Abstract
An oral test supplement increases serum human growth hormone (hGH) levels after acute administration in healthy adults. We investigated the mechanism for the increase in hGH and the effect of continued daily administration of the test supplement on measures of physical fitness and sleep efficiency. In Study 1, serum triiodothyronine (T3) was measured in samples from a prior placebo-controlled, double-blind study in which 16 healthy participants received both placebo and the test supplement in a crossover design; treatment order was randomized, and treatments were separated by a 1-week washout. In Study 2, physical fitness (VO2 max) was measured at baseline and after 2 weeks of daily administration of the test supplement (N = 12 healthy participants). Study 3 assessed daily sleep onset latency and time awake during 3 weeks of daily administration of the test supplement (N = 15 healthy participants). A fall from baseline in T3 was observed with placebo (−6.1 ± 8.5 ng/dL, P = .01). Of note, the change in T3 was smaller with the test supplement (−3.3 ± 10.7 ng/dL, P = not significant) but was not statistically different from placebo. Mean VO2 max increased by 6% from baseline after 2 weeks (P = .02). Sleep-onset latency and time awake during the night were reduced from baseline to week 3 by 22% and 65%, respectively (P = .01 and P = .02). The conservation of T3 levels suggests that the mechanism for increased hGH secretion by the test supplement is through somatostatin inhibition. Furthermore, pilot studies indicated that daily administration of the supplement improved physical fitness and sleep efficiency from baseline, effects consistent with increased endogenous hGH release.
Clinical Trial Registration No. NCT02987868.
Keywords: growth hormone, growth hormone secretagogue, insulin-like growth factor-1, somatostatin
Introduction
Human growth hormone (hGH) is produced and secreted by the pituitary gland in a pulsatile manner; the majority of hGH is released at night.1–3 hGH promotes longitudinal growth in children and influences many other homeostatic functions throughout life, including puberty, body composition, metabolism, physical fitness, and sleep.1,4,5
hGH deficiency (GHD) is a rare disorder characterized by inadequate hGH secretion that occurs in children and adults.6,7 In children, the primary symptoms of GHD are severe short stature and delayed puberty. In adults and children, GHD is associated with low muscle mass, increased adiposity (especially visceral adiposity), decreased bone density, impaired exercise capacity, sleep disturbance, fatigue, impaired lipid metabolism, insulin resistance, infertility, anxiety and depression, and decreased quality of life.6–8 Recombinant hGH replacement is used to increase stature in children with GHD or growth disorders.1,9 In adults with GHD, recombinant hGH replacement improves body composition, exercise performance, bone density, and quality of life.1,6,7,10–15 Recombinant hGH treatment is also used to treat other disorders associated with short stature and HIV wasting syndrome.1
In healthy individuals, hGH levels decrease throughout the lifespan.16 Low levels of hGH have been observed in non-GHD adults, including elderly adults16 and postmenopausal women.17 Low hGH has also been observed in conditions including obesity,18 fibromyalgia,19,20 post-traumatic stress disorder,21 female and male infertility,22,23 impaired sleep,24,25 and low muscle mass (e.g., sarcopenia).26 Treatment with recombinant hGH is not indicated in non-GHD individuals, thus, there is a need for safe therapies that improve endogenous hGH secretion and symptoms associated with low hGH.
We recently reported results of a placebo-controlled crossover clinical trial in which a single oral administration of an amino acid-based test supplement significantly increased endogenous hGH levels in healthy male and female participants 120 min after administration.27 In this study, we conducted a post hoc analysis of serum triiodothyronine (T3) levels in samples from the previous study27 to explore if the test supplement stimulates hGH release by inhibiting somatostatin, the well-established mechanism by which certain amino acids can stimulate hGH release under certain conditions.28–31 Somatostatin reduces T3 levels by inhibiting secretion of thyroid-stimulating hormone (TSH) (given in Fig. 1). We also present the results of two pilot studies designed to characterize the effect of continued administration for at least 2 weeks of the test supplement on functions influenced by hGH: physical fitness and sleep efficiency. Results of these studies will be used to power future controlled intervention trials.
