Abstract
Purpose
To determine the role of histone deacetylase 4 (HDAC4)-controlled chondrocyte hypertrophy in the onset and development of age-related osteoarthritis (OA).
Methods
Morphological analysis of human knee cartilages was performed to observe structural changes during cartilage degeneration. HDAC4 expression was deleted in adult aggrecan (Acan)-CreERT2; HDAC4fl/fl transgenic mice. The onset and development of age-related OA were investigated in transgenic and control mice using hematoxylin and eosin (H&E) and Safranin O staining. Furthermore, the progression of ACLT-induced OA following adenovirus-mediated HDAC4 overexpression was explored in rats. The expression levels of genes related to hypertrophy, cartilage matrix and its digestion, and chondrocyte proliferation were investigated using qPCR. Immunohistochemistry (IHC) was used to explore the mechanisms underlying HDAC4-controlled age-related changes in OA progression.
Results
In human cartilage, we performed morphological analysis and IHC, the results showed that hypertrophy-related structural changes are related to HDAC4 expression. Age-related OA was detected early (OARSI scores 2.7 at 8-month-old) following HDAC4 deletion in 2-month-old mice. Furthermore, qPCR and IHC results showed changes in hypertrophy-related genes Col10a1, Runx2 and Sox9 in chondrocytes, particularly in the expression of Runt-related transcription factor 2 (Runx2, 13.29±0.99 fold). The expression of the main cartilage matrix-related genes Col2a1 and Acan decreased, that of cartilage matrix digestion-related gene MMP-13 increased, while that of chondrocyte proliferation-related genes PTHrP, Ihh and Gli1 changed. In contrast, rat cartilage’s qPCR and IHC results showed opposite outcomes after HDAC4 overexpression.
Conclusion
Based on the results above, we concluded that HDAC4 expression regulates the onset and development of age-related OA by controlling chondrocyte hypertrophy. These results may help in the development of early diagnosis and treatment of age-related OA.
Keywords: osteoarthritis, HDAC4, cartilage, chondrocytes, degeneration
Summary
This study aimed to determine the role of histone deacetylase 4 (HDAC4)-controlled chondrocyte hypertrophy in the onset and development of age-related osteoarthritis (OA). HDAC4 expression was deleted in adult aggrecan (Acan)-CreERT2-HDAC4fl/fl transgenic mice to investigate HDAC4 role in age-related OA. The onset and development of age-related OA were investigated in transgenic and control mice using hematoxylin and eosin and Safranin O staining, whereas the progression of ACLT-induced OA following adenovirus-mediated HDAC4 overexpression was explored in rats. Furthermore, we investigated the expression levels of genes related with hypertrophy, cartilage matrix and its digestion, and chondrocyte proliferation. Age-related OA was detected early and rapidly deteriorated in transgenic mice following HDAC4 deletion in young adulthood. Changes in hypertrophy-related gene expression in chondrocytes, particularly in the expression of Runt-related transcription factor 2 (Runx2) were detected. The expression of cartilage matrix-related genes decreased, that of cartilage matrix digestion-related genes increased, while that of chondrocyte proliferation-related genes decreased. In contrast, opposite outcomes were observed after HDAC4 overexpression in rats. Thus, HDAC4 deletion induced age-related spontaneous OA, while HDAC4 overexpression in cartilage decelerated OA progression. The regulation of chondrocyte hypertrophy is the main mechanism underlying this process. We believe that our study makes a significant contribution to the literature because it improves our understanding of the mechanism underlying age-related OA and may help in the development of early diagnosis and treatment.
Introduction
Age-related changes in cartilage are thought to be major contributors to the etiology of OA.1 As the only cell type within cartilage, the physiologic balance of chondrocytes greatly affects the physicochemical properties of cartilage.
Based on chondrocyte appearance, knee cartilage can be divided into the following four layers from the top: the superficial, transitional (location of all proliferating and a small portion of pre-hypertrophic chondrocytes), radial (location of most prohypertrophic and all hypertrophic chondrocytes), and the calcified zones.2 As we know, the chondrocyte hypertrophy in endochondral bone formation is a crucial step during maturation of growth plate chondrocyte.3 However, previous studies have found that chondrocyte hypertrophy greatly contributes to the degeneration of OA cartilage.1,4 However, the correlation between chondrocyte hypertrophy and OA progression in age-related knee OA has not been fully investigated.
During the development of growth plate, HDAC4 expressed in prehypertrophic and hypertrophic chondrocytes regulates chondrocyte hypertrophy by interacting with and inhibiting the activity of Runt-related transcription factor 2 (Runx2) through MEF2 region of HDAC4.5,6 Similarly expression pattern can be found during the development of OA, as decreased Runx2 inhibition was found to be associated with human osteoarthritis cartilage degeneration.7 However, the significance of HDAC4 expression in age-related OA cartilage remains largely unknown. Furthermore, the parathyroid hormone-related peptide (PTHrP) can repress the transcription of Runx2 and inhibit chondrocyte hypertrophy by activating PP2A to dephosphorylate HDAC4.8 PTHrP also forms a feedback loop with the Indian hedgehog (Ihh), which regulates chondrocyte proliferation and differentiation.9 Glioma-associated oncogene homolog 1 (Gli1), the terminal target of the Hh signaling pathway, regulates the proliferation and differentiation of chondrocytes.10 Besides, HDAC4 represses the expression of matrix metalloprotease 13 (MMP-13), which regulates the degradation of type II collagen and aggrecan, the two main extracellular matrix proteins in cartilage.11 Type X collagen is used as a biomarker for hypertrophic chondrocytes.12 During cartilage aging, sex-determining region Y box 9 (Sox9) plays an essential role in inhibiting chondrocyte transition.4,13 However, the regulation of chondrocyte hypertrophy is complex and cartilage changes in age-related OA progression have been rarely investigated.
In the present study, the objective was to determine the role of HDAC4-controlled chondrocyte hypertrophy in the onset and development of age-related OA and explore the underlying mechanisms.
Materials and Methods
Human Cartilage Samples
Human samples were collected according to protocols approved by the Ethics Committee of the Second Hospital of Shanxi Medical University (No.2021XY019). A total of 15 knee cartilage samples were extracted from the OA biobank of the Second Hospital of Shanxi Medical University in accordance with the approved guidelines. These samples were collected from individuals involved in a car accident or who suddenly died. Cartilage samples were obtained from the weight-bearing area of the tibial plateau. Three tissues per sample were fixed, decalcified, and embedded in paraffin and histopathology assessments for the samples were performed and mean Mankin scores were applied to classify the OA severity. Moreover, immunochemical stainings (IHC) and positive cell ratio analysis were performed on human samples which were in the Nearly Normal group (n = 6; Mankin score: 0–3) and Early Osteoarthritis group (n = 6; Mankin score: 4–7). The samples with Mankin Score over 8 (Moderate OA group; n = 3; Mankin score: 8–11) were excluded from the positive cell ratio analysis because of the incomplete superficial zone in the cartilages.
