Abstract
Introduction:
Lung cancer screening using low-dose computed tomography (LDCT) is now widely recommended for adults who are current or former heavy smokers. It is important to evaluate the impact of screening on patient-centered outcomes. Among current and former smokers eligible for lung cancer screening, we sought to determine the consequences of screening with LDCT, as well as subsequent results, on patient-centered outcomes such as quality of life, distress, and anxiety.
Methods:
We searched the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews (through the 4th Quarter 2012), MEDLINE (2000 to May 31, 2013), reference lists of papers, and Scopus for relevant English-language studies and systematic reviews. To evaluate the effect of LDCT screening on patient-centered outcome, we included only randomized controlled trials (RCT) involving asymptomatic adults. To evaluate the association of particular results and/or recommendations from a screening LDCT with patient-centered outcomes, we included results from RCTs as well cohort studies.
Results:
A total of 8215 abstracts were reviewed. Five publications from two European RCTs and one publication from a cohort study conducted in the U.S. met inclusion criteria. The process of LDCT lung cancer screening was associated with short-term psychologic discomfort in many people but did not impact distress, worry, or health-related quality of life. False positive results were associated with short-term increases in distress that returned to levels that were similar to those among people with negative results. Negative results were associated with short-term decreases in distress.
Conclusions:
As lung cancer screening is implemented in the general population, it will be important to evaluate its association with patient-centered outcomes. People considering lung cancer screening should be aware of the possibility of distress caused by false positive results. Clinicians may want to consider tailoring communication strategies that can decrease the distress associated with these results.
Keywords: lung cancer, screening, patient-centered outcomes
Introduction
It is now widely recommended to consider lung cancer screening using low-dose computed tomography (LDCT) for middle-aged to elderly adults with a history of substantial cigarette smoking.1–6 These recommendations are largely based on the National Lung Screening Trial (NLST) which showed that three annual LDCT screens decreased lung cancer mortality by 20% and overall mortality by 7%.7
LDCT is associated with harms as well.8, 9 The most direct harm to individuals stems from the high rate of false positive LDCT screens. In the NLST, 39% of subjects received at least one positive test, 96% of which were falsely positive. Individuals with false positive results may experience distress as a result of a “near-cancer” diagnosis. Other harms, such as the potential for overdiagnosis and increased risk of radiation-induced cancer, are important as well although are difficult to quantify for individual patients.9, 10
We were particularly interested in understanding the influence of LDCT screening on patient-centered outcomes such as distress, anxiety, and quality of life (QOL). As part of a larger review of the benefits and harms or lung cancer screening conducted for the United States Preventive Service Task Force (USPSTF),9 we conducted a systematic review of evidence that evaluated patient-centered outcomes for people who underwent screening and those who did not, as well as the association of specific LDCT screening findings with these outcomes.
Methods
A standard protocol was developed for this review. A technical report details the methods and includes search strategies and additional evidence tables.11 Key questions addressing the benefits and harms of screening for lung cancer with LDCT were developed by the USPSTF with input from scientific staff at the Agency for Healthcare Research and Quality (AHRQ).11 This report focuses on the association of LDCT lung cancer screening with patient-centered outcomes. Investigators created an analytic framework incorporating the key questions and outlining the patient populations, interventions, outcomes, and harms of LDCT screening for lung cancer. The Preferred Reporting Items for Systematic Reviews and Meta-analyses consensus was followed for the systematic review. 12
Data Sources and Searches
In conjunction with a research librarian, investigators searched the Cochrane Central Register of Controlled Trials and Cochrane Database of Systematic Reviews (through the 4th Quarter 2012), MEDLINE (2000 to May 31, 2013), reference lists of papers, and Scopus for relevant English-language studies and systematic reviews. These dates overlap with those of the previous review of the effectiveness of lung cancer screening. 13
Study Selection
Each abstract was initially reviewed by one investigator and if possibly relevant to the key question then independently reviewed by two investigators to determine eligibility for inclusion. To evaluate the effect of LDCT screening on patient-centered outcomes, we included only randomized controlled trials (RCT) involving asymptomatic adults at high risk of lung cancer because of smoking behaviors that compared screening to no screening. To evaluate the association of specific results and/or recommendations from a screening LDCT with patient-centered outcomes, we included results from RCTs as well as cohort studies that involved asymptomatic adults at high risk of lung cancer because of smoking behaviors.
Data Extraction and Quality Assessment
For each included study, one investigator abstracted details about the patient population, study design, screening procedure, LDCT findings, and patient-centered outcomes which were confirmed by a second investigator. By using predefined criteria for RCTs and cohort studies developed by the USPSTF, 14 two investigators rated the quality of studies (good, fair, poor) and resolved discrepancies by consensus. We assessed the overall quality of the body of evidence (good, fair, poor) using methods developed by the USPSTF on the basis of the number, quality, and size of studies, consistency of results, and directness of evidence.14, 15 When studies reported findings in more than one paper, data from the most recent publication were used unless unique data were presented in a previous publication.