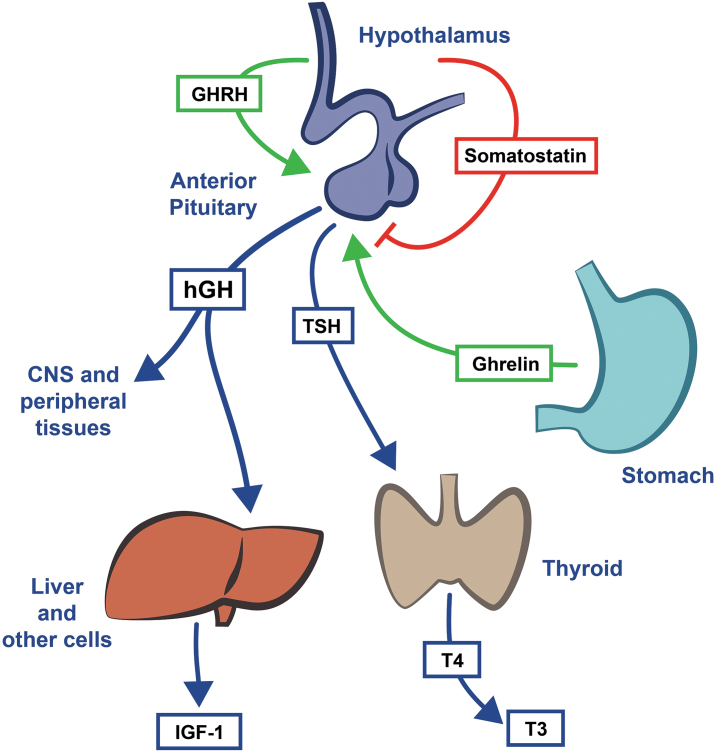
FIG. 1.
Pathways involved in regulation of hGH and downstream effects. hGH exerts direct and indirect (i.e., via IGF-1) effects on CNS and peripheral tissues. Neurons that release GHRH and somatostatin act directly on pituitary cells to influence hGH release. Somatostatin inhibits hGH and TSH release from the pituitary. TSH stimulates production of T4 by the thyroid gland, which is then converted to T3. Ghrelin, a peptide hormone produced by the stomach, stimulates hGH release. hGH stimulates release of IGF-1 from liver and other tissues. CNS, central nervous system, GHRH, growth hormone releasing hormone; hGH, human growth hormone; IGF-1, insulin-like growth factor 1; T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Materials and Methods
Study design and procedures
All studies were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Study 1 was registered at clinicatrials.gov. The Pennington Biomedical Review Board reviewed and approved the protocol (IRB no. 10,036). Written informed consent was obtained from all participants in all studies.
A recent randomized, placebo-controlled, double-blind, crossover clinical trial27 investigated the effects of the test supplement on hGH release in 16 healthy male and female adults. In brief, Study 1 was a post hoc analysis of serum samples obtained from Tam et al., in which all participants received both the test supplement and matching placebo in a randomized order. Study investigators and participants were blinded to treatment order. Each participant reported to the inpatient unit in the morning on two occasions 1 week apart after an overnight fast. Study treatment (test supplement or matching placebo) was administered orally at time 0. Blood was drawn from an IV line at −30, −15, 0, 15, 30, 60, 90, and 120 min. The primary outcome measures from the original study were the percent change in hGH from baseline (0 min) to 120 min and the area under the curve of hGH (0–120 min) over baseline. Participants, investigators, and staff were blinded to treatment. For this analysis, preserved blood samples from all 16 participants were analyzed for serum T3 at time 0 and 120 min using the Siemens Medical Solutions Diagnostics Immulite 2000 (Siemens USA, Los Angeles, CA, USA).
Study 2 included 12 generally healthy participants without confounding medical conditions who agreed to adhere to the study protocol. Participants were recruited by word of mouth. Body weight, percentage body fat (BodPod GS; Cosmed, Rome, Italy), and resting metabolic rate (RMR, indirect calorimetry) were measured after an overnight fast. Daily calorie expenditure was estimated based on measured RMR, estimated lifestyle activity, and estimated exercise. For the testing procedure, participants consumed a standard breakfast (Egg McMuffin; McDonalds, Salt Lake City, UT, USA; 300 calories; 12 g fat; 29 g carbohydrates; 18 g protein), rested for 45 min, and then had a maximal graded exercise test on a treadmill at the University of Utah College of Health testing facility. Physical fitness was measured by monitoring VO2 max using a flowthrough hood metabolic cart (TrueOne® 2400; Parvomedics, Sandy, UT, USA). Study duration was based on a previous study that demonstrated increased energy expenditure in young men after 2 weeks of administration of hGH.32
After baseline measurements, participants were provided with a 2-week supply of the test supplement and instructed to consume 1 dose on an empty stomach (at least 2 h after eating) every night for 2 weeks. Supplement containers and capsules were unmarked, and study site staff and participants were unaware of the nature of the treatment. Participants were also instructed not to change their diet or daily physical activity during the study. After the final dose taken in the evening, participants returned to the same testing facility the following morning and the testing procedure was repeated.
Study 3 included 15 generally healthy participants who were recruited by word of mouth and who expressed a desire for improved sleep. Included participants had a normal score (≤8 units) on the Epworth Sleepiness Scale. After baseline measurements, participants were provided with a 3-week supply of the test supplement and instructed to consume 1 dose on an empty stomach (at least 2 h after eating) every night for 3 weeks. Supplement containers and capsules were unmarked, and study site staff and participants were unaware of the nature of the treatment. Participants reported the daily time that they went to bed, time of final wakening, estimated time to fall asleep, and number of awakenings during sleep and the duration of each. Sleep-onset latency (estimated time to fall asleep) and time awake (length of time awake) were analyzed as indicators of total sleep quality.
