Abstract
Repetitive mild traumatic brain injury (mTBI) has been called the “signature injury” of military service members in the Iraq and Afghanistan wars and is highly comorbid with post-traumatic stress disorder (PTSD). Correct attribution of adverse blast-induced mTBI and/or PTSD remains challenging. Pre-clinical research using animal models can provide important insight into the mechanisms by which blast produces injury and dysfunction—but only to the degree by which such models reflect the human experience. Avoidance of trauma reminders is a hallmark of PTSD. Here, we sought to understand whether a mouse model of blast reproduces this phenomenon, in addition to blast-induced physical injuries. Drawing on well-established work from the chronic stress and Pavlovian conditioning literature, we hypothesized that even while one is anesthetized during blast exposure, environmental cues encountered in the peri-blast environment could be conditioned to evoke aversion/dysphoria and re-experiencing of traumatic stress. Using a pneumatic shock tube that recapitulates battlefield-relevant open-field blast forces, we provide direct evidence that stress is inherent to repetitive blast exposure, resulting in chronic aversive/dysphoric-like responses to previous blast-paired cues. The results in this report demonstrate that, although both single and repetitive blast exposures produce acute stress responses (weight loss, corticosterone increase), only repetitive blast exposure also results in co-occurring aversive/dysphoric-like stress responses. These results extend appreciation of the highly complex nature of repetitive blast exposure; and lend further support for the potential translational relevance of animal modeling approaches currently used by multiple laboratories aimed at elucidating the mechanisms (both molecular and behavioral) of repetitive blast exposure.
Keywords: blast, post-traumatic stress disorder, stress, traumatic brain injury, veteran
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability, affecting every segment of the population, with youth, the elderly, and athletes being most affected. Likewise, following a traumatic event, post-traumatic stress disorder (PTSD) is common and affects 5–10% of the adult population of United States. Moreover, mild traumatic brain injury (mTBI/concussion) is considered the “signature insult” of military service members of Operation Iraqi Freedom/Enduring Freedom/New Dawn (OIF/OEF/OND),1,2 is highly comorbid with PTSD, and is a major source of morbidity among veterans enrolled in the Veterans Administration (VA) healthcare system. Such symptoms are common, with ∼350,000 veteran mTBIs diagnosed since 2000 (estimated post-deployment rates of 10–25%), with an estimated PTSD comorbidity rate of 50–75%.2–4 Blast exposure caused by detonation of high explosives is the primary source of mTBI (accounting for 75% of all TBIs reported by veterans), with multiple exposures more common than a single blast exposure.2,5 Efforts to elucidate the biological processes responsible for the clinical manifestations of blast-related mTBI and PTSD are impeded by several factors that include: (1) high rates of comorbidity, (2) overlapping symptoms, and (3) the initiating insult (i.e. blast exposure) simultaneously inducing both biomechanical/neurological injuries and substantial neuropsychological stress.6,7 Indeed, clinicians have wrestled with this medical conundrum for >100 years since the initial descriptions of “shell shock” among World War I soldiers exposed to artillery bombardments.8,9 There are at least two competing hypotheses that address the apparent connection between mTBI and PTSD following blast exposure: (1) blast exposure is both a TBI-causing event and a PTSD-related stressor or (2) TBI-induced compensatory changes in stress-related brain regions produce outcomes similar in nature to those seen in PTSD and/or render the brain more susceptible to subsequent stressors (or vice versa – PTSD-related trauma damages brain regions involved in post-concussive symptom outcomes). Following mTBI exposure in rodents, we and others have published evidence of post-concussive syndrome-like and PTSD-like outcomes in both rats and mice.10–15 Anesthesia is commonly used in these pre-clinical studies. In these settings, the origin of the precipitating blast-induced trauma (physical injury and/or psychological stress) while animals are anesthetized has been viewed alternately as a complicating factor or a useful reductionistic tool in working toward a better understanding of the mechanisms by which blast exposure results in chronic PTSD-related symptoms.7,15 Although physical injury and neurological damage can result in acute activation of stress pathways, whether blast exposure produces a resulting chronic affective representation of that stress (e.g. PTSD-like aversion/dysphoria to trauma reminders) remains under-studied. Translational research efforts using rodent models can provide much-needed insight into underlying mechanisms by which blast exposure produces dysfunction, but only if these models recapitulate both battlefield exposure conditions and the subsequent injuries and dysfunction displayed by individuals with blast-related mTBI. In this report, we sought to understand the extent to which rodent models of blast exposure reproduce the phenomenon experienced by military service members and veterans with comorbid mTBI and PTSD.
Drawing upon fundamental concepts regarding chronic stress and Pavlovian fear learning,16–22 we postulated that despite the animals being anesthetized during the blast exposure itself, stress responses from events before and after the blast exposure are sufficient to elicit lasting cognitive representations of trauma exposure. We further postulated that such stress responses induce a negative hedonic state with motivational properties that can be associated with neutral cues (odors, visual patterns) to engender subsequent avoidance of and/or re-experiencing-induced stress and dysphoria from those associated cues.16,17,19,22–24 To test these ideas, we examined acute physiological and behavioral stress responses to blast exposure and used place conditioning to measure avoidance and examine behavioral (locomotion and vocalizations) and physiological (corticosterone production) stress responses provoked by re-exposure to blast-related cues. Ultrasonic vocalizations (USVs) are thought to vary with behavioral context, with lower kHz USVs primarily seen during aversive scenarios such as restraint stress or social isolation,25–27 thereby providing a sensitive readout of the animals' affective state.
Using a well-established electronically controlled pneumatic shock tube that models battlefield-relevant open-field blast forces generated by detonation of high explosives,10,11,28–30 we found that whereas both single and repetitive blast exposure result in acute stress responses, only repetitive blast exposure produces chronic aversion and dysphoria to prior blast-paired environmental cues. These results offer new insight regarding how repetitive blast exposure may give rise to PTSD-like symptoms. In addition, these findings support the idea that this animal model simultaneously provokes both neurological and psychological insults, as in military service members with blast-related comorbid mTBI and PTSD, thus demonstrating a previously unappreciated translational aspect of repetitive blast trauma in rodent models.
