Abstract
In this study, ZrO2, TiO2, and Fe3O4 components were synthesized by co-precipitation, sol–gel, and co-precipitation methods, respectively. In addition, solid-state dispersion method was used for synthesizing of ZrO2/TiO2/Fe3O4 ternary nanocomposite. The ZrO2/TiO2/Fe3O4 nanocomposite was characterized by different techniques including XRD, EDX, SEM, BET, FTIR, XPS, EELS, and Photoluminescence (PL). The FTIR analysis of ZrO2/TiO2/Fe3O4 photocatalyst showed strong peaks in the range of 450 to 700 cm−1, which represent stretching vibrations of Zr–O, Ti–O, and Fe–O. The results of FTIR and XRD, XPS analyses and PL spectra confirmed that the solid-state dispersion method produced ZrO2/TiO2/Fe3O4 nanocomposites. The EELS analysis confirmed the pure samples of Fe3O4, TiO2 and ZrO2. The EDAX analysis showed that the Zr:Ti:Fe atomic ratio was 0.42:2.08:1.00. The specific surface area, pores volume and average pores size of the photocatalyst were obtained 280 m2/g, 0.92 cm3/g, and 42 nm respectively. Furthermore, the performance of ZrO2/TiO2/Fe3O4 nanocomposite was evaluated for naproxen removal using the response surface method (RSM). The four parameters such as NPX concentration, time, pH and catalyst concentration was investigated. The point of zero charge of the photocatalyst was 6. The maximum and minimum degradation of naproxen using photocatalyst were 100% (under conditions: NPX concentration = 10 mg/L, time = 90 min, pH = 3 and catalyst concentration = 0.5 g/L) and 66.10% respectively. The stability experiment revealed that the ternary nanocatalyst demonstrates a relatively higher photocatalytic activity after 7 recycles.
Subject terms: Environmental sciences, Nanoscience and technology
Introduction
Environmental pollution by pharmaceutical compounds is considered as one of the most serious issues in recent years1,2. For the treatment of pharmaceutical wastewater and for removing pollutants before those are released into the environment, identification of the most efficient method is a challenge3. Pharmaceutical wastewater can be treated using physical4, chemical, biological5, as well as combined methods6. The Advanced oxidation processes7 including advanced oxidation based on the sulfate radicals8, ultraviolet–visible9, natural sunlight10, Fenton oxidation11, electrochemical12, nanocomposite catalysts13, and sonolysis and sono-Fenton14 have been widely used to remove pollutants from pharmaceutical wastewater with great performance. The formation of radicals during these processes leads to the oxidation of organic pollutants in aqueous solutions. In comparison to other methods, photocatalysis offers several advantages, such as high efficiency, low cost, design of suitable catalysts for specific wastewaters, and high corrosion and temperature stability15–17. Titanium dioxide (TiO2) has been widely used as a catalyst in the degradation of organic compounds and pharmaceutical pollutants since it is a light-sensitive semiconductor (including UV and visible light)18,19. The formation of valence band holes and conduction band electrons during photocatalysis produces oxidation–reduction media in wastewater. It can easily degrade organic compounds and convert them into non-toxic compounds such as CO2 and water20,21. Titanium dioxide is a polymorphic material with three crystalline phases: anatase, rutile, and brookite. The anatase phase is more photocatalytically active than the rutile phase22,23. In order to enhance the TiO2 photocatalyst activity, it is important to use smaller particles (nano size), as smaller particles have higher specific surface areas24. The removal of titanium dioxide nanoparticles after treatment reduces the benefit of this photocatalyst, and immobilization of titanium dioxide (TiO2) onto supporting materials can be performed, but immobilization reduces the specific surface area in comparison with a homogenous catalysts25. On the other hand, fast recombination of generated electron–hole pairs can decrease the activity of titanium dioxide photocatalyst26. Therefore, some other semiconductors such as ZrO2 are used to improve of the activity of TiO2. ZrO2 doping can slow down the electron–hole pair recombination, strengthen the material and increase surface area and anatase to rutile crystal phases ratio27,28. ZrO2-TiO2 photocatalyst has been used to degrade organic compounds, which in this compound, ZrO2 acts as support or photocatalyst in the system29.
The addition of a small amount of ZrO2 to TiO2 can increase the surface area because ZrO2 inhibits anatase to rutile phase transitions, densification, and crystallite growth by providing dissimilar boundaries30. It was observed that changes in surface chemistry, particularly acidity improve the photocatalyst activity31. In this binary catalyst, holes trap such as the hydroxyl groups, prevent electron–hole recombination and oxidation–reduction reactions, and increase quantum yield31. In addition to ZrO2 nanoparticles, it is necessary to be added another compound to these binary metal oxides to improve the surface area of the TiO2 photocatalyst as well as recovery of TiO2 from treated wastewater32,33. Magnetic nanoparticles, such as Fe3O4, are suitable for this purpose. A Fe3O4@TiO2 photocatalyst has been synthesized to degrade Bisphenol A under visible and long-wavelength UV light irradiation. Moderate iron loading reduced hole-pair separation effectively, and shifted the bandgap in the visible range. Further, the magnetic properties of Fe3O4 play a critical role in recovering the used catalyst from the solution, which facilitates practical applications of the Fe3O4@TiO2 photocatalyst34. Fe3O4@SiO2@g-C3N4/TiO2 nanocomposite was used to remove dye pollutants. It has been found that the 10 wt.% g-C3N4/TiO2 composite catalyst can degrade over 91% of anionic and cationic dyes35. Therefore, it is possible to combine the three compounds to degrade toxic and harmful pollutants in wastewater. In recent years, non-steroidal anti-inflammatory drugs (NSAIDs) have been increasingly used for the treatment of COVID-1936,37. Therefore, it is predicted that pharmaceutical-produced wastewater containing NSAIDs such as naproxen will increase and it will be very important to develop an effective method of degradation these compounds from the wastewater. The degradation of naproxen by H2O2-modified titanate nanomaterial and Bi-modified titanate nanobulks under visible light irradiation has been successfully accomplished38,39. In previous works, each of these metal oxides was used separately as a catalyst to remove various contaminants40–43, but the interaction of the three metal oxides of Fe3O4, TiO2 and ZrO2 together and its function to remove pharmaceutical contaminants can produce a synergistic effect. The difference in physical and chemical properties of each component of these nanophotocatalyst makes it unique. A nanocomposite with magnetic, stable, and high photocatalytic properties was finally synthesized44,45. TiO2 has a band gap of 3.2 eV for anatase, and 3.0 eV for rutile46,47. The ZrO2 is used as a photocatalyst in heterogeneous reactions due to its semiconductor properties (type n). The band gap energy of ZrO2 varies from 3.25 to 5.1 eV depending on the sample preparation48–55. Its common band gap energy is 5 eV55. It has a wide bandgap and a highly negative flat-band potential, which can be used for hydrogen production through water decomposition55,56. Magnetic properties of Fe3O4 nanoparticles make recyclable nanocomposites possible. Furthermore, the excitation range of nanocomposites synthesized from Fe3O4 can be used in the visible light range due to their unique optical properties.
The Response surface methodology (RSM) is a widely used mathematical and statistical method for modeling and analyzing a process in which a response is affected by multiple variables, and its goal is to optimize the response57,58. Synthesis of Fe3O4/TiO2/ZrO2 nanocomposite can improve the photocatalytic property of catalyst. The synthesis of these nanoparticles individually can have some disadvantages such as a wide energy gap, activity only in UV, lower photocatalytic activity, However, their composite synthesis will offer advantages such as higher magnetic properties, recyclable, higher stability and activity, and enhanced photocatalytic activity59–67. The Box–Behnken design (BBD) was used in this study due to its rotatable or nearly rotatable second-order design. The percentage of naproxen degradation was selected as the experimental design response68,69.
The current work is focused on the synthesis of a new ZrO2/TiO2/Fe3O4 nano-photocatalyst with high photocatalytic activity and reusability for the pharmaceutical wastewater treatment. Its performance has been evaluated in the removal of naproxen from pharmaceutical wastewater under UV light. The synthesised photocatalyst has been characterized with methods such as X-ray diffraction (XRD), Energy Dispersive X-Ray (EDX), Scanning Electron Microscope (SEM), Brunauer–Emmett–Teller (BET), Fourier Transform Infrared Spectroscopy (FTIR), X-ray Photoelectron Spectroscopy (XPS), Electron energy-loss spectroscopy (EELS), and Photoluminescence (PL). We investigated various factors including the initial concentration of naproxen, initial pH of the solution, photocatalyst dosage, and time until the optimal degradation efficiency was obtained using the RSM method.
Experimental and methods
Materials
Titanium (IV) iso-propoxide 97%, zirconyl(IV) chloride octahydrate (ZrOCl2.8H2O, 98%), and Iron (II) chloride tetrahydrate (≥ 99%) and iron (III) chloride hexahydrate(≥ 99%) were used as a precursors for the synthesis of TiO2, ZrO2, and Fe3O4 respectively. Ethanol was obtained from Sigma-Aldrich and utilized without any further processing. Deionized water (DI-water) was also used in the experiment. Sulphuric acid (H2SO4, 0.1 M) and sodium hydroxide (NaOH, 0.1 M) were used for the adjustment of the pH of the solution. (S)-6 methoxy α-methyl 2-naphthaleneaceticb sodium salt was purchased from Sigma Aldrich and its aqueous solution used for degradation experiments. In Table 1, naproxen's physical and chemical properties are shown.
Table 1.
Physical and chemical properties of naproxen.
| Property | Value |
|---|---|
| Molecular structure |
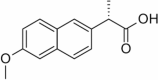
|
| Solubility | 15.9 mg/L at 25 °C |
| Melting point | 152–154 °C |
| Log Kow | 3.18 |
| pKa | 4.15 |
Synthesis of nanoparticles
ZrO2 nanoparticles
The co-precipitation method was used for the synthesis of ZrO2 nanoparticles. Firstly, ZrOCI2.8H2O was dissolved in deionized water. Then, 2 M NaOH solution was added to the solution in order to achieve a solution with a pH of 10. The solution was gently stirred for 1 h. After that, the precipitate was filtered, washed with distilled water to reach neutral pH. The obtained powder was dried in an oven at 60 °C for 24 h and then calcined at 700 °C for 10 h70.
TiO2 nanoparticles
Sol–Gel method was used for the preparation of TiO2 nanoparticles. 15 mL Titanium (IV) isopropoxide was added into 60 mL ethanol. A magnetic stirrer was used to mix the solution for 30 min. 10 mL deionized water in a drop-wise fashion was added into the mixture until hydrolysis reaction occurred in the system. The obtained white gel was dried at 100 °C and later calcined at 450 °C for 2 h71.
Fe3O4 nanoparticles
Known amounts of FeCl3.6H2O (0.605 g) and FeCl2.4H2O (0.215 g) were dissolved in deionized water. Then, the obtained solution was placed in an ultrasonic homogenizer for 5 min. After that, 60 mL of 1 M NaOH was added dropwise into solution under ultrasonic at 70 °C. After 60 min, the brownish powder was obtained at a pH of 13.4 and it was separated from the solution using a centrifuge. Finally, it was calcined at 300 °C for 1 h72.
ZrO2/TiO2/Fe3O4 nano-photocatalyst
ZrO2/TiO2/Fe3O4 photocatalysts were prepared using solid-state dispersion method. ZrO2, TiO2, and Fe3O4 nanoparticles were mixed at a ratio of 4:8:1 in ethanol solution. The resultant ternary oxide was stirred at a constant rate of 300 rpm for 15 min. Then, ethanol was removed from a mixture of nanocomposite by evaporation. Then, the product was dried at 110 °C and calcined at 450 °C for 6 h to obtain ZrO2/TiO2/Fe3O4 nanocomposite photocatalyst60. The mixing ratio of used components in the synthesized nanocomposite was selected based on the highest efficiency of naproxen degradation under the same operation conditions. The best ratio was achieved (4:8:1) of the ZrO2, TiO2, Fe3O4 compounds.
Photocatalyst characterization
The mineralogical analysis of the prepared nanoparticles was characterized by X-ray diffraction (Inel France, Equinox 3000) with Cu Kα radiation. SEM(AIS2100). It was used to determine the surface morphology and microstructure of ZrO2/TiO2/Fe3O4 nanocomposite photocatalyst. The Fourier-transform infrared spectroscopy (VERTEX 70, Bruker, USA) in the wavelength range from 400 to 4000 cm−1 was used to analyze the chemical bonding in a photocatalyst. Texture properties of nanoparticles was investigated using TriStar-II-Series, Micromeritics Instrument Corporation, USA. The specific surface area of photocatalysts was determined via the N2 adsorption–desorption and Brunauer–Emmett–Teller (BET) model. Barrett-Joyner–Halenda (BJH) method was used to calculate the size of pores and pore size distribution using adsorption–desorption curves. Powder samples for XPS analysis are prepared in the form of pellets in the laboratory (the powder should be large enough to cover a surface of 1.5 × 1.5 cm), then the samples should be placed in a vacuum chamber after preparation. A PHI Perkin-Elmer Model 5400 instrument was used to record XPS spectra. The EELS analysis was obtained using a sub-nanometer probe size using the GIF2000 Filter. The PL Spectroscopy was recorded by Avaspec-2048-TEC model spectrometer.
Point of zero charge determination
An amphoteric oxide method was used to determine the point of zero charge of the photocatalyst73. In order to determine the point of zero charge (PZC), 0.25 g of the synthesized photocatalyst was added into the solution with the same ionic strength and different pH values. The synthesized nanocatalyst was added to 50 mL of 0.1 M NaNO3 solution. The pH of solution was adjusted in the range of 3–9 using 0.1 M H2SO4 and 0.1 M NaOH. The pH of solution was measured before and after 24 h mixing and those were called pHi and pHf respectively. The plot of pH changes (ΔpH) as a function of initial pHi was used for the determination of PZC73.