Test supplement
The test supplement consists of 4 capsules (2.9 g total dose). Each capsule contained a blend of 374.8 mg l-lysine hydrochloride, 177.8 mg l-arginine hydrochloride, 169.2 mg oxo-proline, 250 μg N-acetyl-l-cysteine, 250 μg l-glutamine, and 125 μg Schizonepeta (aerial parts). The purity and potency of each component and the final composition and stability of the test supplement was verified by a third-party laboratory. An inert placebo was used that was indistinguishable from test supplement.
Statistical analysis
Study 1
The change in mean T3 from baseline (t = 0 min) to endpoint (t = 120 min) was analyzed for the placebo versus test supplement treatment groups with a crossover Wilcoxon rank sum test. Specifically, the difference between the change scores in period 1 and period 2 were compared in the two randomization groups. This procedure accounts for period effects since period 1 is always subtracted from period 2, and for the randomization groups, as described in Senn.33 The mean T3 at baseline (t = 0) and end of study (t = 120 min) were compared with Wilcoxon signed rank tests.
Study 2
The mean VO2 max at baseline (day 0) and end of study (day 14) were compared with a paired t-test.
Study 3
The reported minutes to fall asleep (sleep-onset latency), and minutes awake during the night, over 3 weeks, were modeled with a mixed-effects Poisson model with random subject-specific intercepts and a fixed-slope effect (this model allows the intercepts [sleep latency or time awake at baseline] to vary across participants but assumes a common slope for all participants, that is, the effect of the test supplement was assumed to be the same). An overdispersion term was included in both models. All available data were included in the analyses. Mixed-effect Poisson models were fit to the individual data per night.
Statistical significance was set at P < .05 for all studies.
Results
Study 1
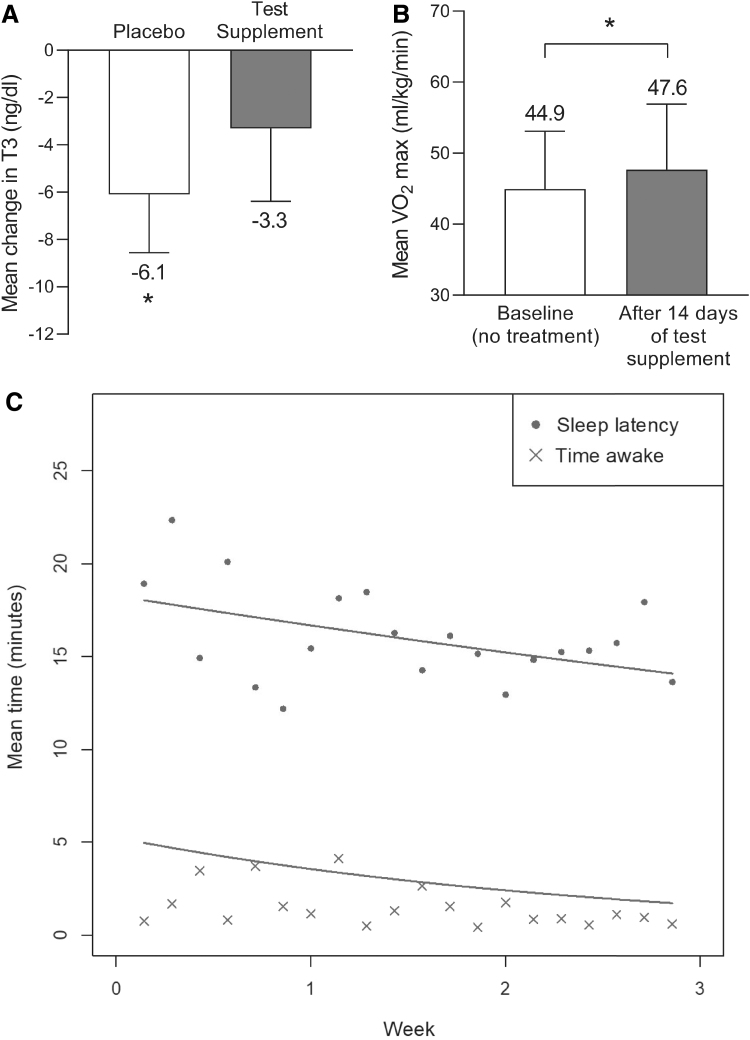
Sixteen healthy participants (12 men and 4 women) were included in the study, received both placebo and the test supplement, and completed all study visits. Mean ± standard deviation (SD) age was 32 ± 14 years (range: 18–62 years) and body mass index (BMI) was 26.4 ± 5.0 kg/m2. Mean T3 levels at baseline (time t = 0) were slightly higher in the test supplement group (Table 1). In the placebo group, morning T3 levels fell significantly from baseline to 120 min by (mean ± SD) −6.1 ± 8.5, a 6% decrease (P = .01; Table 1 and Fig. 2A). In the test supplement group, the mean change from baseline in morning T3 was smaller (mean ± SD change from 0 to 120 min: −3.3 ± 10.7 ng/dL, a 3% decrease from baseline) but was not statistically significant from placebo. Of note, this was a secondary analysis of a previous study that was designed to detect a change in hGH between placebo and the test supplement (as described previously);27 it was not powered to detect a statistically significant change in T3 between treatment groups.