Methods
Animals and mouse model of blast overpressure (BOP)
Male C57Bl/6 mice (Jackson Laboratory) 3–4 months of age (weight 22–35 g; mean 27.0 ± 0.2 g) were used. All animal experiments were conducted in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines and were approved by the VA Puget Sound Institutional Animal Care and Use Committees. The shock tube (Baker Engineering and Risk Consultants, San Antonio, TX) was designed to generate BOP that mimic open-field high explosive detonations encountered by military service members in combat, and the design and modeling characteristics have been described in detail elsewhere.10,28,29
Briefly, mice were anesthetized with isoflurane (induced at 5% and maintained at 2–3%), secured on a gurney, and placed in the shock tube oriented perpendicular to the oncoming blast wave (ventral body surface facing the oncoming shock wave).31 Sham (control) animals received anesthesia only for a duration matched to blast animals. Repeated blast/sham exposures occurred successively over the course of 3 days (one per day). Following exposure, mice were immediately removed from the shock tube and anesthesia was discontinued (anesthesia duration ranged from 3 to 4 min). Mice were then placed in a warm enclosure for observation during recovery. The BOP peak intensity (psi), initial pulse duration (ms), and impulse (psi·ms) used were in keeping with mild to moderate blast exposure31,32 (20.23 psi ±0.08 psi; 5.797 ms ±0.017 ms; 0.037 psi × ms × 0.000 psi × ms) (Fig. 1A). Under these experimental conditions, the survival rate was 96%, with blast-exposed mice appearing comparable to sham-exposed mice by inspection 2–4 h post-blast exposure as previously reported.10,11,28,29 Animals were weighed daily prior to sham/blast exposure and at 24 and 72-h post-exposure. There were no statistically significant weight differences between 1x sham (n = 9) and 3x sham (n = 18)-treated mice (24 h post-exposure: Student's unpaired t test, t[25] = 0.355, p > 0.05;. 72 h post-exposure: Student's unpaired t test, t[25] = 0.9368, p > 0.05); therefore, 1x and 3x sham animals at each time point were pooled together for subsequent weight analyses.
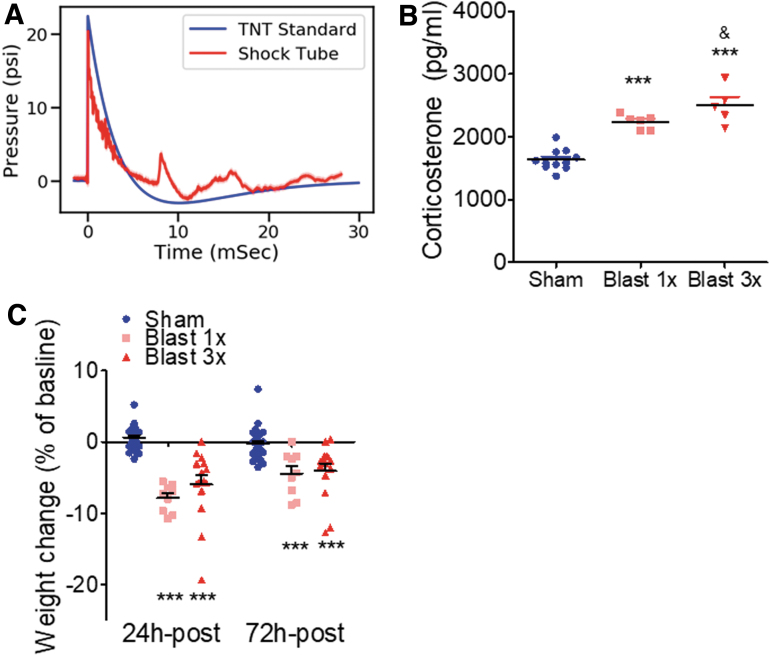
FIG. 1.
Blast-induced acute stress response. (a) Time versus pressure plot (averaged over 350 blasts) measuring the static blast overpressure (measured 5 cm above the animal). Note close correspondence with the superimposed Friedlander waveform expected from an open-field detonation of ∼20 kg of TNT at a distance of 7.8 m. (b) Blast exposure results in blood corticosterone increase. ***p ≤ 0.0001: sham versus blast. (c) Blast exposure results in acute weight loss that lasts at least 3 days. ***p ≤ 0.0001: sham versus blast. Color image is available online.
A subset of animals was euthanized 30 min after the last exposure, and trunk blood was collected. Serum samples were processed to assay corticosterone levels using an enzyme-linked immunosorbent assay (ELISA) kit as per manufacture protocol (Arbor Assays, Ann Arbor, MI). There were no statistically significant corticosterone differences between 1x sham (n = 6) and 3x sham (n = 6)-treated mice (Student's unpaired t test, t[10] = 0.355, p > 0.05;. 72 h post-exposure: Student's unpaired t test, t[25] = 0.836, p > 0.05); therefore, 1x and 3x sham animals were pooled together for subsequent corticosterone analyses.
Odorant conditioning paradigm
See Figure 2A for experimental schematic. One week prior to sham/blast exposure, animals were first pre-exposed to a Plexiglas T-Maze (66 cm long × 40 cm wide × 15 cm high) for 5 min. A stainless-steel mesh tea-ball (Amazon) containing a clean quarter Nestletts (PharmaServ, Framingham, MA) was also placed into each home cage to allow for pre-exposure. The following week, sham/blast exposure occurred on 3 consecutive days. On each day of sham/blast exposure, animals received a stainless-steel mesh tea-ball (Amazon) containing one-quarter Nestletts (PharmaServ, Framingham, MA) with 20 μL of imitation almond extract (Kroger, Cincinnati, OH) in their home cage 5 min prior to sham/blast exposure. The Nestletts and scent were refreshed on each morning of sham/blast exposure and the tea-ball remained in place until 24 h after the final sham/blast exposure. One month following exposure, animals were tested for odorant-conditioning in the T-Maze with a tea-ball containing one-quarter Nestlett with 20 μL imitation almond odorant cue placed in the left arm of the maze and a tea-ball containing one-quarter Nestlett with 20 μL saline placed in the opposite arm of the maze. Animals were placed in the long arm of the T-maze and given 5 min to explore the entire maze. Latency to enter and time spent in each of the two distal ends of the short arms was recorded and analyzed using Anymaze (Stoelting, Wood Dale, IL). There were no statistically significant differences between 1x sham (n = 5) and 3x sham (n = 6)-treated mice (saline corner time: Student's unpaired t test, t[9] = 0.537, p > 0.05; odor corner time: Student's unpaired t test, t[9] = 0.036, p > 0.05; saline corner latency: Student's unpaired t test, t[9] = 0.712, p > 0.05; odor corner time: Student's unpaired t test, t[9] = 1.113, p > 0.05); therefore, 1x and 3x sham animals were pooled together for subsequent odorant-pairing analyses.