Data Synthesis and Analysis
Values and ranges for summary statistics are reported based on information provided by the study authors. Trial results could not be meaningfully combined because of heterogeneity of the outcome measures.
External Review
The draft report, from which the current analysis is based, was reviewed by content experts, USPSTF members, AHRQ Project Officers, and collaborative partners.11
Results
A total of 8215 abstracts were reviewed. Five publications from two RCTs16–20 and one publication from a cohort study21 were included (Figure 1). In general the quality of these studies was fair. Table 1 includes details about the screening studies. Quality ratings are reported in eTables 1 and 2.
Figure 1:
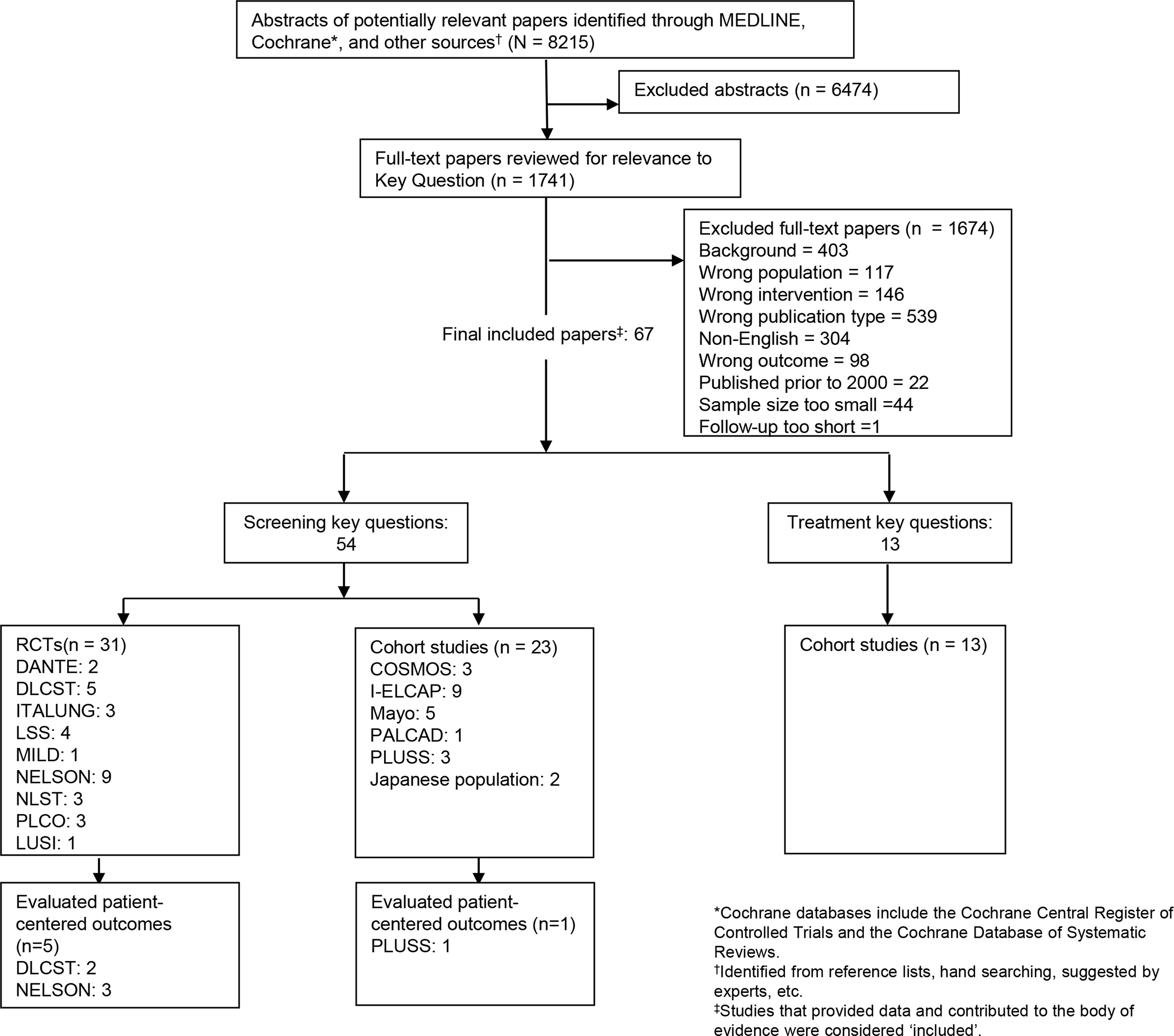
Selection of Studies
Table 1:
Summary of included studies (n=4).
