A or 5-HT2C receptor abundance. However, there was a significant inverse correlation between 5-HT2A receptor availability and basal PKC activity (r=−0.515, P=0.041), with a trend for significant association with activated PKC (−0.435, P=0.092).
Table 4.
Correlations between PKA and PKC activity and 5-HT receptors
| Group | 5-HT receptor |
PKA |
PKC |
||
|---|---|---|---|---|---|
| Basal | Activated | Basal | Activated | ||
| Total | 5-HT1A | 0.009 | 0.030 | 0.093 | 0.067 |
| 5-HT2A | −0.365* | −0.348* | −0.297 | −0.230 | |
| 5-HT2C | 0.238 | 0.300† | −0.045 | −0.007 | |
| Depressed | 5-HT1A | 0.210 | 0.213 | 0.341 | 0.255 |
| 5-HT2A | −0.421† | −0.560* | 0.112 | 0.143 | |
| 5-HT2C | 0.432† | 0.437† | −0.097 | −0.134 | |
| Control | 5-HT1A | −0.362 | −0.357 | −0.123 | −0.154 |
| 5-HT2A | −0.051 | 0.031 | −0.515* | −0.435† | |
| 5-HT2C | 0.090 | 0.175 | −0.009 | 0.100 | |
P<0.05.
P>0.05 and <0.10.
5-HT receptors in depressed suicide versus non-suicide groups
Within the depressed sample, there were no statistically significant differences between those who died by suicide (n=11) and those who died by other causes (n=9) for any of the 5-HT receptors. Similarly, the groups did not show any differences in basal or activated PKA or PKC.
Effects of age, sex, PMI, and pH on 5-HT receptors and kinases
There were no statistically significant correlations between age, PMI, or pH on availability of 5-HT1A, 5-HT2A, or 5-HT2C receptors or either PKA or PKC activity in the total sample, depressed, or control groups, although there was a trend for a significant correlation between age and 5-HT1A receptors in the depressed sample (r=−437, P=0.058). There were no significant differences by sex on any 5-HT receptors or either PKA or PKC in the total, depressed, or control samples, although the number of females was small (n=3 in depressed and controls).
Increased 5-HT2A receptor availability still held when males only (n=17 per group) were analyzed. Males were increased at 233% of matched controls. Females were a mean of only 127% of controls, but, as noted earlier, the sample size was too low to draw any conclusions. When only depressed males were analyzed, the significant inverse correlation between 5-HT2A receptor protein and activated PKA still held (r=−0.583, P=0.014), with a trend for basal PKA (r=−0.474, P=0.058).
DISCUSSION
In this study, we evaluated the abundance of specific 5-HT receptors and activity of two key enzymes, PKA and PKC, in human postmortem brain tissue specimens from PFC (BA10), which produced several significant findings. First, like a number of prior studies (Turecki et al., 1999; Stanley and Mann; Pandey et al., 2002; Hrdina and Du, 2001; Hrdina et al., 1993; Arranz et al., 1994; Arango et al., 1990, 1997), we found a significant increase in 5-HT2A receptors in frontal cortex specimens in depressed persons relative to controls. The mean value for 5-HT2A receptors was increased to 157% in the depressed sample; when the depressed individuals were compared against their matched controls, the increase was 217%. Increased 5-HT2A receptors have significant implications with regard to depression vulnerability. As noted earlier, activation of 5-HT2A receptors has been shown to increase anxiety (Mora et al., 1997; Graeff et al., 1996), which is reversed by disruption (Weisstaub et al., 2006), inhibition (Sibille et al., 1997), or blockade (Kleven et al., 1997; Griebel et al., 1997). Anxiety is an important part of the complex of features of depression (Brown et al., 1998); anxious temperament (i.e. trait neuroticism) and anxiety disorders also are known to increase the risk for depression (Jorm et al., 2000; Hettema et al., 2006). Hence, increased availability of 5-HT2A receptors, which may increase anxious responding, may mediate depressive vulnerability in some individuals.
Although brain 5-HT2A receptors have been shown to be elevated in depression, the causal pathway is obscure. Both genetic variation of the 5-HT2A receptor (Turecki et al., 1999) and decreased 5-HT innervation (Pandey et al., 2002) have been hypothesized as possible causal models. However, to our knowledge, the relationship between kinase activity and 5-HT2A receptor abundance has not been previously investigated. PKA activity was decreased in the samples from depressed persons relative to controls, as demonstrated in other postmortem studies in PFC (Pandey et al., 2005, 2007). Consistent with the overall findings, Dwivedi et al. (2004a) have found that stress-induced learned helplessness in rats results in both decreased PKA and increased 5-HT2A receptor (Dwivedi et al., 2005) availability in frontal cortex.