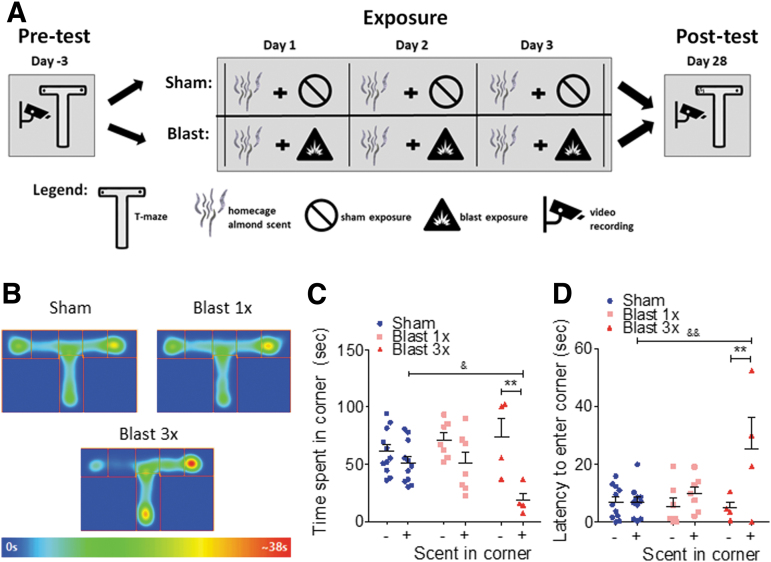
FIG. 2.
Blast-induced odorant aversion. (a) Schematic of odorant-blast aversion paradigm. (b) Heat maps of odorant aversion post-test (odorant placed in left corner of T). (c) Significant aversion to repetitive blast/odor pairings 1 month post-injury, with no aversion seen from sham or a single blast/odor pairing. (d) Latency to enter to odorant corner is significantly increased 1 month post repetitive blast/odor pairings. Two-way repeated measures analysis of variance (RM ANOVA) post-hoc Bonferroni Multiple Comparison Test. **p ≤ 0.001: + odor corner versus – odor corner, &p ≤ 0.05 and &&p ≤ 0.001: sham versus blast odor corner. Error bars are mean ± standard error of the mean (SEM). Color image is available online.
Place conditioning paradigm
Figure 3A illustrates the experimental design. A balanced three-compartment conditioning apparatus was used as described previously.17 One week prior to sham/blast exposure, animals were pre-tested by placing individual animals in the small central compartment and allowing exploration of the entire apparatus for 20 min. Time spent in each compartment was recorded and analyzed using Anymaze. Mice were randomly assigned an a.m. and p.m. box (either gray walls or vertical black and white strip walls). The following week, sham/blast exposure occurred on 3 consecutive days. On each day of exposure, in the morning, animals were first placed in their a.m.-pairing chamber containing distinct visual cues for 10 min, and then were immediately given a sham exposure. In the afternoon, animals were placed in their p.m.-pairing chamber containing a different set of distinct visual cues for 10 min and then were immediately given a blast or sham exposure (depending on group assignment). Place conditioning was assessed at 1 and 3 months following repetitive exposure by allowing the mice to roam freely in all three compartments. Time spent in each compartment was recorded and analyzed using Anymaze (Stoelting, Wood Dale, IL). Place conditioning scores were calculated by subtracting the time spent in the p.m.-paired compartment from the time spent in the a.m.-paired compartment.
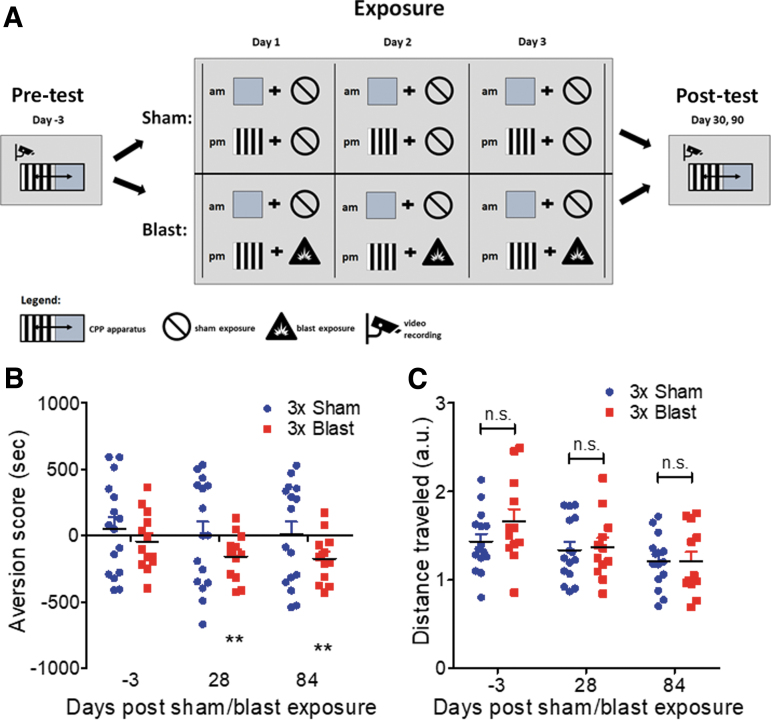
FIG. 3.
Blast-induced place aversion. (a) Schematic of blast-induced place aversion paradigm. (b) Significant aversion to blast-paired but not sham-paired cues at 1 month and 3 months post-injury. (c) No difference in locomotion during place aversion post-test across groups and time points. Student's one-sample t test versus theoretical of 0 (no aversion). Two-way analysis of variance (ANOVA) post-hoc Bonferroni. **p ≤ 0.001: sham versus blast or neutral versus paired. Values represent mean ± standard error of the mean (SEM). Color image is available online.