Optimization of the photocatalyst Activity
In order to optimize photocatalyst activity, the RSM has been used. Design Expert 8.0 software (Stat-Ease, Inc., USA) was used to design experiments and analyze mathematical modeling. Each experiment was performed in duplicate. Box–Behnken design (BBD) was applied in this study because of its rotatable or nearly rotatable second-order design. The “y” was the response (the percentage of naproxen degradation). We evaluated four independent variables, including catalyst loading dosage, initial concentration of naproxen, time and pH value in order to find an optimal condition:
| 1 |
Photocatalyst activity
ZrO2/TiO2/Fe3O4 Photocatalyst was used for the degradation of naproxen in a pharmaceutical synthetic solution. The experiments were conducted based on the conditions in Table 2. In this study, the effects of solution pH, time, initial naproxen concentration, and photocatalyst loading dose on naproxen degradation were evaluated. To determine the adsorption behaviour of the photocatalyst, the solution kept was in the dark for half an hour. The reactor was irradiated immediately after adding the photocatalyst. For each experiment, a 150 W UV light source has been used for irradiation (The intensity was kept as 15 W/m2). At the end of each experiment, centrifugation with 12,000 rpm for 20 min have been performed for each withdrawn sample followed by absorbance measurement using UV‐Vis (Shimadzu UV2401PC) at λ = 230 nm. The efficiency of designed photocatalysts for the degradation of naproxen was calculated as follows:
| 2 |
Table 2.
Operating parameters for degradation of naproxen experiment.
| Factor | Name | Units | Minimum | Maximum | Coded low | Coded high |
|---|---|---|---|---|---|---|
| A | NPX concentration | mg/L | 10.00 | 30.00 | − 1 ↔ 10.00 | + 1 ↔ 30.00 |
| B | Time | Min | 30.00 | 90.00 | − 1 ↔ 30.00 | + 1 ↔ 90.00 |
| C | pH | – | 3.00 | 9.00 | − 1 ↔ 3.00 | + 1 ↔ 9.00 |
| D | Catalyst concentration | g/L | 0.1000 | 0.5000 | − 1 ↔ 0.10 | + 1 ↔ 0.50 |
where Ci and Cf are the naproxen concentration before and after degradation reaction respectively.
Reusable photocatalytic properties
Reusability tests were conducted for ZrO2/TiO2/Fe3O4 Photocatalyst. Briefly, the selected photocatalyst being used in the degradation test of naproxen was separated from solution using a 1.3 Tesla magnet. Used photocatalysts were rinsed in distilled water and irradiated under UV light for 12 h. In order to evaluate the reusability of a photocatalyst, seven successive experimental runs were conducted.
Results and discussion
Characterization
XRD analysis
The constituting phase, crystalline size, and crystalline structures of the ZrO2, TiO2, and Fe3O4, nanoparticles, and ZrO2/TiO2/Fe3O4 nanocomposite characterized using XRD, which are depicted in Fig. 1. For ZrO2, the primary characteristic diffraction peaks appeared at 2θ = 30°, 35°, 50.8°, 60.1°, and 63° were corresponds to crystal planes of (1 1 1), (0 0 2), (0 2 2), (3 1 1), and (2 2 2). The majority of these crystalline phases are monoclinic. The transition from tetragonal to monoclinic can occur with an increase in the calcination temperature which increases the crystallite size of the samples74. According to Fig. 1 for TiO2 sample, there is good agreement between the results of this study and the XRD pattern for the titanium dioxide phase reported in the literature75–77. The diffraction peaks at 2 thetha values of 25.32°, 37.90°, 48.09°, 54.10°, 55.15°, 62.85°, 68.99°, 70.49°, and 75.12° were belonged to crystal planes of (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1), (2 0 4), (1 1 6), (2 2 0), (2 1 5)78. All the peaks observed in the diffraction pattern of TiO2 nanoparticles are in good agreement with anatase (JCPDS No. 00–001-0562). Figure 1 clearly shows magnetite formation with well-defined crystallinity in Fe3O4. All of the diffraction peaks are related to (2 2 0), (3 1 1), (2 2 2), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) crystal planes and those were belonged to Fe3O4. The results confirmed the occurrence of inverse spinel structure in Fe3O4. The XRD peaks of impurities were not observed, which means that the synthesized Fe3O4 nanoparticles are pure79. Finally, in the XRD pattern of ZrO2/TiO2/Fe3O4 nanocomposite, the main peaks related to all three compounds were observed. The results showed that the sample has spinal reverse cube of Fe3O4 structure. The ZrO2 is amorphous phase and the phase of TiO2 is tetragonal (Anatase). The average crystallite sizes were calculated by Scherrer formula:
| 3 |
where λ is X-ray wavelength, β is the full width at half maximum of the diffraction line and θ is the diffraction angle80. The average crystallite size of TiO2, ZrO2, Fe3O4 and ZrO2/TiO2/Fe3O4 was found to be 18.75 nm, 22.29 nm, 13.27 and 33.42 nm respectively. The most important cause of difference in particle size of synthesized samples can be the difference in their synthesis method81.
Figure 1.
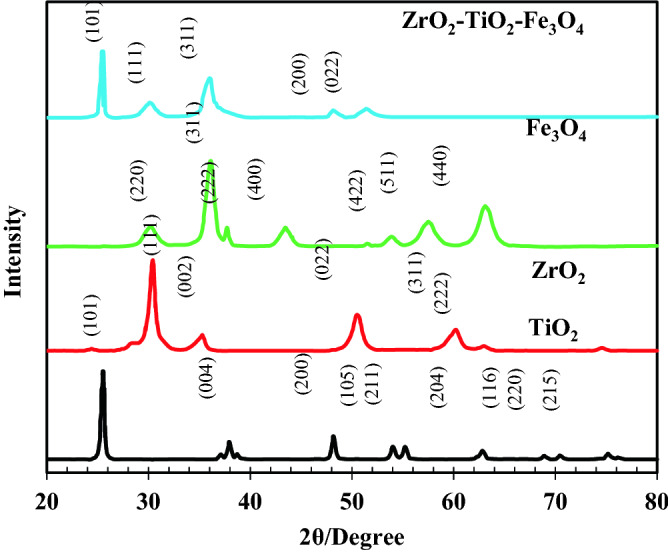
The XRD patterns of ZrO2, TiO2, Fe3O4, and ZrO2/TiO2/Fe3O4 nanocomposite.
EDX analysis
EDAX analysis confirmed the presence of all elements, including Zr, Ti, O and Fe in the synthesized photocatalyst and there is no evidence of any other element. The inserted table in Fig. 2 presents an elemental composition of the photocatalyst. The results showed that the atomic ratio of Zr:Ti:Fe was 0.42:2.08:1.00. The synthesized nanocomposite had different peaks at 0.70, 6.39, and 6.92 keV which are related to Lα, Kα, and Kβ of Fe and 0.52 keV is Kα of oxygen. Peaks at 2.04 (Lα) and 2.26 (Lβ) keV are for zirconium. Fe3O4 particles are larger than TiO2 particles based on the results obtained.
Figure 2.
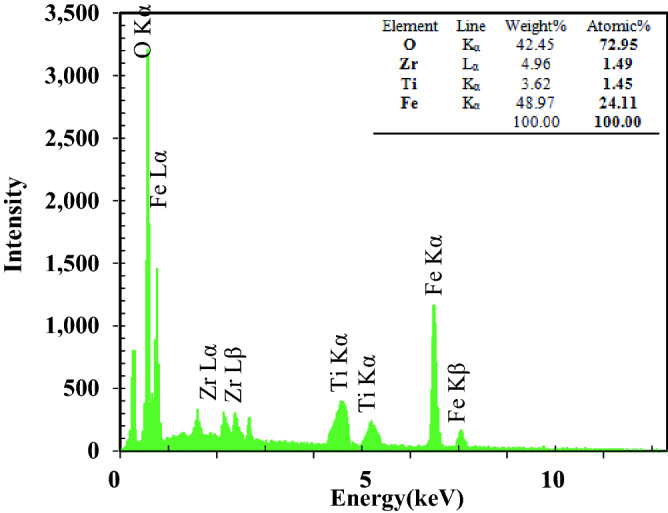
EDX spectrum. (Insert: Element analysis of synthesized nanocomposite).
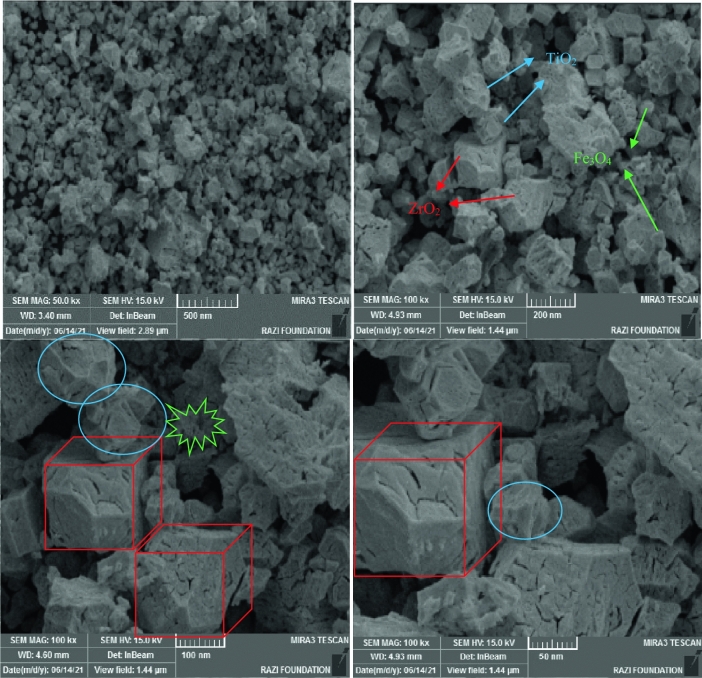
SEM analysis
SEM analysis was used to determine the morphology and size of nanoparticles of ZrO2/TiO2/Fe3O4 nanocomposite (see in Fig. 3). In Fig. 3, ZrO2 and TiO2 nanoparticles are almost cubic and spherical respectively, while Fe3O4 does not have a specific shape.
Figure 3.
SEM image of ZrO2/TiO2/Fe3O4 nanocomposite.
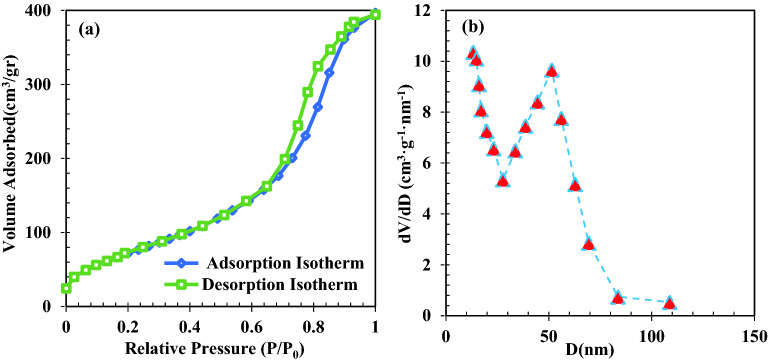
BET analysis
Figure 4a and b show the porosity distribution curve and pore size distribution of ZrO2/TiO2/Fe3O4 nanocomposite. The specific surface area (SSA) was calculated using the N2 isotherms. The BET-SSA, the volume of pores, the average size of pores of the photocatalyst obtained were 280 m2/g, 0.92 cm3/g, and 42 nm respectively. The sample is mesoporous as the diameter of pores is larger than 2 nm. Hysteresis loop of types IV-H1 was obtained for the sample which is related to cylindrical and spherical pores in the sample82. In terms of pore size distribution, wide pore size distribution was observed, especially, pores with size larger than 3 nm had significant volume in the sample. It was found that the pore size larger than 3 nm is appropriate for the reactants penetration into the porous media of photocatalyst83,84.
Figure 4.
(a) N2 adsorption–desorption hysteresis and (b) pore size distribution of ZrO2/TiO2/Fe3O4 photocatalyst.
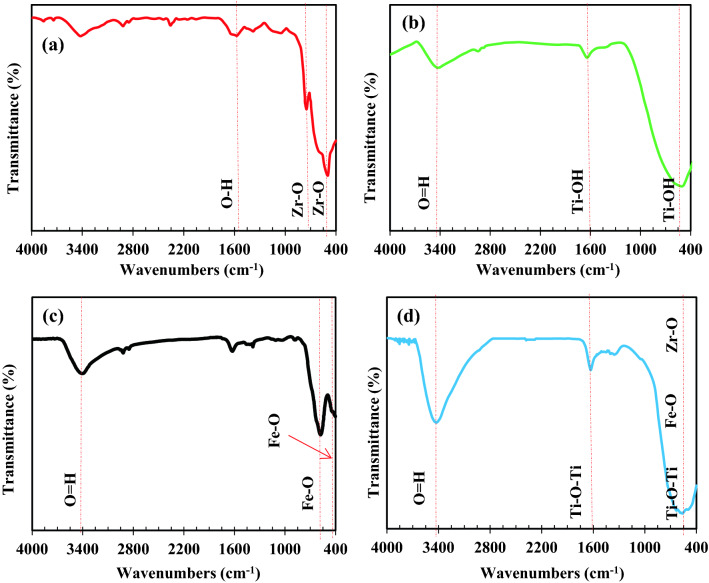
FTIR analysis
Figure 5a–d shows the FTIR analysis of ZrO2, TiO2, Fe3O4, and ZrO2/TiO2/Fe3O4 photocatalysts. As for ZrO2, the strong broad peak at 498–502 cm−1 region is attributed to the vibration mode of groups. The peaks around 754 cm−1 are related to the Zr–O stretching vibrations in ZrO2. The IR at 1553 cm−1 attributed to stretching of O–H groups, which indicates the adsorbed moisture85. For TiO2 nanoparticles, TiO2 network bonds and deformation vibrations of stretching mode of Ti–OH peaks were observed at 483 cm−1 and 1623 cm−1 respectively. It can be related to the absorption of water on the TiO2 surface. Asymmetrical and symmetrical stretching vibration of hydroxyl groups (–OH) was observed at 3405 cm−1. The obtained results are consistent with those reported in the literature86,87. According to Fig. 5c, the stretching vibration of the O–H can be attributed to the absorption band 3444 cm−1. The peaks at 419 cm−1 and 589 cm−1 were attributed the stretching vibration of the Fe–O. The strong peaks in the 450–700 cm−1 range were attributed to stretching vibrations of Zr–O, Ti–O, and Fe–O in ZrO2/TiO2/Fe3O4 photocatalyst. The results confirm the presence of all three nanoparticles in the ZrO2/TiO2/Fe3O4 photocatalyst.