| Study, recruitment years | Population/Eligibility | No. of screening rounds | Psychosocial Outcome (Instrument) |
|---|---|---|---|
| Randomized Controlled Trials | |||
| DLCST16, 17, 2004–2006 | • Age: 50–70 years • Current or Former Smokers (quit after age 50 and <10 years prior) • ≥20 pack-years |
• 5 | • Anxiety, sense of dejection, negative impact on behavior and sleep, busy to take mind of things, less interest in sex (COS, COS-LC) • Focus on airway symptoms, introvert, stigmatization, harm of smoking, self-blame (COS-LC) • Redeemed AD or AX prescriptions |
| NELSON18–20, 1st phase: 2003-NR 2nd phase: October 2005-NR | • Age: 50–75 years • Current Smokers or Former Smokers (≤10 years prior) • >15 cig/day for >25 years or >10 cig/day for >30 years |
• 3 | • Health-related quality of life (EQ-5D, SF-12) • Anxiety (STAI-6) • Distress (IES) |
| Cohort | |||
| PLuSS21, Jan 2002- Apr 2005 | • Age: 50–79 years • Current Smokers or Former smokers (≤10 years prior) • ≥1/2 pack/day for ≥25 years |
• 2 | • Anxiety (STAI) • Fear (PCQ) |
Abbreviations: AD = antidepressant, AX = anxiolytic, COS-LC = Consequences of Screening in Lung Cancer, CT = computed tomography, DLCST = Danish Lung Cancer Screening Trial, EQ-5D = EuroQol questionnaire, IES = Impact of Event Scale, Mayo = Mayo Clinic Lung Cancer Genetic Epidemiology Registry, NA = not applicable, NELSON = NEderlands Leuvens Longkanker Screenings Onderzoek, No. = number, NR= not reported, PCQ = Psychological Consequences Questionnaire, PLuSS = Pittsburgh Lung Screening Study, SF-12 = 12-item Short Form, STAI = Full version Spielberger State-Trait Anxiety Inventory, STAI-6 = Six-item Spielberger State-Trait Anxiety Inventory
eTable 1.
Quality Ratings for Studies reporting on the Influence of LDCT Screening on Patient-Centered Outcomes
| Trial, Year | Randomization adequate? | Allocation concealment adequate? | Groups similar at baseline? | Maintain Comparable Groups? | Eligibility criteria specified? | Outcome assessors masked? | Care provider masked? | Patient masked? | Attrition, crossovers, adherence, & contamination reported? | Loss to follow-up: differential/high? | Intention-to-screen (ITS) analysis? | Post-randomization exclusions? | Outcomes Pre-specified? | Funding source reported? | External validity | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| DLCST, 201214 | Yes | NR | Yes | Yes | Yes | NR | NR | No | Yes | No | Yes | No | Yes | Yes | Fair | Fair |
| DLCST, 201215 | Yes | NR | Yes | Yes | Yes | NR | NR | No | Yes | No | Yes | No | Yes | Yes | Fair | Fair |
| NELSON, 201118* | Yes | NR | Yes | Yes | Yes | NR | NR | No | Yes | Yes | Yes | No | Yes | Yes | Fair | Fair |
This study also reported on the association of LDCT screening findings with patient-centered outcomes.
Abbreviations: DLCST = Danish Lung Cancer Screening Trial; LDCT = low-dose computed tomography; NELSON = Nederlands-Leuvens Longkanker Screenings Onderzoek; NR = Not reported
eTable 2.
Quality Ratings for Studies reporting on the Association of LDCT Screening Findings with Patient-Centered Outcomes
| Trial, Year | Did the study attempt to enroll a random sample or consecutive patients meeting inclusion criteria (inception cohort)? | Were the groups comparable at baseline? | Did the study use accurate methods for ascertaining exposures, potential confounders, and outcomes? | Were outcome assessors and/or data analysts blinded to treatment? | Did the article report attrition? | Did the study perform appropriate statistical analyses on potential confounders? | Is there important differential loss to follow-up or overall high loss to follow-up? | Were outcomes pre-specified and defined, and ascertained using accurate methods? | Quality |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| NELSON, 200816 | Yes | NR | Yes | No | Yes | No | No | Yes | Fair |
| NELSON, 201017 | Yes | Yes | Yes | No | Yes | Yes | NR | Yes | Fair |
| PLuSS, 200819 | Yes | No | Yes | No | Yes | Yes | No | Yes | Fair |
Abbreviations: DLCST = Danish Lung Cancer Screening Trial; LDCT = low-dose computed tomography; NELSON = Nederlands-Leuvens Longkanker Screenings Onderzoek; NR = Not reported; PLuSS = Pittsburgh Lung Screening Study
Influence of LDCT Screening
Two reports each from the Danish Lung Cancer Screening Trial (DLCST) and the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) study evaluated patient-centered outcomes.16–18, 20 The DLCST compared LDCT with no screening22 and enrolled healthy men and women ages 50 to 70 years, who were current or former (quit after age 50 and < 10 years prior) smokers with 20 pack-years or greater smoking history.22 All subjects were administered the Consequences of Screening (COS) scale (includes items on anxiety, negative impact on behavior, dejection, and sleep) and Consequences of Screening in Lung Cancer (COS-LC) scale (includes items on self-blame, focus on airway symptoms, stigmatization, introvert, harm of smoking, and anxiety)23 at two time points: prior to randomization and at the time of the second LDCT.16 Subjects with positive LDCT results for lung cancer (including false positives) were excluded. Prior to randomization, there were no differences in the COS scores between screen and control subjects. More subjects in the control arm did not complete the second survey than LDCT subjects (92% vs. 97%). Control subjects at baseline had worse scores in the anxiety, behavior, dejection, self-blame, focus on symptoms, and introvert domains of the COS and COS-LC surveys. Subjects in both arms reported statistically significant increases in several scales, including the negative impact on behavior, dejection, and sleep scales, but the degree of change was similar in both groups. This study did not report on the minimally important difference (the smallest change that a patient would consider as significant) of the COS or COS-LC scales or domains.
