Abstract
Purpose:
Fighting cancer is a costly battle, and understanding the relationship between patient-reported financial toxicity (FT) and health outcomes can help inform interventions for post-treatment cancer survivors.
Methods:
Stage I-III solid tumor, insured U.S. cancer survivors (N=103) completed a survey addressing FT (as measured by the standardized COST measure) and clinically relevant health outcomes (including health-related quality of life [HRQOL] and adherence to recommended survivorship health behaviors). Univariate and multivariate analyses were used to assess demographic and disease-specific correlates of FT, and to assess the predictive value of FT on HRQOL and adherence to survivorship health behaviors.
Results:
Approximately 18% of respondents noted FT levels associated with significant financial burden. In univariate analyses, after correcting for multiple comparisons, greater FT was associated with unpartnered status, non-retirement and lower level of educational attainment. Greater FT was also significantly associated with HRQOL components of anxiety, fatigue, pain, physical functioning, and social functioning. FT was not significantly associated with any measured survivorship health behaviors. In multivariate analyses, FT was found to be a meaningful predictor of patient-reported anxiety, fatigue, physical functioning, and social functioning above and beyond theoretically and statistically relevant demographic characteristics.
Conclusions:
Although overall levels of FT were lower among cancer survivors in this sample, as compared with active treatment patients assessed in previous studies, financial burden continued to be a concern for a significant minority of cancer survivors and was associated with components of reduced HRQOL. Further research is needed to understand FT among underinsured survivors and those treated in community oncology settings.
Implications for Cancer Survivors:
Incorporation of FT assessment into survivorship care planning could enhance clinical assessment of survivors’ FT vulnerability, help address the dynamic and persistent challenges of survivorship, and help identify those most in need of intervention across the cancer care continuum.
Keywords: Financial Toxicity, Cancer, Survivor, Patient-Reported Outcomes, Health-Related Quality of Life, Survivorship Health Behaviors
Fighting cancer is a costly battle. Each year in the United States, individuals diagnosed with cancer spend nearly $4 billion in direct, out-of-pocket costs for treatment [1]. Even treatment regimens that are covered by private or government-issued insurance can cost up to $10,000 in out-of-pocket costs related to deductibles, premiums, co-pays, and out-of-network costs [1]. Many cancer patients also lose income due to disability and other treatment-related costs (e.g., specialized care not covered by insurance, travel and lodging near treatment, caregiving, symptom management) [1,2]. Within the context of cancer treatment and post-treatment survivorship, the totality of these costs and their consequences has been labeled financial toxicity (FT) [2–5]. The impact of FT is significant; in one study, first-year cancer care costs for Medicare patients totaled 20% of income for many patients [6]. Another report from Washington State noted that cancer survivors were 2.65 times more likely to file for bankruptcy compared with those who had no history of cancer [7]. Within both of those studies [6,7] and various other studies [4,8,9], cancer patients who were female gender and of younger age tended to experience greater financial toxicity. Financial toxicity is also a byproduct of advances in cancer treatment. As more treatment regimens include costly molecular testing and precision-medicine therapies (that may not be covered under insurance), financial burden is increasingly shifted to patients [10]. In fact, cancer-specific differences in duration and cost of treatment [11] suggest that FT may differ across cancer types, with one recent article identifying lung cancer patients as having the highest cancer-related cost expenditures among common cancer types [12].
A multitude of studies from North America as well as European countries with third-party health care payer systems have documented the negative impact of financial burden on cancer prognosis [3,13,14]. In an effort to strengthen methodological consensus and bring clarity to the subjective experience of FT, Witte et al. (2019) recently conducted a systematic review of the patient-reported methodologies used across 43 studies and identified six key domains associated with the FT construct: 1) active financial spending; 2) use of passive financial resources; 3) psychosocial responses; 4) support seeking; 5) coping with care; 6) coping with one’s lifestyle [13]. Understanding the relationship between patient-reported FT and its health consequences can inform the identification and targeting of potentially high-risk patients at the time of diagnosis and throughout the treatment trajectory.
The development and psychometric evaluation of the measure, COmprehensive Score for financial Toxicity (COST) [15,16], has provided an opportunity to assess FT from a patient-centered perspective and in a standardized manner. This measure, developed by de Souza and Colleagues (2014) [15], assesses several, although not all, key domains associated with the multifaceted FT construct outlined by Witte et al. [13], including subjective financial satisfaction, financial worry, out-of-pocket costs, and personal control over finances related to cancer. Initial psychometric testing has confirmed that COST is a reliable and well-validated measure of financial toxicity. Thus far, a handful of studies have used the COST scale to assess FT in patients undergoing active cancer treatment [16–20]. However, limited data exist to assess the distribution of COST scores and the relationship between FT and clinically meaningful health indicators (including quality of life and health behaviors) among post-treatment cancer survivors, indicating a specific gap in our understanding of the global impact of FT across the cancer care continuum.
The current report investigated patient-reported FT and its health-related quality of life and health behavior correlates in a diverse cohort of cancer survivors. Addressing these relationships allows better understanding of the clinical utility of COST as a patient-reported outcome, a potential focus of intervention, and a predictor of quality of life and survivorship health behavioral indicators. Specifically, we focused on post-treatment, Stage I-III solid tumor (breast, colorectal, head and neck, lung, prostate) cancer survivors, who were treated in an NCI-designated comprehensive cancer center and insured at the time of treatment. Our first aim addressed demographic and disease-specific factors associated with higher levels of FT. Our second aim focused on the impact of financial toxicity on health-related quality of life and health behaviors that are consistent with cancer survivorship guidelines, both in univariate analyses and after controlling for demographic predictors.