Figure 5.
FTIR spectra of (a) ZrO2, (b) TiO2, (c) Fe3O4, (d) of ZrO2/TiO2/Fe3O4 photocatalyst.
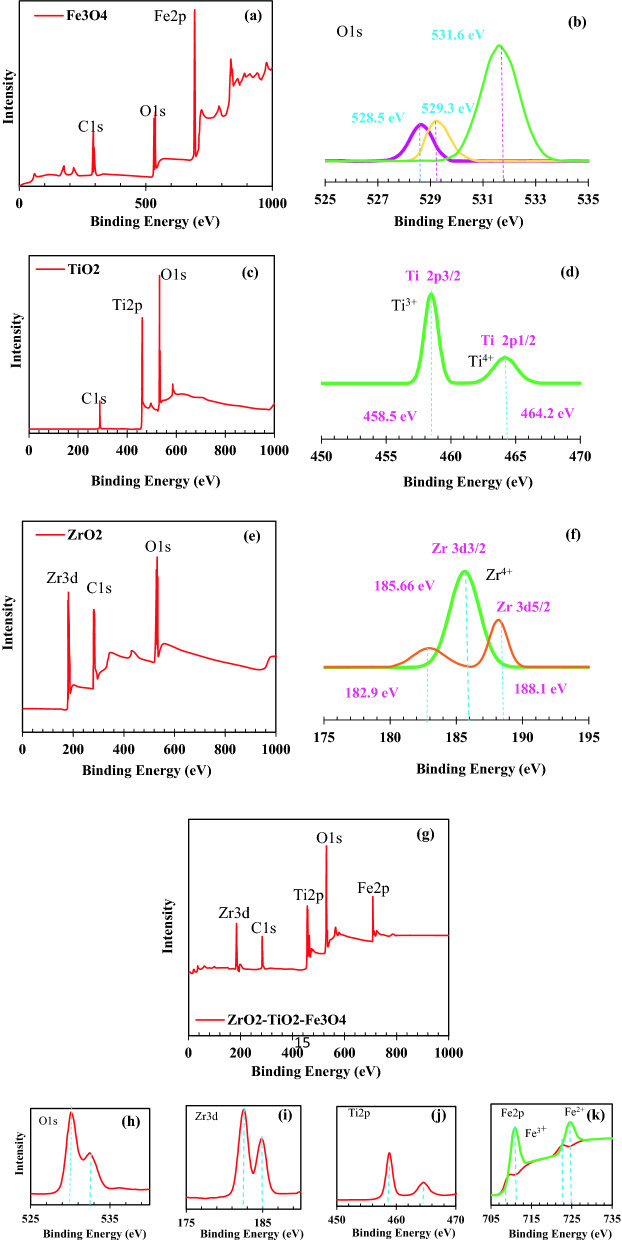
XPS analysis
For further investigation of the nature of the synthesized nanomaterial, we performed high-resolution X-ray photoelectron spectroscopy (XPS) on the synthesized ZrO2, TiO2 and Fe3O4 pure nanoparticles and the ZrO2/TiO2/Fe3O4 nanocomposite. The XPS spectra of pure and nanocomposite samples are shown in Fig. 6. Figure 6a and b show the XPS results of high-resolution Fe3O4 nanoparticles. Three components of the Fe–O bond can be seen in the XPS spectrum. After photoexcitation, the splitting of O1s at 531.6 eV indicates the formation of a Fe–O bond. The XPS peak of C1s at 292.6 eV and O1s at 531.6 eV indicate the formation of Fe–C and Fe–O bonds following particle photoexcitation (see Fig. 6a and b)88. Figure 6c and d show the XPS analysis of the pure TiO2 elemental composition. According to the XPS spectrum, elements Ti, C, and O are present on the surface of the TiO2 nanoparticles. Fe3O4 and TiO2 nanoparticle samples contained carbon resulting from carbon-based contaminants. The XPS peaks of Fig. 6c show C1s at 288.27 eV and O1s at 532.1 eV and Ti2p at 461.1 eV. Figure 6d shows the peaks of 458.5 eV and 464.2 eV, which correspond to Ti 2p3/2 and Ti 2p1/2, respectively. The peak position between Ti 2p3/2 and Ti 2p1/2 at 464.2 eV indicates the presence of Ti4+ oxidation state89,90. Figure 6e and f show the XPS spectrum of ZrO2 nanoparticles, which indicate the presence of relevant elements. The Zr3d level spectra of Fig. 6f show the Zr3d5/2 and Zr3d3/2 peaks at binding energies of 185.66 eV and 188.1 eV, respectively. The energy difference of 2.44 eV between the two peaks indicates the presence of Zr+491. The third suitable peak for the shoulder that appears at the base of Zr 3d3/2 can be attributed to the lack of oxygen, which can be due to under-coordinated Zr sites of very small ZrO2 nanoparticles92. XPS analysis was investigated to accurately determine the surface composition and chemical state of ZrO2/TiO2/Fe3O4 nanocomposite (As shown in Fig. 6g–k). As shown in Fig. 6g, the major peaks of Zr3d, C1s, Ti2p, O1s, and Fe2p are shown at 184.33, 282.59, 458.05, 530.18, and 708.37 eV, respectively. These results, in addition to confirming the presence of three nanoparticles ZrO2, Fe3O4 and TiO2, showed that ZrO2, Fe3O4 and TiO2 are mainly present as separate phases in the ZrO2/TiO2/Fe3O4 composite. Figure 6h shows that the binding energy of O1s appears at 530.08 eV, which proves the existence of oxygen in the crystal lattice (O2)93. In Fig. 6i, the dual peaks of Zr 3d with binding energies at 182.52 eV and 184.87 eV correspond to the chemical states Zr 3d3/2 and Zr 3d5/2, respectively, indicating zirconium in the + 4 oxidation state94. In addition, two peaks at 182.52 and 184.87 eV indicate the presence of Zr-Ti chemical bonds in the composites95. In Fig. 6j, the Ti 2p peaks at 458.84 eV and 464.58 eV correspond to the chemical states Ti 2p1/2 and Ti 2p3/2, respectively. Ti peaks confirm the presence of Ti4+ oxidation state in the nanocomposite. Meanwhile, the peak of 464.58 eV can be attributed to Zr-Ti chemical bonds96. The presence of chemical bonds Fe–O, Zr-O, Ti–O and Zr-Ti, Ti-Fe and Zr-Fe indicates a phase contact between Fe3O4, TiO2 and ZrO2. In Fig. 6k for Fe3O4, the major peaks at 710.96 eV and 724.59 eV are attributed to Fe3+ 2p3/2 and Fe3+ 2p1/2, respectively. The bond energy of Fe2+ 2p3/2 and Fe2+ 2p1/2 has a dual peak at 708.96 eV and 721.52 eV, respectively. Results of Fe3O4 XPS analysis are consistent with Fe2p spectrum97.
Figure 6.
XPS analysis of Nanoparticles (a) Fe3O4, (b) The O1s level spectrum, (c) TiO2, (d) The Ti 2p level spectrum, (e) ZrO2, (f) The Zr 3d level spectrum, and (g) Nanocomposite of ZrO2/TiO2/Fe3O4, XPS spectrum of (h) The O1s level (i) The Zr 3d level (j) The Ti 2p level and (k) The Fe 2p level.
EELS analysis
The electron energy-loss spectroscopy (EELS) analysis was used to identify the pure samples more accurately. The combination of EELS and statistical analysis can provide more information on the differentiation of Fe3O4 and Fe2O3 spinel structures. Figure 7a shows that the energy-loss peaks at 70.96 eV and 72.19 eV can only be attributed to Fe3O4 phase, since the expected value for gamma- Fe2O3 (70.2 eV) is significantly different. As shown in Fig. 7a, the EELS spectra of ZrO2 nanoparticles are obtained (obtained peaks for ZrO2 are 1.4, 14.8, 27.28 and 43.72 eV respectively). The synthesized ZrO2 nanoparticles can be proved according to the obtained peaks from the analysis. As shown in Fig. 7, the Ti structure in the EELS spectrum for Ti4+, indicates a lower oxidation state which has edges shift slightly towards the lower energy loss. Here, the blue curve is the fine structure of the Ti4+ state. EELS was obtained using a sub-nanometer probe size using the GIF2000 Filter.
Figure 7.
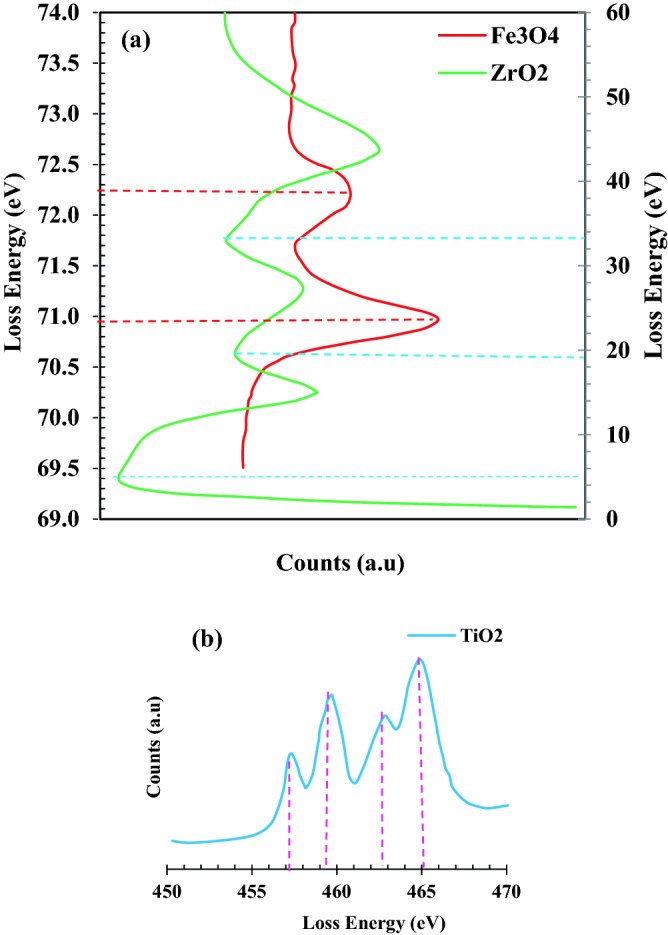
EELS spectrum for Structures of (a) Fe3O4, (b) ZrO2 and TiO2.