Table 1.
Mean Triiodothyronine Levels After Administration of the Test Supplement in Study 1
| Placebo | Test supplement | |
|---|---|---|
| T3 at baseline (ng/dL) | 106.2 ± 17.0 | 100.9 ± 19.4 |
| T3 at 120 mina (ng/dL) | 100.1 ± 15.9 | 97.6 ± 18.4 |
| Change in T3 from baseline to 120 min (ng/dL) | −6.1 ± 8.5 | −3.3 ± 10.7 |
| 95% Confidence interval | −10.6 to −1.5 | −9.0 to 2.4 |
| P value for change from baseline | .01 | .09 |
| P value for difference from placebo | NS |
Mean serum T3 levels at baseline (t = 0) and end of study (t = 120 min) in the placebo and test supplement intervention groups were compared using a crossover Wilcoxon signed rank test (N = 16). Data are given as mean and SD unless otherwise indicated.
Mean and standard error of the mean change from baseline (t = 0) to t = 120 min.
NS, not significant; T3, triiodothyronine; SD, standard deviation.
FIG. 2.
Effect of the test supplement on T3, VO2 max, and sleep quality. (A) Mean ± SEM change from baseline (t = 0) to endpoint (t = 120 min) in T3 observed following administration of placebo or the test supplement (treatment was a crossover design in which all individuals, N = 16, received the placebo or test supplement, treatment order was randomized and separated by a 1-week washout period). (B) Mean ± SD VO2 max observed in the same individuals, N = 12, at baseline before treatment and after 14 days of administration of the test supplement. (C) Geometric mean for sleep onset latency and time awake are shown for each day from day 0 to week 3 in healthy individuals, N = 15. Lines represent the population-level predicted regression lines. Mixed-effect Poisson models were fit to the individual data per night. Sleep onset latency model: log(μ(t)) = 2.905 – 0.091t, where μ is the mean sleep onset latency at time t. Time awake model: log(μ(t)) = 1.657 – 0.390t, where μ is the mean sleep onset latency at time t. *p < 0.05 vs. baseline. SD, standard deviation; SEM, standard error of the mean.
Study 2
Twelve healthy participants (seven men and five women) were enrolled in the study. Baseline mean ± SD age: 31 ± 6 years (range: 22–39 years), body weight: 81.2 ± 16.1 kg, body fat: 26.0 ± 8.2%, BMI: 25.7 kg/m2, RMR: 1687 ± 230 kcal/day, and estimated caloric expenditure: 2367 ± 322. After 14 days of administration of the test supplement, there was a statistically significant increase in mean VO2 max from baseline to end of study of (mean ± standard error of the mean [SEM]) 2.7 ± 1.0 mL/kg/min, a 6% increase from baseline (Table 2 and Fig. 2B).
Table 2.
Mean VO2 max at Baseline and After 14 Days of Administration of the Test Supplement in Study 2
| Test supplement | |
|---|---|
| Baseline VO2 max (mL/kg/min) | 44.9 ± 8.1 |
| Day 14 VO2 max (mL/kg/min) | 47.7 ± 9.3 |
| Change in VO2 max* (mL/kg/min) | 2.7 ± 1.0 |
| 95% Confidence interval | 0.5 to 5.0 |
| P value for change from baseline | .02 |
Study participants administered the test supplement for 14 days. Data are given as mean and SD unless otherwise indicated. Mean VO2 max at baseline (day 0) and end of study (day 14) were compared with a paired t-test (N = 12).
Mean and standard error of the mean change from baseline (day 0) to end of study (day 14).
VO2 max, maximum oxygen uptake.
Study 3
Fifteen healthy participants (10 men and 5 women) were enrolled in the study. Baseline mean ± SD age was 33 ± 7 years (range: 22–48 years) and BMI was 26.2 ± 3.6 kg/m2. A repeated-measures overdispersed Poisson regression model was fit to the sleep-onset latency and time awake data. This model accounts for the dependence between repeated measures on subjects and models the non-normally distributed counts of minutes to fall asleep and time awake better than a linear regression. Sleep-onset latency model: log(μ(t)) = 2.905 – 0.091t, where μ is the mean sleep-onset latency at time t. Time awake model: log(μ(t)) = 1.657 – 0.390t, where μ is the mean sleep-onset latency at time t. Sleep-onset latency decreased from a predicted mean ± SEM of 18.0 ± 1.9 min at baseline to 14.1 ± 1.5 min at day 20, a statistically significant decrease (P = .01) of 22% (Fig. 2C). The predicted mean ± SEM time awake also decreased from 5.0 ± 1.7 min at baseline to 1.7 ± 0.6 min at week 20, a 65% decrease that was statistically significant (P = .02; Fig. 2C).