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Shanxi Medical University (No.20180571). Acan-CreERT2 transgenic mice (Stock no.019148, the Jackson Lab. Bar Harbor ME, USA)14 and HDAC4fl/fl transgenic mice (purchased from biocytogen Beijing) were obtained.5 The offspring of these transgenic mice will have the Acan-CreERT2 recombinase construct and loxP-flanked HDAC4 mutation. Transgenic mice were bred by sibling mating. Male mice were chosen for further study. Genotyping of mice was performed using conventional PCR and the horizontal strip electrophoresis was performed to examine and select the proper offspring transgenic mice. Due to the dwarfism and early death of HDAC4-ablation mice at birth and long period of age-related research,5 2-month-old (as young adult mice) was chosen as the injection time for age-related OA induction to avoid the possible skeleton development influence of gene knockout.15,16
Before performing further experiments, 2-month-old wild-type (n = 3) and Acan-CreERT2; HDAC4fl/fl transgenic mice (n = 3) were compared to examine the possible genetic recombination influences on cartilage development and bone structure. Then, mice with Acan-CreERT2 and HDAC4fl/fl mutations were randomly divided into two groups: mice in the AggH-Tam group (n = 10) were injected with Tamoxifen (TM, Sigma-Aldrich) to knock out the HDAC4 gene, whereas mice in the AggH-Oil group (n = 10) were injected with corn oil (oil, Sigma-Aldrich) as a control. The HDAC4fl/fl littermates were injected with TM (H-Tam group, n = 10) as a control for the TM itself (it is reported that long term and high-dose of tamoxifen injection could cause osteoporosis in female mice17). The TM was dissolved in oil (10 mg/mL) and injected at 2-month-old. 0.1 mL/10 g body weight/per day was intraperitoneally injected into mice for 5 days. Oil was injected in the same manner in AggH-Oil group mice. After the injection, 2 randomly selected mice in the same cage of AggH-Tam group were executed monthly. Therefore, sampling rate was between 30% to 50% (4–6 pups per litter). Mice in other groups were spared because age-related changes in mice were hardly ever observed before 12-month-old.4 SafO staining was performed to observe the changes in both knee joint and identify the onset of OA (representative pictures are shown bimonthly). Combined with the previous work,18 the onset of OA was identified. The cartilages of both hip joints were collected and 8 mice per group were randomly selected to measure the rate of HDAC4-deletion by PCR. The right hind limbs of mice were collected and 6 mice per group were randomly selected to explore the percentage of HDAC4 positive cells. Furthermore, histological analysis was performed on all mice to quantify the degree of cartilage degradation. The knee cartilages of the left hind limb were collected and 3 mice per group were randomly selected. To explore the mechanism underlying the onset of age-related OA, the expression of OA-related genes was further analyzed using real-time quantitative PCR (RT-qPCR) and IHC was performed on the samples of the right hind limb.
We further tested the expression level of OA-related genes in HDAC4-overexpressing chondrocytes. Given the operation of intra-articular injection in the narrow space of mice knee may lead to the injury of cartilage by mistaken, anterior cruciate ligament transection (ACLT) was performed on the right hind limb of 2-month-old male rats (A model of the onset and progression of OA), followed by intra-articular injection of an HDAC4 gene-containing adenoviral vector (HDAC4-Ad). The rats were randomly divided into four groups. For the ACLT+HDAC4-Ad group, rats (n = 9) received the HDAC4-Ad injection. For the ACLT+GFP (green fluorescent protein) group, rats (n = 9) received an empty adenoviral vector (GFP-Ad) at the same time. Rats in the Sham group (n = 9) underwent a sham procedure instead of ACLT, and the rats in the Control group (n = 9) received no intervention. The virus titer was 1×109 PFUs per knee for the injection. The intra-articular injection was taken 48 hours after surgery, and every 3 weeks thereafter. The right hind limbs of the rats (n = 36) were collected 3 months after the procedure. Rt-PCR (4 mice per group), histological and IHC (5 mice per group) were performed to explore the underlying mechanism.
Radiography
To examine the possible genetic recombination influences on cartilage development and bone structure of transgenic and control mice, the X-ray images of mice knee anteroposterior radiographs were taken using a small-animal X-ray radiography system (UltraFocus, Faxitron, Tucson, AZ, USA volatage, 37kv; time, 4s) and the bone structure (including growth plate region) of mice knee were evaluated using Micro-Ct (GE Revolution 256-row spiral CT; tube voltage 120kv; pitch, 0.985:1; thickness, 0.450 mm; 3D reconstruction work-station, GE AW4.7).
Histological Analysis for Samples
After the samples were collected, knee joints were fixed in 10% phosphate-buffered formalin at room temperature for 24 h. Samples containing bones were decalcified in 0.5M EDTA solution (four months for human samples, two months for rat samples, and nine days for mouse samples) before paraffin embedding. Histological sections were cut from the tibial plateau and femur on the frontal plane, and 5–10 coronal sections of 6 μm thickness were collected throughout the depth of the joint. Safranin-O staining (SafO) (0.2%) and counterstaining with Fast Green (0.2%) were performed to assess glycosaminoglycan production. The sections were also stained with H&E and cartilage degeneration was observed. Histological assessment of slides was performed using the modified Mankin score (for humans and rats) and the Osteoarthritis Research Society International (OARSI) score (for mice). Mankin Score (commonly used for human and rats samples) assess structure (0 to 6 points), cellularity (0 to 3 points), matrix staining (0 to 4 points), and tidemark integrity (0 to 1 points), up to a maximum score of 14 points. Based on the Mankin Scores, human samples were further classified into the Nearly Normal (Mankin Score: 0–3), Early OA (Mankin Score: 4–7), Moderated OA (Mankin Score: 8–11) and Late OA (Mankin Score: 12–14).19 For mice samples, sections were stained with SafO staining and rated according to the OARSI scoring system (commonly used for mice), a 0–6 subjective scoring system.20 The samples were analyzed by three experienced assessors independently. Furthermore, the mean score of the three calculated scores was recorded as the final score of the mouse.
RT-qPCR Analysis for the Cartilage of Animals
Total RNA was extracted from cartilage (snap-frozen and grinded in liquid nitrogen) using TRIzol (Invitrogen Corp., CA, USA). 1 μg total RNA was transcribed into cDNA using the PrimeScriptTM RT-PCR Kit (Takara, Dalian, China) in a total volume of 20 μL. RT-qPCR was conducted using the SYBR Premix Ex TaqTM (Takara Dalian, China) and the IQ5 PCR Detection System (Bio-Rad Laboratories, Hercules, CA). The following cycling conditions were used: 10 min at 95℃, 40 cycles at 98℃ for 10s, 57.5℃ for 30s, and 72℃ for 30s. The last steps of PCR are performed to acquire the dissociation curve and to validate the specificity of PCR amplicons. Relative mRNA levels were calculated based on the x=2-ΔΔCt formula. Three technical replicates were used for every sample and an average was taken. Non template control was added each time in all the assays. 18S rRNA (for mice16) and GAPDH (for rats21) was used to normalize the gene expression levels. The primers were designed using Primer-BLAST (NCBI primer designing tool). The primer sequences are listed in Supplementary Table S1.