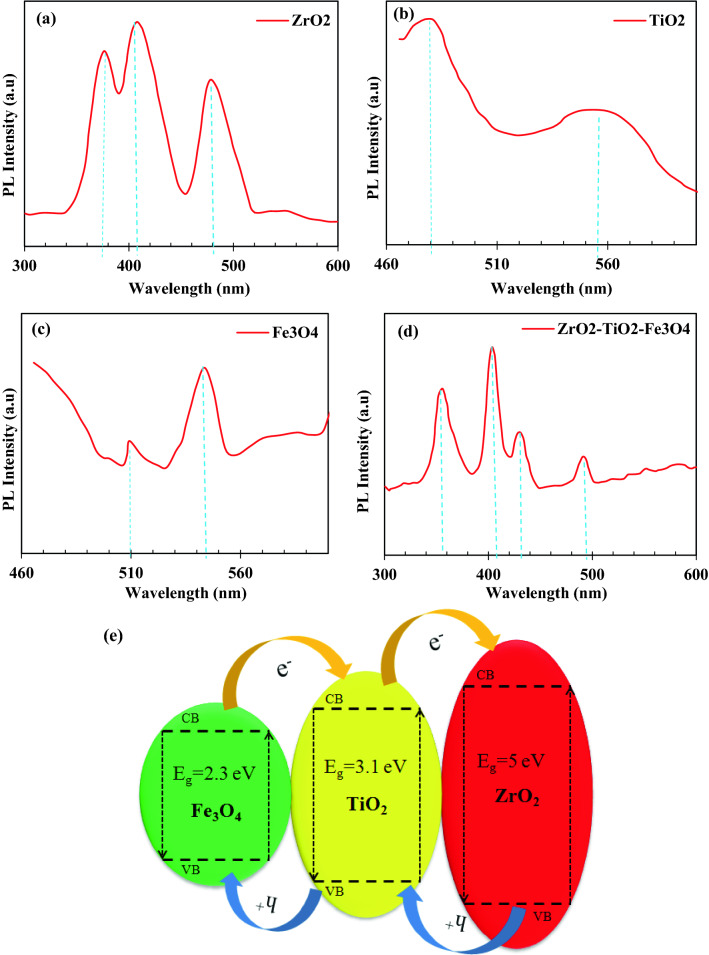
Photoluminescence spectra
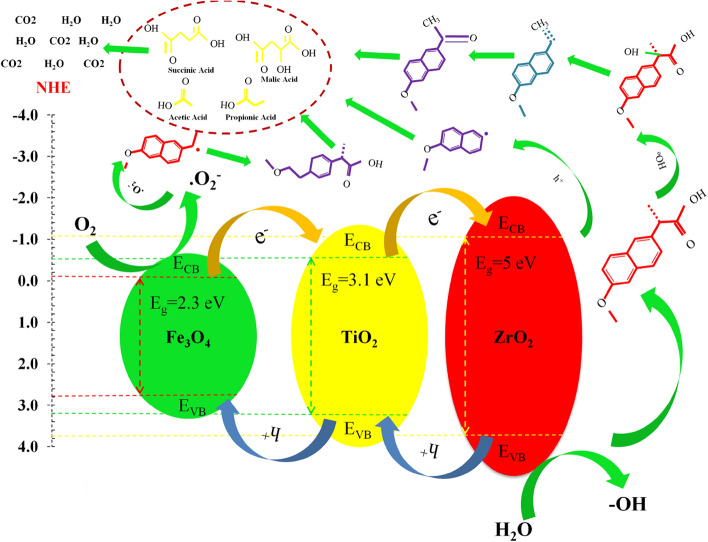
The light absorption of photocatalysts significantly affects the activity of photocatalysts. Also, the spectrum of PL (Photoluminescence) and their intensity are closely related to its photocatalytic activity. Figure 8a–d shows the photoluminescence spectra (PL) of pure ZrO2, TiO2 and Fe3O4 samples and the ZrO2/TiO2/Fe3O4 nanocomposite at room temperature in the wavelength range of 300–600 nm with an excitation wavelength of 293 nm. As shown in Fig. 8a–c the pure ZrO2, TiO2 and Fe3O4 samples show significant PL signals at 376, 407, 478, and 480, 556, 509 and 543 nm, respectively. Among all samples, the TiO2 sample shows the highest intensity. The obtained PL spectrum for pure ZrO2 nanoparticles is almost similar to the results previously reported98. According to previous research99–102, the presence of these peaks in the visible range is probably due to the presence of oxygen vacancies, defects, surface states and other structural impurities. A high PL intensity of pure ZrO2 appears to indicate that the ZrO2 surface states are much lower, hence easy electron transfer can occur between VB and CB of ZrO2 even with a low-energy laser excitation source (325 nm)103. Additionally, when Fe3O4 and TiO2 are loaded with ZrO2 as ternary oxide catalysts, the PL intensity of ZrO2 decreases abruptly (Fig. 8d). The formation of a chemical interaction between pure oxides when they accumulate together (–Ti–O–Fe–), (–Ti–O–Zr–) and (–Fe–O–Zr–), as shown, has a profound effect on the photocatalytic properties104. A PL spectrum of the physically mixed pure ZrO2, TiO2, and Fe3O4 catalysts in different ratios further confirms the synergistic effect of the ZrO2/TiO2/Fe3O4 ternary system and the presence of (–Ti–O–Fe–), (–Ti–O–Zr–) and (–Fe–O–Zr–). The peaks shown at 403, 427, and 490 nm under the excitation wavelength of 293 nm for the ZrO2/TiO2/Fe3O4 nanocomposite must be due to the interference of trap states between the gaps, such as surface defects and oxygen vacancies (Fig. 8d)105. Preparation for solid samples (powder) was performed for PL (Photoluminescence Spectroscopy) analysis. The PL Spectroscopy was recorded by Avaspec-2048-TEC model spectrometer. The valence band (VB) and conduction band (CB) are shown in Fig. 8e. In this figure, the photocatalyst energy bands of Fe3O4/TiO2/ZrO2 are shown in (CB) and (VB). The VB determines the energy levels of electrons in the VB of an atomic structure. The combination of three metal oxides can be improved electromagnetic separation and narrowing of the photocatalyst band gap. In Fe3O4/TiO2/ZrO2 semiconductor, electrons migrate to CB whereas; positive holes are created on VB. The OH is formed through the reaction of charge carriers with adsorbed compounds on the photocatalyst surface. In Fe3O4/TiO2/ZrO2 nanocomposite, the electron–hole separation may occur between ZrO2 and TiO2. The surface energy of Fe3O4 for VB and CB was placed in TiO2 band gap and surface energy of TiO2 was placed in ZrO2 band gap (Fig. 8e). In the excitation of electrons from three catalysts, the majority of electrons migrate from CB of ZrO2 to CB of TiO2, then to CB of Fe3O4. As a result, electron–hole recombination is prevented in ZrO2 and occurs in Fe3O4.
Figure 8.
Photoluminescence (PL) spectra of (a) ZrO2, (b) TiO2, (c) Fe3O4 nanoparticles and (d) ZrO2/TiO2/Fe3O4 nanocomposite, (e) Energy level and electron–hole pair separation/transfer in ZrO2/TiO2/Fe3O4 nanocomposite.
Photocatalyst performance
The experimental work was designed and the obtained actual results, as well as predicted values, were provided in Table 3. Box–Behnken design for RSM was used for the experimental design. Statistical investigation of experimental data was performed using linear, two-factor interaction, quadratic, and cubic models. The results were given in Table 4. According to Table 4 and R2 values, the quadratic model was found the most accurate model for the prediction of experimental data in terms of naproxen removal. Its predicted R2 value was found to be 0.9901 which means the developed model equation is able to predict the results accurately. Therefore, the following quadratic polynomial model in terms of coded factors was proposed for investigation of naproxen degradation from pharmaceutical synthetic solution as a function of operating parameters:
| 4 |
where A, B, C, D are photocatalyst dose (g/L), time (min), solution initial pH, and initial concentration of naproxen (mg/L). The variables were defined at three levels including − 1, 0, and + 1.
Table 3.
Operational parameters range in the designed experiments and actual and predicted results in terms of naproxen removal.
| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response | |
|---|---|---|---|---|---|---|
| A:NPX concentration | B:Time | C:pH | D:catalyst concentration | Naproxen Removal | ||
| Actual Value | Predicted Value | |||||
| mg/L | min | – | g/L | % | % | |
| 1 | 10 | 90 | 3 | 0.1 | 87.3 | 87.6 |
| 2 | 10 | 90 | 9 | 0.5 | 96.8 | 97.2 |
| 3 | 30 | 90 | 9 | 0.1 | 72.1 | 72.8 |
| 4 | 10 | 60 | 6 | 0.3 | 92.3 | 91.8 |
| 5 | 20 | 60 | 3 | 0.3 | 88.8 | 89.1 |
| 6 | 20 | 60 | 6 | 0.3 | 89.8 | 89.9 |
| 7 | 20 | 60 | 6 | 0.3 | 90.5 | 89.9 |
| 8 | 20 | 60 | 6 | 0.3 | 89 | 89.9 |
| 9 | 30 | 90 | 3 | 0.5 | 95.4 | 95.3 |
| 10 | 20 | 60 | 6 | 0.1 | 86.8 | 86.1 |
| 11 | 30 | 30 | 9 | 0.5 | 79.2 | 79.2 |
| 12 | 10 | 30 | 9 | 0.5 | 88.9 | 88.9 |
| 13 | 10 | 30 | 3 | 0.5 | 93.5 | 93.1 |
| 14 | 10 | 90 | 3 | 0.5 | 100 | 100 |
| 15 | 20 | 60 | 6 | 0.3 | 89.6 | 89.9 |
| 16 | 30 | 30 | 9 | 0.1 | 66.1 | 65.8 |
| 17 | 30 | 30 | 3 | 0.1 | 73.8 | 73.8 |
| 18 | 10 | 30 | 9 | 0.1 | 77.5 | 77.8 |
| 19 | 30 | 90 | 3 | 0.1 | 79.9 | 79.7 |
| 20 | 30 | 30 | 3 | 0.5 | 89.6 | 89.5 |
| 21 | 30 | 60 | 6 | 0.3 | 82.8 | 83.0 |
| 22 | 10 | 30 | 3 | 0.1 | 80.5 | 80.5 |
| 23 | 10 | 90 | 9 | 0.1 | 86.5 | 86.3 |
| 24 | 20 | 60 | 6 | 0.3 | 90.2 | 89.9 |
| 25 | 20 | 60 | 6 | 0.3 | 89.3 | 89.9 |
| 26 | 30 | 90 | 9 | 0.5 | 86.3 | 86.1 |
| 27 | 20 | 30 | 6 | 0.3 | 83.8 | 84.1 |
| 28 | 20 | 60 | 9 | 0.3 | 83.8 | 83.2 |
| 29 | 20 | 60 | 6 | 0.5 | 99.8 | 100 |
| 30 | 20 | 90 | 6 | 0.3 | 92.1 | 91.4 |
Significant values are in bold.
Table 4.
Statistical investigation based on linear, two-factor interaction, quadratic, and cubic models.
| Source | Sequential p-value |
Lack of fit p-value |
Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|---|
| Linear | < 0.0001 | 0.0002 | 0.8158 | 0.7680 | |
| 2FI | 0.6041 | 0.0002 | 0.8050 | 0.5781 | |
| Quadratic | < 0.0001 | 0.5274 | 0.9952 | 0.9901 | Suggested |
| Cubic | 0.5874 | 0.3447 | 0.9948 | 0.9248 | Aliased |
Significant values are in bold.
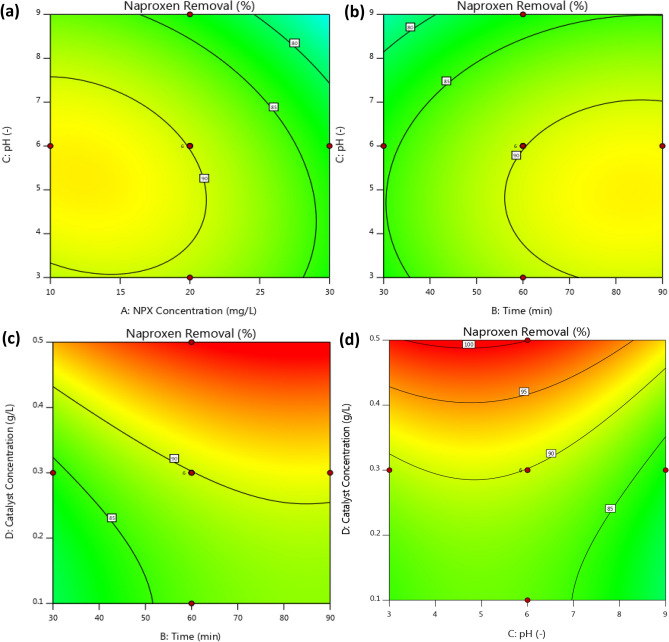
Figure 9 illustrates the individual effects of operational parameters on naproxen removal. The contour lines represent lines of equal response and can be visualized as response contours two factors at a time. In this study, contour lines mean the performance of photocatalyst for naproxen removal at different operating parameters. The contour map reflects the cross-interaction between two variables by keeping the other variable constant. Interaction of initial pH and naproxen concentration for photocatalyst dose of 0.3 g/L and time of 60 min was shown in Fig. 9a. At a pH value of 6, increasing the initial concentration of naproxen from 10 to 30 mg/L led to the reduction of naproxen removal from 91.81 to 82.9%. Naproxen removal is reduced due to solution turbidity and a decrease in light absorption by the photocatalyst. Also, the amount of hydroxyl radical in the solution decreases. Furthermore, the photocatalyst surface is covered by pollutants with increasing its concentration from 10 to 30 mg/L which decreases photon penetration into the photocatalyst. Therefore, the photocatalyst was unable to generate enough electron–hole pairs, resulting in a reduced removal of naproxen. In the interaction between time and initial pH of the solution (Fig. 9b), the photocatalyst dosage and initial concentration of naproxen were considered 0.3 g/L and 20 mg/L respectively. Firstly, the efficiency of naproxen degradation was increased from 90.10 to 91.34% with increasing pH of the solution from 3 to 6, then, it was decreased to 84.9% at a pH value of 9. These changes are related to the oxidation potential and surface charge of the photocatalyst. The pH of the media can have a significant impact on the adsorption and desorption of pollutants on the photocatalyst surface.
Figure 9.
The 2D contour plots of RSM for investigation the degradation percentage of naproxen.
The amount of pKa is 4.15 for the naproxen. At pH values higher than 4.15, naproxen has a negative charge, otherwise, it has a positive charge. The neutral charge point of photocatalyst is 6. Therefore, it has a positive charge at pH below 6, whereas the surface charge of photocatalyst is negative at pH above 6. When the pH value is between 4.15 and 6, the naproxen and photocatalyst have opposite charges, so the adsorption of naproxen on the photocatalyst surface increases in this range. It is for this reason that maximum degradation occurs at pH 6 rather than pH 3 or 9. When the pH solution was 6, an increase in time from 30 to 60 min increased naproxen removal from 84.10 to 91.34%. The time and photocatalyst dosage interaction at constant initial pH and concentration of naproxen of 6 and 20 mg/L was shown in Fig. 9c. In Fig. 9c, and for a 60-min experiment run, there was an increase in naproxen degradation from 86.2 to 100% when the photocatalyst dosage was increased from 0.1 to 0.5 g/L. Photocatalyst dosage had a significant effect on naproxen degradation, increasing the number of available active sites for the generation of hydroxyl radicals. However, too much photocatalyst in the system can reduce naproxen degradation as a result of agglomeration of photocatalyst nanoparticles. The reduction in active site of the photocatalyst resulted from agglomeration. It was found that a decrease in pH can increase naproxen degradation (Fig. 9d), and the greatest degradation was obtained at pH 3. The solution pH had a complicated effect on the photocatalytic oxidation reaction. The optimum pH value is highly dependent on the type of pollutant and point of zero charges (PZC) of the photocatalyst. According to Fig. 10, it is 6 for the synthesized photocatalyst. At pH = pHPZC, the surface charge is neutral. The surface charge can be positive or negative at pH < pHPZC and pH > pHPZC. The amount of adsorption is highly dependent on the surface charge of photocatalyst and pollutant and it can be controlled by a change in solution pH.
Figure 10.