Cue conditioning and re-exposure paradigm
See Figure 4A for an experimental schematic. Sham/blast exposure occurred on 3 consecutive days. On each day of exposure, animals were first placed in a pairing chamber containing distinct visual cues (randomly assigned to gray or black and white striped walls) for 10 min, and then were immediately given a sham or blast exposure (depending on group assignment). One month following repetitive exposure, the animals were re-exposed to either a neutral chamber or the chamber previously paired with blast or sham for 10 min, and movement (via Anymaze [Stoelting, Wood Dale, IL]), and ultrasonic vocalizations were recorded. Blood was collected from the submandibular vein 1 day prior and 30 min after removal from the pairing chamber. Plasma samples were processed to assay corticosterone levels using an ELISA kit as per manufacture protocol (Arbor Assays, Ann Arbor, MI). USVs were recorded using a Petterson microphone (Norway, model M500-384) and Avisoft SASLab Lite recording software and were manually analyzed using RavenLite (Cornell lab of Ornithology).
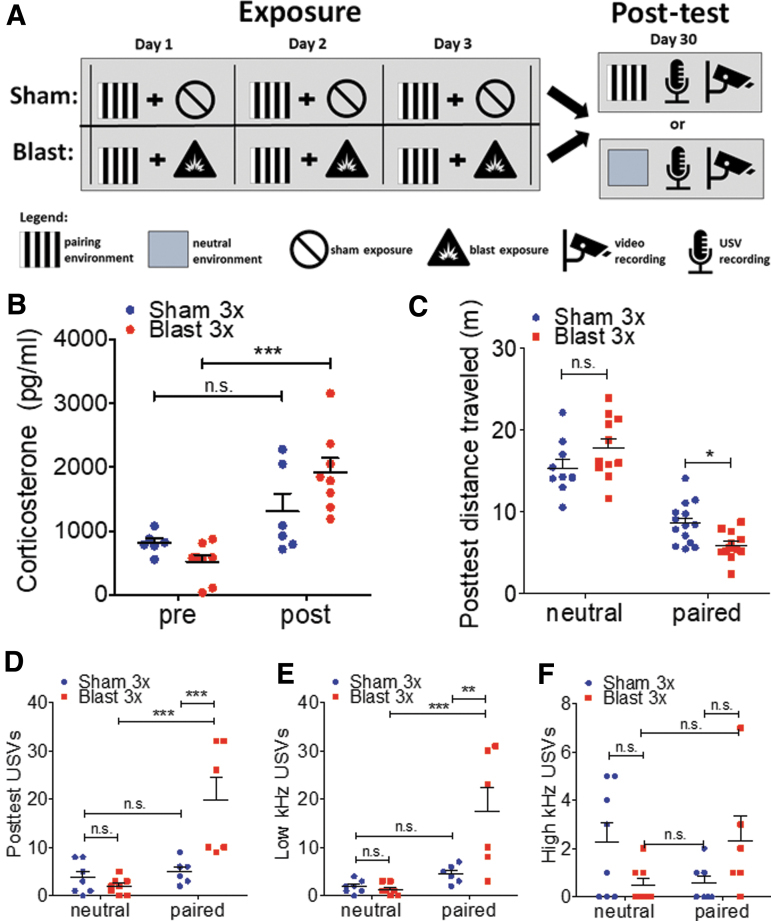
FIG. 4.
Blast-induced stress responses to cue re-exposure. (a) Schematic of blast-induced environmental pairing and re-exposure paradigm. (b) Significant increase in plasma corticosterone following re-exposure to blast-paired but not sham-paired cues 1 month post-injury. (c) Significant decrease in locomotion during re-exposure to blast-paired but not sham-paired or neutral cues 1 month post-injury. (d–f) Aversive (low kHz) ultrasonic vocalizations are significantly increased following re-exposure to blast-paired but not sham-paired or neutral cues 1 month post-injury. Student's one-sample t test versus hypothetical mean = 1.0. Two-way analysis of variance (ANOVA) post-hoc Bonferroni. *p ≤ 0.05, **p ≤ 0.001, and ***p ≤ 0.0001: sham versus blast or neutral versus paired. Values represent mean ± standard error of the mean (SEM). Color image is available online.
Statistical analysis
Where appropriate, data were analyzed using: (1) two-tailed Student's t tests and (2) one-way or two-way (between/within subjects design) repeated or non-repeated measures analysis of variance (ANOVA), followed by Newman–Keuls Multiple Comparison Tests or Bonferroni post-hoc tests, respectively. Reported p values denote two-tailed probabilities of p ≤ 0.05 and non-significance (n.s.) indicates p > 0.05. Statistical analyses were conducted using Python, Graph Pad Prism 4.0 (GraphPad Software, Inc., La Jolla, CA), and SPSS software (IBM, Armonk, NY).
Results
Acute stress responses after blast exposure
Using well-established methods,10,11,28–30 male C57Bl/6 adult mice were exposed to one or three BOPs using a pneumatic shock tube delivering a peak static pressure of 20.23 psi ±0.08 psi, positive phase duration of 5.80 ms ±0.02 ms; 0.037 psi × ms ±0.000 psi × ms (Fig. 1A).
Stressful events commonly elicit release of corticosterone (in rodents); therefore, we measured the level of corticosterone in blood 30 min following exposure to one or three BOPs (or sham exposure). There were no statistically significant differences between 1x sham and 3x sham-treated mice. Therefore, 1x and 3x sham animals were pooled together for subsequent corticosterone analysis (see Methods for sham comparison statistics). In accordance with an acute stress response, blast exposure resulted in a significant increase in blood corticosterone (one-way ANOVA: F[2,21] = 41.79, p < 0.0001, Bonferroni's Multiple Comparison Test post-hoc: sham n = 11, blast 1x n = 6, blast 3x n = 5). Post-hoc analyses demonstrate significant corticosterone increases in both 1x and 3x blast animals as compared with sham control. Likewise, there was a significant increase in corticosterone in 3x blast animals as compared with 1x blast animals (Fig. 1B). Stress exposure in mice commonly results in weight loss; therefore, we measured body weight at baseline, 24 h, and 72 h following sham or blast exposure. There were no statistically significant differences between 1x sham and 3x sham-treated mice. Therefore, 1x and 3x sham animals at each time point were pooled together for subsequent weight analyses (see Methods for sham comparison statistics). In accordance with an acute stress response, blast exposure resulted in acute weight loss (two-way repeated measures [RM] ANOVA: interaction effect F[2,46] = 12.95, p < 0.0001, Bonferroni's Multiple Comparison Test post-hoc: sham n = 25, blast 1x n = 9, blast 3x n = 15). Post-hoc analyses demonstrate significant weight loss for both 1x and 3x blast animals at both 24 h and 72 h following exposure (Fig. 1C).