Immunohistochemistry
Immunohistochemistry (IHC) analysis was performed to explore the temporal and spatial specificity of related genes. The Super SensitiveTM Polymer-HRP Detection Kit (ZSGB-BIO, Beijing, China) was used for immunohistochemical analysis. The antibodies used are shown in Supplemental Table S2. Sections were deparaffinized and endogenous peroxidase was blocked; antigens were retrieved using 5 mg/mL hyaluronidase in PBS for 30 min at 37°C. The sections were then incubated with the primary antibody at 37°C for 2 h, then treated sequentially with a biotinylated secondary antibody and SP conjugate, and finally developed using DAB chromogen (Zymed Laboratories/Invitrogen, Frederick, MD). Images were taken using a Nikon E800 microscope (Nikon, Melville, NY, USA). The percentage of positive cells was defined as the proportion of positive cells per 100 cells in 10 representative positive fields. All samples were reviewed separately by two experienced researchers blinded to the sample data. In the case of disagreement, sections were further reviewed to reach an agreement.
Statistical Analysis
The data were analyzed and graphs were generated using GraphPad Prism 5.0 (LaJolla, CA, USA) and expressed as the mean±standard deviation. Normal distribution was checked by D’Agostino and Pearson normality test. The positive cell ratio in different human cartilage zones was compared using the Student’s t-test. For data analysis of different mouse and rat groups, one-way analysis of variance (ANOVA) with Sidak’s Multiple Comparison Test was used for normally distributed data. For data without a normal distribution, the Kruskal Wallis test was used for comparing 3 or more groups with Dunn’s multiple comparisons test to account for multiple comparisons.
Results
Consistent Chondrocyte Hypertrophy During Human OA Development
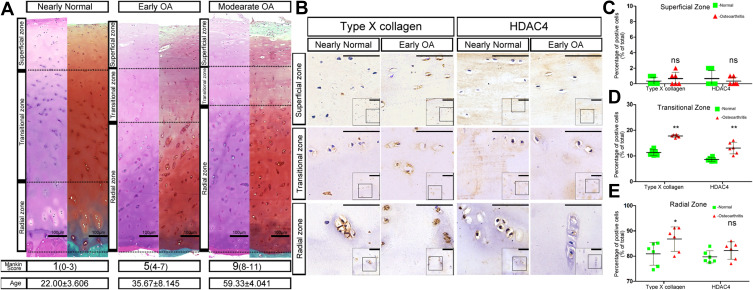
SafO and H&E stainings were used and Mankin scores were calculated to categorize the samples with different ages according to OA severity into the following four groups (Figure 1A). The SafO results further showed that the knee cartilage exhibited typical OA signs with the onset and development of OA (from Early OA to Late OA). As shown in Figure 1A, increasing fibrous degeneration and proteoglycan loss can be seen in the superficial cartilage zone with increasing cartilage age and OA progression. The superficial and transitional zones of aged cartilage become thinner whereas the radial zone becomes thicker. Interestingly, the average age of different OA levels was increased along with OA severity.
Figure 1.
Chondrocyte hypertrophy during human OA development. (A) HE and SafO staining of knee cartilage at different osteoarthritis (OA) stages. The different Mankin Score ranges which classified OA stages were listed in brackets. The superficial zones of human cartilage were wearing off and the radial zones were thickening along with the increase of OA severity. Interestingly, the average age of the samples in different level of OA was also increasing. The dashed lines depict the boundaries between the superficial, transitional and radial zones. The average age of the human samples in different stages of OA was shown as mean±SD. Scale bars:100μm. (B) Immunohistochemistry for histone deacetylase 4 (HDAC4) and type X collagen of Nearly Normal and Early OA patient knee cartilage. Type X collagen expression is localized in the transitional and radial zones, paralleling the staining pattern of HDAC4. Scale bars:50μm. (C–E) Positive cell ratio of type X collagen and HDAC4 in different zones of normal and OA patient knee cartilage. (C) superficial zone; (D) transitional zone; (E) radial zone, n=6 for both groups; *P <0.05,**P <0.01, Student’s t-test.
Abbreviation: ns, not significant.
Immunohistochemical staining showed that type X collagen and HDAC4 were mainly expressed in the radial zone of normal cartilage whereas low expression was observed in the transitional zone. Although the expression of type X collagen and HDAC4 was still located in the same areas as those of normal cartilage, the positive staining was stronger in the OA cartilage (Figures 1B and C). Furthermore, the positive cell rate of type X collagen and HDAC4 showed that although the increased expression of HDAC4 in the normal cartilage radial zone was not significant compared to that in OA cartilage, the positive cell rate of type X collagen and HDAC4 was increased in the transitional and radial zones of OA cartilage (Figure 1D and E).
Generation and Evaluation of Experimental Mice
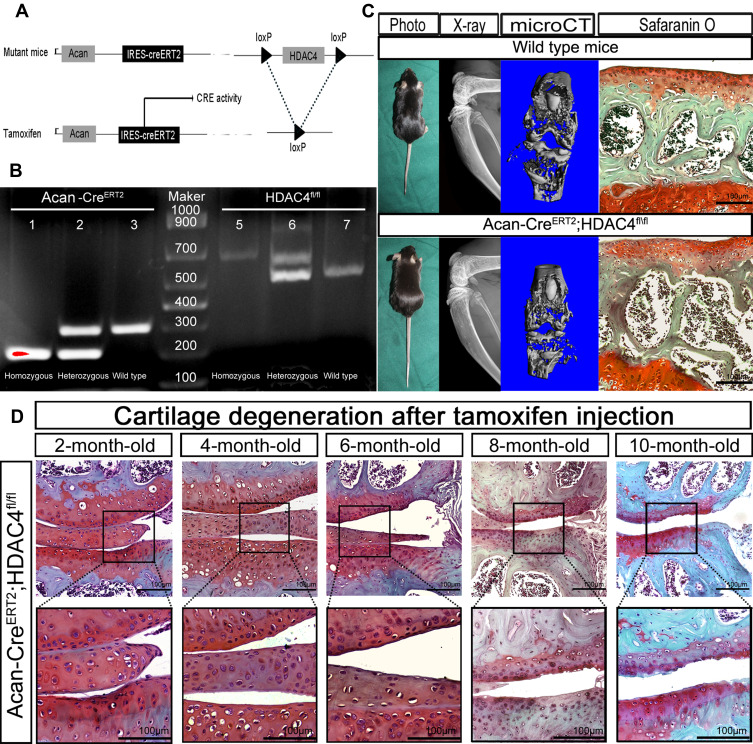
The role of HDAC4 during OA progression was further elucidated in adult knees of HDAC4 knockout transgenic mice. Following TM injection, the loxP site of the HDAC4 fl/fl homozygous mice was identified and folded to knock out the HDAC4 gene (Figure 2A). Genotyping was used to confirm the presence of Acan-CreERT2 and homozygous HDAC4 fl/fl mutants (Figure 2B). Photographs of 2-month-old Acan-CreERT2-HDAC4fl/fl and wild-type mice were taken to compare body size. In addition, X-ray imaging, microCT and SafO staining, used to examine the possible influence of genetic recombination on the natural growth of the trabecula bone and cartilage structure, showed that transgenic mice have no obvious deformity when compared to wild-type mice at the same age (Figure 2C). These results demonstrated that Acan-CreERT2-HDAC4fl/fl mice are the best candidates for the study of age-related changes in the knee joint. After TM injection in 2-month-old AggH-Tam mice, early OA changes were first observed in 8-month-old mice (Figure 2D), confirming that early-onset age-related spontaneous OA was related to a decrease in HDAC4 expression.