Method
Sample and Participant Selection
Participants included 103 individuals who had been previously treated for Stage I-III solid tumor cancers at an NCI-designated comprehensive cancer center in the Western region of the United States. A detailed description of patient eligibility, contacts, and participation is presented in Figure 1. Briefly, 812 individuals were identified through the cancer center’s patient registry as having a confirmed diagnosis of a solid Stage I-III tumor and having been at least one-month into post-treatment cancer survivorship. These potentially eligible individuals were sent letters explaining the study and allowing them to opt out of the contact process. Over a 6-month period, trained, bilingual study personnel associated with the cancer center’s Behavioral Measurement Intervention and Shared Resources (BMISR) shared resource attempted to contact the 718 individuals who had not opted out via telephone. Ultimately, 288 cancer survivors were successfully contacted via phone, and of those, 121 (42%) provided study consent, and 103 (85%) completed the telephone survey. All recruitment and study methods were approved by the appropriate Institutional Review Board (Approval Number: 1804441636).1
Figure 1.
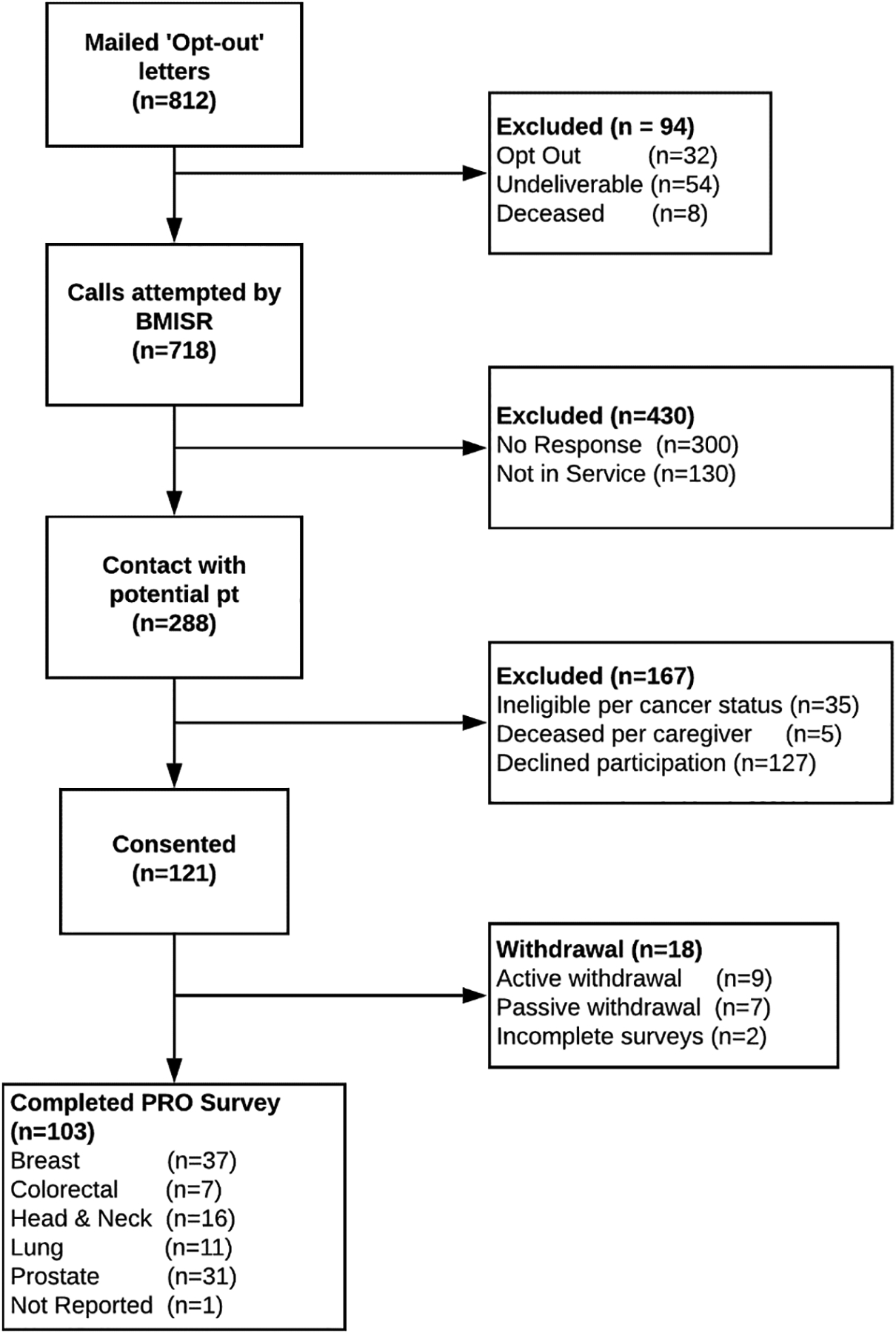
CONSORT Diagram for Patient-Reported Financial Toxicity Among Insured Cancer Survivors Study. Study participant flow chart following CONSORT guidelines. ‘Opt-Out’ letters= letters that were mailed to potentially eligible participants explaining the study and allowing them to opt out of the study contact process.
Assessments and Measures
Demographics and disease history.