Chronic aversion to cues previously paired with blast
To further investigate potential blast-induced stress responses, we employed a conditioned odorant paradigm in mice that paired sham or blast exposure with a neutral odorant cue (Fig. 2A). The neutral odorant cue (almond scent) was placed in the home cage 5 min prior to the first sham or blast exposure and remained in place (scent refreshed daily) until 24 h after the final sham or blast exposure. Finally, conditioning to the paired-odorant cue was assessed 1 month following exposure. There were no statistically significant differences between 1x sham and 3x sham-treated mice. Therefore, 1x and 3x sham animals at each time point were pooled together for subsequent odorant pairing analyses (see Methods for sham comparison statistics). The panels in Figure 2B show occupancy heat maps for each group during the post-test (odorant is placed in left corner of T-maze) 1 month following the exposure/odorant pairings. Mice developed a significant aversion to the odorant when subsequently presented alone in one corner of the testing chamber (left corner of T-maze) (two-way RM ANOVA: main effect of location F[1,19] = 11.83, p = 0.003, Bonferroni's Multiple Comparison Test post-hoc: sham n = 11, blast 1x n = 7, blast 3x n = 4) (Fig. 2C). Post-hoc analyses demonstrated significant aversion following only repetitive (3x, one per day) but not single (1x) blast or sham exposure. Odorant-blast pairings also increased the latency to enter the odorant corner in a blast number-dependent manner (two-way RM ANOVA: main effect of location F[1,19] = 8.798, p = 0.008, Bonferroni's Multiple Comparison Test post-hoc: sham n = 11, blast 1x n = 7, blast 3x n = 4) (Fig. 2D).
To assess the generality of these observations, the ability of repetitive blast exposure to induce place aversion was investigated using a modified place conditioning paradigm in mice that pairs sham and blast exposure with distinct visual cues (Fig. 3A). On each day of exposure, in the morning, animals were first placed in a pairing chamber containing distinct visual cues for 10 min, and then were immediately given a sham exposure (e.g., anesthesia only). Then, in the afternoon, animals were placed in a pairing chamber containing a different set of distinct visual cues for 10 min and then were immediately given a blast or sham exposure. Conditioned aversion to the blast-paired compartment was evident 1 month post-blast exposure and was sustained until at least 3 months post-blast exposure (one-sample t test vs. a theoretical mean of 0 [no aversion]; sham baseline: t[15] = 1.372, p = 0.1903, sham 1 month: t[14] = 1.479, p = 0.1613, sham 3 months: t[13] = 0.5922, p = 0.5639; blast baseline: t[14] = 0.1919, p = 0.8506 blast 1 month: t[14] = 3.346, p = 0.0048, blast 3 months: t[12] = 3.256, p = 0.00679) (Fig. 3B). Despite significant place aversion, blast exposure did not affect total distance traveled during the post-test, suggesting that aversion was not a non-specific effect of locomotion changes (two-way RM ANOVA: interaction F[2,50] = 0.931, p = 0.401, Bonferroni's Multiple Comparison Test post-hoc: sham n = 16, blast 3x n = 15) (Fig 3C.). Taken together, these data demonstrate that a single blast exposure was sufficient to produce acute stress responses. However, only repetitive blast exposure induced significant conditioned aversion.
Dysphoric and aversive behavioral response to visual cues previously paired with blast
In order to investigate whether cues previously paired with blast or sham exposure elicited distinct physiological and behavioral responses during re-exposure, we extended the modified place conditioning paradigm described previously to allow for re-exposure to either a neutral visual environment or one that was previously paired with exposure (Fig. 4A). On each day of exposure, animals were first placed in a pairing chamber containing distinct visual cues for 10 min, and were then immediately given a sham or blast exposure. One month following repetitive exposure, animals were re-exposed to either a neutral chamber or the chamber previously paired with exposure (blast or sham) for 10 min, movement and USVs were recorded, and blood was collected (pre/post re-exposure) for subsequent plasma corticosterone analysis. Differential blood corticosterone responses were detected pre/post re-exposure to sham/blast-paired cues (two-way ANOVA: interaction F[1,12] = 7.998, p = 0.0152, Bonferroni's Multiple Comparison Test post-hoc: sham n = 6, blast 3x n = 8) (Fig. 4B). Post-hoc analyses demonstrate a significant corticosterone increase in blast but not sham animals (pre/post re-exposure). Differential behavioral responses were also demonstrated following re-exposure to neutral versus sham/blast-paired cues (two-way ANOVA: interaction effect F[1,43] = 9.462, p = 0.0036, Bonferroni's Multiple Comparison Test post-hoc: sham n = 13, blast 3x n = 11) (Fig. 4C). Post-hoc analyses demonstrated a significant decrease in distance traveled during re-exposure to blast-paired cues as compared with sham-paired cues with no difference when re-exposed to neutral cues. In addition to movement, we also recorded USVs during re-exposure as an additional read-out of affective state. Total USVs were significantly increased in blast-exposed mice during re-exposure to the sham/blast-paired but not neutral-paired cues (two-way ANOVA: interaction effect F[1,23] = 9.714, p = 0.0049, Bonferroni's Multiple Comparison Test post-hoc: sham n = 8, blast 3x n = 6) (Fig. 4D). In line with an aversive/dysphoric stress response to reminders of blast exposure, low kHz USVs were specifically increased during re-exposure to the sham/blast paired but not neutral-paired cues (two-way ANOVA: interaction effect F[1,23] = 8.820, p = 0.0069, Bonferroni's Multiple Comparison Test post-hoc: sham n = 8, blast 3x n = 6) (Fig. 4E). Post-hoc analyses demonstrated a significant increase in low kHz USVs during re-exposure to blast-paired cues as compared with sham-paired cues, with no difference when re-exposed to neutral cues. Although a significant interaction effect was also seen in the number of high kHz USVs (two-way ANOVA: interaction effect F[1,23] = 5.206, p = 0.0321, Bonferroni's Multiple Comparison Test post-hoc: sham n = 8, blast 3x n = 6) (Fig. 4F), post-hoc analyses did not demonstrate significant differences between cues or blast and sham exposure.