Figure 2.
Generation and evaluation of Acan-CreERT2-HDAC4fl/fl mice. (A) Schematic representation of the Acan-CreERT2-HDAC4fl/fl transgenic mice. The upper panel shows the offspring of mice with histone deacetylase 4 (HDAC4) homologous recombination and Acan-Cre recombination. The bottom panel shows the deletion of the HDAC4 gene after the activation of the Cre enzyme by tamoxifen injection. (B) Acan-CreERT2-HDAC4fl/fl mouse genotyping using PCR. Acan-CreERT2 (lanes 1, 2 and 3): lane 1, Cre homozygous (200 bp); lane 2, Cre heterozygous (200 bp and 299 bp); lane 3, wild type Acan (299 bp), HDAC4fl/fl (lanes 5, 6 and 7): lane 4, HDAC4fl/fl homozygous (620 bp); lane 5, HDAC4fl/fl heterozygous (620 and 520 bp); lane 6, HDAC4 wild type (520 bp), Marker: lane 4, 1000 bp DNA ladder. (C) Phenotype of 2-month-old Acan-CreERT2-HDAC4fl/fl and wild-type mice. X-ray. Micro Ct and SafO stain images shows no apparent deformation (the defection of bone structure between the articular surface and tibia represent the cartilage zone of mice epiphyseal line). Scale bars:100μm. (D) Early osteoarthritis (OA) is shown in 8-month-old HDAC4-null mice. After the tamoxifen injection at 2-months-old, the mutant mouse knee shows early OA characterization at 8-months-old. n=18. 2 mice/month. Scale bars:100μm.
HDAC4 Knock Out Mice Show Age-Related Early OA
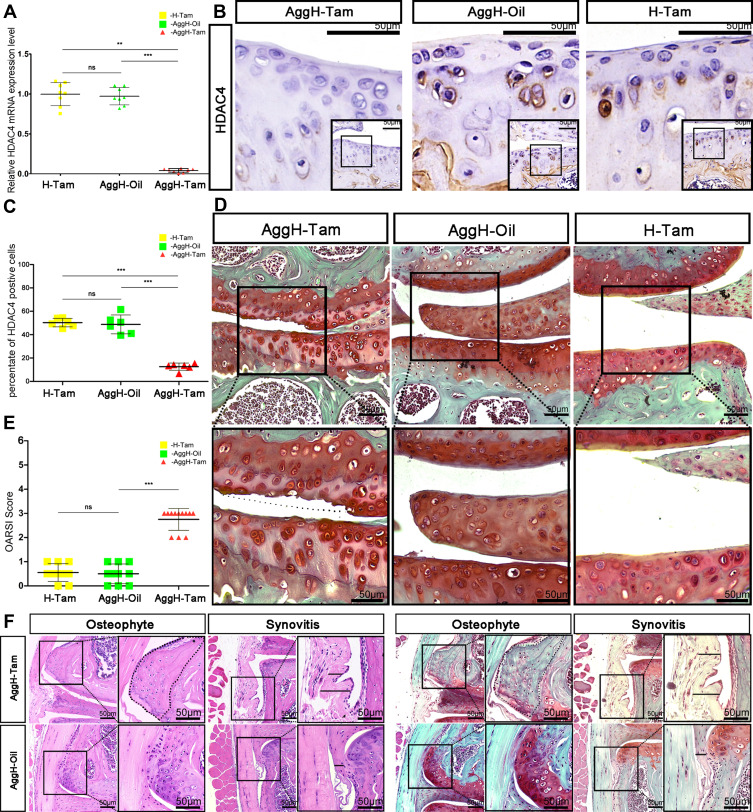
After tamoxifen injection in 2-month-old mice, the gene expression level of HDAC4 in adult mouse cartilage was reduced by 99.5% (Figure 3A). By the age of 8-months-old, HDAC4 cartilage expression could be hardly observed in the AggH-Tam group compared to that of the other two groups. The restricted hypertrophy chondrocyte localization makes positive cell ratio the best method to measure the expression of HDAC4. Statistical analysis results demonstrated that the positive cell rate of HDAC4 expression was reduced by 87% (0.1262±0.03078 with 95% CI: 0.09389 to 0.1585) (Figure 3B and C). Subsequently, AggH-Tam group mice showed typical cartilage wear and absence of SafO staining. However, no obvious signs of OA were observed in the AggH-Oil and H-Tam groups (Figure 3D). OARSI scores show that AggH-Oil and H-Tam group mice had a mean score of 0.5, while AggH-Tam group mice had a higher mean OARSI score of 2.7, classified as early OA (Figure 3E). Furthermore, the samples of 8-month-old AggH-Tam group mice had thicker synovial membranes. Besides, 10% of the 10-month-old samples showed changes in osteophytes (Figure 3F). These results showed that HDAC4 deletion induced the onset of age-related OA and might accelerate the progression of early OA. However, the underlying mechanisms remain unclear.
Figure 3.
Eight-month-old mice experienced early osteoarthritis (OA) after histone deacetylase 4 (HDAC4) knock out. (A) The relative mRNA expression of HDAC4 was significantly reduced after tamoxifen injection in 2-month-old mice. (B) Positive HDAC4 staining was hardly seen in the cartilage of 8-month-old AggH-Tam mice. Scale bars:50μm. (C) Quantitative analysis of HDAC4 expression showed that HDAC4 expression was consistently reduced up to the age of 8-months. (D) Safranin O staining of 8-month-old mice in all three groups showing OA changes in the AggH-Tam group. The dotted line shows where the surface of the cartilage should be. Scale bars:50μm. (E) Osteoarthritis Research Society International (OARSI) scores for all three groups showing significant OA changes in AggH-Tam group mice at the early stage of OA progression. (F) Osteophytes and thickened synovium were observed in 8-month-old AggH-Tam group mice. The dotted line shows the outgrowing osteophyte of the knee joint. The black line in the image indicates the thickened synovium. Scale bars:50μm.