To characterize this sample of cancer survivors, demographic and disease histories were collected including: (a) general demographics (gender, race, ethnicity, age, marital status, employment, insurance, and highest level of education), and (b) cancer history (type of cancer, stage at diagnosis, time since completing treatment, and types of treatment).
Financial toxicity.
Financial toxicity (FT) was measured using the COmprehensive Score for Financial Toxicity measure (COST – Facit, Version 1) [16]. The COST measure was developed and validated in 2017 [15,16], and remains one of the only standardized and well-validated patient-reported outcome measures of financial toxicity [21]. Respondents were asked to reflect on their experiences over the past 7 days and to rate the degree to which they felt that each of 11 financial statements was important using a 4-point Likert scale, with 0 being ‘Not at all agree’ and 4 being ‘Very much agree.’ Items 2, 3, 4, 5, 8, 9, and 10 were reverse-scored. Total COST score (range: 0–44) was calculated as a summation of the 11-item measure, with higher scores indicating less financial toxicity. Although the COST measure is not associated with specific norms or cut-off scores, previous studies have identified scores less than 22 as indicating significant financial toxicity [14,19].
Health-related quality of life (HRQOL).
PROMIS-29 Profile (v2.0) [22] is a health-related quality of life assessment that is part of the larger set of PROMIS (Patient-Reported Outcomes Measurement Information System) instruments [23,24] funded by the National Institutes of Health (NIH) and normalized to the U.S. adult population. PROMIS-29 consists of 29 items that cover the most relevant health-specific domains for individuals with chronic illness: anxiety, depression, fatigue, pain interference, sleep disturbance, physical function, and ability to participate in social roles and activities. Each domain is measured via four items using a 5-point Likert scale. Higher scores on PROMIS-29 subscales (score range: 4–20) indicate that a greater amount of that subscale domain is being measured. Specifically, for the symptom-oriented domain subscales (Anxiety-4a, Depression-4a, Fatigue-4a, and Pain Interference-4a, Sleep Disturbance-4a), higher scores signify a worse symptom profile. For the function-oriented domain subscales (Physical Functioning-4a, Social Functioning-4a), higher scores signify better functioning. PROMIS instruments are standardized, well-validated, and efficient [25], and have been applied with increasing interest among researchers across a variety of health conditions and patient populations.
Survivorship health behaviors.
The American Cancer Society (ACS) [26], The National Comprehensive Cancer Network (NCCN) [27,28], the Centers for Disease Control and Prevention (CDC) [29], and other cancer survivorship organizations have identified specific health behavior guidelines that should be addressed consistently as part of cancer survivorship. These include recommendations on alcohol consumption reduction, healthy diet, physical activity, sun safety, smoking cessation, and weight management. Our study team used the specific recommendations from the NCCN to develop a set of six patient-reported questions that mapped on to each of the health behaviors (see Table 1). Responses were dichotomized in a manner consistent with NCCN recommendations [27,28].2
Table 1.
Survivorship Health Behaviors, Research Study Questions, and Responses
| Survivorship Health Behaviors per NCCN[28] | Research Study Questions | Dichotomized Responses |
|---|---|---|
|
Alcohol Use Limit intake to no more than one drink per day for a woman and two drinks per day for a man. |
On average, how many alcoholic beverages do you currently drink per week? | < 5 drinks per week or > 5 drinks per week |
|
Healthy Diet Eat a diet that is at least 50% plant-based, with the majority of food being vegetables, fruit, and whole grains. |
Generally speaking, how healthy do you consider your diet to be? | Not Healthy or Healthy |
|
Physical Activity Overall volume of weekly activity should be at least 150 minutes of moderate-intensity activity or 75 min of vigorous physical activity. |
Would you say that you get 150 minutes of moderate physical activity (e.g., walking) or 75 minutes of vigorous physical activity (e.g., running) per week? | No or Yes |
|
Sun Screen Use Practice sun safety. Utilize sunscreen with SPF of at least 30 and apply generously ever two hours while outside. |
When you go outside on a warm sunny day for MORE than one hour, how often do you where sunscreen? | Never or Sometimes/Always |
|
Tobacco Use Avoid use or stop using all tobacco products. |
Are you a current, former, or never smoker? | Current or Never/Former Smoker |
|
Weight Management All survivors should be encouraged to achieve and maintain a normal body mass index and strive for metabolic health. |
Currently, would you classify your weight as underweight, overweight, or healthy? | Under/Overweight or Healthy |
Data Analysis
Aim 1: Identify demographic and disease characteristics associated with FT among post-treatment, Stage I-III solid tumor cancer survivors.
Demographic and disease characteristics were represented using appropriate descriptive statistics (means and standard deviations; frequencies and percentages) based on the type of variable and measurement. Correlations and/or t-tests were conducted to assess relationships between financial toxicity (COST scores) and demographic and disease characteristics, utilizing the Holm-Bonferroni (H-B) correction to reduce type I errors from multiple comparisons (n=15 demographic and disease variables) related to aim 1. Tests were considered significant when the H-B familywise error rate was less than 0.05 [30]. A one-way ANOVA analysis was utilized to determine whether financial toxicity varied across cancer types with Tukey’s Honestly Significant Difference (HSD) post-hoc test to determine how financial toxicity mean scores differed between each of the 5 cancer types. Results of the one-way ANOVA were also corrected for multiple comparisons using H-B.
Aim 2: Document the relationship between patient-reported FT, HRQOL, and adherence to health behavior survivorship guidelines.