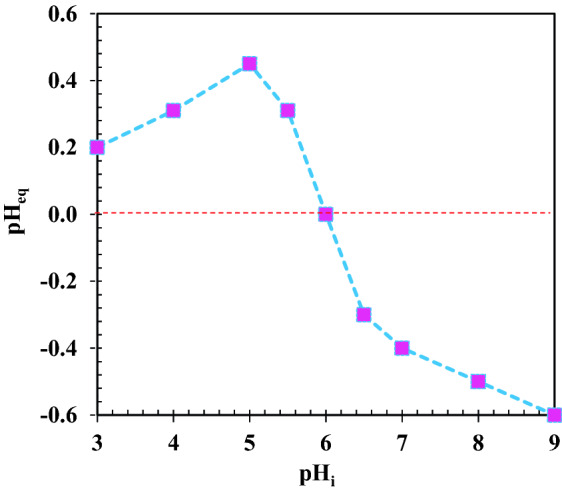
Point of zero charge ZrO2/TiO2/Fe3O4 photocatalyst.
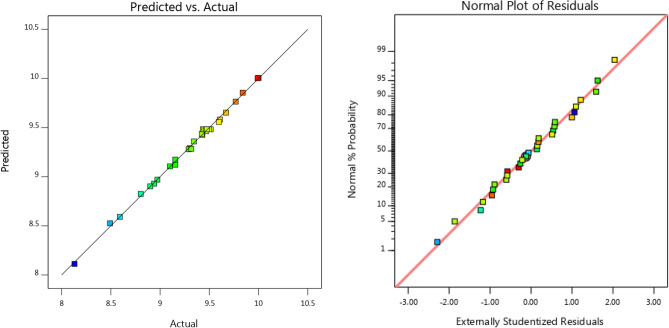
The analysis of variance (ANOVA) results obtained for the present model are summarized in Table 5. The results suggested that a quadratic model is significant because of its high F-value (431.34) and very low p-value (< 0.001). The calculated higher F-value and lower p-value for the photocatalyst dosage (D factor) in comparison with other factors indicated that the photocatalyst dosage is the most important parameter in the system. The results in Table 5 showed that the proposed statistical model is accurately fitted to experimental data. The accuracy of the proposed model should be investigated in detail. Figure 11 shows a linear relationship between experimental data and predicted values in terms of naproxen removal and it confirms the accuracy of the proposed model as all the data accumulated around a 45-degree line. The normal probability plot of the residuals was shown in Fig. 11b and it can be clearly seen a linear scattering of modeling data. The linear scattering means normal distribution of errors in a defined matrix for experimental design. Therefore, the probability of random error intervention and effect of the sequence of experiments is considerably decreased in the proposed model.
Table 5.
ANOVA results for the quadratic model.
| Source | Sum of Squares | df | Mean Square | F-value | p-value | |
|---|---|---|---|---|---|---|
| Model | 5.28 | 14 | 0.3770 | 431.34 | < 0.0001 | Significant |
| A-NPX Concentration | 1.02 | 1 | 1.02 | 1170.41 | < 0.0001 | |
| B-Time | 0.6622 | 1 | 0.6622 | 757.51 | < 0.0001 | |
| C-pH | 0.4464 | 1 | 0.4464 | 510.73 | < 0.0001 | |
| D-Catalyst Concentration | 2.32 | 1 | 2.32 | 2654.05 | < 0.0001 | |
| AB | 0.0027 | 1 | 0.0027 | 3.09 | 0.0990 | |
| AC | 0.1119 | 1 | 0.1119 | 128.00 | < 0.0001 | |
| AD | 0.0355 | 1 | 0.0355 | 40.67 | < 0.0001 | |
| BC | 0.0059 | 1 | 0.0059 | 6.75 | 0.0202 | |
| BD | 0.0013 | 1 | 0.0013 | 1.50 | 0.2391 | |
| CD | 0.0067 | 1 | 0.0067 | 7.61 | 0.0146 | |
| A2 | 0.0500 | 1 | 0.0500 | 57.22 | < 0.0001 | |
| B2 | 0.0353 | 1 | 0.0353 | 40.41 | < 0.0001 | |
| C2 | 0.1073 | 1 | 0.1073 | 122.76 | < 0.0001 | |
| D2 | 0.0672 | 1 | 0.0672 | 76.84 | < 0.0001 | |
| Residual | 0.0131 | 15 | 0.0009 | |||
| Lack of Fit | 0.0088 | 10 | 0.0009 | 1.02 | 0.5274 | Not significant |
| Pure Error | 0.0043 | 5 | 0.0009 | |||
| Cor Total | 5.29 | 29 |
Figure 11.
(a) The relationship of predicted and actual values of the RSM model for naproxen removal; (b) the externally studentized residuals versus normal % probability distribution.
Optimization using RSM
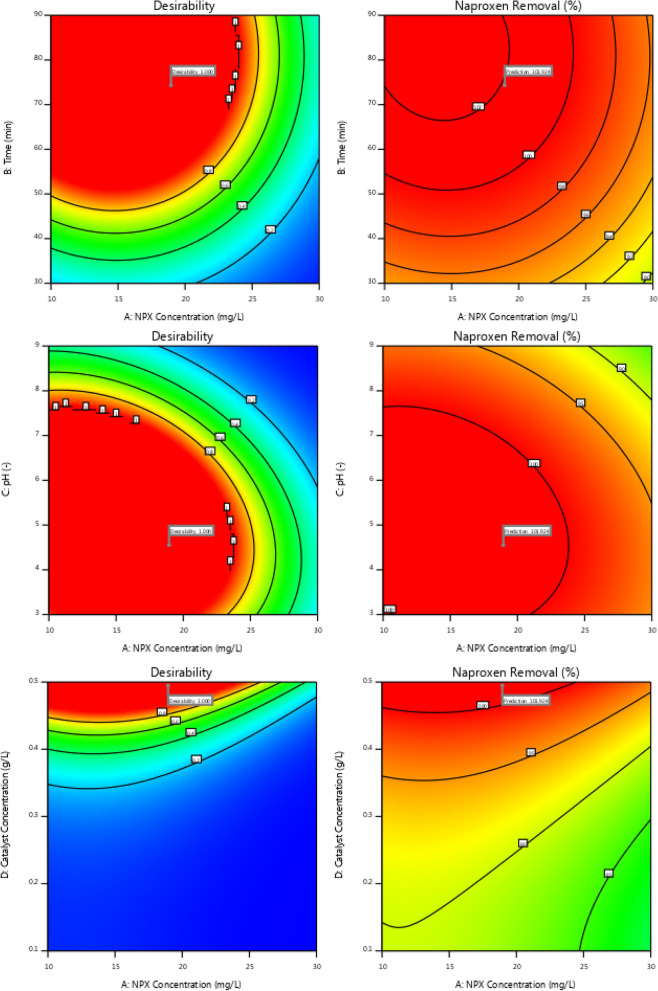
An optimization was performed in order to find the optimal operating conditions for the removal of naproxen completely from pharmaceutical synthetic solutions. According to the proposed model, the following independent operating parameters are required for complete removal of naproxen (101.92%): initial naproxen concentration = 18.95 mg/L, initial pH value = 4.55, photocatalyst dosage = 0.49 g/L, time = 74.31 min. Figure 12 illustrates different operating conditions that can be used to achieve complete degradation of naproxen.
Figure 12.
A range of independent variables of pH, photocatalyst dosage, time, and initial naproxen concentration.
Reusability ZrO2/TiO2/Fe3O4 photocatalyst
The regeneration and re-use of the used or spent photocatalyst is very critical for determining the applicability of the process applied. The used photocatalyst was separated from the synthetic solution using 1.3 Tesla magnetic. Then, it was used for the treatment of pharmaceutical synthetic solution as given in Fig. 13. As can be seen in Fig. 13, the degradation efficiency only display partial reduction after seven cycles for degradation of naproxen, indicating excellent reusability of the synthesized photocatalyst as after 7 runs, its performance for naproxen degradation was decreased only 12%. Additionally, the photocatalyst was placed in distilled water under UV light for 12 h in order to regenerate it. After doing the regeneration process, the photocatalyst was able to degrade the naproxen by about 98.4%. However, after three regenerations, the activity of the photocatalyst decreased by 8.4%. The decrease in performance of photocatalyst may be related to the blocking of active pores due to precipitation of non-sensitive chemicals to light or destroying of effective nanoparticles during the recovery process. Each experiment was replicated three times and an error bar was shown in Fig. 13.
Figure 13.
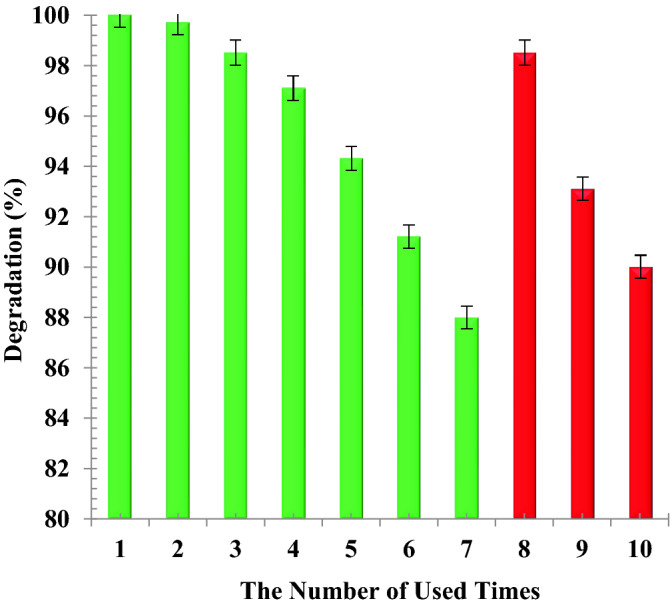
Reusability and regeneration of ZrO2/TiO2/Fe3O4 photocatalyst for naproxen degradation.
Analysis study of naproxen removal kinetics
The removal of naproxen using heterogeneous photocatalyst is divided into two main stages: physical adsorption reaction and chemical reaction. In order to describe the photocatalytic degradation rate of naproxen by plotting ln(C0/C) versus time (t), at different concentrations, the Langmuir–Hinshelwood (LH) kinetic model was used106:
| 5 |
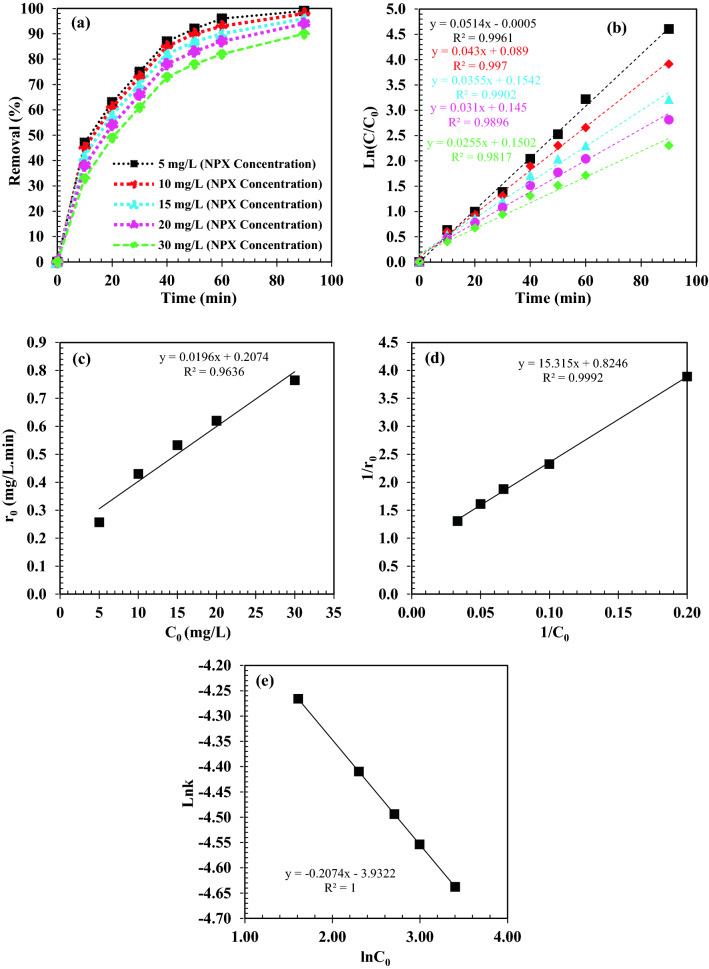
where Ci(mg/L), the initial concentration of naproxen and C(mg/L), the concentration at time (t) after irradiation, and k is a pseudo-first-order rate constant. As shown in Fig. 14, the pseudo-first-order rate constant was determined based on a straight-line slope. According to the results of Fig. 14b, the maximum reaction rate at the initial concentration of 5 mg/L naproxen is 0.0514 min−1, which is about 2 times higher than the obtained reaction rate at the initial concentration of 30 mg/L naproxen. The obtained results in Fig. 14b show that reaction rates decrease with increasing concentration. The naproxen absorbed amount qe (mg/g) by ZrO2/TiO2/Fe3O4 nanocomposite was calculated using the following equation (Eq. (6)):
| 6 |
where Ci and Ce are the initial and equilibrium concentrations of naproxen (mg/L), respectively. The volume of naproxen solution (ml) is V and the mass of ZrO2/TiO2/Fe3O4 nanocomposite (mg) is m. The following equation was used to calculate the percentage of naproxen adsorption (%) (Eq. 7):
| 7 |
Figure 14.
(a) The effect of initial concentrations on NPX removal(%), (b) The effect of initial concentrations of NPX on photo degradation, (c) The initial reaction rate (r0) as a function of NPX initial concentration (C0); (d) L–H model of photocatalytic NPX degradation by ZrO2/TiO2/Fe3O4 ; (e) The effect of different initial concentrations on the NPX photo degradation rate constant.