HDAC4 Deletion Results in Knee Cartilage Chondrocyte Hypertrophy in Mice
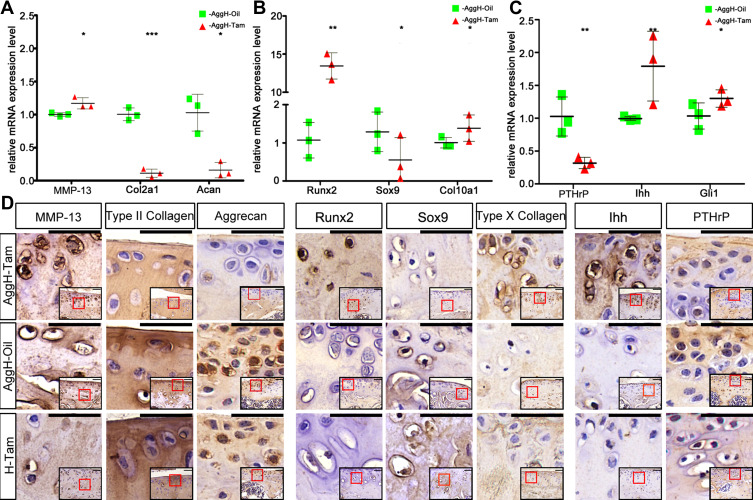
We performed qPCR and immunochemistry to analyze age-related OA gene expression (Figure 4). The relative mRNA expression levels of the inherent cartilage components aggrecan and type II collagen were decreased, and the expression of MMP-13 was increased (Figure 4A). The same trend was also observed in protein level changes. The relative mRNA expression levels of hypertrophy biomarkers, such as Runx2 and Col10a1, were also increased, and Sox9 expression was decreased (Figure 4B). Decreased positive staining for Sox9 and increased positive staining for Runx2 and type X collagen were also observed using immunohistochemistry. Interestingly, the mRNA and protein expression levels of Runx2 were much higher than those of the other genes (Figure 4C). The relative expression of PTHrP was also analyzed using qPCR, showing that its expression decreased. Further results showed Ihh and Gli1 mRNA and protein upregulation.
Figure 4.
Histone deacetylase 4 (HDAC4) deletion induced diverse changes in osteoarthritis (OA)-related genes in mice. (A–C) HDAC4 knockout led to significant differences in the relative mRNA expression of OA-related genes between the AggH-Tam and AggH-Oil groups in 8-month-old. Data are shown as the mean ± SD. The results were normalized to 18S mRNA. *P < 0.05, **p < 0.01, ***p < 0.001. (D) Matrix metalloprotease 13 (MMP-13), type II collagen, aggrecan, Runt-related transcription factor 2 (Runx2), SRY-box transcription factor 9 (Sox9), type X collagen, Indian hedgehog (Ihh), and parathyroid hormone-related protein (PTHrP) immunochemical staining of the different groups. And the relative locations were indicated by the red frame in the bottom right corner of each picture. Scale bars: 50 μm.
OA Progression Was Decelerated by HDAC4 Overexpression in Rats
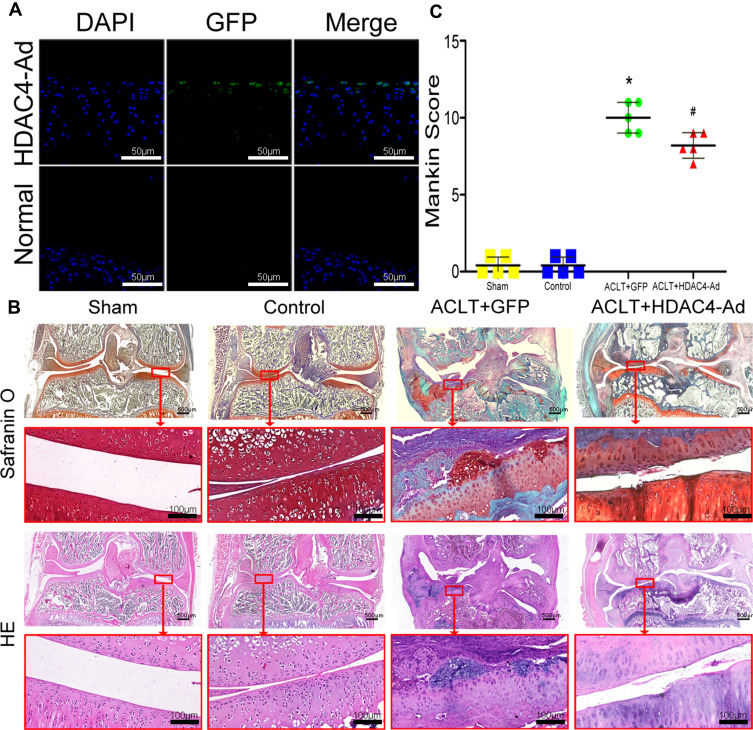
HDAC4 was overexpressed in knee cartilage chondrocytes to further investigate the effects of HDAC4. However, as the small space of the mouse knee joint cavity was inconvenient for injection, rats were used for further studies (Supplemental Figure 1A). After OA modeling (Supplemental Figure 1B) and grouping as previously described. The frozen sections of knee cartilage showed that the chondrocytes expressed exogenous HDAC4 three days after the injection (Figure 5A). Despite the possible bias caused by the inconsistency of the rat model, SafO staining results successfully showed that ACLT+GFP group mice had advanced OA (Figure 5B, upper panel). Wear of the cartilage surface, hyperplasia of the synovial membrane, and osteophytes of the tibia could be observed. Combined with the results of HE staining, Mankin score grading was performed (Figure 5B, bottom panel, and Figure 5C). With the injection of HDAC4, ACLT+HDAC4-Ad group rats only exhibited mild OA progression, and the Mankin score showed a decrease compared to that of the ACLT+GFP group.
Figure 5.
Osteoarthritis (OA) progression decelerated after the intra-articular injection of histone deacetylase 4 (HDAC4) in rats. (A) Fluorescence microscopy images of HDAC4-green fluorescent protein (GFP) chondrocytes with DAPI staining. Scale bars:50μm. (B) HE and SafO staining of rat knee joints at three months after the procedure. Mice in the Control group receive no intervention. Typical OA was observed in the ACLT+GFP group. Milder OA was observed in the ACLT+HDAC4-Ad group. Scale bars for Up panel images were 500μm. And Scale bars for down panel images were 100μm. (C) Mankin scores were analyzed to quantify the degree of OA. With the intra-articular injection of HDAC4, the progression of OA was decelerated. *P < 0.05, ACLT+GFP vs Control; #P < 0.05, ACLT+HDAC4-Ad vs ACLT+GFP; n = 5 pre group.