In the present study, a statistically significant association was found between cyclic AMP–stimulated PKA activity and 5-HT2A receptor abundance. This suggests three possible causal models. First, as discussed earlier, PKA is involved in heterologous regulation of 5-HT2A receptors; phosphorylation of 5-HT2A receptors leads to internalization, followed by dephosphorylation and either recycling to the cell surface or degradation (Van Oekelen et al., 2003; Roth et al., 1998). Therefore, decreased phosphorylation of cell surface receptors could increase availability, as in this study. Second, increased 5-HT2A receptors could lead to reduced PKA, although the mechanism is unclear. Finally, an independent factor could be involved in the regulation of both PKA and 5-HT2A receptors. For example, increased glucocorticoid activity has been shown to decrease PKA availability (Dwivedi and Pandey, 2000) and increase 5-HT2A receptor responsiveness (Umeda et al., 2007; Katagiri et al., 2001; Dwivedi and Pandey, 2000) in rats. It is therefore possible that increased glucocorticoids induce both findings, given the fact that hypothalamic–pituitary–adrenal axis activity is enhanced in many depressed patients (Plotsky et al., 1998).
It should be noted that only a portion of the variance of 5-HT2A (approximately 31%) in the depressed sample is accounted for by PKA activity. Therefore, other factors, such as reduced 5-HT availability, may also contribute to the reduction. However, the relationship between 5-HT2A abundance and PKA activity in the depressed sample is potentially significant since, essentially, none of the 5-HT2A variance was explained by PKA in the controls. In addition, since this is a static finding in postmortem tissue, a direct causal link between PKA and 5-HT2A variance cannot be definitively established, only inferred.
We did not find a decrease in PKC activity in the depressed group, unlike prior reports (Pandey et al., 1997, 1998; Coull et al., 2000; Akin et al., 2005). The reason for this discrepancy is unclear. Our group has reported reduced PKC in peripheral fibroblasts from living depressed persons (Akin et al., 2005), and other groups have shown low PKC in both peripheral and brain tissue samples (Pandey et al., 1997, 1998). Most brain tissue studies have been done in samples from BA8 or 9, whereas the current study examined BA10. To our knowledge, only one previous study examined PKC in this region, and found no differences in levels of PKC isoforms in samples from depressed persons (Hrdina et al., 1998), which is consistent with the current results.
We did find a significant association between basal PKC activity and 5-HT2A receptor availability, but only in the control group. Agonist binding to 5-HT2A receptors leads to coupling of Gq/11 proteins and activation of phospholipase C, which hydrolyzes phosphatidylinositol-4,5-bisphosphate (PI) catalyzing the formation of inositol-triphosphate and diacylglycerol. The latter binds to and activates PKC and homologous phosphorylation of 5-HT2A receptors by PKC leads to down-regulation (Saucier et al., 1998). Hence the availability of the 5-HT2A receptor is dependent, in part, on PKC activity, as was found in the control group. The reason for the lack of correlation in the depressed sample is unclear. One possible explanation is a decreased availability of 5-HT in depressed subjects, leading to lessened homologous desensitization via PKC. Alternatively, previous research in our laboratory (Akin et al., 2004) has shown reduced PI hydrolysis after activation of 5-HT2A receptors in cultured fibroblasts from depressed patients relative to controls. Therefore, a second possible explanation is uncoupling of 5-HT2A receptors from post-receptor mechanisms in the depressed subjects, which would obviate the relationship between receptor and intracellular signaling.
We did not find significant differences between brains from depressed subjects versus controls in 5-HT1A or 5-HT2C receptor abundance. Prior studies have shown lower 5-HT1A receptors in specific brain regions (Stockmeier, 2003; Drevets et al., 2007). For example, using positron emission tomography imaging, Drevets et al. (2007) showed reduced 5-HT1A ligand binding in medial temporal cortex and raphe nuclei, but not in other brain regions (Drevets et al., 1999, 2007). Also consistent with the current study (Stockmeier et al., 1997), did not find any differences in [3H]8-OH-DPAT binding to 5-HT1A receptors in BA10. A recent postmortem study in BA10 found reduced 5-HT1A in females only, which may also be consistent with our results since our sample was predominantly male; it should be pointed out, however, that the absolute mean values in males and females did not differ substantially in the present study. In fact, both males and females were slightly higher than their matched controls (116.3% and 109.3% respectively).