No adverse events were reported by participants in Studies 1, 2, and 3 and no other tolerability or safety findings were observed.
Discussion
We recently reported that the test supplement significantly increased mean endogenous hGH levels by 682% two hours after administration.27 Here, we evaluated the mechanism for the increase in hGH following a single administration. We also investigated whether repeated daily administration of the test supplement would demonstrate improvements in conditions associated with low hGH, physical fitness: and sleep disturbance.12,32,34,35
We hypothesized that the amino acid-based test supplement would inhibit somatostatin, resulting in an increase in T3 levels. This hypothesis is based on the amino acid-based composition of the test supplement. It is well established that inhibition of somatostatin is the mechanism by which some amino acids can stimulate hGH release from the pituitary gland under certain conditions.28–31 The increase in T3 observed in Study 1 is consistent with our hypothesis. In brief, pituitary hGH release is primarily influenced by levels of growth hormone-releasing hormone (GHRH), ghrelin, and somatostatin (illustrated in Fig. 1).1,3 GHRH and ghrelin stimulate hGH secretion, whereas somatostatin inhibits hGH release directly and indirectly (by antagonizing the effects of ghrelin or GHRH). Because somatostatin also inhibits TSH release (TSH stimulates production of thyroxine by the thyroid gland, which is then converted to T3), T3 levels are an indicator of somatostatin release.
In the placebo group, T3 levels decreased from baseline to 120 min, consistent with the normal reduction in T3 that occurs in the morning.36 After administration of the test supplement, there was no statistically significant change in T3 levels from baseline to 120 min. This suggests that the test supplement may conserve T3 levels by preventing the physiological fall in T3 levels that occurs in the morning. If the test supplement increased hGH release through an alternative mechanism (i.e., increase in ghrelin or GHRH), we would have expected to observe a reduction from baseline in T3 after administration of both the placebo and the test supplement. Because T3 measurements represent a downstream proxy for changes in somatostatin release, the conservative changes in T3 observed here would be expected to be a result of the multistep process. Furthermore, the lack of a statistically significant difference in the mean change in T3 between treatment groups may instead reflect a limitation of the statistical power of the study design. The post hoc power was calculated using PASS 202037 Tests for Differences Between Two Means in a 2 × 2 Cross-Over Design procedure using the observed difference in means (2.8 ng/dL) and the observed SD of the paired differences (11.6). The power for a sample size of 16 is 14.7%. Thus, it is unlikely that we would have observed a statistically significant change with this sample size.
Studies 2 and 3 investigated the effects of the test supplement on functions that are influenced by hGH. Results of Study 2 indicated that physical fitness, measured by VO2 max, improved by 6% over baseline after 2 weeks of daily administration of the test supplement. The mean baseline and end of study VO2 max (44.9 ± 8.1 vs. 47.7 ± 9.3) are comparable with gas exchange data obtained in other studies using the same testing procedures and comparable populations or study duration.38,39 Furthermore, the increase from baseline in VO2 max observed in this study is similar to observed improvements in VO2 max as a result of exercise.38,39 In addition, 2 weeks of administration of hGH has been shown to be sufficient to increase energy expenditure in healthy men.32 Because participants in our study were specifically instructed not to change their diet or physical activity, the reason for the observed changes in VO2 max (i.e., increased hGH release or other mechanism) warrants further study.
In Study 3, administration of the test supplement for 3 weeks was associated with a progressive improvement in sleep efficiency as indicated by reduced sleep-onset latency and time awake, both with an average decrease of ∼0.25 min/day over 3 weeks of daily administration, or ∼4–5 min over the 21-day study. The decrease in sleep-onset latency is similar to the 4-min decrease in sleep-onset latency produced by melatonin in a meta-analysis and warrants further investigation.40
By inhibiting somatostatin tone, the test supplement may be especially effective in individuals with low hGH associated with elevated somatostatin tone, such as advanced age,41,42 sleep disruption,5,21,25 fibromyalgia,20 infertility,43 or in postmenopausal women.17 In support of this hypothesis, administration of the test supplement to individuals with suboptimal control of fibromyalgia and low-normal insulin-like growth factor 1 (IGF-1) (indicating low hGH), resulted in an increase in IGF-1 and improvement in fibromyalgia symptoms.44,45 Additional work is necessary to elucidate if there is a positive effect of the test supplement in these and other conditions associated with low hGH, that is, post-traumatic stress disorder, impaired cognition, mood, fertility, quality of life, and sarcopenia.21,26,46 As advanced age is associated with reduced physical fitness35 and changes in sleep patterns, including reduced sleep efficiency,47 further investigation of the test supplement on these parameters in elderly individuals may yield positive results.