Expression Level Changes After HDAC4-Ad Transfection in Rats
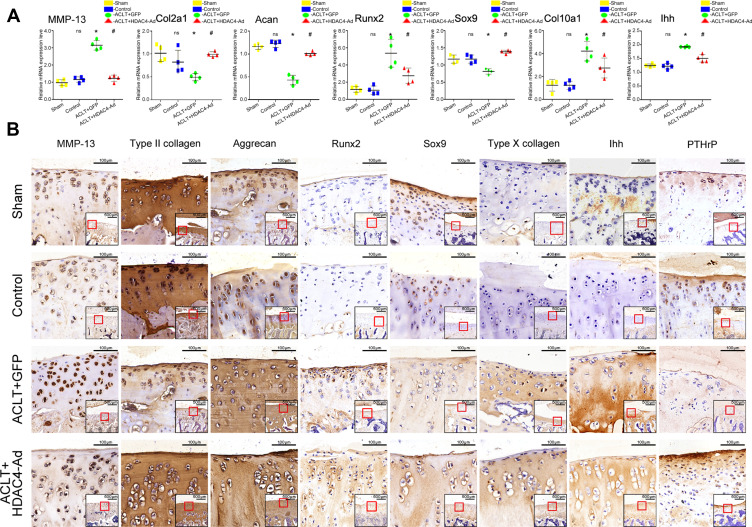
To further explore the mechanism underlying the slower OA progression induced by HDAC4 overexpression (Figure 6), qPCR and immunohistochemical staining of OA-related genes were performed based on the previous work.21 The expression of COL2A1 and Aggrecan was decreased in ACLT-induced OA progression, but increased when HDAC4-Ad was intra-articularly injected after the modeling. The expression of MMP-13 was increased in the ACLT+GFP and ACLT+HDAC4-Ad groups. However, compared to the ACLT+GFP group, the MMP-13 expression levels were decreased in the ACLT+HDAC4-Ad groups. An increase in Sox9 expression and a decrease in Runx2 and Col10a1 expression were only observed in ACLT+GFP and ACLT+HDAC4-Ad group mice with OA progression. However, in the ACLT+HDAC4-Ad group, the Runx2 and Col10a1 levels were higher than those in the ACLT+GFP group, whereas the Sox9 levels were lower. The downregulation of PTHrP and upregulation of Ihh in the ACLT+GPF and ACLT+HDAC4-Ad groups were observed with the onset of OA. However, compared to the ACLT+GFP group, Ihh expression was decreased and PTHrP expression was increased in the ACLT+HDAC4-Ad group. Interestingly, the changes in the mRNA and protein expression levels of Runx2, MMP-13, and Col10a1 were much more dramatic than those of other genes.
Figure 6.
Expression level changes of osteoarthritis (OA)-related genes after histone deacetylase 4 (HDAC4)-Ad transfection in rats. (A) qPCR analysis of OA-related genes after HDAC4-Ad transfection. Collagen type II alpha 1 chain (COL2A1) and aggrecan (Acan) expression were reduced in the ACLT+GFP group. Furthermore, the expression levels were relatively increased in the ACLT+HDAC4-Ad group when compared to the ACLT+GFP group. Matrix metalloprotease 13 (MMP-13), Runt-related transcription factor 2 (Runx2), the alpha chain of type X collagen (Col10a1), and Indian hedgehog (Ihh) expression was significantly increased in the ACLT+GFP group. Moreover, the expression levels were relatively decreased in the ACLT+HDAC4-Ad group when compared to the ACLT+GFP group. Data are shown as the mean±SD. ns, P > 0.05, Control vs Sham; *P < 0.05, ACLT+GFP vs Control; #P< 0.05, ACLT+HDAC4-Ad vs ACLT+GFP; n = 4 pre group. The results were normalized to GAPDH mRNA. (B) Immunochemical analysis of OA-related genes after transfection with HDAC4-Ad. These results were consistent with those of qPCR analysis. COL2A1 and Acan expression were reduced in the ACLT+GFP group. Moreover, the expression levels were relatively increased in the ACLT+HDAC4-Ad group when compared to the ACLT+GFP group. MMP-13, Runx2, Col10a1, and Ihh expression was increased in the ACLT+GFP group. Furthermore, the expression levels were relatively decreased in the ACLT+HDAC4-Ad group when compared to the ACLT+GFP group. The images show the immunohistochemical staining of cartilage. Scale bars: 100μm. And the relative location was indicated by the red frame in the bottom right corner of the picture. Scale bar: 500μm.
Discussion
In this study, we used Acan-CreERT2-HDAC4fl/fl transgenic mice to knock out HDAC4 expression and spontaneous age-related OA was induced. Combined with the results of HDAC4-Ad intra-articular injection, chondrocyte hypertrophy caused by HDAC4 downregulation was identified as the major cause of age-related OA.
At the beginning of this study, we addressed the preliminary relationship between HDAC4 expression and age-related OA. Besides the previous reports,1,22,23 we further found a decrease in superficial zone thickness, and an increase in transitional zone thickness, before extended wear of the cartilage surface. This tendency continued with cartilage aging and OA progression (Figure 1A). Although hypertrophy-related biomarkers were normally investigated in the development of growth plate, their contributions to the chondrocyte degeneration during OA progresses have been reported in multiple articles.5,7,11,22,24 In this study, as hypertrophic chondrocytes are mainly present in the transitional zone of the cartilage,2 it is believed that chondrocytes undergo hypertrophy during the onset of age-related spontaneous OA. Furthermore, the expression and localization of HDAC4 parallels that of the generalized hypertrophy biomarker-type X collagen (Figure 1D and E).10 These results strongly suggest that there might be a correlation between HDAC4 expression and age-dependent chondrocyte hypertrophy during OA.
To investigate the role of HDAC4 in age-related OA, AcanERT2-HDAC4fl/fl transgenic mice were used (Figure 2A and B). Immunohistochemistry and qPCR results confirmed that HDAC4 was deleted in adulthood and kept low-expression till 8-months old (Figure 3B and C). Further results identified early stage OA at 8-months-old (Figure 2D). However, wild-type mice usually show early signs of OA at 12-month-old or never. Furthermore, spontaneous OA in some mice was only classified as moderate by the time of 15-month old.16 These results suggest that HDAC4 deletion could early induce age-related spontaneous OA. SafO staining showed cartilage surface defects in AggH-Tam group mice classified as early-stage OA while no obvious changes were observed in the other two control groups (Figure 3D and E). The results of thicker synovium and osteophytes further indicated that the progression of OA might accelerate with HDAC4 deletion (Figure 3F). These results further demonstrated that HDAC4 deletion early induced the onset of early age-related spontaneous OA.