To our knowledge, only one study (Pandey et al., 2006) has examined 5-HT2C receptors in human postmortem brain tissue in suicide victims versus controls; the suicide sample showed higher 5-HT2C availability in BA8/9 (dorsolateral PFC) but not in hippocampus or choroid plexus. The fact that the present study did not show differences in depressed or suicide samples in BA10 suggests that the differences previously shown may be regionally specific, even within the cortex.
Previous research has suggested that elevated 5-HT2A receptor binding is present in suicide victims, and may not depend on the presence of MDD per se (Turecki et al., 1999; Stockmeier et al., 1997; Rosel et al., 2000; Pandey et al., 2002; Hrdina and Du, 2001; Du et al., 2000; Arango et al., 1997). In the present study, there were no differences in the depressed sample between those who died by suicide versus other causes. Elevated 5-HT2A receptors may, in fact, not be specific to MDD, but may be a vulnerability factor in people otherwise predisposed to being depressed. 5-HT2A receptor activation is associated with anxiety-related symptoms and behaviors in both animal and human models (Mora et al., 1997; Graeff et al., 1996). Anxiety has been shown to be associated with risk for depression (Kendler et al., 2003; Kendler, 1996) and suicide (Hawgood and De Leo, 2008). Elevations in 5-HT2A receptor abundance may be associated with increases in stress-related dysphoric responses which may increase risk for depression, but may also enhance risk for suicide in either depressed or non-depressed samples.
This study is limited by the fact that it only involved 20 depressed and control samples, all were from Caucasians, and all but three in each group were from men. Therefore, the results may not be representative of all depressed patients. The samples were only from BA10, and may not reflect the state in other brain regions. In addition, diagnostic classification was made retrospectively and by second-hand report. However, the data are consistent with those from previous studies, which support the findings.
CONCLUSION
In conclusion, the present study found an elevation in 5-HT2A receptors in human postmortem brain tissue specimens from BA10 in depressed relative to control groups. This elevation was inversely correlated with PKA activity, suggesting that abnormalities of PKA may, at least in part, explain the abnormalities in 5-HT2A receptors. Further research is needed to more completely understand the causal pathways for these findings.
Acknowledgments—
The project described was supported by grant award numbers MH073630 (R.C.S.), MH34007 (E.S.-B.), and MH084053 (D.A.L.) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Abbreviations:
- BA
Brodmann area
- CREB
cyclic AMP response element binding protein
- CREB-P
phosphorylated cyclic AMP response element binding protein
- ECL
enhanced chemiluminescence
- EDTA
ethylenediaminetetraacetic acid
- MDD
major depression
- PFC
prefrontal cortex
- PI
phosphatidylinositol-4,5-bisphosphate
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PMI
postmortem interval
- SDS
sodium dodecyl sulfate
- SSRI
5-HT selective reuptake inhibitor
- Tris
tris(hydroxymethyl)aminomethane
- [3H]8-OH-DPAT
[3H]8-hydroxy-2-(di-n-propyl)-aminotetralin
REFERENCES
- Akin D, Manier DH, Sanders-Bush E, Shelton RC (2004) Decreased serotonin 5-HT2A receptor-stimulated phosphoinositide signaling in fibroblasts from melancholic depressed patients. Neuropsychopharmacology 29:2081–2087. [DOI] [PubMed] [Google Scholar]
- Akin D, Manier DH, Sanders-Bush E, Shelton RC (2005) Signal transduction abnormalities in melancholic depression. Int J Neuropsychopharmacol 8:5–16. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ (1990) Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry 47:1038–1047. [DOI] [PubMed] [Google Scholar]
- Arango V, Huang YY, Underwood MD, Mann JJ (2003) Genetics of the serotonergic system in suicidal behavior. J Psychiatr Res 37:375–386. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ (2001) Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 25:892–903. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ (1997) Postmortem findings in suicide victims. Implications for in vivo imaging studies. Ann N Y Acad Sci 836:269–287. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J (1994) Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry 35(7):457–463. [DOI] [PubMed] [Google Scholar]
- Blier P, Haddjeri N, Szabo ST, Dong J (2001) Enhancement of serotoninergic function: a sometimes insufficient cause of antidepressant action. Hum Psychopharmacol 16:23–27. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH (1998) Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol 107:179–192. [DOI] [PubMed] [Google Scholar]
- Coull MA, Lowther S, Katona CL, Horton RW (2000) Altered brain protein kinase C in depression: a postmortem study. Eur Neuropsychopharmacol 10:283–288. [DOI] [PubMed] [Google Scholar]