DLCST investigators also examined the new prescription of anti-depressant and anxiolytic medications as recorded in the Danish National Prescription Registry among all control and LDCT subjects.17 Subjects were followed for up to three years after randomization and censored from analysis if they died, emigrated, or were diagnosed with lung cancer. No differences were found between the screen or control group in terms of prescriptions for antidepressant or anxiolytic medications (hazard ratio [HR] 1.00 [95% CI 0.90 to 1.12]), even when adjusted for important confounders such as age, sex, and previous prescription of antidepressant and anxiolytic medications.
Several reports from the NELSON trial, which compares LDCT with no screening,24 described patient-centered outcomes. NELSON is being conducted in the Netherlands and Belgium, enrolling male and female former and current smokers (≥15 cigarettes/day for >25 years, or >10 cigarettes/day for >30 years, and if a former smoker, quit ≤10 years prior to enrollment), ages 50 to 75 years.
One report included a subset of 351 subjects consecutively randomized to the LDCT arm who were asked to complete surveys that assessed discomfort, HRQOL (12-item Short Form (SF-12) and EuroQOL questionnaire (EQ-5D)), anxiety (6-item State-Trait Anxiety Inventory (STAI-6)), and lung cancer-specific distress (Impact of Event Scale (IES)) after the LDCT.18 These surveys were completed 1 week before the LDCT, 1 week afterward (before receiving results), and 6 months afterward. Subjects with positive results, growth of a suspicious lesion noted at the time of a repeat scan, indeterminate results at baseline without being informed of repeat results, and those who did not receive the initial LDCT or refused subsequent questionnaires were excluded. The response rate for each questionnaire was over 90% and 77% returned all three. Many participants reported discomfort in connection with having to wait for the results of the CT scan (46%) and dreading those results (50%). In general, the median HRQOL, anxiety, and distress scores did not appear to change more than the minimally important difference over time. Compared to respondents who did not report discomfort waiting for CT results, those reporting discomfort had worse anxiety and distress scores (p <0.01) which exceeded the minimally important difference at all three assessments.
NELSON investigators also evaluated differences between screened and control subjects. An initial sample of 1466 subjects from both arms was surveyed at three time intervals: before randomization, 2 months after baseline screening (screen group only), and at 2 year follow-up.20 They evaluated HRQOL (SF-12, EQ-5D), anxiety (STAI-6), lung cancer-specific distress (IES). Response rates were over 85% for each survey except for the 2-year survey sent to the control subjects which had a 65% response rate. Between the baseline and 2 year follow up surveys, there were no significant differences in any psychosocial outcome scores between the screen and control groups.
Influence of LDCT Result
Two reports from NELSON included information on the association of LDCT findings and patient-centered outcomes. Several outcomes, including HRQOL (SF-12, EQ-5D), anxiety (STAI-6), lung cancer-specific distress (IES), were measured at four time intervals: before randomization, 1 week before the LDCT, within 1 day after the LDCT (before result), and 2 months after the LDCT (before the 3 month follow-up CT for patients with indeterminate results).19 This study included most of the same subjects as reported in the report from NELSON that evaluated long-term outcomes among screened and control patients.20 Response to each questionnaire was 88% or higher and 71% returned all four questionnaires. Scores on the HRQOL and anxiety instruments showed no clinically relevant changes over time. At 2 months after baseline, distress scores significantly increased more than the minimally important difference after an indeterminate result, whereas these scores showed a significant decrease after a negative result. Differences in distress scores between indeterminate and negative result groups were both significant and more than the minimally important difference (p<0.01) at 2 months after baseline.
This group also reported on long-term associations with LDCT findings.20 There was a temporary increase in mean distress scores after an indeterminate LDCT result (4.0 [95% CI 2.8 to 5.3] before randomization, 7.8 [95% CI 6.5 to 9.0] 2 months after baseline LDCT, and 4.5 [95% CI 3.3 to 5.8] 2 years after baseline LDCT). Participants with negative results had decreases in mean distress scores at 2 months after the baseline LDCT (4.1 [95% CI 3.4 to 4.8], to 2.6 [95% CI 2.0 to 3.3] and 3.5 [95% CI 2.9 to 4.2] at the same time points). Between groups, these differences were more than the minimally important difference and statistically significant at the 2 month survey (p<0.01). At the 2 year follow-up survey, distress scores between groups were no longer significantly different. Other statistically significant differences in outcome scores were not more than the minimally important difference.