In this study, we report that the increase in hGH previously demonstrated27 after administration of the test supplement likely occurs through inhibition of somatostatin. In addition, results of studies 2 and 3 indicated a positive effect of the test supplement on sleep and physical fitness and are consistent with similar effects observed after administration of recombinant hGH administration, GHRH, and other hGH secretagogues (e.g., ghrelin mimetics).10,48 Potential advantages of the test supplement over these and other treatments are that the side effects of ghrelin mimetics (increased appetite and weight gain) would be avoided.48 Evidence that the test supplement acts through normal physiological pathways (somatostatin inhibition) further supports the observed favorable tolerability profile and would indicate an absence of pharmacological treatment effects such as hGH overdose or tachyphylaxis. For these reasons, addition of the test supplement to standard care is a possible treatment strategy, as has been demonstrated in a recent study showing improvement in fibromyalgia symptoms with administration of the test supplement in addition to standard care in individuals with fibromyalgia.44 Consistent with previous results,27 no adverse tolerability or safety findings were observed in any studies.
The effect of the test supplement on T3 levels was a post hoc analysis of a previous placebo-controlled crossover study in which each participant received the test supplement and matching placebo in a randomized order; both investigators and participants were blinded to treatment.27 Strengths of this study include the crossover and double-blind design. However, the post hoc analysis of the difference in the mean change from baseline in T3 levels between the placebo and test supplement is limited in that it was inadequately powered to detect a change in T3 between treatment groups. Larger studies designed to detect a change in T3 are needed. Studies 2 and 3 were not placebo-controlled; however, capsules were unmarked and study site staff and participants were unaware of the nature of the treatment. The current results support conduct of placebo-controlled studies with larger sample sizes and use of standardized questionnaires (e.g., the Pittsburgh Sleep Quality Index49) and complementary objective observational sleep models to validate our findings.
These results indicate that the test supplement enhances hGH release through inhibition of somatostatin. Furthermore, repeated treatment with the test supplement for up to 3 weeks was associated with an improvement in physical fitness (VO2 max) and a self-reported improvement in sleep efficiency, results that are consistent with increased hGH release.
Authors' Contributions
A.L.H. and F.L.G. designed the studies. J.R. and F.L.G. collected data and/or ran samples. C.K., J.R., and F.L.G. took part in data analysis. All authors had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. All authors were involved in data interpretation, critically reviewed the article, and have given final approval of the version to be published.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
A.L.H. is an employee of Bydex Management, LLC. C.K. is a consultant for Basic Research, LLC. F.L.G. is a consultant for Beachbody, Basic Research, LLC, Eisai, Inc., General Nutrition Corporation, Melior Discoveries, and Techenterprises, is on advisory boards for Baronova, Inc., Curves-Jenny Craig, Gelesis, Microbiome Therapeutics, Novo Nordisk, Novartis, Plensat, and Zafgen, holds stock or stock options in Microbiome Therapeutics, Plensat, and Zafgen, and holds patents in Neuroquest. C.S.T. and J.R. have no conflict of interest.
Funding Information
Funding for this work was provided by Sierra Research Group, LLC, Salt Lake City, UT, a wholly owned subsidiary of Basic Research Holdings, LLC, Salt Lake City, UT. The authors thank the study volunteers for participating in the study and the PEAK Fitness Testing Facility at the University of Utah College of Health for performing the standard Maximal Aerobic Fitness Test procedures. We also thank Alok Gupta, MD (currently at Permanente Medical Group, Pinole, CA) for clinical assistance with the conduct of Study 1. Medical writing assistance was provided by Sonja K. Billes (August Scientific) and funded by the Sponsor. C.S.T. is also supported by a National Health and Medical Research Centre Early Career Fellowship from Australia (No. 1037275). F.L.G. is supported in part by 1U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health that funds the Louisiana Clinical and Translational Science Center.