- Devous MD Sr, Trivedi MH, Rush AJ (2001) Regional cerebral blood flow response to oral amphetamine challenge in healthy volunteers. J Nucl Med 42:535–542. [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C (1999) PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 46:1375–1387. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Thase ME, Moses-Kolko EL, Price J, Frank E, Kupfer DJ, Mathis C (2007) Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol 34:865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD (2000) Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. Am J Med Genet 96:56–60. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2002) [(3)H]cAMP binding sites and protein kinase a activity in the prefrontal cortex of suicide victims. Am J Psychiatry 159:66–73. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Payappadoudar GV, Rizavi HS (2005) Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology 48:204–214. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Shukla PK, Rizavi HS, Lyons J (2004a) Altered protein kinase a in brain of learned helpless rats: effects of acute and repeated stress. Biol Psychiatry 56:30–40. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Pandey GN (2000) Adrenal glucocorticoids modulate [3H]cyclic AMP binding to protein kinase A (PKA), cyclic AMP-dependent PKA activity, and protein levels of selective regulatory and catalytic subunit isoforms of PKA in rat brain. J Pharmacol Exp Ther 294:103–116. [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, Sarosi A, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2004b) Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry 55:234–243. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for axis I DSM-IV disorders–patient edition (with psychotic screen) (SCID-I/P) (W/psychotic screen) (version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Gobert A, Rivet JM, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas JP, Cistarelli L, Melon C, Millan MJ (2000) Serotonin(2C) receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: a combined dialysis and electrophysiological analysis in the rat. Synapse 36(3):205–221. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF (1996) Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54:129–141. [DOI] [PubMed] [Google Scholar]
- Griebel G, Perrault G, Sanger DJ (1997) A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacology 36:793–802. [DOI] [PubMed] [Google Scholar]
- Gurevich I, Tamir H, Arango V, Dwork AJ, Mann JJ, Schmauss C (2002) Altered editing of serotonin 2C receptor pre-mRNA in the prefrontal cortex of depressed suicide victims. Neuron 34:349–356. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, de Montigny C, Blier P (1997) Modulation of the firing activity of noradrenergic neurones in the rat locus coeruleus by the 5-hydroxtryptamine system. Br J Pharmacol 120(5):865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawgood J, De Leo D (2008) Anxiety disorders and suicidal behaviour: an update. Curr Opin Psychiatry 21:51–64. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS (2006) A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry 163:857–864. [DOI] [PubMed] [Google Scholar]
- Hrdina P, Faludi G, Li Q, Bendotti C, Tekes K, Sotonyi P, Palkovits M (1998) Growth-associated protein (GAP-43), its mRNA, and protein kinase C (PKC) isoenzymes in brain regions of depressed suicides. Mol Psychiatry 3:411–418. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu TB, Sotonyi P, Palkovits M (1993) 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: increase in 5-HT2 sites in cortex and amygdala. Brain Res 614:37–44. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Du L (2001) Levels of serotonin receptor 2A higher in suicide victims? Am J Psychiatry 158:147–148. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP (2003) Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. J Neurochem 87(1):182–194. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY (2001) 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem 76:1521–1531. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Meltzer HY (1999) R(+)-8-OH-DPAT, a serotonin(1A) receptor agonist, potentiated S(−)-sulpiride-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens but not striatum. J Pharmacol Exp Ther 291:1227–1232. [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A (2007) Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry 12:522–543. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B (2000) Predicting anxiety and depression from personality: is there a synergistic effect of neuroticism and extraversion? J Abnorm Psychol 109:145–149. [DOI] [PubMed] [Google Scholar]
- Katagiri H, Kagaya A, Nakae S, Morinobu S, Yamawaki S (2001) Modulation of serotonin2A receptor function in rats after repeated treatment with dexamethasone and L-type calcium channel antagonist nimodipine. Prog Neuropsychopharmacol Biol Psychiatry 25:1269–1281. [DOI] [PubMed] [Google Scholar]