As shown in Fig. 14 when the initial concentration of naproxen was increased from 5 to 30 mg/L, the initial photodegradation rate (r0) also gradually increased from 0.26 to 0.77 mg/L. It was shown that naproxen photocatalytic degradation occurs on ZrO2/TiO2/Fe3O4 nanocomposite surfaces and that the rate of photo degradation increases as a function of increasing of adsorption. As the initial concentration of naproxen increases, the coverage of naproxen molecules on the surface of ZrO2/TiO2/Fe3O4 nanocomposite also increases accordingly. As a result, the electron transfer efficiency of naproxen molecules to the adsorbed surface and the charge produced by the light increases, which leads to an increase in the initial optical decomposition rate (r0). As a result, the electron transfer efficiency of naproxen molecules, which leads to an increase in the initial photodegradation rate (r0), increases with increasing absorbed surface and the produced charge by light. For different initial concentrations, the kinetic constant of naproxen photo degradation gradually decreased (from 0.0514 to 0.0255 min−1), while the R2 correlation coefficient also decreased from 0.9961 to 0.9817. The relationship between the reaction rate (k) and the initial concentration of the substrate during the photocatalytic process can be generally explained by the following experimental formula (Eq. 8):
| 8 |
| 9 |
where n is the correlation index and [NPX] is the initial concentration (NPX) (mg.L−1).
Linear regression was used to analyze the relationship between the kinetic constant of NPX photo degradation and its initial concentration (5–30 mg/L). Figure 14 shows that the relationship between reaction rate k and NPX concentration is as follows:
| 10 |
The results showed that the adsorption of naproxen on the surface of ZrO2/TiO2/Fe3O4 nanoparticles is clearly time-dependent. As shown in Fig. 14a most naproxen removal occurred within the initial 50 min, and after 50 min, naproxen removals occurred almost in the flat part of the graph. The highest percentage of naproxen removal is at the initial concentration of 5 mg/L of solution. In the initial 50 min, two mechanisms were involved in the removal of naproxen: the free active sites at the adsorbent surface, and the generation of hydroxyl ions. Subsequently, a slow rate of naproxen removal was observed.
Different kinetic models were investigated to analyze the kinetic data and determine the kinetic mechanism of naproxen adsorption on the surface of ZrO2/TiO2/Fe3O4 nanocomposite which is shown in Table 6.
Table 6.
Equations of adsorption kinetic models for naproxen adsorption on ZrO2/TiO2/Fe3O4 nanocomposite.
| Model | Equation | Parameter | References | |
|---|---|---|---|---|
| Elovich | qt = (1/β)(Ln(αβ)) + (1/β)Ln(t) | R2 | 0.9202 | 107–109 |
| α | 67.378 | |||
| β | 0.313 | |||
| Weber and Moris | qt = C + Kint(t)1/2 | R2 | 0.9377 | 107–110 |
| Kint | 1.984 | |||
| C | 10.57 | |||
| Pseudo 2nd order (McKay-Ho) | (t/qt) = 1/(K2.qe2) + (1/qe).t | R2 | 0.9814 | 111,112 |
| K2 | 0.013 | |||
| Calculated qe | 26.4 | |||
| Experimental qe | 25.7 | |||
| Pseudo 1st order (Lagergeren) | Log(qe − qt) = Log qe − (K1/2.303).t | R2 | 0.9891 | 111,113 |
| K1 | 0.085 | |||
| Calculated qe | 17.12 | |||
qe: the amount of adsorbed NPX by ZrO2/TiO2/Fe3O4 nanocomposite (mg/g) [at equilibrium].
qt: the amount of adsorbed NPX by ZrO2/TiO2/Fe3O4 nanocomposite (mg/g) [at determined time interval t].
K1: rate constant of pseudo 1st order adsorption process (min−1).
K2: the rate constant of pseudo 2nd order adsorption process (g mg−1 min−1).
α: the initial adsorption rate constant (mg/g min).
β: constant related to surface coverage and the activation energy for chemisorptions (g/mg).
Kint: the intraparticle rate constant (mg/g min1/2).
C: value about the boundary thickness.
Elovich model shows less linearity in the regression coefficient R2; which was found to be 0.9202, On the other hand, the correlation coefficient value for a Langmuir–Hinshelwood (LH) kinetic model was obtained 0.9992 for NPX and greater than all other adsorption kinetic models. The calculated qe value was found to be 26.4 mg/g for NPX, which was very close to the obtained experimental values (shown in Table 6). According to the obtained results, it is expected that NPX and its degradation intermediates may be further degraded by reactive species, thus leading to ring openings and eventually oxidation to CO2 and H2O. As can be seen in Fig. 15, the possible degradation pathways of NPX are proposed based on the identification of intermediates and mineralization results. The photocatalytic degradation of NPX by ZrO2/TiO2/Fe3O4 mainly refers to three main pathways including decarboxylation and hydroxylation39. Three possible degradation pathways through oxidation processes are shown in Fig. 15. In the oxidation pathway I, NPX degradation was initiated by the electrophilic additive interaction between the naphthalene ring of NPX and ·OH114–116. In pathways II and III, NPX oxidation was performed by h+ and ·O2− and carbon-based radical species were formed by decarboxylation117. All produced intermediates during the process were decomposed by ring-opening reactions to malic acid, succinic acid, propionic acid and acetic acid, and finally mineralized to CO2 and H2O39,118,119.
Figure 15.
Photo degradation reaction mechanism of NPX by ZrO2/TiO2/Fe3O4.
In order to confirm mineralization, we investigated total organic carbon (TOC) in the degradation of naproxen at ambient temperature and pH = 3. The amount of TOC was decreased with increasing irradiation time (Fig. 16). In this study, the mineralization of naproxen is confirmed using ZrO2/TiO2/Fe3O4 photocatalysts. TOC removal was obtained 87% after 90 min irradiation. Naproxen may be oxidized to CO2, H2O, and some small molecules, according to TOC results.
Figure 16.
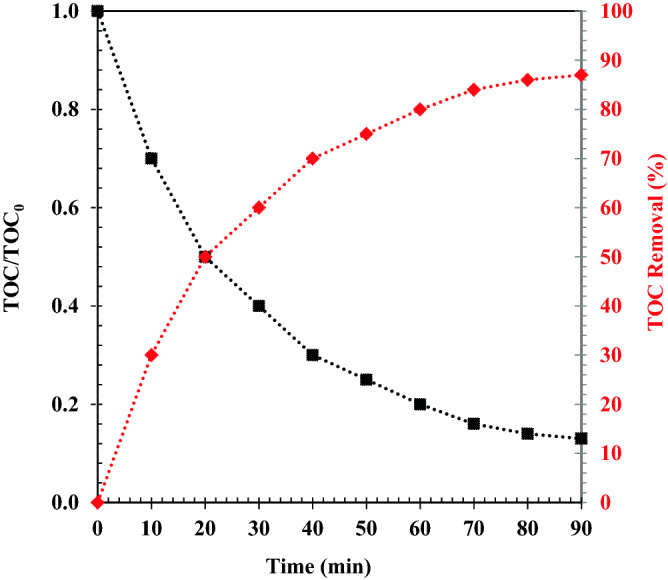
Total organic carbon (TOC) in naproxen wastewater as a function of time and the ZrO2/TiO2/Fe3O4 photocatalytic activity.
As shown in Table 7, in comparison with previous studies, the synthesized photocatalyst in this study had the highest naproxen removal efficiency. The synthesis of ternary nanocomposites confirmed the excellent synergistic effect of these three types of nanoparticles on the photocatalytic process. Based on the Table 7, the synthesized photocatalyst has high specific surface area. In addition, it’s proper capability in acidic environment increases efficiency and absorption over time for the removal of naproxen at low dosage of catalyst in comparison with other similar synthesized photo catalysts.
Table 7.
Comparison of naproxen removal efficiency in the presence of synthesized photocatalyst under optimum process conditions.
| Type of photocatalyst | Surface areas (m2 g−1) | NPX concentration (mg/L) | Irradiation time (min) | Catalyst concentration (g/L) | pH | Degradation efficiency (%) | Average pore volume (cm3 g−1) | References |
|---|---|---|---|---|---|---|---|---|
| ZrO2/TiO2/Fe3O4 | 280 | 10 | 90 | 0.5 | 3 | 100 | 0.92 | Present work |
| TiO2 | 55 | 0.184 | 120 | 0.1 | 6.15 | 40 | – | 120 |
| MoS2–CeO2–ZrO2 | 39.45 | 11.51 | 40 | 0.5 | 5.8 | 21 | – | 121 |
| P25–TiO2/TEOS | – | 5 | 600 | 0.003 | 6 | 94 | – | 122 |
| HTNM | 53.67 | 0.5 | 180 | 1.5 | 7 | 99.9 | 0.32 | 39 |
| ZnO | – | 40 | 120 | 0.5 | 7 | 98.7 | – | 123 |
| Fe3O4/MWCNTs | 144 | 10 | – | 0.4 | 7 | 83 | 0.24 | 124 |
| N-doped TiO2/SiO2/Fe3O4 | 232.41 | 9.33 | 217.08 | 0.06 | 4.29 | 96.32 | – | 125 |
Conclusion
In summary, we synthesized a ternary ZrO2/TiO2/Fe3O4 nanocomposite system using a solid-state dispersion method. Furthermore, its photocatalytic activity towards naproxen degradation was investigated. The effect of operating parameters including initial naproxen concentration, initial pH, photocatalyst dosage, and time on the degradation of naproxen was investigated. Characterization results confirmed the formation of the ternary nanocomposite. The existence of cylindrical and spherical pores in the sample was proved because its N2 adsorption–desorption hysteresis followed IV-H1 type hysteresis. Based on optimization results using the RSM method, the optimal conditions for the complete removal of naproxen were determined as initial naproxen concentration of 18.95 mg/L, initial pH of 4.55, photocatalyst dosage of 0.49 g/L, and time of 74.31 min. Reusability results showed 12% reduction in naproxen degradation after 7 runs. It was found that Langmuir–Hinshelwood's (LH) kinetic model was linear. The regression coefficient (R2) was obtained 0.9992 and it was greater than all other adsorption kinetic models.
Author contributions
M.H.Z.: conceptualization, methodology, experimental, resources, reviewing and editing, original draft preparation. A.M.-Z.: conceptualization, methodology, experimental, resources, supervision, reviewing and editing.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lumbaque EC, et al. Degradation of pharmaceuticals in wastewater matrices through solar light-driven photocatalyst prepared from petrochemical waste. Environ. Sci. Pollut. Res. 2021;28(19):24124–24137. doi: 10.1007/s11356-020-12142-8. [DOI] [PubMed] [Google Scholar]
- 2.Gao J, et al. Using prescription and wastewater data to estimate the correction factors of atenolol, carbamazepine, and naproxen for wastewater-based epidemiology applications. Environ. Sci. Technol. 2021;55(11):7551–7560. doi: 10.1021/acs.est.1c00931. [DOI] [PubMed] [Google Scholar]
- 3.Petrovic M, Radjenovic J, Barcelo D. Advanced oxidation processes (AOPs) applied for wastewater and drinking water treatment. Elimination of pharmaceuticals. Holist. Approach Environ. 2011;1(2):63–74. [Google Scholar]
- 4.Liu Z-H, Kanjo Y, Mizutani S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—Physical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009;407(2):731–748. doi: 10.1016/j.scitotenv.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Wang S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manage. 2016;182:620–640. doi: 10.1016/j.jenvman.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Mojiri A, et al. Removal performance and optimisation of pharmaceutical micropollutants from synthetic domestic wastewater by hybrid treatment. J. Contam. Hydrol. 2020;235:103736. doi: 10.1016/j.jconhyd.2020.103736. [DOI] [PubMed] [Google Scholar]
- 7.Khan AH, et al. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020;269:122411. doi: 10.1016/j.jclepro.2020.122411. [DOI] [Google Scholar]
- 8.Kilic MY, et al. Photochemical treatment of tyrosol, a model phenolic compound present in olive mill wastewater, by hydroxyl and sulfate radical-based advanced oxidation processes (AOPs) J. Hazard. Mater. 2019;367:734–742. doi: 10.1016/j.jhazmat.2018.06.062. [DOI] [PubMed] [Google Scholar]
- 9.Biń AK, Sobera-Madej S. Comparison of the advanced oxidation processes (UV, UV/H2O2 and O3) for the removal of antibiotic substances during wastewater treatment. Ozone Sci. Eng. 2012;34(2):136–139. doi: 10.1080/01919512.2012.650130. [DOI] [Google Scholar]
- 10.Zhang Q, et al. Efficient degradation of typical pharmaceuticals in water using a novel TiO2/ONLH nano-photocatalyst under natural sunlight. J. Hazard. Mater. 2021;403:123582. doi: 10.1016/j.jhazmat.2020.123582. [DOI] [PubMed] [Google Scholar]
- 11.Li C, et al. Degradation characteristics of four major pollutants in chemical pharmaceutical wastewater by Fenton process. J. Environ. Chem. Eng. 2021;9(1):104564. doi: 10.1016/j.jece.2020.104564. [DOI] [Google Scholar]
- 12.Feng L, et al. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013;228:944–964. doi: 10.1016/j.cej.2013.05.061. [DOI] [Google Scholar]
- 13.Peng G, et al. Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem. Eng. J. 2018;341:361–370. doi: 10.1016/j.cej.2018.02.064. [DOI] [Google Scholar]
- 14.Adityosulindro S, et al. Sonolysis and sono-Fenton oxidation for removal of ibuprofen in (waste) water. Ultrason. Sonochem. 2017;39:889–896. doi: 10.1016/j.ultsonch.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y, Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015;1(3):167–176. doi: 10.1007/s40726-015-0015-z. [DOI] [Google Scholar]
- 16.Giannakoudakis DA, et al. Boosting the photoactivity of grafted titania: Ultrasound-driven synthesis of a multi-phase heterogeneous nano-architected photocatalyst. Adv. Func. Mater. 2021;31(1):2007115. doi: 10.1002/adfm.202007115. [DOI] [Google Scholar]
- 17.Chen W, et al. Direct Z-scheme 2D/2D MnIn2S4/g-C3N4 architectures with highly efficient photocatalytic activities towards treatment of pharmaceutical wastewater and hydrogen evolution. Chem. Eng. J. 2019;359:244–253. doi: 10.1016/j.cej.2018.11.141. [DOI] [Google Scholar]
- 18.Vaiano V, et al. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015;261:3–8. doi: 10.1016/j.cej.2014.02.071. [DOI] [Google Scholar]
- 19.Elmolla ES, Chaudhuri M. The feasibility of using combined TiO2 photocatalysis-SBR process for antibiotic wastewater treatment. Desalination. 2011;272(1):218–224. doi: 10.1016/j.desal.2011.01.020. [DOI] [Google Scholar]