Univariate relationships between patient-reported financial toxicity (COST scores), HRQOL (measured by PROMIS-29), survivorship health behaviors (dichotomized NCCN recommendations), and demographic and disease characteristics were analyzed using Pearson correlations. The Holm-Bonferroni correction was used to account for multiple tests within the HRQOL univariate analyses (n=7), and the survivorship health behavior univariate analyses (n=6) [30].
To determine whether financial toxicity (utilizing the COST measure) predicted HRQOL and survivorship health behaviors after controlling for conceptually and statistically significant predictor variables, multiple linear regression and multiple logistic regression models were performed. Specifically, the HRQOL and survivorship health behavior variables that were statistically significant based on H-B corrected univariate analyses were entered as dependent variables into separate regression models. Each regression model included the same set of covariates that were chosen based on theoretical relevance (age and gender [4,8,9]) and statistical associations from univariate H-B corrected demographic and disease variables (p<.05) such that the regression blocks for each model included: Block 1= gender, age; Block 2= partner status, retirement status, educational attainment; Block 3= COST score. Model comparison was performed between the model that only included demographic predictor variables (i.e., Blocks 1 and 2) and the model that included demographic predictor variables and the COST variable (i.e., Block 1= gender, age; Block 2= partner status, retirement status, educational attainment; Block 3= COST score) to evaluate whether COST explained a statistically significant amount of additional variance in the dependent HRQOL and survivorship health behaviors scores. The H-B correction was not applied to multivariate analyses.
Results
Descriptive Analyses
Demographic characteristics for the 103 cancer survivors who completed the cross-sectional phone survey are presented in Table 2. The sample included approximately equal numbers of men and women and was primarily Non-Hispanic White. The majority of participants were age 65 or older (65%), partnered (69%), and retired (63%). All participants reported having insurance during and after cancer treatment, with approximately half of survivors holding private insurance (53%; the remainder reported government-sponsored coverage, including Medicare, Medicaid, and/or VA/Tricare). Educational attainment varied widely across participants with approximately half reporting a 4-year college degree or higher (53%).
Table 2.
Demographic and Disease Characteristics of the Sample (N=103) Associated with FT
| Characteristic | Number | Percentage | Association with FT (r) | p-value with Holm-Bonferroni correction |
|---|---|---|---|---|
| Gender | −.08 | p = .405 | ||
| Male | 53 | 52% | ||
| Female | 49 | 48% | ||
| Other | 1 | |||
| Race | −.05 | p = .585 | ||
| White | 95 | 92.2% | ||
| Black | 2 | 1.9% | ||
| Mixed | 4 | 3.9% | ||
| Other | 2 | 1.9% | ||
| Ethnicity | −.26 | p = .008 | ||
| Non-Hispanic | 92 | 89.3% | ||
| Hispanic | 11 | 10.7% | ||
| Non-Hispanic White (NHW) | .23 | p = .017 | ||
| NHW | 88 | |||
| Other | 15 | |||
| Age, y | .19 | p = .056 | ||
| Age | M=67.28 | SD=10.12 | ||
| Partnered | .35 | p = .003 * | ||
| Yes | 71 | 68.9% | ||
| No | 32 | 31.1% | ||
| Employment | .30 | p = .002 * | ||
| Retired | 65 | 63.1% | ||
| Not Retired (partially employed or unemployed) | 38 | 36.9% | ||
| Insurance Type | .24 | p = .013 | ||
| Private Insurance | 55 | 53.4% | ||
| Other Insurance | 48 | 46.6% | ||
| Educational Attainment | .39 | p < .000 * | ||
| < High School | 6 | 6.1% | ||
| High School/GED | 19 | 19.2% | ||
| Two-year College | 21 | 21.2% | ||
| Four-year College | 24 | 24.2% | ||
| Post-Graduate Degree | 29 | 29.3% | ||
| Disease Characteristics | ||||
| Cancer Type | ||||
| Breast | 37 | 36.3% | ||
| Colorectal | 7 | 6.9% | ||
| Head and Neck | 16 | 15.7% | ||
| Lung | 11 | 10.8% | ||
| Prostate | 31 | 30.4% | p = .043 | |
| Months Since Completing TX | .09 | p = .341 | ||
| Months | M=28.16 | SD=16.27 | ||
| Chemotherapy | −.21 | p = .035 | ||
| Yes | 33 | 32% | ||
| No | 69 | 67% | ||
| Don’t Know | 1 | |||
| Radiation Therapy | −.12 | p = .239 | ||
| Yes | 68 | 66% | ||
| No | 35 | 34% | ||
| Surgery | .03 | p = .788 | ||
| Yes | 77 | 25.3% | ||
| No | 26 | 25.2% | ||
| Hormone Therapy | .12 | p = .411 | ||
| Yes | 23 | 22.3% | ||
| No | 79 | 76.7% |
Note: M= Mean and SD= Standard Deviation.
p < .05 after Holm-Bonferroni (H-B) Correction
Disease characteristics of the cancer survivors are also presented in Table 2. Cancer types included breast (36%), colorectal (7%), head & neck (16%), lung (11%), and prostate (30%). All participants (100%) reported having completed cancer treatment. Participants varied in time since completion of cancer treatment from less than one month to 5–7 years, with almost half of survivors reporting completion of treatment at least three years earlier (49%). In relation to anti-cancer treatment, the majority of participants reported radiation therapy (66%) and surgery (77%); only 32% reported receiving chemotherapy and 23% reported receiving hormone therapy.