Discussion
Blast-induced mTBI is currently defined as cellular and/or structural damage to the brain, which can cause adverse somatic, vestibular, cognitive, and affective symptoms.2,33 PTSD is an anxiety disorder that develops after exposure to potentially life-threatening stress (such as blast) and persistent symptoms include re-experiencing, avoidance, and hyperarousal.21,34,35 As such, blast-induced mTBI is commonly associated with the development of battlefield PTSD, and the two conditions share several overlapping symptoms. Indeed, some have postulated that post-concussive symptoms following mTBI are non-specifically related to physical blast injury, and instead better explained by psychological trauma consistent with PTSD.6,12 Because battlefield blast exposures can simultaneously provoke both psychological and neurological insults, understanding the basis for chronic symptoms in dual-diagnosis (e.g., mTBI and PTSD) veterans has remained challenging.
Translational research efforts using rodent models are thought to be free from many of the confounding variables associated with human exposures in the battlefield, providing much needed insight into underlying mechanisms by which blast exposure produces dysfunction, but only in so far as these models are sufficiently translationally relevant. Drawing from long-established chronic stress and Pavlovian conditioning research,16–22 the results herein strongly argue that the conditions surrounding the immediate blast exposures per se are sufficient to drive Pavlovian learning and to generate subsequent PTSD-like behavioral outcomes. Specifically, these data directly support the notion that blast exposure in this animal model can give rise to stress responses (both physiological and behavioral) that elicit subsequent PTSD-like avoidance, intrusive symptoms, and mood alteration (e.g. dysphoria).36,37 We utilized place conditioning as an objective measure of behavior and to infer a relationship between the aversion exhibited at time of testing to previous stress and dysphoria at the time of conditioning. Indeed, Land and coworkers16 operationally defined dysphoria as the emotional response to a sustained stimulus that creates aversion and is thought to represent the underlying emotional state at the time of stimulus exposure. Importantly, we demonstrate chronic effects that persist for at least 3 months following blast exposure, an important requirement to differentiate PTSD from acute stress disorder in humans as per the Diagnostic and Statistical Manual of Mental Disorders (DSM). Although PTSD-like aversion-related outcomes were only seen in animals exposed to repetitive blast, acute stress effects related to increased corticosterone and weight loss were apparent following a single blast exposure, raising the possibility that a single blast might model aspects of acute stress disorder without progressing to full PTSD-like outcomes.
In most animal studies, blast exposure is performed under anesthetized, and therefore potentially restricting conditions, so that the animals will form cognitive associations with the immediate blast exposure. Under such conditions, it is well established that blast exposure produces chronic PTSD-like behavioral responses such as increased anxiety and exaggerated startle response, raising the possibility that cellular/structural insults to the brain caused by blast may be sufficient to drive pathogenic processes leading to PTSD-like symptoms with limited attention to psychological trauma and/or stress.15 Some studies have used an additional stressor at the time of injury (e.g., dual exposure) in order to study mTBI/PTSD comorbidity.38–41 The findings in this report demonstrate that repetitive blast exposure alone can generate traumatic stress responses sufficient to drive subsequent aversion and dysphoria, despite anesthesia and without the requirement of an additional, experimentally administered stressor.
In a typical pre-clinical Pavlovian fear/stress conditioning experiment, a rodent receives repeated presentations of a conditioning stimulus (CS) (e.g., an environmental chamber, odor, or auditory cue) that coincide with presentation of an unconditioned stimulus (US) (e.g. shock, predator odor, or social defeat). Subsequently, the animal will display a variety of conditioned responses upon later re-exposure to the CS (e.g., avoidance, freezing, increased heart rate and blood pressure, corticosterone release, USVs). Importantly, the typical blast exposure paradigm provides opportunities for Pavlovian learning (even in the absence of experimenter-delivered conditioned stimuli). For example, animals must first be transported from the vivarium to a blast holding room where they stay until transferred to the blast exposure room. Once transferred to the blast exposure room, animals are placed in an anesthesia induction chamber, anesthetized, exposed to blast, and then allowed to recover. Overall, although the actual blast exposure under anesthesia might only last 3–5 min, the entire episode outside of the vivarium might last anywhere from 10 min to hours (depending on location of vivarium vs. blast holding and exposure rooms). In the current study, we exposed animals to discrete Pavlovian cues (i.e, an almond scent or a distinct visual environment), but we also postulate that additional cues available surrounding events such as transport to and wait time in the blast holding room, transport to the blast exposure room, and placement in the anesthesia induction chamber might also serve as potential conditioning stimuli without intentional experimenter administration or manipulation. As such, long-standing cognition and behavior literature supports the idea that being awake and conscious during a traumatic event is not required for subsequent aversion and PTSD-like behavioral outcomes (e.g., drug-facilitated sexual assault, post-intensive care syndrome, and conditioned taste aversion to anesthetics.42–45) Indeed, we take precautions to limit unnecessary re-exposure to potential blast-related cues (for example, we use different colored drapes and gowns on blast exposure days vs. behavioral testing days, and we do not use the holding or blast rooms for any other procedures).
The idea that sedation can be used to dissect psychological trauma from neurological and cellular injury is inherently attractive from a reductionist viewpoint, especially when considering the contemporary significance of an as-yet-unresolved controversy regarding the underlying causes of the “shell shock” symptoms first described during World War I.46,47 The data herein demonstrate that repetitive blast is capable of provoking PTSD-like behavioral outcomes related to aversion and dysphoria, as well as the traumatic brain insults that we and others have reported previously in this mouse model.10–15 Therefore, on one hand this suggests that animal blast models can, like veterans with blast-related mTBI/PTSD, present a more complex biological puzzle than one might hope for. On the other hand, and perhaps more importantly, the findings in this report are encouraging because they demonstrate a previously unappreciated level of translational relevance in this animal model. Indeed, using blast-paired Pavlovian cues, it may be possible to conduct increasingly relevant studies of trauma re-exposure effects on mTBI- and PTSD-related adverse outcomes such as sleep quality, substance use and misuse, and aggression, facilitating understanding of the mechanisms of blast-induced mTBI/PTSD-like outcomes and speeding progress toward better treatments for veterans and service members with long-term blast-related health concerns.