We included one cohort study. Among a subset of subjects in the Pittsburgh Lung Screening Study (PLuSS), the authors evaluated state/trait anxiety, fear of cancer, and perceived risk of lung cancer over time (prior to initial screening, 1 to 2 weeks after the result, 6 months after baseline screen, and 12 months after the baseline screen).21 The PLuSS was conducted in the U.S. and enrolled 3642 men and women ages 50 to 79 years who were current or former smokers with at least one half pack/day for 25 years and had quit less than 10 years prior to enrollment. Overall, subjects with negative results had no change in anxiety. For subjects with indeterminate or suspicious results, state anxiety increased 1 to 2 weeks after the result was known and then decreased to baseline at 1 year.
Discussion
In summary, we found six studies of fair-quality that evaluated psychosocial consequences among individuals undergoing LDCT screening. In European trials, screening did not appear to significantly impact overall health-related quality of life and no long-term differences in anxiety or distress were reported. In the short-term, the studies suggested participants with positive or indeterminate results for lung cancer had increased distress compared to their baseline level while those with negative results often had decreased distress. Overall HRQOL and anxiety did not appear to substantially change over time or differ in association with LDCT results. Over the long-term, distress appeared to be similar for participants with both indeterminate and negative results. In general, it appears distress increases after a positive result and then returns to similar levels to those with negative results.
Similar to our findings, a systematic review of patients with false positive mammograms found that increased worry was the most common consequence.25 In general however, there is a paucity of data regarding the association of cancer screening procedures with patient-centered outcomes.26 It is also important to consider how distress and worry from lung cancer screening might lead to other adverse outcomes such as non-adherence to recommendations and negative health behaviors, as postulated by the Biobehavioral Model of Cancer Stress.27
While it is important to quantify adverse outcomes that are associated with the lung cancer screening process, it is unknown if these consequences can be modified or are intrinsically linked to unalterable aspects of the process. Given the high rate of false positive results7 from LDCT screening and the evidence that distress is associated with these results, strategies that decrease the risk for a positive result may be important. Careful consideration of eligibility criteria28, 29, optimizing the balance of sensitivity versus specificity of LDCT result reporting30, and following diagnostic algorithms 31 are potential interventions.
As false positive results will never be eliminated, optimizing communication processes, such as discussions of risk, benefits, values and preferences, notification of the result and its implications, and follow-up plans, may also improve patient-centered outcomes 32. Communication is a cornerstone of the patient-clinician relationship and is a critical component of high quality, patient-centered care.33 The Institute of Medicine and the National Cancer Institute emphasize the importance of improving communication strategies as a means of improving patient outcomes.34, 35 Improving patient and clinician knowledge is a core domain of patient-centered communication36 and it is recommended that information exchange focus on plain language, describe absolute instead of relative risks, and include tables and graphs.37 However, a report from the NELSON study found that participant’s knowledge about screening, which was in general higher than non-participants,38 was not associated with patient-centered outcomes.39 Thus, attention to additional elements of quality communication, such as shared decision making and considering the patient’s values and preferences, may be important.
As LDCT screening is incorporated into general practice, it will be important to continue to evaluate its influence on patient-centered outcomes. Volunteers in screening trials have different demographic and socioeconomic characteristics than otherwise screening-eligible people in the general population.40 Results from the DLCST showed that eligible non-participants had more negative psychosocial characteristics than participants, even after adjustment for important sociodemographic characteristics.41 Finally, procedures for communication of results differ in screening trials compared to routine practice. Notably, studies from two cohorts of patients in the U.S. with incidental pulmonary nodules found that many of these patients also appeared to experience distress.42–44
While not the focus of our review, several of the included studies reported findings that suggested certain demographic and behavioral characteristics were associated with patient-centered outcomes. For instance, in the NELSON study, levels of HRQOL were worse for women, current smokers, and subjects with more pack-years of smoking.19, 20 Subjects with higher perceived risk of lung cancer also reported higher levels of distress.45 In the PLuSS, current smokers had higher levels of anxiety, fear of cancer and perceived risk of cancer, women reported higher levels of fear of cancer, and married participants and those in higher education classes had lower anxiety levels.21 Efforts to improve patient-centered outcomes should consider focused efforts among these categories of patients at higher risk for negative outcomes.
Our review and the results have several limitations. Despite identifying over 8000 papers in our search, we may have missed relevant results. Most of the results come from European screening trials so it is unclear how generalizable these results will be to people in the U.S. who undergo screening as part of routine care. In addition, we found patient-centered outcomes were reported from only four of twenty screening studies that were included in our related review that focused on mortality and other outcomes.9 Finally, we did not find evidence regarding how people view the tradeoffs between the potential mortality benefit and risks of harm that are engendered from lung cancer screening.