Associations between FT and Demographic and Disease Characteristics
The average score for patient-reported financial toxicity was 32.5 (SD = 9.46) and scores ranged from 6 – 44, approximately one standard deviation higher (indicating lower FT) than mean COST scores in the de Souza paper of advanced cancer patients undergoing active treatment [31]. Based on previously reported COST score cut-offs (<22), we found that 18% of our sample reported FT scores consistent with significant financial burden. As noted in Table 2, greater FT was significantly associated with unpartnered status, non-retirement, and lower level of educational attainment. The strength of associations between FT and unpartnered status (r=.30), between FT and non-retirement (r=.35), and between FT and lower level of education (r=.39) are all considered moderately strong, and therefore, constitute not only statistical significance but also practical significance as related to financial toxicity. After H-B correction, results from the one-way ANOVA suggested that survivors did not differ significantly on financial toxicity as a function of cancer type, F (4, 97) = 2.57, p = .043.
Associations between FT and HRQOL and Adherence to Survivorship Guidelines
HRQOL.
As noted in Table 3, greater FT was significantly associated with anxiety, fatigue, pain interference, physical and social functioning. The strength of associations between FT and anxiety (r=−.34), FT and fatigue (r=−.41), FT and pain interference (r=−.27), FT and physical functioning (r=.32), and FT and social functioning (r=−.31) are all considered moderately strong and, therefore, constitute both statistical significance and practical significance with regard to financial toxicity. Results of the multiple linear regressions for each of these domains indicated that, after controlling for relevant demographic characteristics (regression blocks: Block 1= gender, age; Block 2= partner status, retirement status, educational attainment; Block 3= COST score), patient-reported financial toxicity (COST) continued to be associated with anxiety, fatigue, physical functioning, and social functioning but was no longer a statistically significant predictor of pain interference (Table 4). As Table 4 indicates, at an alpha = .05 level, there was a statistically significant difference between the model that included demographic predictor variables only (i.e., Blocks 1 and 2) and the model that included demographic predictor variables and the COST variable (i.e., Block 1= gender, age; Block 2= partner status, retirement status, educational attainment; Block 3= COST score) for anxiety, fatigue, physical functioning, and social functioning because the F-test of change in R2 was significant (Anxiety, p=.012; Fatigue, p=.001; Physical Functioning, p=.020; Social Functioning, p=.013). These results indicate that inclusion of the COST predictor variable in the model explained a statistically significant additional amount of variance in the dependent variables (Anxiety, Fatigue, Physical Functioning, and Social Functioning) above and beyond that contributed by the other predictors of gender, age, partner status, retirement status, and educational attainment. Thus, adding financial toxicity (i.e., COST) to the regression models explained 6% of additional variance predicted in anxiety scores, 10% of the additional variance in predicted fatigue scores, 5% of additional variance in predicted physical functioning scores, and 6% of additional variance in predicted social functioning scores (see Table 4).
Table 3.
Correlations between of HRQOL, Health Behaviors, and Financial Toxicity
| Characteristic | M (SD) | Range | Association with FT (r) | p-value with Holm-Bonferroni correction |
|---|---|---|---|---|
| HRQOL | ||||
| Anxiety | 6.14 (3.05) | 4–17 | −.34 −.21 −.41 |
p = .001* |
| Depression | 5.40 (2.34) | 4–13 | p = .031 | |
| Fatigue | 8.91 (4.11) | 4–20 | p < .000* | |
| Sleep | 9.20 (3.32) | 4–20 | −.25 | p = .010 |
| Pain | 7.78 (3.90) | 4–20 | −.27 | p = .006* |
| Physical Functioning | 17.57 (4.07) | 4–20 | .31 | p =.001* |
| Social Functioning | 7.24 (4.07) | 4–20 | −.31 | p =.002* |
| Health Behaviors | ||||
| Alcohol | .20 (.27) | 0–1 | .03 | p =.042 |
| Smoking | .92 (.27) | 0–1 | .20 | p =.047 |
| Diet | .58 (.50) | 0–1 | .26 | p =.008 |
| Weight | .46 (.50) | 0–1 | .14 | p =.161 |
| Physical Activity | .74 (.44) | 0–1 | .18 | p =.065 |
| Sunscreen | .55 (.50) | 0–1 | .04 | p =.730 |
Note: M= Mean and SD= Standard Deviation.
p < .05 after Holm-Bonferroni (H-B) Correction
Table 4.
Multiple Regression Analyses from Five Separate Models Using COST Score to Predict Health-Related Quality of Life (HRQOL) (N=103)
| HRQOL Outcomes | B | SE B | β | ΔR2 | p-value | |
|---|---|---|---|---|---|---|
| Anxiety | −.09 | .04 | −.28 | .06 | p = .012* | |
| Fatigue | −.16 | .05 | −.36 | .10 | p = .001* | |
| Pain Interference | −.07 | .06 | −.15 | .02 | p = .206 | |
| Physical Functioning | .11 | .05 | .27 | .05 | p = .020* | |
| Social Functioning | −.17 | .05 | −.28 | .06 | p = .013* |
Note: All analyses included covariates entered in the following order: Block 1= gender, age; Block 2= partner status, retirement status, educational attainment; Block 3= COST score. F-test of change in R2 (ΔR2) indicates that the average additional variance explained by using the additional predictor (COST) explained a statistically significant additional amount of variance in the dependent variable above and beyond that contributed by the other predictors of gender, age, partner status, retirement status, and educational attainment. H-B correction was not utilized within multivariate analyses.