- Kendler KS (1996) Major depression and generalised anxiety disorder. Same genes, (partly) different environments: revisited. Br J Psychiatry Suppl 68–75. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC (2003) The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60:929–937. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Assie MB, Koek W (1997) Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther 282:747–759. [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL (2000) Neural correlates of episodic retrieval success. Neuroimage 12:276–286. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, Li SJ (2005) Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage 28:904–914. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR (2003) Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci 23:8788–8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS (2005) Differential anterior prefrontal activation during the recognition stage of a spatial working memory task. Cereb Cortex 15:1742–1749. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Huang M, Prus A, Dai J, Meltzer H (2005) ACP-103, a 5-HT2A/2C inverse agonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Psychopharmacology 183:144–153. [DOI] [PubMed] [Google Scholar]
- Lowry DH, Rosebrough NJ, Farr AL, Randall RJ, Wuarin J, Schibler U (1990) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275. [PubMed] [Google Scholar]
- Manier DH, Eiring A, Shelton RC, Sulser F (1996) Beta-adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacology 15:555–561. [DOI] [PubMed] [Google Scholar]
- Manier DH, Shelton RC, Ellis TC, Peterson CS, Eiring A, Sulser F (2000) Human fibroblasts as a relevant model to study signal transduction in affective disorders. J Affect Disord 61:51–58. [DOI] [PubMed] [Google Scholar]
- Manier DH, Shelton RC, Sulser F (2001) Cross-talk between PKA and PKC in human fibroblasts: what are the pharmacotherapeutic implications? J Affect Disord 65:275–279. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brent DA, Arango V (2001) The neurobiology and genetics of suicide and attempted suicide: a focus on the serotonergic system. Neuropsychopharmacology 24:467–477. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY (1991) Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect 85:181–194. [DOI] [PubMed] [Google Scholar]
- Mora PO, Netto CF, Graeff FG (1997) Role of 5-HT2A and 5-HT2C receptor subtypes in the two types of fear generated by the elevated T-maze. Pharmacol Biochem Behav 58:1051–1057. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Herrick-Davis K, Dilley GE, Meltzer HY, Overholser JC, Stockmeier CA, Emeson RB, Sanders-Bush E (2001) RNA editing of the human serotonin 5-HT2C receptor: alterations in suicide and implications for serotonergic pharmacotherapy. Neuropsychopharmacology 24:478–491. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Yamadori A, Frith CD, Burgess PW (2007) Differential involvement of regions of rostral prefrontal cortex (Brodmann area 10) in time- and event-based prospective memory. Int J Psychophysiol 64:233–246. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Kumari R, Janicak PG (1998) Protein kinase C in platelets of depressed patients. Biol Psychiatry 44:909–911. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA (1997) Protein kinase C in the postmortem brain of teenage suicide victims. Neurosci Lett 228:111–114. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Faludi G, Sarosi A, Palkovits M (2006) Regional distribution and relative abundance of serotonin(2c) receptors in human brain: effect of suicide. Neurochem Res 31:167–176. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Mondal AC, Shukla PK, Conley RR (2005) Brain region specific alterations in the protein and mRNA levels of protein kinase A subunits in the postmortem brain of teenage suicide victims. Neuropsychopharmacology 30:1548–1556. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR (2007) Cyclic AMP response element-binding protein in postmortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol 10:621–629. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR, Tamminga C (2003a) Altered expression and phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in postmortem brain of suicide victims with or without depression. J Psychiatr Res 37:421–432. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Pandey SC, Ren X, Dwivedi Y, Janicak PG (2003b) Serotonin receptors in platelets of bipolar and schizoaffective patients: effect of lithium treatment. Psychopharmacology (Berl) 170:115–123. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Pandey SC, Pesold C, Roberts RC, Conley RR, Tamminga CA (2002) Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. Am J Psychiatry 159:419–429. [DOI] [PubMed] [Google Scholar]
- Perez J, Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R (1999) Abnormalities of cyclic adenosine monophosphate signaling in platelets from untreated patients with bipolar disorder. Arch Gen Psychiatry 56:248–253. [DOI] [PubMed] [Google Scholar]
- Perez J, Tardito D, Racagni G, Smeraldi E, Zanardi R (2001) Protein kinase A and Rap1 levels in platelets of untreated patients with major depression. Mol Psychiatry 6:44–49. [DOI] [PubMed] [Google Scholar]