In conclusion, limited, fair quality evidence suggests lung cancer screening with low-dose computed tomography was associated with short-term psychologic discomfort in many people but does not impact distress, worry, or health-related quality of life. False positive results are associated with short-term increases in distress that return to levels that are similar to those among people with negative results. Negative results are associated with short-term decreases in distress. In lieu of evidence regarding how to improve patient-centered outcomes among screened people, clinicians may want to focus on decreasing the chance of false positive results and optimize communication strategies that emphasize adequate information exchange, consideration of values and preferences, and shared decision making.
Table 2:
Influence of LDCT Screening with Patient-Centered Outcomes.
| Study cohort selection | Population CT vs. Control | Instrument Interval (Response Rate, LDCT vs. Control) | LDCT Group | Control Group | Statistics |
|---|---|---|---|---|---|
| DLCST16, n=3925 CT group (excludes screen positive/false-positive) vs. control group* | • Men: 57% vs. 55% • Age (mean): 57 vs. 57 |
• Before LDCT (100% vs. 100%) • 12 month (97% vs 92%) |
• COS-LC#: Negative psychosocial factors mean increase from baseline (t-test, p<0.0001)† | • COS-LC#: Negative psychosocial factors mean increase from baseline (t-test, p<0.0001)† | • †No difference in increase between groups (t-test, p NS) • No difference in self-rated health between groups |
| DLCST17, n=4104 CT group vs. control group | • Men: 56% vs. 55% • Age (mean): 57 vs. 57 • Previous AD or AX prescription: 13% vs. 14% |
• Includes 3 years follow-up after initial CT | • Use of AD or AX: 30.9% (634/2052) | • Use of AD or AX: 31.4% (644/2052) | • HR: 1.00 (95 % CI, 0.90–1.12, p NR) Multivariable adjusted |
| NELSON18, n=351 Convenience sample from baseline cohort of screen negative CT group (excludes screen positive/indeterminate) | • Men: 50.9% • Age (mean): 60.3 |
• Before LDCT (92%) • 1 week (94%) • 6 month (90%) |
• 46% (148/319) reported discomfort from waiting and 50% (162/321) dreading CT result | • No control group | • No intra-group differences in IES, EQ-5D, SF-12, STAI-6 scores over time |
| NELSON20, n=1466 CT group vs. control group (sample from overall cohort) | • Men: 46% vs. 50% • Age (mean): 58 vs. 58 |
• Before LDCT (90% vs. 86%) • 2 years (89% vs. 65%) |
IES score (mean)#: • NR • NR |
IES score (mean)#• NR • NR |
• No difference in scores between CT vs. control • Repeated measures ANOVA |
COS-LC: sense of dejection, negative impact on behavior and sleep, busy to take mind off things, and less interest in sex all increased.
5.2% higher dropout rate in control vs. CT group
Abbreviations: AD = antidepressant medication, ANOVA = analysis of variance, AX = anxiolytic medication, COS-LC = Consequences of Screening in Lung Cancer, CT = computed tomography, DLCST = Danish Lung Cancer Screening Trial, EQ-5D = EuroQol questionnaire, IES = Impact of Event Scale, NELSON = NEderlands Leuvens Longkanker Screenings Onderzoek, NS = non-significant, NR = not reported, SF-12 = 12-item Short Form, STAI-6 = Six-item Spielberger State-Trait Anxiety Inventory.
Table 3.
Association of LDCT Screening Result with Patient-Centered Outcomes.