Survivorship health behaviors.
In terms of health behavioral outcomes, FT was not found to be associated with any of the survivorship health behaviors after utilizing the H-B correction (see Table 3). Therefore, none of the survivorship health behaviors were evaluated using multiple logistic regression (see Table 4).
Discussion
Broadening attention from the immediate to the longer-term post-treatment period has expanded our understanding of unmet needs for cancer survivors. The current analyses further addressed the continuing financial burden during cancer survivorship by utilizing a standardized metric of financial toxicity (FT) and exploring associations with quality of life and health behaviors. Based on suggested cut-offs from previous studies [14,19], 18% of our sample experienced significant FT well into the post-treatment cancer survivorship period. Overall, our results indicated lower levels of financial toxicity (FT) in this population of cancer survivors, compared to studies of populations receiving active cancer treatment. Both this relatively low percentage and higher average COST scores (approximately one standard deviation higher than studies of active treatment patients) suggest several possibilities. Financial burden associated with treatment may dissipate over time as oncologic needs become less acute, at least among insured survivors in the United States. Alternatively, the composition of our sample of cancer survivors (65% of whom were over the age of 65 and 63% of whom were retired) may not adequately reflect the experience of younger adult cancer survivors who are known to experience unique financial stressors such as returning to the workforce and providing for their young children [32]. Yet, it remains notable that, although patient-reported financial burden may diminish across the cancer care continuum and may differ between survivorship cohorts, our data suggest that FT remains a persistent challenge for a significant minority of post-treatment cancer survivors.
Patterns of association between FT and cancer survivors’ demographic and disease characteristics provide a window into identifying predictors of financial vulnerability and a framework for establishing potential risk categories. Surprisingly, time since treatment was not associated with FT. However, greater financial toxicity was associated with unpartnered status (single, divorced, or widowed), employed status (i.e., not retired), and lower levels of education. Many of these predictors reflect broad social determinants of health quality that are often linked to socioeconomic status and social support [33], emphasizing the need to assess for these sociodemographic factors when identifying patients most at risk for poor health outcomes and in need of intervention. Our findings that being employed (i.e., not retired), unpartnered, and of lower educational attainment predict higher financial burden are of particular interest because they highlight the need to target assessments and interventions to patient populations that may lack critical resources associated with post-retirement savings [34]. Although our H-B corrected findings suggested that financial burden did not vary across cancer type, we believe that future evaluation of how cancer type and treatment may differentially impact FT remains a worthy research pursuit, especially since previous studies have observed worse symptom burden, greater psychosocial distress, greater cost expenditures, and functional impairment in patients being treated for lung cancer compared to other common cancer types [12,35–37]. Although the dominant survivorship care planning templates (e.g., ASCO, Journey Forward, etc.) have not directly acknowledged or assessed the potential impact of FT on HRQOL [38], clinicians, patients, and patient-advocates alike are in support of incorporation of FT as an explicit and critical component of cancer care planning [39]. In fact, an ongoing clinical trial is currently using a patient-reported financial impact assessment tool to prospectively assess financial distress in metastatic colorectal cancer patients with a goal of enhancing supportive care for patients with the greatest financial need [40]. The results of our study highlight the potential risk factors associated with FT that could be assessed both during treatment and during survivorship care planning. Specifically, recognition of patient demographic characteristics and cancer type as potential risk factors could enhance real-time clinical assessment of survivors’ FT vulnerability during cancer clinic visits across the cancer continuum.
Our second set of study goals addressed relationships between FT, HRQOL, and health-enhancing behaviors during cancer survivorship. None of the survivorship health behavior outcomes were associated with FT in univariate analyses after correcting for multiple testing (H-B correction). This was somewhat surprising, given known associations between financial burden and health behaviors in non-oncology populations [41,42]. More research is needed to expand our understanding of potential connections between FT and survivors’ adherence to recommended health behavior guidelines. Our study’s findings that focused on HRQOL were more expansive. Our models that controlled for relevant demographic variables demonstrated that greater FT was statistically associated with higher patient-reported anxiety, fatigue, and social functioning and lower patient-reported physical functioning. Although true directionality cannot be assessed with cross-sectional data, these findings suggest that financial burden may impact health-related quality of life long into the post-treatment period for cancer survivors. Given the relationship between poorer HRQOL and cancer recurrence [43], these data underscore the importance of assessing and addressing FT well into the period of post-cancer survivorship.
By outlining the potential detrimental outcomes associated with FT and identifying the demographic and disease characteristics that often place cancer survivors at particular risk, our findings set the stage for the development and targeting of interventions aimed at ameliorating financial burden in the cancer care setting. In recent years, there has been increased attention toward intervening on FT with cancer patients but research interventions have been limited [44]. Many cancer centers have hired financial navigators to help patients in need obtain discounted drug prices (e.g., drug discount programs such as 340b). Although the impact of implementation of a financial navigator within a cancer setting requires further investigation [45], the potential for enhancing health outcomes through financial counseling with cancer patients is clearly worth pursuing [46]. Although multiple intervention studies within oncology as well as general medical settings have attempted to address FT using enhanced price transparency during medical decision-making, there remains potential for greater optimization of cost-effective medical decision-making [47,48]. Of interest, a recently published equity intervention used the COST measure to address financial burden among cancer clinical trial participants by reimbursing them for nonclinical expenses such as transportation and lodging [49]. Future interventions designed to reduce FT within the United States should consider the potential impact of broad health care reform on financial burden and implement FT interventions that have the potential to be most practicable within a changing health care system [50].