Acknowledgments
We thank Greg Elder for insightful comments on the manuscript and also thank Scott Ng-Evans, Benjamin Land, Traci J Webber, Cindy Pekow, Kari Koszdin, and Monica Foley for considerable technical assistance and veterinary care.
Funding Information
This work was supported by Department of Veteran Affairs (VA) Basic Laboratory Research and Development (BLR&D) Career Development Award 1IK2BX003258 (A.G.S.), VA BLR&D Merit Review Award 5I01BX002311 (D.G.C.), VA Clinical Science Research and Development Career Development Award #CX-001787 (G.E.T.), VA Rehabilitation Research and Development Service Merit Review Award #B77421 (E.R.P.), University of Washington Friends of Alzheimer's Research (D.G.C., E.R.P.), UW Royalty Research Fund (D.G.C.), and the VA Northwest Mental Illness Research Education and Clinical Center (J.S.M., M.M.R., E.R.P.).
Disclaimer
The views expressed in this scientific presentation are those of the author(s) and do not reflect the official policy or position of the United States government or the Department of Veterans Affairs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. O'Neil, M.E., Carlson, K., Storzbach, D., Brenner, L., Freeman, M., Quinones, A., Motu'apuaka, M., Ensley, M., and Kansagara, D. (2013). in Complications of Mild Traumatic Brain Injury in Veterans and Military Personnel: A Systematic Review Washington, DC: Department of Veterans Affairs. [PubMed] [Google Scholar]
- 2. Tanielian, T., and Jaycox, L.H. (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, their Consequences, and Services to Assist Recovery. The RAND Center for Military Health Policy Research: Santa Monica, CA. [Google Scholar]
- 3. Hoge, C.W., McGurk, D., Thomas, J.L., Cox, A.L., Engel, C.C., and Castro, C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463. [DOI] [PubMed] [Google Scholar]
- 4. DVBIC (2019). DoD Numbers for Traumatic Brain Injury Worldwide. Washington, DC: Defense and Veterans Brain Injury Center, Department of Defense. [Google Scholar]
- 5. Owens, B.D., Kragh, J.F.Jr., Wenke, J.C., Macaitis, J., Wade, C.E., and Holcomb, J.B. (2008). Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J. Trauma 64, 295–299. [DOI] [PubMed] [Google Scholar]
- 6. Bryant, R. (2011). Post-traumatic stress disorder vs traumatic brain injury. Dialogues Clin. Neurosci. 13, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hendrickson, R.C., Schindler, A.G., and Pagulayan, K.F. (2018). Untangling PTSD and TBI: challenges and strategies in clinical care and research. Curr. Neurol. Neurosci. Rep. 18, 106. [DOI] [PubMed] [Google Scholar]
- 8. Jones, E., Fear, N.T., and Wessely, S. (2007). Shell shock and mild traumatic brain injury: a historical review. Am. J. Psychiatry 164, 1641–1645 [DOI] [PubMed] [Google Scholar]
- 9. Jones, E. (2006). Historical approaches to post-combat disorders. Philos. T. R. Soc. B 361, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schindler, A.G., Meabon, J.S., Pagulayan, K.F., Hendrickson, R.C., Meeker, K.D., Cline, M., Li, G., Sikkema, C., Wilkinson, C.W., Perl, D.P., Raskind, M.R., Peskind, E.R., Clark, J.J., and Cook, D.G. (2017). Blast-related disinhibition and risk seeking in mice and combat Veterans: potential role for dysfunctional phasic dopamine release. Neurobiol. Dis. 106, 23–34. [DOI] [PubMed] [Google Scholar]
- 11. Meabon, J.S., Huber, B.R., Cross, D.J., Richards, T.L., Minoshima, S., Pagulayan, K.F., Li, G., Meeker, K.D., Kraemer, B.C., Petrie, E.C., Raskind, M.A., Peskind, E.R., and Cook, D.G. (2016). Repetitive blast exposure in mice and combat veterans causes persistent cerebellar dysfunction. Sci. Transl. Med. 8, 321–326 [DOI] [PubMed] [Google Scholar]
- 12. Elder, G.A., Dorr, N.P., De Gasperi, R., Gama Sosa, M.A., Shaughness, M.C., Maudlin-Jeronimo, E., Hall, A.A., McCarron, R.M., and Ahlers, S.T. (2012). Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 29, 2564–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perez-Garcia, G., De Gasperi, R., Gama Sosa, M.A., Perez, G.M., Otero-Pagan, A., Tschiffely, A., McCarron, R.M., Ahlers, S.T., Elder, G.A., and Gandy, S. (2018). PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perez-Garcia, G., Gama Sosa, M.A., De Gasperi, R., Lashof-Sullivan, M., Maudlin-Jeronimo, E., Stone, J.R., Haghighi, F., Ahlers, S.T., and Elder, G.A. (2016). Exposure to a predator scent induces chronic behavioral changes in rats previously exposed to low-level blast: implications for the relationship of blast-related TBI to PTSD. Front. Neurol. 7, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perez-Garcia, G., Gama Sosa, M.A., De Gasperi, R., Tschiffely, A.E., McCarron, R.M., Hof, P.R., Gandy, S., Ahlers, S.T., and Elder, G.A. (2019). Blast-induced “PTSD”: Evidence from an animal model. Neuropharmacology 145, 220–229. [DOI] [PubMed] [Google Scholar]
- 16. Land, B.B., Bruchas, M.R., Lemos, J.C., Xu, M., Melief, E.J., and Chavkin, C. (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schindler, A.G., Li, S.A., and Chavkin, C. (2010). Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappa-opioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology 35, 1932–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruchas, M.R., Land, B.B., and Chavkin, C. (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heinrichs, S.C., and Koob, G.F. (2004) Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J. Pharmacol. Exp. Ther. 311, 427–440. [DOI] [PubMed] [Google Scholar]
- 20. Li, S.S.Y., and McNally, G.P. (2014). The conditions that promote fear learning: Prediction error and Pavlovian fear conditioning. Neurobiol. Learn. Mem. 108, 14–21. [DOI] [PubMed] [Google Scholar]
- 21. Richter-Levin, G., Stork, O., and Schmidt, M. V. (2019). Animal models of PTSD: a challenge to be met. Mol Psychiatry 24, 1135–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chavkin, C., Xu, M., Land, B.B., Redila, V., and Bruchas, M.R. (2006). Kappa opioid receptor activation by endogenous opioids mediates the dysphoric properties of stress. Neuropsychopharmacology 31, S40–S40. [Google Scholar]