| Study cohort selection | Population Abnormal vs. Negative LDCT | Instrument Interval (Overall Response Rate) | Abnormal LDCT | Negative LDCT | Statistics |
|---|---|---|---|---|---|
| NELSON19, n=733 Convenience sample from baseline cohort of screen indeterminate vs. negative CT groups | • Men: 50% vs. 46% • Age (mean): 58 vs. 58 |
• Before LDCT (91%) • 1 week pre-LDCT (94%) • 1 day post- LDCT (93%) • 2 month (88%) |
IES score (mean): • 4.5† • 6.3 • 4.9 • 8.3†* |
IES score (mean): • 4.1 • 5.8 • 4.5 • 2.4†* |
• †p<0.05 for increase and decrease • *p<0.01 for difference • No differences in SF-12, EQ-5D, or STAI-6 Repeated measures ANOVA - unadjusted |
| NELSON20, n=733 Convenience sample from baseline cohort of screen indeterminate vs. negative CT groups | • Men: NR • Age: NR |
• Before LDCT (NR) • 2 month (NR) • 2 years (NR) |
IES score (mean): • 4.0 • 7.8 • 4.5 |
IES score (mean): • 4.1 • 2.6 • 3.5 |
• Interaction time × result (p<0.01) • Significant difference at 2 months • No difference at 2 years • No differences in SF-12, EQ-5D, or STAI-6 Repeated measures ANOVA - adjusted |
| PLuSS 21, n=400 Convenience sample from baseline cohort categorized into indeterminate, suspicious vs. negative CT groups | Indeterminate vs. Suspicious vs. Negative • Men: 41% vs. 58% vs. 56% • Age (mean): 60 vs. 61 vs. 60 |
• Overall Response Rate: 85%β • Before LDCT • 1–2 week • 6 month • 12 month • Baseline • 1–2 week • 6 month • 12 month • Baseline • 1–2 week • 6 month • 12 month |
Indeterminate: STAI-6 score (mean)#: • 34.4 • 37.7 • 37.3 • 35.3 Suspicious: PCQ score (mean)#: • 6.4 • 8.5 • 7.4 • 7.1 Suspicious: Perceived % risk of cancer (mean)#: • 18.6 • 34.5 • 30.3 • 31.2 |
Negative: STAI-6 score (mean) • 35.9 • 35.9 • 34.4 • 35.1 PCQ score (mean) • 7.0 • 7.0 • 6.5 • 6.7 Perceived % risk of cancer (mean): • 17.1 • 11.2 • 13.1 • 13.1 Among negative group only perceived % risk of cancer changed over time (p NR) |
• #p<0.001 Increase from baseline, then decrease in scores over time • Anxiety (STAI-6): higher in current smokers (p<0.03) • Fear of cancer (PCQ): higher in women (p<0.03) and current smokers (p<0.001) • Perceived % risk of cancer: higher in current smokers (p<0.001) Multivariable adjusted |
Cancer thoughts categorized as “not at all” or “some”
-Per authors: There were no significant differences between those not missing a survey and those missing a survey.
Abbreviations: ANOVA = analysis of variance, CT = computed tomography, EQ-5D = EuroQol questionnaire, IES = Impact of Event Scale, Mayo = Mayo Clinic Lung Cancer Genetic Epidemiology Registry, NELSON= NEderlands Leuvens Longkanker Screenings Onderzoek, NR = not reported, PCQ = Psychological Consequences Questionnaire, PLuSS = Pittsburgh Lung Screening Study, SF-12 = 12-item Short Form, STAI-6 = Spielberger State-Trait Anxiety Inventory.
Acknowledgements
Note: This research was supported by the Agency for Healthcare Research and Quality under Contract No. HHSA-290-2007-10057-I-EPC3, Task Order No. 13. Dr. Slatore is a Core Investigator in Health Services Research and Development at the Portland VA Medical Center and was supported by a VA HSR&D Career Development Award. The research reported here was also supported by resources from the Portland VA Medical Center, Portland, Oregon. The Department of Veterans Affairs did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest with the results presented in this manuscript.
Note: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the U.S Government.
References
- 1.Moyer VA. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine 2013;N/A:N/A–N/A. [DOI] [PubMed] [Google Scholar]
- 2.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and Harms of CT Screening for Lung Cancer: A Systematic ReviewBenefits and Harms of CT Screening for Lung Cancer. Jama 2012:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network: NCCN guidelines on lung cancer screening. Available at http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf. Accessed June 27, 2012
- 4.Jaklitsch MT, Jacobson FL, Austin JH, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012;144:33–38. [DOI] [PubMed] [Google Scholar]
- 5.Samet JM, Crowell R, San Jose Estepar R, et al. American Lung Association: Providing guidance on lung cancer screening to patients and physicians. Available at http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening-guidelines/lung-cancer-screening.pdf. Accessed June 27, 2012 2012.