- Perez J, Zanardi R, Mori S, Gasperini M, Smeraldi E, Racagni G (1995) Abnormalities of cAMP-dependent endogenous phosphorylation in platelets from patients with bipolar disorder. Am J Psychiatry 152:1204–1206. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB (1998) Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 21:293–307. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Acconcia S, Ceglia I, Invernizzi RW, Samanin R (2002) Stimulation of 5-hydroxytryptamine (5-HT(2C)) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J Neurochem 82:93–100. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, Robbins TW (1999) Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci 19:9029–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosel P, Arranz B, San L, Vallejo J, Crespo JM, Urretavizcaya M, Navarro MA (2000) Altered 5-HT(2A) binding sites and second messenger inositol trisphosphate (IP(3)) levels in hippocampus but not in frontal cortex from depressed suicide victims. Psychiatry Res 99:173–181. [DOI] [PubMed] [Google Scholar]
- Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K (1998) Serotonin 5-HT2A receptors: molecular biology and mechanisms of regulation. Crit Rev Neurobiol 12:319–338. [DOI] [PubMed] [Google Scholar]
- Saucier C, Albert PR (1997) Identification of an endogenous 5-hydroxytryptamine2A receptor in NIH-3T3 cells: agonist-induced down-regulation involves decreases in receptor RNA and number. J Neurochem 68:1998–2011. [DOI] [PubMed] [Google Scholar]
- Saucier C, Morris SJ, Albert PR (1998) Endogenous serotonin-2A and -2C receptors in Balb/c-3T3 cells revealed in serotonin-free medium: desensitization and down-regulation by serotonin. Biochem Pharmacol 56:1347–1357. [DOI] [PubMed] [Google Scholar]
- Schmauss C (2003) Serotonin 2C receptors: suicide, serotonin, and runaway RNA editing. Neuroscientist 9:237–242. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Manier DH, Peterson CS, Ellis TC, Sulser F (1999) Cyclic AMP-dependent protein kinase in subtypes of major depression and normal volunteers. Int J Neuropsychopharmacol 2:187–192. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Manier DH, Sulser F (1996) cAMP-dependent protein kinase activity in major depression. Am J Psychiatry 153:1037–1042. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Papakostas GI (2008) Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand 117:253–259. [DOI] [PubMed] [Google Scholar]
- Sibille E, Sarnyai Z, Benjamin D, Gal J, Baker H, Toth M (1997) Antisense inhibition of 5-hydroxytryptamine(2a) receptor induces an antidepressant-like effect in mice. Mol Pharmacol 52:1056–1063. [DOI] [PubMed] [Google Scholar]
- Simon JS, Nemeroff CB (2005) Aripiprazole augmentation of antidepressants for the treatment of partially responding and nonresponding patients with major depressive disorder. J Clin Psychiatry 66:1216–1220. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Shayegan DK (2003) The psychopharmacology of ziprasidone: receptor-binding properties and real-world psychiatric practice. J Clin Psychiatry 64(Suppl 19):6–12. [PubMed] [Google Scholar]
- Stanley M, Mann JJ (1983) Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet 1:214–216. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA (2003) Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter [review]. J Psychiatr Res 37:357–373. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY (1997) Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology 16(2):162–173. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G (1998) Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci 18:7394–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Blier P (2001) Serotonin (1A) receptor ligands act on norepinephrine neuron firing through excitatory amino acid and GABA(A) receptors: a microiontophoretic study in the rat locus coeruleus. Synapse 42:203–212. [DOI] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC (2008) Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol 19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochigi M, Iwamoto K, Bundo M, Sasaki T, Kato N, Kato T (2008) Gene expression profiling of major depression and suicide in the prefrontal cortex of postmortem brains. Neurosci Res 60:184–191. [DOI] [PubMed] [Google Scholar]
- Turecki G, Briere R, Dewar K, Antonetti T, Lesage AD, Seguin M, Chawky N, Vanier C, Alda M, Joober R, Benkelfat C, Rouleau GA (1999) Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. Am J Psychiatry 156:1456–1458. [DOI] [PubMed] [Google Scholar]
- Umeda Y, Amano M, Suemaru K, Yamaguchi T, Kitamura Y, Gomita Y, Kawasaki H, Araki H (2007) The influence of hyperactivity of the hypothalamic-pituitary-adrenal axis and hyperglycemia on the 5-HT2A receptor-mediated wet-dog shake responses in rats. Acta Med Okayama 61:311–317. [DOI] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten WH, Leysen JE (2003) 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci 72:2429–2449. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, Felder C, Gatley SJ, Ding YS, Hitzemann R, Pappas N (2000) Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 157:75–80. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA (2006) Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313(5786):536–540. [DOI] [PubMed] [Google Scholar]