- 20.Han F, et al. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A. 2009;359(1):25–40. doi: 10.1016/j.apcata.2009.02.043. [DOI] [Google Scholar]
- 21.Da Dalt S, Alves AK, Bergmann CP. Photocatalytic degradation of methyl orange dye in water solutions in the presence of MWCNT/TiO2 composites. Mater. Res. Bull. 2013;48(5):1845–1850. doi: 10.1016/j.materresbull.2013.01.022. [DOI] [Google Scholar]
- 22.Luttrell T, et al. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2014;4:1–8. doi: 10.1038/srep04043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matos J, et al. Influence of anatase and rutile phase in TiO2 upon the photocatalytic degradation of methylene blue under solar irradiation in presence of activated carbon. Water Sci. Technol.: J. Int. Assoc. Water Pollut. Res. 2014;69(11):2184–2190. doi: 10.2166/wst.2014.127. [DOI] [PubMed] [Google Scholar]
- 24.Xu N, et al. Effects of particle size of TiO2 on photocatalytic degradation of methylene blue in aqueous suspensions. Ind. Eng. Chem. Res. 1999;38:373–379. doi: 10.1021/ie980378u. [DOI] [Google Scholar]
- 25.Shan AY, Ghazi TIM, Rashid SA. Immobilisation of titanium dioxide onto supporting materials in heterogeneous photocatalysis: A review. Appl. Catal. A. 2010;389(1):1–8. doi: 10.1016/j.apcata.2010.08.053. [DOI] [Google Scholar]
- 26.Xu Z, et al. Structure, luminescence properties and photocatalytic activity of europium doped-TiO2 nanoparticles. J. Mater. Sci. 2005;40(6):1539–1541. doi: 10.1007/s10853-005-0599-6. [DOI] [Google Scholar]
- 27.McManamon C, Holmes JD, Morris MA. Improved photocatalytic degradation rates of phenol achieved using novel porous ZrO2-doped TiO2 nanoparticulate powders. J. Hazard. Mater. 2011;193:120–127. doi: 10.1016/j.jhazmat.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval S, et al. Europium-doped TiO2 hollow nanoshells: Two-photon imaging of cell binding. Chem. Mater. 2012;24(21):4222–4230. doi: 10.1021/cm302642g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, et al. ZrO2-modified mesoporous nanocrystalline TiO2–xNx as efficient visible light photocatalysts. Environ. Sci. Technol. 2006;40(7):2369–2374. doi: 10.1021/es052000a. [DOI] [PubMed] [Google Scholar]
- 30.Jung KY, Park SB. Photoactivity of SiO2/TiO2 and ZrO2/TiO2 mixed oxides prepared by sol–gel method. Mater. Lett. 2004;58(22):2897–2900. doi: 10.1016/j.matlet.2004.05.015. [DOI] [Google Scholar]
- 31.Fu X, et al. Enhanced photocatalytic performance of ittania-based binary metal oxides: TiO2/SiO2 and TiO2/ZrO2. Environ. Sci. Technol. 1996;30(2):647–653. doi: 10.1021/es950391v. [DOI] [Google Scholar]
- 32.Rao Y, et al. Photocatalytic activity of G-TiO2@Fe3O4 with persulfate for degradation of alizarin red S under visible light. Chemosphere. 2021;266:129236. doi: 10.1016/j.chemosphere.2020.129236. [DOI] [PubMed] [Google Scholar]
- 33.Izadpanah M, et al. Aquatic center sewage reclamation and water reuse, using an integrated system combining adsorption, RO membrane system, and TiO2/Fe3O4 photocatalytic oxidation. J. Environ. Chem. Eng. 2021;9(1):104957. doi: 10.1016/j.jece.2020.104957. [DOI] [Google Scholar]
- 34.Chu A-C, et al. Magnetic Fe3O4@TiO2 nanocomposites to degrade bisphenol A, one emerging contaminant, under visible and long wavelength UV light irradiation. J. Environ. Chem. Eng. 2021;9(4):105539. doi: 10.1016/j.jece.2021.105539. [DOI] [Google Scholar]
- 35.Narzary S, et al. Visible light active, magnetically retrievable Fe3O4@SiO2@g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants. J. Environ. Chem. Eng. 2020;8(5):104373. doi: 10.1016/j.jece.2020.104373. [DOI] [Google Scholar]
- 36.Zhao D, et al. Use of nonsteroidal anti-inflammatory drugs for COVID-19 infection: Adjunct therapy? Cardiol. Rev. 2020;28(6):303–307. doi: 10.1097/CRD.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 37.Yousefifard M, et al. Non-steroidal anti-inflammatory drugs in management of COVID-19; A systematic review on current evidence. Int. J. Clin. Pract. 2020;74(9):e13557. doi: 10.1111/ijcp.13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan G, et al. Visible-light-driven photocatalytic degradation of naproxen by Bi-modified titanate nanobulks: Synthesis, degradation pathway and mechanism. J. Photochem. Photobiol. A. 2020;386:112108. doi: 10.1016/j.jphotochem.2019.112108. [DOI] [Google Scholar]
- 39.Fan G, et al. Photocatalytic degradation of naproxen by a H2O2-modified titanate nanomaterial under visible light irradiation. Catal. Sci. Technol. 2019;9(17):4614–4628. doi: 10.1039/C9CY00965E. [DOI] [Google Scholar]
- 40.Gutierrez AM, Dziubla TD, Hilt JZ. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health. 2017;32(1–2):111–117. doi: 10.1515/reveh-2016-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marjani A, et al. Synthesis of alginate-coated magnetic nanocatalyst containing high-performance integrated enzyme for phenol removal. J. Environ. Chem. Eng. 2021;9(1):104884. doi: 10.1016/j.jece.2020.104884. [DOI] [Google Scholar]
- 42.Sarkar S, Chakraborty S, Bhattacharjee C. Photocatalytic degradation of pharmaceutical wastes by alginate supported TiO2 nanoparticles in packed bed photo reactor (PBPR) Ecotoxicol. Environ. Saf. 2015;121:263–270. doi: 10.1016/j.ecoenv.2015.02.035. [DOI] [PubMed] [Google Scholar]
- 43.Ghenaatgar A, Tehrani RM, Khadir A. Photocatalytic degradation and mineralization of dexamethasone using WO3 and ZrO2 nanoparticles: Optimization of operational parameters and kinetic studies. J. Water Process Eng. 2019;32:100969. doi: 10.1016/j.jwpe.2019.100969. [DOI] [Google Scholar]
- 44.Radu T, et al. Evaluation of physico-chemical properties and biocompatibility of new surface functionalized Fe3O4 clusters of nanoparticles. Appl. Surf. Sci. 2020;501:144267. doi: 10.1016/j.apsusc.2019.144267. [DOI] [Google Scholar]
- 45.Das D, et al. Preparation, physico-chemical characterization and catalytic activity of sulphated ZrO2–TiO2 mixed oxides. J. Mol. Catal. A: Chem. 2002;189(2):271–282. doi: 10.1016/S1381-1169(02)00363-1. [DOI] [Google Scholar]
- 46.Chen X, Mao SS. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007;107(7):2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- 47.Reyes-Coronado D, et al. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology. 2008;19(14):145605. doi: 10.1088/0957-4484/19/14/145605. [DOI] [PubMed] [Google Scholar]
- 48.Navio J, Colón G. Heterogeneous photocatalytic oxidation of liquid isopropanol by TiO2, ZrO2 and ZrTiO4 powders. Stud. Surf. Sci. Catal. 1994;82:721–728. doi: 10.1016/S0167-2991(08)63468-0. [DOI] [Google Scholar]
- 49.Wiemhöfer HD, Vohrer U. Spectroscopy and thermodynamics of electrons in yttria-stabilized zirconia. Ber. Bunsenges. Phys. Chem. 1992;96(11):1646–1652. doi: 10.1002/bbpc.19920961123. [DOI] [Google Scholar]
- 50.Preusser S, Stimming U, Wippermann K. An optical and electrochemical investigation of ZrO2 thin films (from nm to mm thickness) Electrochim. Acta. 1994;39(8–9):1273–1280. doi: 10.1016/0013-4686(94)E0047-4. [DOI] [Google Scholar]
- 51.Bendoraitis J, Salomon R. Optical energy gaps in the monoclinic oxides of hafnium and zirconium and their solid solutions1. J. Phys. Chem. 1965;69(10):3666–3667. doi: 10.1021/j100894a513. [DOI] [Google Scholar]
- 52.Newmark A, Stimming U. Photoelectrochemical studies of passive films on zirconium and amorphous iron–zirconium alloys. Langmuir. 1987;3(6):905–910. doi: 10.1021/la00078a006. [DOI] [Google Scholar]
- 53.Sato S, Kadowaki T. Photocatalytic activities of metal oxide semiconductors for oxygen isotope exchange and oxidation reactions. J. Catal. 1987;106(1):295–300. doi: 10.1016/0021-9517(87)90235-1. [DOI] [Google Scholar]
- 54.Botta SG, et al. Photocatalytic properties of ZrO2 and Fe/ZrO2 semiconductors prepared by a sol–gel technique. J. Photochem. Photobiol. A. 1999;129(1–2):89–99. doi: 10.1016/S1010-6030(99)00150-1. [DOI] [Google Scholar]
- 55.Sayama K, Arakawa H. Photocatalytic decomposition of water and photocatalytic reduction of carbon dioxide over zirconia catalyst. J. Phys. Chem. 1993;97(3):531–533. doi: 10.1021/j100105a001. [DOI] [Google Scholar]
- 56.Sayama K, Arakawa H. Effect of carbonate addition on the photocatalytic decomposition of liquid water over a ZrO2 catalyst. J. Photochem. Photobiol. A. 1996;94(1):67–76. doi: 10.1016/1010-6030(95)04204-0. [DOI] [Google Scholar]
- 57.Aydar AY. Utilization of Response Surface Methodology in Optimization of Extraction of Plant Materials. In: Silva V, editor. Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes. BoD; 2018. pp. 157–169. [Google Scholar]
- 58.Khuri AI, Mukhopadhyay S. Response surface methodology. Wiley Interdiscip. Rev.: Comput. Stat. 2010;2(2):128–149. doi: 10.1002/wics.73. [DOI] [Google Scholar]
- 59.Díaz-Uribe C, et al. Photocatalytic activity of Ag–TiO2 composites deposited by photoreduction under UV irradiation. Int. J. Photoenergy. 2018;2018:1–8. doi: 10.1155/2018/6080432. [DOI] [Google Scholar]
- 60.Kambur A, Pozan GS, Boz I. Preparation, characterization and photocatalytic activity of TiO2–ZrO2 binary oxide nanoparticles. Appl. Catal. B. 2012;115:149–158. doi: 10.1016/j.apcatb.2011.12.012. [DOI] [Google Scholar]
- 61.Polisetti S, Deshpande PA, Madras G. Photocatalytic activity of combustion synthesized ZrO2 and ZrO2–TiO2 mixed oxides. Ind. Eng. Chem. Res. 2011;50(23):12915–12924. doi: 10.1021/ie200350f. [DOI] [Google Scholar]
- 62.Davar F, Majedi A, Abbasi A. Synthesis of Fe3O4@ZrO2 core–shell nanoparticles through new approach and its solar light photocatalyst application. J. Mater. Sci.: Mater. Electron. 2017;28(6):4871–4878. [Google Scholar]
- 63.Shojaei AF, Shams-Nateri A, Ghomashpasand M. Comparative study of photocatalytic activities of magnetically separable WO3/TiO2/Fe3O4 nanocomposites and TiO2, WO3/TiO2 and TiO2/Fe3O4 under visible light irradiation. Superlattices Microstruct. 2015;88:211–224. doi: 10.1016/j.spmi.2015.09.014. [DOI] [Google Scholar]
- 64.Fallah Shojaei A, Shams-Nateri A, Ghomashpasand M. Magnetically recyclable Fe3+/TiO2@ Fe3O4 nanocomposites towards degradation of direct blue 71 under visible-light irradiation. Micro Nano Lett. 2017;12(3):161–165. doi: 10.1049/mnl.2016.0620. [DOI] [Google Scholar]
- 65.El Ghandoor H, et al. Synthesis and some physical properties of magnetite (Fe3O4) nanoparticles. Int. J. Electrochem. Sci. 2012;7(6):5734–5745. [Google Scholar]
- 66.Taghizadeh MT, Vatanparast M. Ultrasonic-assisted synthesis of ZrO2 nanoparticles and their application to improve the chemical stability of Nafion membrane in proton exchange membrane (PEM) fuel cells. J. Colloid Interface Sci. 2016;483:1–10. doi: 10.1016/j.jcis.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 67.Reddy CV, et al. Synthesis and characterization of pure tetragonal ZrO2 nanoparticles with enhanced photocatalytic activity. Ceram. Int. 2018;44(6):6940–6948. doi: 10.1016/j.ceramint.2018.01.123. [DOI] [Google Scholar]
- 68.Aslan N, Cebeci Y. Application of Box–Behnken design and response surface methodology for modeling of some Turkish coals. Fuel. 2007;86(1–2):90–97. doi: 10.1016/j.fuel.2006.06.010. [DOI] [Google Scholar]
- 69.Berkani M, et al. Combinatıon of a Box–Behnken design technique with response surface methodology for optimization of the photocatalytic mineralization of CI Basic Red 46 dye from aqueous solution. Arab. J. Chem. 2020;13(11):8338–8346. doi: 10.1016/j.arabjc.2020.05.013. [DOI] [Google Scholar]
- 70.Chintaparty CR. Influence of calcination temperature on structural, optical, dielectric properties of nano zirconium oxide. Optik. 2016;127(11):4889–4893. doi: 10.1016/j.ijleo.2016.02.014. [DOI] [Google Scholar]
- 71.Mushtaq K, et al. Synthesis and characterization of TiO2 via sol-gel method for efficient photocatalytic degradation of antibiotic ofloxacin. Inorg. Nano-Metal Chem. 2020;50(7):580–586. doi: 10.1080/24701556.2020.1722695. [DOI] [Google Scholar]
- 72.He Q, et al. A novel biomaterial—Fe3O4:TiO2 core–shell nano particle with magnetic performance and high visible light photocatalytic activity. Opt. Mater. 2008;31(2):380–384. doi: 10.1016/j.optmat.2008.05.011. [DOI] [Google Scholar]
- 73.Mullet M, et al. A simple and accurate determination of the point of zero charge of ceramic membranes. Desalination. 1999;121(1):41–48. doi: 10.1016/S0011-9164(99)00006-5. [DOI] [Google Scholar]
- 74.Heshmatpour F, Aghakhanpour RB. Synthesis and characterization of nanocrystalline zirconia powder by simple sol–gel method with glucose and fructose as organic additives. Powder Technol. 2011;205(1):193–200. doi: 10.1016/j.powtec.2010.09.011. [DOI] [Google Scholar]
- 75.Zhang W, et al. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000;33(8):912. doi: 10.1088/0022-3727/33/8/305. [DOI] [Google Scholar]
- 76.Mahshid S, et al. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloy. Compd. 2009;478(1–2):586–589. doi: 10.1016/j.jallcom.2008.11.094. [DOI] [Google Scholar]
- 77.Theivasanthi, T. & M. Alagar, Titanium dioxide (TiO2) nanoparticles XRD analyses: An insight. arXiv preprint arXiv:1307.1091 (2013).