References
- 1. Ranke MB, Wit JM: Growth hormone—past, present and future. Nat Rev Endocrinol 2018;14:285–300. [DOI] [PubMed] [Google Scholar]
- 2. Giustina A, Veldhuis JD: Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev 1998;19:717–797. [DOI] [PubMed] [Google Scholar]
- 3. Olarescu NC, Gunawardane K, Hansen TK, Moller N, Jorgensen JOL: Normal physiology of growth hormone in adults. In: Endotext (Feingold KR, et al., eds.). 2000. [Google Scholar]
- 4. Bidlingmaier M, Strasburger CJ: Growth hormone. Handb Exp Pharmacol 2010:187–200. [DOI] [PubMed] [Google Scholar]
- 5. Van Cauter E, Latta F, Nedeltcheva A, et al. : Reciprocal interactions between the GH axis and sleep. Growth Horm IGF Res 2004;14 Suppl A: S10–S17. [DOI] [PubMed] [Google Scholar]
- 6. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine S: Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1587–1609. [DOI] [PubMed] [Google Scholar]
- 7. Thomas JD, Monson JP: Adult GH deficiency throughout lifetime. Eur J Endocrinol 2009;161 Suppl 1:S97–S106. [DOI] [PubMed] [Google Scholar]
- 8. Copinschi G, Nedeltcheva A, Leproult R, et al. : Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiency. J Clin Endocrinol Metab 2010;95:2195–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen DB, Cuttler L: Clinical practice. Short stature in childhood—challenges and choices. N Engl J Med 2013;368:1220–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chikani V, Cuneo RC, Hickman I, Ho KK: Growth hormone (GH) enhances anaerobic capacity: Impact on physical function and quality of life in adults with GH deficiency. Clin Endocrinol (Oxf) 2016;85:660–668. [DOI] [PubMed] [Google Scholar]
- 11. Cuneo RC, Salomon F, Wiles CM, Hesp R, Sonksen PH: Growth hormone treatment in growth hormone-deficient adults. I. Effects on muscle mass and strength. J Appl Physiol (1985) 1991;70:688–694. [DOI] [PubMed] [Google Scholar]
- 12. Chikani V, Cuneo RC, Hickman I, Ho KK: Impairment of anaerobic capacity in adults with growth hormone deficiency. J Clin Endocrinol Metab 2015;100:1811–1818. [DOI] [PubMed] [Google Scholar]
- 13. Salomon F, Cuneo RC, Hesp R, Sonksen PH: The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med 1989;321:1797–1803. [DOI] [PubMed] [Google Scholar]
- 14. Barake M, Klibanski A, Tritos NA: Effects of recombinant human growth hormone therapy on bone mineral density in adults with growth hormone deficiency: A meta-analysis. J Clin Endocrinol Metab 2014;99:852–860. [DOI] [PubMed] [Google Scholar]
- 15. Morselli LL, Nedeltcheva A, Leproult R, et al. : Impact of GH replacement therapy on sleep in adult patients with GH deficiency of pituitary origin. Eur J Endocrinol 2013;168:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corpas E, Harman SM, Blackman MR: Human growth hormone and human aging. Endocr Rev 1993;14:20–39. [DOI] [PubMed] [Google Scholar]
- 17. Bernardi F, Petraglia F, Seppala M, et al. : Somatotropic axis and body weight in pre-menopausal and post-menopausal women: Evidence for a neuroendocrine derangement, in absence of changes of insulin-like growth factor binding protein concentrations. Hum Reprod 1998;13:279–284. [DOI] [PubMed] [Google Scholar]
- 18. Veldhuis JD, Liem AY, South S, et al. : Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab 1995;80:3209–3222. [DOI] [PubMed] [Google Scholar]
- 19. Bennett RM: Disordered growth hormone secretion in fibromyalgia: A review of recent findings and a hypothesized etiology. Z Rheumatol 1998;57 Suppl 2:72–76. [DOI] [PubMed] [Google Scholar]
- 20. Paiva ES, Deodhar A, Jones KD, Bennett R: Impaired growth hormone secretion in fibromyalgia patients: Evidence for augmented hypothalamic somatostatin tone. Arthritis Rheum 2002;46:1344–1350. [DOI] [PubMed] [Google Scholar]
- 21. van Liempt S, Vermetten E, Lentjes E, Arends J, Westenberg H: Decreased nocturnal growth hormone secretion and sleep fragmentation in combat-related posttraumatic stress disorder; potential predictors of impaired memory consolidation. Psychoneuroendocrinology 2011;36:1361–1369. [DOI] [PubMed] [Google Scholar]
- 22. Homburg R, Singh A, Bhide P, Shah A, Gudi A: The re-growth of growth hormone in fertility treatment: A critical review. Hum Fertil (Camb) 2012;15:190–193. [DOI] [PubMed] [Google Scholar]
- 23. Lee HS, Park YS, Lee JS, Seo JT: Serum and seminal plasma insulin-like growth factor-1 in male infertility. Clin Exp Reprod Med 2016;43:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Cauter E, Leproult R, Plat L: Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000;284:861–868. [DOI] [PubMed] [Google Scholar]
- 25. Obal F Jr., Krueger JM: The somatotropic axis and sleep. Rev Neurol (Paris) 2001;157:S12–S15. [PubMed] [Google Scholar]