This study is not without limitations. Our sample focused exclusively on an insured population treated at a single, NCI-designated cancer center in the United States. In addition, given our generally low response rate (40%), our data may reflect a response bias that does not fully capture the full range of survivor demographics or experiences [51,52]. As such, our data may underestimate FT in a larger cohort of younger and/or more diverse cancer survivors who are underinsured, being treated in community oncology settings, and less willing to participate in research. Although the COST measure is standardized and validated within the United States, the measure itself is limited in its comprehensive assessment of the six key domains identified in a recent systematic review[13]. Specifically, it would have been helpful to consider how the use of a second measure might have provided additional information on FT domains that are missing from COST such as behavioral coping around cancer care and lifestyle decision-making [13]. Further, the COST measure may not be the appropriate choice for cancer patients and survivors outside of the United States, as it does not directly take into account third-party payment structures [13]. As a cross-sectional study, we cannot make definitive statements about causality. In addition, conclusions from the multiple linear regressions should be interpreted somewhat cautiously as they represent findings with small effect sizes from a relatively small sample (n=103) and H-B correction was not used. Therefore, despite this study’s use of the Holm-Bonferroni correction for multiple testing for all univariate analyses, caution should still be applied to extrapolation of our findings into interpretations that infer directional pathways. Despite these limitations, the merits of this study – use of standardized, well-validated measurement [53] in a post-treatment oncology sample – suggest that our findings provide a worthy contribution to the FT literature.
In conclusion, using well-validated patient-report instruments, we demonstrated that FT was a meaningful predictor of patient-reported anxiety, fatigue, physical functioning, and social functioning in that, even after controlling for relevant demographic characteristics, the addition of financial toxicity (i.e., COST) to the regression models explained an additional 5–10 percent of variance predicted scores for anxiety, fatigue scores, physical functioning, and social functioning. These findings have implications for future research focused on financial toxicity within the context of cancer survivorship and interventions to enhance health outcomes for cancer survivors. These findings also have implications for clinical settings, including the potential to incorporate a measure of patient-reported FT into survivorship care planning and other documents to understand dynamic and ongoing challenges faced by cancer survivors and identify those most in need of intervention.
Acknowledgments:
We wish to thank all study participants for their valuable contributions. Research reported in this publication utilized the Behavioral Measurement and Interventions Shared Resource (BMISR) which is supported by the National Cancer Institute of the National Institutes of Health under award number P30 CA023074.
Funding:
Research reported in this publication was supported by a grant from the Merck Foundation Alliance to Advance Patient-Centered Cancer Care awarded to Heidi Hamann, PhD.
Footnotes
Survey data were stored in REDCap (Research Electronic Data Capture) and securely hosted on HIPAA-compliant servers.
The NCCN recommendation for alcohol consumption was not fully consistent with the dichotomization of the study’s alcohol question because the study question asked for alcohol consumption > or < 5 drinks per week and the NCCN recommendation for alcohol consumption is ‘no more than 1 drink per day for women and 2 drinks per day for men’.
Disclosures and declarations
The authors declare that they have no conflict of interest.
References
- 1.American Cancer Society. The Costs of Cancer | American Cancer Society Cancer Action Network. https://www.fightcancer.org/policy-resources/costs-cancer. Accessed 2019 Sep 20.
- 2.National Cancer Institute. Financial Toxicity (Financial Distress) and Cancer Treatment (PDQ®): Patient Version. Natl. Cancer Inst 2017. [Google Scholar]
- 3.Gilligan AM, Alberts DS, Roe DJ, Skrepnek GH. Death or Debt? National Estimates of Financial Toxicity in Persons with Newly-Diagnosed Cancer. Am J Med. Elsevier; 2018;131:1187–1199.e5. [DOI] [PubMed] [Google Scholar]
- 4.Gordon LG, Merollini KMD, Lowe A, Chan RJ. A Systematic Review of Financial Toxicity Among Cancer Survivors: We Can’t Pay the Co-Pay. Patient - Patient-Centered Outcomes Res. 2017;10:295–309. [DOI] [PubMed] [Google Scholar]
- 5.Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park). NIH Public Access; 2013;27:80–1, 149. [PMC free article] [PubMed] [Google Scholar]
- 6.Narang AK, Nicholas LH. Out-of-Pocket Spending and Financial Burden Among Medicare Beneficiaries With Cancer. JAMA Oncol. American Medical Association; 2017;3:757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood). NIH Public Access; 2013;32:1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones SMW, Yi JC, Jim HSL, Loren AW, Majhail NS, Uberti J, et al. Age and gender differences in financial distress among hematopoietic cell transplant survivors. Support Care Cancer. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabroff KR, Dowling EC, Guy GP, Banegas MP, Davidoff A, Han X, et al. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34:259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6:166–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute: Division of Cancer Control and Population Sciences. Financial Burden of Cancer Care. 2019.