- 23. Bruchas, M.R., Land, B.B., Aita, M., Xu, M., Barot, S.K., Li, S., and Chavkin, C. (2007). Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J. Neurosci. 27, 11614–11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edwards, S., Baynes, B.B., Carmichael, C.Y., Zamora-Martinez, E.R., Barrus, M., Koob, G.F., and Gilpin, N.W. (2013). Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry 3, e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonasera, S.J., Schenk, A.K., Luxenberg, E.J., Wang, X., Basbaum, A., and Tecott, L.H. (2015). Mice lacking serotonin 2C receptors have increased affective responses to aversive stimuli. Plos One 10, e0142906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grimsley, J.M., Sheth, S., Vallabh, N., Grimsley, C.A., Bhattal, J., Latsko, M., Jasnow, A., and Wenstrup, J.J. (2016). Contextual modulation of vocal behavior in mouse: newly identified 12 kHz “mid-frequency” vocalization emitted during restraint. Front. Behav. Neurosci. 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simola, N., and Granon, S. (2019). Ultrasonic vocalizations as a tool in studying emotional states in rodent models of social behavior and brain disease. Neuropharmacology 159, 107420. [DOI] [PubMed] [Google Scholar]
- 28. Huber, B.R., Meabon, J.S., Hoffer, Z.S., Zhang, J., Hoekstra, J.G., Pagulayan, K.F., McMillan, P.J., Mayer, C.L., Banks, W.A., Kraemer, B.C., Raskind, M.A., McGavern, D.B., Peskind, E.R., and Cook, D.G. (2016) Blast exposure causes dynamic microglial/macrophage responses and microdomains of brain microvessel dysfunction. Neuroscience 319, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huber, B.R., Meabon, J.S., Martin, T.J., Mourad, P.D., Bennett, R., Kraemer, B.C., Cernak, I., Petrie, E.C., Emery, M.J., Swenson, E.R., Mayer, C., Mehic, E., Peskind, E.R., and Cook, D.G. (2013). Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J. Alzheimers Dis. 37, 309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Logsdon, A.F., Meabon, J.S., Cline, M.M., Bullock, K.M., Raskind, M.A., Peskind, E.R., Banks, W.A., and Cook, D.G. (2018). Blast exposure elicits blood-brain barrier disruption and repair mediated by tight junction integrity and nitric oxide dependent processes. Sci Rep 8, 11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koliatsos, V.E., Cernak, I., Xu, L., Song, Y., Savonenko, A., Crain, B.J., Eberhart, C.G., Frangakis, C.E., Melnikova, T., Kim, H., and Lee, D. (2011). A mouse model of blast injury to brain: initial pathological, neuropathological, and behavioral characterization. J. Neuropathol. Exp. Neurol. 70, 399–416. [DOI] [PubMed] [Google Scholar]
- 32. Cernak, I., Merkle, A.C., Koliatsos, V.E., Bilik, J.M., Luong, Q.T., Mahota, T.M., Xu, L., Slack, N., Windle, D., and Ahmed, F.A. (2011). The pathobiology of blast injuries and blast-induced neu.rotrauma as identified using a new experimental model of injury in mice. Neurobiol. Dis. 41, 538–551. [DOI] [PubMed] [Google Scholar]
- 33. Warden, D. (2006) Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 34. Crocq, M.A., and Crocq, L. (2000) From shell shock and war neurosis to posttraumatic stress disorder: a history of psychotraumatology. Dialogues Clin. Neurosci. 2, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association: Washington, DC. [Google Scholar]
- 36. Wessa, M., and Karl, A. (2007). Stress sensitization and fear learning in posttraumatic stress disorder. Psychophysiology 44, S8–S8. [Google Scholar]
- 37. Gewirtz, J.C., and Davis, M. (2000). Using Pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn. Mem. 7, 257–266. [DOI] [PubMed] [Google Scholar]
- 38. Klemenhagen, K.C., O'Brien, S.P., and Brody, D.L. (2013). Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. Plos One 8, :e74510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ojo, J.O., Greenberg, M.B., Leary, P., Mouzon, B., Bachmeier, C., Mullan, M., Diamond, D.M., and Crawford, F. (2014) Neurobehavioral, neuropathological and biochemical profiles in a novel mouse model of co-morbid post-traumatic stress disorder and mild traumatic brain injury. Front. Behav. Neurosci. 8, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kwon, S.K., Kovesdi, E., Gyorgy, A.B., Wingo, D., Kamnaksh, A., Walker, J., Long, J.B., and Agoston, D.V. (2011). Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Front. Neurol. 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamnaksh, A., Kovesdi, E., Kwon, S.K., Wingo, D., Ahmed, F., Grunberg, N.E., Long, J., and Agoston, D.V. (2011). Factors affecting blast traumatic brain injury. J. Neurotrauma 28, 2145–2153. [DOI] [PubMed] [Google Scholar]
- 42. Rawal, G., Yadav, S., and Kumar, R. (2017). Post-intensive care syndrome: An overview. J. Transl. Intern. Med. 5, 90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin, J.Y., Arthurs, J., and Reilly, S. (2017). Anesthesia-inducing drugs also induce conditioned taste aversions. Physiol. Behav. 177, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jaffe, A.E., Blayney, J.A., Bedard-Gilligan, M., and Kaysen, D. (2019) Are trauma memories state-dependent? Intrusive memories following alcohol-involved sexual assault. Eur. J. Psychotraumatol. 10, 1634939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaffe, A.E., Steel, A.L., DiLillo, D., Hoffman, L., Gratz, K.L., and Messman-Moore, T.L. (2017). Victim alcohol intoxication during a sexual assault: relations with subsequent PTSD symptoms. Violence Vict. 32, 642–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mott, F.W. (1916). The effects of high explosives upon the central nervous system. Lancet 1, 441–449. [Google Scholar]
- 47. Shively, S.B., and Perl, D.P. (2012). Traumatic brain injury, shell shock, and posttraumatic stress disorder in the military-past, present, and future. J. Head Trauma Rehabil. 27, 234–239. [DOI] [PubMed] [Google Scholar]