- 26. Roubenoff R, Rall LC, Veldhuis JD, et al. : The relationship between growth hormone kinetics and sarcopenia in postmenopausal women: The role of fat mass and leptin. J Clin Endocrinol Metab 1998;83:1502–1506. [DOI] [PubMed] [Google Scholar]
- 27. Tam CS, Johnson WD, Rood J, Heaton AL, Greenway FL: Increased human growth hormone after oral consumption of an amino acid supplement: Results of a randomized, placebo-controlled, double-blind, crossover study in healthy subjects. Am J Ther 2020;27:e333–e337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alba-Roth J, Muller OA, Schopohl J, von Werder K: Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab 1988;67:1186–1189. [DOI] [PubMed] [Google Scholar]
- 29. Ghigo E, Arvat E, Valente F, et al. : Arginine reinstates the somatotrope responsiveness to intermittent growth hormone-releasing hormone administration in normal adults. Neuroendocrinology 1991;54:291–294. [DOI] [PubMed] [Google Scholar]
- 30. Merimee TJ, Rabinowtitz D, Fineberg SE: Arginine-initiated release of human growth hormone. Factors modifying the response in normal man. N Engl J Med 1969;280:1434–1438. [DOI] [PubMed] [Google Scholar]
- 31. Low MJ: Neuroendocrinology. In: Williams Book of Endocrinology, 13th Edition (Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds.). Elsevier, Philadelphia, PA, 2016, p. 1936. [Google Scholar]
- 32. Hansen M, Morthorst R, Larsson B, et al. : Effects of 2 wk of GH administration on 24-h indirect calorimetry in young, healthy, lean men. Am J Physiol Endocrinol Metab 2005;289:E1030–E1038. [DOI] [PubMed] [Google Scholar]
- 33. Senn S: Cross-Over Trials in Clinical Research. John Wiley & Sons Ltd., West Sussex, England, 1993. [Google Scholar]
- 34. Rudman D, Feller AG, Nagraj HS, et al. : Effects of human growth hormone in men over 60 years old. N Engl J Med 1990;323:1–6. [DOI] [PubMed] [Google Scholar]
- 35. Vahl N, Jorgensen JO, Jurik AG, Christiansen JS: Abdominal adiposity and physical fitness are major determinants of the age associated decline in stimulated GH secretion in healthy adults. J Clin Endocrinol Metab 1996;81:2209–2215. [DOI] [PubMed] [Google Scholar]
- 36. Russell W, Harrison RF, Smith N, et al. : Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 2008;93:2300–2306. [DOI] [PubMed] [Google Scholar]
- 37. PASS 2020 Power Analysis and Sample Size Software. NCSS, LLC., Kaysville, UT.2020. ncss.com/software/pass (accessed August 28, 2020).
- 38. Astorino TA, Allen RP, Roberson DW, Jurancich M: Effect of high-intensity interval training on cardiovascular function, VO2max, and muscular force. J Strength Cond Res 2012;26:138–145. [DOI] [PubMed] [Google Scholar]
- 39. Vehrs PR, Keller DM, George JD, Hager RL, Fellingham GW: Monitoring VO2max during fourteen weeks of endurance training using the CardioCoach. J Strength Cond Res 2007;21:62–66. [DOI] [PubMed] [Google Scholar]
- 40. Brzezinski A, Vangel MG, Wurtman RJ, et al. : Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med Rev 2005;9:41–50. [DOI] [PubMed] [Google Scholar]
- 41. Ceda GP, Ceresini G, Denti L, et al. : Alpha-glycerylphosphorylcholine administration increases the GH responses to GHRH of young and elderly subjects. Horm Metab Res 1992;24:119–121. [DOI] [PubMed] [Google Scholar]
- 42. Muller EE, Rigamonti AE, Colonna Vde G, Locatelli V, Berti F, Cella SG: GH-related and extra-endocrine actions of GH secretagogues in aging. Neurobiol Aging 2002;23:907–919. [DOI] [PubMed] [Google Scholar]
- 43. Spiliotis BE: Growth hormone insufficiency and its impact on ovarian function. Ann N Y Acad Sci 2003;997:77–84. [DOI] [PubMed] [Google Scholar]
- 44. Pekarovics S, Romans J, Beres A, Kelly C, Heaton A, Greenway F: SUN-439 improvement in insulin-like growth factor-1 and clinical symptoms: Results of an open-label, single-arm study of a human growth hormone-enhancing amino acid supplement. J Endocr Soc 2019;3(Supplement_1). [Google Scholar]
- 45. Pekarovics S, Romans J, Billes SK, Heaton A, Greenway FL: LB SUN-57 Increased IGF-1 and improvement in fibromyalgia symptoms with an hGH secretagogue: Result of three case studies. Endocr Rev 2017;38. [Google Scholar]
- 46. Baker LD, Barsness SM, Borson S, et al. : Effects of growth hormone-releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: Results of a controlled trial. Arch Neurol 2012;69:1420–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV: Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 2004;27:1255–1273. [DOI] [PubMed] [Google Scholar]
- 48. White HK, Petrie CD, Landschulz W, et al. : Effects of an oral growth hormone secretagogue in older adults. J Clin Endocrinol Metab 2009;94:1198–1206. [DOI] [PubMed] [Google Scholar]
- 49. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ: The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]