- 78.Sridevi A, et al. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow 86. Inorg. Chem. Commun. 2021;130:108715. doi: 10.1016/j.inoche.2021.108715. [DOI] [Google Scholar]
- 79.Zhuang L, et al. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Sci. Rep. 2015;5(1):9320. doi: 10.1038/srep09320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klug HP, Alexander LE. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials. Wiley; 1974. [Google Scholar]
- 81.Patra JK, Baek K-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014;2014:417305. doi: 10.1155/2014/417305. [DOI] [Google Scholar]
- 82.Laha SC, et al. Cerium containing MCM-41-type mesoporous materials and their acidic and redox catalytic properties. J. Catal. 2002;207(2):213–223. doi: 10.1006/jcat.2002.3516. [DOI] [Google Scholar]
- 83.Rahmani Vahid B, Haghighi M. Biodiesel production from sunflower oil over MgO/MgAl2O4 nanocatalyst: Effect of fuel type on catalyst nanostructure and performance. Energy Convers. Manage. 2017;134:290–300. doi: 10.1016/j.enconman.2016.12.048. [DOI] [Google Scholar]
- 84.Nayebzadeh H, et al. Fabrication of carbonated alumina doped by calcium oxide via microwave combustion method used as nanocatalyst in biodiesel production: Influence of carbon source type. Energy Convers. Manage. 2018;171:566–575. doi: 10.1016/j.enconman.2018.05.081. [DOI] [Google Scholar]
- 85.Vivekanandhan S, et al. Acrylamide assisted polymeric citrate route for the synthesis of nanocrystalline ZrO2 powder. Mater. Chem. Phys. 2010;120(1):148–154. doi: 10.1016/j.matchemphys.2009.10.038. [DOI] [Google Scholar]
- 86.Abazari R, Mahjoub AR, Sanati S. A facile and efficient preparation of anatase titania nanoparticles in micelle nanoreactors: Morphology, structure, and their high photocatalytic activity under UV light illumination. RSC Adv. 2014;4(99):56406–56414. doi: 10.1039/C4RA10018B. [DOI] [Google Scholar]
- 87.Antić Ž, et al. Multisite luminescence of rare earth doped TiO2 anatase nanoparticles. Mater. Chem. Phys. 2012;135(2):1064–1069. doi: 10.1016/j.matchemphys.2012.06.016. [DOI] [Google Scholar]
- 88.Gangopadhyay P, et al. Magnetite-polymethylmethacrylate core–shell nanocomposites: Applications in all-optical magnetometers. Nonlinear Opt. Quantum Opt.: Concepts Mod. Opt. 2010;41(1):87–104. [Google Scholar]
- 89.Hariharan D, et al. Visible light active photocatalyst: Hydrothermal green synthesized TiO2 NPs for degradation of picric acid. Mater. Lett. 2018;222:45–49. doi: 10.1016/j.matlet.2018.03.109. [DOI] [Google Scholar]
- 90.Su C, et al. Fabrication of Ag/TiO2 nanoheterostructures with visible light photocatalytic function via a solvothermal approach. CrystEngComm. 2012;14(11):3989–3999. doi: 10.1039/c2ce25161b. [DOI] [Google Scholar]
- 91.Rivera V, et al. Near-infrared light emission of Er3+-doped zirconium oxide thin films: An optical, structural and XPS study. J. Alloy. Compd. 2015;619:800–806. doi: 10.1016/j.jallcom.2014.09.007. [DOI] [Google Scholar]
- 92.Xu X, et al. Size dependence of defect-induced room temperature ferromagnetism in undoped ZnO nanoparticles. J. Phys. Chem. C. 2012;116(15):8813–8818. doi: 10.1021/jp3014749. [DOI] [Google Scholar]
- 93.Zheng W, et al. Interpenetrated networks between graphitic carbon infilling and ultrafine TiO2 Nanocrystals with patterned macroporous structure for high-performance lithium ion batteries. ACS Appl. Mater. Interfaces. 2017;9(24):20491–20500. doi: 10.1021/acsami.7b02345. [DOI] [PubMed] [Google Scholar]
- 94.Chary KVR, et al. Characterization and reactivity of copper oxide catalysts supported on TiO2−ZrO2. J. Phys. Chem. B. 2005;109(19):9437–9444. doi: 10.1021/jp0500135. [DOI] [PubMed] [Google Scholar]
- 95.Li M, et al. Hierarchically macro–mesoporous ZrO2–TiO2 composites with enhanced photocatalytic activity. Ceram. Int. 2015;41(4):5749–5757. doi: 10.1016/j.ceramint.2014.12.161. [DOI] [Google Scholar]
- 96.Zhao J, et al. Solvothermal synthesis, characterization and photocatalytic property of zirconium dioxide doped titanium dioxide spinous hollow microspheres with sunflower pollen as bio-templates. J. Colloid Interface Sci. 2018;529:111–121. doi: 10.1016/j.jcis.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 97.Ran F-Y, et al. Angle-resolved photoemission study of Fe3O4 (0 0 1) films across Verwey transition. J. Phys. D Appl. Phys. 2012;45(27):275002. doi: 10.1088/0022-3727/45/27/275002. [DOI] [Google Scholar]
- 98.Lai L-J, Su C-S. Luminescence excitation and near edge X-ray absorption spectra of Er2O3 dopant on zirconia ceramics. Mater. Chem. Phys. 2000;62(2):148–152. doi: 10.1016/S0254-0584(99)00172-8. [DOI] [Google Scholar]
- 99.Luo S, et al. Synthesis and low-temperature photoluminescence properties of SnO2 nanowires and nanobelts. Nanotechnology. 2006;17(6):1695. doi: 10.1088/0957-4484/17/6/025. [DOI] [PubMed] [Google Scholar]
- 100.He JH, et al. Beaklike SnO2 nanorods with strong photoluminescent and field-emission properties. Small. 2006;2(1):116–120. doi: 10.1002/smll.200500210. [DOI] [PubMed] [Google Scholar]
- 101.Liqiang J, et al. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells. 2006;90(12):1773–1787. doi: 10.1016/j.solmat.2005.11.007. [DOI] [Google Scholar]
- 102.Liqiang J, et al. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004;177(10):3375–3382. doi: 10.1016/j.jssc.2004.05.064. [DOI] [Google Scholar]
- 103.Ramakrishna G, et al. Dynamics of interfacial electron transfer from photoexcited quinizarin (Qz) into the conduction band of TiO2 and surface states of ZrO2 nanoparticles. J. Phys. Chem. B. 2004;108(15):4775–4783. doi: 10.1021/jp036623r. [DOI] [Google Scholar]
- 104.Wu J-C, et al. Nonoxidative dehydrogenation of ethylbenzene over TiO2–ZrO2 catalysts: II. The effect of pretreatment on surface properties and catalytic activities. J. Catal. 1984;87(1):98–107. doi: 10.1016/0021-9517(84)90172-6. [DOI] [Google Scholar]
- 105.Kumari L, et al. Controlled hydrothermal synthesis of zirconium oxide nanostructures and their optical properties. Cryst. Growth Des. 2009;9(9):3874–3880. doi: 10.1021/cg800711m. [DOI] [Google Scholar]
- 106.Kumar KV, Porkodi K, Rocha F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008;9(1):82–84. doi: 10.1016/j.catcom.2007.05.019. [DOI] [Google Scholar]
- 107.Dada A, et al. Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012;3(1):38–45. doi: 10.9790/5736-0313845. [DOI] [Google Scholar]
- 108.Ahmed MJ. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons. J. Environ. Manage. 2017;190:274–282. doi: 10.1016/j.jenvman.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 109.Husein DZ. Adsorption and removal of mercury ions from aqueous solution using raw and chemically modified Egyptian mandarin peel. Desalin. Water Treat. 2013;51(34–36):6761–6769. doi: 10.1080/19443994.2013.801793. [DOI] [Google Scholar]
- 110.Kavitha D, Namasivayam C. Experimental and kinetic studies on methylene blue adsorption by coir pith carbon. Biores. Technol. 2007;98(1):14–21. doi: 10.1016/j.biortech.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 111.Erhayem M, et al. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am. J. Anal. Chem. 2015;6(01):1. doi: 10.4236/ajac.2015.61001. [DOI] [Google Scholar]
- 112.Ho Y-S, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34(5):451–465. doi: 10.1016/S0032-9592(98)00112-5. [DOI] [Google Scholar]
- 113.Lagergren SK. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898;24:1–39. [Google Scholar]
- 114.Arany E, et al. Degradation of naproxen by UV, VUV photolysis and their combination. J. Hazard. Mater. 2013;262:151–157. doi: 10.1016/j.jhazmat.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 115.Dulova N, Kattel E, Trapido M. Degradation of naproxen by ferrous ion-activated hydrogen peroxide, persulfate and combined hydrogen peroxide/persulfate processes: The effect of citric acid addition. Chem. Eng. J. 2017;318:254–263. doi: 10.1016/j.cej.2016.07.006. [DOI] [Google Scholar]
- 116.Chi H, et al. Hydroxylamine enhanced degradation of naproxen in Cu2+ activated peroxymonosulfate system at acidic condition: Efficiency, mechanisms and pathway. Chem. Eng. J. 2019;361:764–772. doi: 10.1016/j.cej.2018.12.114. [DOI] [Google Scholar]
- 117.Wang F, et al. Novel ternary photocatalyst of single atom-dispersed silver and carbon quantum dots co-loaded with ultrathin g-C3N4 for broad spectrum photocatalytic degradation of naproxen. Appl. Catal. B. 2018;221:510–520. doi: 10.1016/j.apcatb.2017.09.055. [DOI] [Google Scholar]
- 118.Gao Y-Q, et al. Kinetics and mechanistic investigation into the degradation of naproxen by a UV/chlorine process. RSC Adv. 2017;7(53):33627–33634. doi: 10.1039/C7RA04540A. [DOI] [Google Scholar]
- 119.Jallouli N, et al. UV and solar photo-degradation of naproxen: TiO2 catalyst effect, reaction kinetics, products identification and toxicity assessment. J. Hazard. Mater. 2016;304:329–336. doi: 10.1016/j.jhazmat.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 120.Méndez-Arriaga F, Gimenez J, Esplugas S. Photolysis and TiO2 photocatalytic treatment of naproxen: Degradation, mineralization, intermediates and toxicity. J. Adv. Oxid. Technol. 2008;11(3):435–444. [Google Scholar]
- 121.Talukdar K, et al. Rational construction of CeO2–ZrO2@MoS2 hybrid nanoflowers for enhanced sonophotocatalytic degradation of naproxen: Mechanisms and degradation pathways. Compos. B Eng. 2021;215:108780. doi: 10.1016/j.compositesb.2021.108780. [DOI] [Google Scholar]
- 122.Zhang H, et al. Photocatalytic degradation of four non-steroidal anti-inflammatory drugs in water under visible light by P25-TiO2/tetraethyl orthosilicate film and determination via ultra performance liquid chromatography electrospray tandem mass spectrometry. Chem. Eng. J. 2015;262:1108–1115. doi: 10.1016/j.cej.2014.10.019. [DOI] [Google Scholar]
- 123.Sabouni R, Gomaa H. Photocatalytic degradation of pharmaceutical micro-pollutants using ZnO. Environ. Sci. Pollut. Res. Int. 2019;26(6):5372–5380. doi: 10.1007/s11356-018-4051-2. [DOI] [PubMed] [Google Scholar]
- 124.Huaccallo-Aguilar Y, et al. Naproxen removal by CWPO with Fe3O4/multi-walled carbon nanotubes in a fixed-bed reactor. J. Environ. Chem. Eng. 2021;9(2):105110. doi: 10.1016/j.jece.2021.105110. [DOI] [Google Scholar]
- 125.Amini Z, et al. Synthesis of N-doped TiO2/SiO2/Fe3O4 magnetic nanocomposites as a novel purple LED illumination-driven photocatalyst for photocatalytic and photoelectrocatalytic degradation of naproxen: Optimization and different scavenger agents study. J. Environ. Sci. Health Part A. 2019;54(12):1254–1267. doi: 10.1080/10934529.2019.1673609. [DOI] [PubMed] [Google Scholar]