- 6.Wender R, Fontham ET, Barrera E Jr., et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013;63:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris RP, Sheridan SL, Lewis CL, et al. The Harms of Screening: A Proposed Taxonomy and Application to Lung Cancer Screening. JAMA Intern Med 2013. [DOI] [PubMed] [Google Scholar]
- 9.Humphrey LL, Deffebach M, Pappas M, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: A Systematic Review to Update the U.S. Preventive Services Task Force Recommendation. Ann Intern Med 2013;159:411–420. [DOI] [PubMed] [Google Scholar]
- 10.Patz EF Jr., Pinsky P, Gatsonis C, et al. Overdiagnosis in Low-Dose Computed Tomography Screening for Lung Cancer. JAMA Intern Med 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphrey L, Deffebach M, Pappas M, et al. Screening for Lung Cancer: Systematic Review to Update the U.S Preventive Services Task Force Recommendation; 2013. [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humphrey LL, Teutsch S, Johnson M. Lung cancer screening with sputum cytologic examination, chest radiography, and computed tomography: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2004;140:740–753. [DOI] [PubMed] [Google Scholar]
- 14.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Preventive Services Task Force. Procedure Manual. 2011. Available at http://www.uspreventiveservicestaskforce.org/uspstf08/methods/procmanual.htm. Accessed April 3, 2013
- 16.Aggestrup LM, Hestbech MS, Siersma V, et al. Psychosocial consequences of allocation to lung cancer screening: a randomised controlled trial. BMJ Open 2012;2:e000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaerlev L, Iachina M, Pedersen JH, et al. CT-Screening for lung cancer does not increase the use of anxiolytic or antidepressant medication. BMC Cancer 2012;12:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Bergh KA, Essink-Bot ML, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113:396–404. [DOI] [PubMed] [Google Scholar]
- 19.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Short-term health-related quality of life consequences in a lung cancer CT screening trial (NELSON). Br J Cancer 2010;102:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van den Bergh KA, Essink-Bot ML, Borsboom GJ, et al. Long-term effects of lung cancer computed tomography screening on health-related quality of life: the NELSON trial. Eur Respir J 2011;38:154–161. [DOI] [PubMed] [Google Scholar]
- 21.Byrne MM, Weissfeld J, Roberts MS. Anxiety, fear of cancer, and perceived risk of cancer following lung cancer screening. Med Decis Making 2008;28:917–925. [DOI] [PubMed] [Google Scholar]
- 22.Ashraf H, Tonnesen P, Holst Pedersen J, et al. Effect of CT screening on smoking habits at 1-year follow-up in the Danish Lung Cancer Screening Trial (DLCST). Thorax 2009;64:388–392. [DOI] [PubMed] [Google Scholar]
- 23.Brodersen J, Thorsen H, Kreiner S. Consequences of screening in lung cancer: development and dimensionality of a questionnaire. Value Health 2010;13:601–612. [DOI] [PubMed] [Google Scholar]
- 24.van der Aalst CM, van den Bergh KA, Willemsen MC, et al. Lung cancer screening and smoking abstinence: 2 year follow-up data from the Dutch-Belgian randomised controlled lung cancer screening trial. Thorax 2010;65:600–605. [DOI] [PubMed] [Google Scholar]
- 25.Brewer NT, Salz T, Lillie SE. Systematic review: the long-term effects of false-positive mammograms. Ann Intern Med 2007;146:502–510. [DOI] [PubMed] [Google Scholar]
- 26.Heleno B, Thomsen MF, Rodrigues DS, et al. Quantification of harms in cancer screening trials: literature review. BMJ 2013;347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. Am Psychol 1994;49:389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med 2013;369:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henschke CI, Yip R, Yankelevitz DF, et al. Definition of a positive test result in computed tomography screening for lung cancer: a cohort study. Ann Intern Med 2013;158:246–252. [DOI] [PubMed] [Google Scholar]
- 31.NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer; Version 3.2014. Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed March 19, 2014
- 32.Anticipate and Communicate: Ethical management of incidental and secondary findings in the clinical, research, and direct-to-consumer contexts. In: issues. PCftsob, ed.Washington, D.C.: 2013. Available at http://bioethics.gov/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf. [DOI] [PubMed] [Google Scholar]
- 33.Simpson M, Buckman R, Stewart M, et al. Doctor-patient communication: the Toronto consensus statement. BMJ 1991;303:1385–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Epstein RM, Street RLJ. Patient-Centered Communication in Cancer Care: Promoting Healing and Reducing Suffering. National Cancer Institute, NIH Publication No. 07-6225. Bethesda, MD. 2007. [Google Scholar]
- 35.Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 36.Mead N, Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med 2000;51:1087–1110. [DOI] [PubMed] [Google Scholar]
- 37.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst 2011;103:1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Bergh KAM, Essink-Bot ML, van Klaveren RJ, et al. Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. Eur Respir J 2009;34:711–720. [DOI] [PubMed] [Google Scholar]
- 39.van den Bergh KA, Essink-Bot ML, van Klaveren RJ, et al. Informed decision making does not affect health-related quality of life in lung cancer screening (NELSON trial). Eur J Cancer 2010;46:3300–3306. [DOI] [PubMed] [Google Scholar]
- 40.Aberle DR, Adams AM, Berg CD, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hestbech MS, Siersma V, Dirksen A, et al. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011;73:325–331. [DOI] [PubMed] [Google Scholar]
- 42.Slatore CG, Press N, Au DH, et al. What the heck is a “nodule”? A qualitative study of Veterans with pulmonary nodules. AATS; 2013;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiener RS, Gould MK, Woloshin S, et al. “What do you mean, a spot?”: A qualitative analysis of patients’ reactions to discussions with their doctors about pulmonary nodules. Chest 2013;143:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiener RS, Gould MK, Woloshin S, et al. ‘The thing is not knowing’: patients’ perspectives on surveillance of an indeterminate pulmonary nodule. Health Expect 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunge EM, van den Bergh KA, Essink-Bot ML, et al. High affective risk perception is associated with more lung cancer-specific distress in CT screening for lung cancer. Lung Cancer 2008;62:385–390. [DOI] [PubMed] [Google Scholar]