Furthermore, qPCR and IHC were performed to detect the gene and protein expression levels of several OA-related genes after HDAC4 deletion (Figure 4). Type II collagen, encoded by the col2a1 gene, is one of the ECM molecules responsible for the vast majority of the mechanical properties associated with cartilage.11,24 Aggrecan, encoded by the Acan gene, fills the interstices of the collagen network and provides compressibility and elasticity to the collagen.13 MMP-13 is considered the most important catabolic enzyme targeting type II collagen.13 The expression of aggrecan was also decreased by the upregulation of MMP-13 in cartilage.25 In this study, following HDAC4 deletion-induced age-related spontaneous OA, the mRNA expression level of MMP-13 was increased, and the mRNA expression levels of type II collagen and aggrecan were decreased. Similar immunohistochemistry results were obtained. Furthermore, it has been reported that hypertrophic chondrocytes are characterized by the expression of type X collagen.12,26 In this study, the mRNA and protein expression levels of type X collagen in the AggH-Tam group were increased compared to the levels in the other groups. These results are consistent with the increasing chondrocyte hypertrophy observed in aging human cartilage. Sox9 is a transcription factor required for chondrocyte differentiation and cartilage formation.27 A recent study showed that the expression of Sox9 follows an age-dependent manner in degenerated cartilage.4 In this study, the expression of Sox9 in the cartilage of AggH-Tam group mice was decreased, which is consistent with the age-dependent expression of Sox9. It has also been reported that chondrocyte hypertrophy can be induced by Runx2 overexpression, which eventually leads to cartilage degeneration.28 In this study, the expression of Runx2 was increased during the induction of age-related OA by HDAC4 deletion. Interestingly, the expression level of Runx2 was much higher than that of the other OA-related genes we tested. It has been reported that HDAC4 inhibits the expression and activity of Runx2 by directly binding to its MEF2 domain.5 Therefore, HDAC4 deletion released the inhibition of Runx2 and led to a large increase in Runx2 expression. It is well documented that the feedback loop formed by Ihh and PTHrP regulates the balance between chondrocyte proliferation and maturation.9,12 With HDAC4 deletion-induced OA, the expression of PTHrP decreased while that of Ihh increased. Furthermore, as the downstream target of PTHrP-Ihh signaling, the expression of Gli1 was increased. These results indicated that the effects of HDAC4 deletion on the PTHrP-Ihh feedback loop resulted in chondrocyte hypertrophy.
The effects of HDAC4 and the underlying mechanism during the onset of OA were further explored using HDAC4-Ad intra-articular injection in rats. Although the exogenous HDAC4 only worked on the superficial zone of the rats’ cartilage, the expression level of HDC4 in the chondrocytes was upregulated by the intra-articular injection of HDAC4-Ad. After the over-expression of HDAC4 was confirmed by immunofluorescence (Figure 5A), the severity of ACLT-induced OA was decreased at 3 months after injection (Figure 5B and C). Compared to the OA caused by HDAC4 deletion, although there are some inconsistencies in ACLT induced OA, the expression of HDAC4 was increased by the intra-articular injection. The qPCR and IHC were performed to examine the expression level of OA-related genes (Figure 6). The results showed that the mRNA and protein expression levels of type II collagen, aggrecan, Sox9, and PTHrP were decreased, while those of MMP-13, Runx2, type X collagen, and Ihh were increased. Furthermore, Runx2 expression was much lower than that of other genes, as we anticipated. Combined with the results of Runx2 and type x collagen expression in HDAC4 deletion mice, these results proved that HDAC4 controls the onset of age-related spontaneous OA by regulating chondrocyte hypertrophy. During the process, the expression of catabolic enzymes was increased, and the inherent components of cartilage were digested and downregulated. With the increase of HDAC4 expression, the expression of PTHrP increased while that of Ihh decreased. These results indicated that chondrocyte proliferation was increased. Forever, with the HDAC4 deletion-induced OA, the expression of PTHrP decreased while that of Ihh increased. Furthermore, as the downstream target of PTHrP-Ihh signaling, the expression of Gli1 was increased. These results above were consistent with the reported work of Dr. Gu et al.21 This work further indicated that the effects of HDAC4 deletion on the PTHrP-Ihh feedback loop might also contribute to chondrocyte hypertrophy.
There were several limitations in our study. Firstly, we mainly focused on the HDAC4 deletion induced age-related OA which in the early stages. The later time point for HDAC4 deletion was primarily investigated to observe the development of OA. Therefore, future research shall extend the observation period to the late OA. Secondly, although the ACLT rat model was used to avoid the possible injury of intra-articular injection operation in this study, it is not the best candidate for the research of age-related OA. Better OA model might be beneficial for further research of age-related OA.
Conclusion
Above all, our study showed that HDAC4 deletion in young adult mice induced age-related spontaneous OA, while HDAC4 overexpression in cartilage decelerated OA progression. The regulation of chondrocyte hypertrophy is the main mechanism underlying this process. These results improve our understanding of the mechanism underlying age-related OA and may help in the development of early diagnosis and treatment. Interestingly, genetic ablation of HDAC4 caused spontaneous ossification and thicker synovium was observed. However, as the mechanisms underlying age-related OA are complex, further studies are required to confirm these hypotheses.
Funding Statement
Supported by the National Natural Science Foundation of China (grant 8217250), the Natural Science Foundation of Shanxi Fundamental Research Project (project 20210302123263 and project 20210302123270) and Shanxi Scholarship Council of China (project HGKY2019099). The funding source had no active role in the study.
Abbreviations
OA, osteoarthritis; HDAC4, histone deacetylase 4; Acan, aggrecan; TM, Tamoxifen; H&E, hematoxylin and eosin; ACLT, anterior cruciate ligament transection; IHC, immunohistochemistry; OARSI, Osteoarthritis Research Society International; SafO, Safranin-O staining; Runx2, Runt-related transcription factor 2; Sox9, sex-determining region Y box 9; PTHrP, parathyroid hormone-related peptide; Ihh, Indian hedgehog; Gli1, Glioma-associated oncogene homolog 1; MMP-13, metalloprotease 13.
Data Sharing Statement
The data of this article which used to support the findings are available from the corresponding author upon request.
Ethics Approval
Human samples were collected according to protocols approved by the Ethics Committee of the Second Hospital of Shanxi Medical University. The samples were extracted from the OA biobank of the Second Hospital of Shanxi Medical University in accordance with the approved guidelines which meet the standard of the Declaration of Helsinki and written informed consent was obtained. The experiments of mice and rats were conducted in strict compliance with the regulations of “the Care and Use of Laboratory Animals Guidelines” published by the Ethics Committee of the second hospital of Shanxi Medical University and all protocols were approved.
Disclosure
No potential conflicts of interest relevant to this article were reported.
References
- 1.O’Brien MS, McDougall JJ. Age and frailty as risk factors for the development of osteoarthritis. Mech Ageing Dev. 2019;180:18021–18028. [DOI] [PubMed] [Google Scholar]
- 2.Weiss C, Rosenberg L, Helfet AJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg Am. 1968;50(4):663–674. doi: 10.2106/00004623-196850040-00002 [DOI] [PubMed] [Google Scholar]
- 3.Nishimori S, Lai F, Shiraishi M, et al. PTHrP targets HDAC4 and HDAC5 to repress chondrocyte hypertrophy. JCI Insight. 2019;4. doi: 10.1172/jci.insight.97903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M, Theleman JL, Lygrisse KA, Wang J. Epigenetic mechanisms underlying the aging of articular cartilage and osteoarthritis. Gerontology. 2019;65(4):387–396. doi: 10.1159/000496688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega RB, Matsuda K, Oh J, et al. Histone deacetylase 4 controls chondrocyte hypertrophy during skeletogenesis. Cell. 2004;119(4):555–566. doi: 10.1016/j.cell.2004.10.024 [DOI] [PubMed] [Google Scholar]
- 6.Nishimori S, Wein MN, Kronenberg HM. PTHrP targets salt-inducible kinases, HDAC4 and HDAC5, to repress chondrocyte hypertrophy in the growth plate. Bone. 2021;142:142115709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao K, Wei L, Zhang Z, et al. Decreased histone deacetylase 4 is associated with human osteoarthritis cartilage degeneration by releasing histone deacetylase 4 inhibition of runt-related transcription factor-2 and increasing osteoarthritis-related genes: a novel mechanism of human osteoarthritis cartilage degeneration. Arthritis Res Ther. 2014;16(6):491. doi: 10.1186/s13075-014-0491-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papaioannou G, Mirzamohammadi F, Lisse TS, Nishimori S, Wein MN, Kobayashi T. MicroRNA-140 provides robustness to the regulation of hypertrophic chondrocyte differentiation by the PTHrP-HDAC4 pathway. J Bone Miner Res. 2015;30(6):1044–1052. doi: 10.1002/jbmr.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong L, Huang X, Karperien M, Post JN. The regulatory role of signaling crosstalk in hypertrophy of MSCs and human articular chondrocytes. Int J Mol Sci. 2015;16(8):19225–19247. doi: 10.3390/ijms160819225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu T, Xu G, Gu Z, Wu H. Hedgehog signal expression in articular cartilage of rat temporomandibular joint and association with adjuvant-induced osteoarthritis. J Oral Pathol Med. 2017;46(4):284–291. doi: 10.1111/jop.12497 [DOI] [PubMed] [Google Scholar]
- 11.Nakatani T, Chen T, Partridge NC. MMP-13 is one of the critical mediators of the effect of HDAC4 deletion on the skeleton. Bone. 2016;90:90142–90151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–3. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y, Sinkeviciute D, He Y, et al. The minor collagens in articular cartilage. Protein Cell. 2017;8(8):560–572. doi: 10.1007/s13238-017-0377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henry SP, Jang CW, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Generation of aggrecan-CreERT2 knockin mice for inducible Cre activity in adult cartilage. Genesis. 2009;47(12):805–814. doi: 10.1002/dvg.20564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang B, Mamidi MK, Samsa WE, et al. Targeted and sustained Sox9 expression in mouse hypertrophic chondrocytes causes severe and spontaneous osteoarthritis by perturbing cartilage homeostasis. Am J Transl Res. 2020;12(3):1056–1069. [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Wei X, Wang D, et al. Positive effects of a young systemic environment and high growth differentiation factor 11 levels on chondrocyte proliferation and cartilage matrix synthesis in old mice. Arthritis Rheumatol. 2020;72(7):1123–1133. doi: 10.1002/art.41230 [DOI] [PubMed] [Google Scholar]
- 17.Perry MJ, Gujra S, Whitworth T, Tobias JH. Tamoxifen stimulates cancellous bone formation in long bones of female mice. Endocrinology. 2005;146(3):1060–1065. doi: 10.1210/en.2004-1114 [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Wei X, Lv Z, et al. Cyclic equibiaxial tensile strain alters gene expression of chondrocytes via histone deacetylase 4 shuttling. PLoS One. 2016;11(5):e0154951. doi: 10.1371/journal.pone.0154951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afara IO, Prasadam I, Moody H, Crawford R, Xiao Y, Oloyede A. Near infrared spectroscopy for rapid determination of Mankin score components: a potential tool for quantitative characterization of articular cartilage at surgery. Arthroscopy. 2014;30(9):1146–1155. doi: 10.1016/j.arthro.2014.04.097 [DOI] [PubMed] [Google Scholar]
- 20.Nielsen AW, Klose-Jensen R, Hartlev LB, et al. Age-related histological changes in calcified cartilage and subchondral bone in femoral heads from healthy humans. Bone. 2019;129:115037. [DOI] [PubMed] [Google Scholar]
- 21.Gu X, Li F, Gao Y, Che X, Li P. HDAC4 mutant represses chondrocyte hypertrophy by locating in the nucleus and attenuates disease progression of posttraumatic osteoarthritis. BMC Musculoskelet Disord. 2022;23(1):8. doi: 10.1186/s12891-021-04947-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Sun Y, Ge Q, Teng H, Jiang Q. Histone deacetylase 4 alters cartilage homeostasis in human osteoarthritis. BMC Musculoskelet Disord. 2014;15:15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer KA, Andriacchi TP, Garcia Aznar JM. The nature of age-related differences in knee function during walking: implication for the development of knee osteoarthritis. PLoS One. 2016;11(12):e0167352. doi: 10.1371/journal.pone.0167352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurie LE, Kokubo H, Nakamura M, Saga Y, Funato N, Kim J-E. The transcription factor hand1 is involved in Runx2-Ihh-regulated endochondral ossification. PLoS One. 2016;11(2):e0150263. doi: 10.1371/journal.pone.0150263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:11529–11543. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Zhou Q, Liang QQ, et al. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthritis Cartilage. 2009;17(1):100–106. doi: 10.1016/j.joca.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 27.Prasadam I, Zhou Y, Shi W, Crawford R, Xiao Y. Role of dentin matrix protein 1 in cartilage redifferentiation and osteoarthritis. Rheumatology. 2014;53(12):2280–2287. doi: 10.1093/rheumatology/keu262 [DOI] [PubMed] [Google Scholar]
- 28.Catheline SE, Hoak D, Chang M, et al. Chondrocyte-Specific RUNX2 overexpression accelerates post-traumatic osteoarthritis progression in adult mice. J Bone Miner Res. 2019;34(9):1676–1689. doi: 10.1002/jbmr.3737 [DOI] [PMC free article] [PubMed] [Google Scholar]