- 12.Park J, Look KA. Health Care Expenditure Burden of Cancer Care in the United States. Inq (United States). 2019;56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witte J, Mehlis K, Surmann B, Lingnau R, Damm O, Greiner W, et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Ann Oncol. 2019;30:1061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ezeife DA, Morganstein BJ, Lau S, Law JH, Le LW, Bredle J, et al. Financial Burden Among Patients With Lung Cancer in a Publically Funded Health Care System. Clin Lung Cancer. 2018; [DOI] [PubMed] [Google Scholar]
- 15.de Souza JA, Yap BJ, Hlubocky FJ, Wroblewski K, Ratain MJ, Cella D, et al. The development of a financial toxicity patient-reported outcome in cancer: The COST measure. Cancer. 2014;120:3245–53. [DOI] [PubMed] [Google Scholar]
- 16.de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araújo FS, Hlubocky FJ, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda K, Gyawali B, Ando M, Sugiyama K, Mitani S, Masuishi T, et al. A prospective survey of comprehensive score for financial toxicity in Japanese cancer patients: report on a pilot study. [DOI] [PMC free article] [PubMed]
- 18.Huntington SF, Weiss BM, Vogl DT, Cohen AD, Garfall AL, Mangan PA, et al. Financial toxicity in insured patients with multiple myeloma: a cross-sectional pilot study. Lancet Haematol. 2015;2:e408–16. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig M, West M, Matthews J, Stokan M, Yoojin Kook YK, Gallups S, et al. Financial Toxicity Among Women With Metastatic Breast Cancer. Oncol Nurs Forum. 2019;46:83–91. [DOI] [PubMed] [Google Scholar]
- 20.Allcott N, Dunham L, Levy D, Carr J, Stitzenberg K. Financial burden amongst cancer patients treated with curative intent surgery alone. Am J Surg. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2017;109:djw205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Person-Centered Assessment Resource. HealthMeasures: Transforming How Health is Measured. http://www.healthmeasures.net/search-view-measures?task=Search.search. Accessed 2019 Apr 5.
- 23.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group During its First Two Years. Med. Care 2007. pp. S3--S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11:304–14. [PMC free article] [PubMed] [Google Scholar]
- 25.PROMIS Cooperative Group. PROMIS® instrument development and validation scientific standards version 2.0. 2013;0:1–72. [Google Scholar]
- 26.American Cancer Society. Survivorship Care Plans. https://www.cancer.org/treatment/survivorship-during-and-after-treatment/survivorship-care-plans.html. Accessed 2019 Apr 6.
- 27.National Comprehensive Cancer Network I. NCCN Guidelines: Survivorship. Am J Clin Pathol. 2012;137:516–42.22431528 [Google Scholar]
- 28.Scott Baker K, Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Hudson M, et al. NCCN Guidelines Panel Disclosures Continue NCCN Guidelines Version 2.2019 Survivorship. 2019.
- 29.Centers for Disease Control and Prevention. Staying Healthy After Cancer Treatment. https://www.cdc.gov/cancer/survivors/life-after-cancer/staying-healthy-after-cancer-treatment.htm. Accessed 2019 Apr 6.
- 30.Holm S Board of the Foundation of the Scandinavian Journal of Statistics A Simple Sequentially Rejective Multiple Test Procedure Author ( s ): Sture Holm Published by : Wiley on behalf of Board of the Foundation of the Scandinavian Journal of Statistics Stable U. Scand J Stat. 1978;6:65–70. [Google Scholar]
- 31.de Souza JA, Yap BJ, Wroblewski K, Blinder V, Araújo FS, Hlubocky FJ, et al. Measuring financial toxicity as a clinically relevant patient-reported outcome: The validation of the COmprehensive Score for financial Toxicity (COST). Cancer. 2017;123:476–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: Contributions from psychosocial oncology research. Am Psychol. 2015;70:159–74. [DOI] [PubMed] [Google Scholar]
- 33.Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. American Public Health Association; 2010;100 Suppl 1:S186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Cancer Society. Special Issues for Young Adults With Cancer. https://www.cancer.org/cancer/cancer-in-young-adults/special-issues.html. Accessed 2019 Aug 1.
- 35.Lehto RH. Psychosocial challenges for patients with advanced lung cancer: interventions to improve well-being. Lung Cancer Targets Ther. 2017;Volume 8:79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamann HA, Ostroff JS, Marks EG, Gerber DE, Schiller JH, Lee SJC. Stigma among patients with lung cancer: a patient-reported measurement model. Psychooncology. 2014;23:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriram N, Mills J, Lang E, Dickson HK, Hamann HA, Nosek BA, et al. Attitudes and Stereotypes in Lung Cancer versus Breast Cancer. Gorlova OY, editor. PLoS One. 2015;10:e0145715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coughlin SS, Dean LT. Cancer survivorship care plans, financial toxicity, and financial planning alleviating financial distress among cancer survivors. Support Care Cancer. 2019;27:1969–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zafar SY, Newcomer LN, McCarthy J, Fuld Nasso S, Saltz LB. How Should We Intervene on the Financial Toxicity of Cancer Care? One Shot, Four Perspectives. Am Soc Clin Oncol Educ B. 2017;35–9. [DOI] [PubMed] [Google Scholar]
- 40.ClinicalTrials.Gov. S1417CD Financial Impact Assessment Tool in Patients With Metastatic Colorectal Cancer - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02728804. Accessed 2019 Jul 31.
- 41.Sweet E, Nandi A, Adam EK, McDade TW. The high price of debt: household financial debt and its impact on mental and physical health. Soc Sci Med. NIH Public Access; 2013;91:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drentea P, Lavrakas PJ. Over the limit: the association among health, race and debt. Soc Sci Med. Pergamon; 2000;50:517–29. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez JL, Hawkins NA, Berkowitz Z, Li C. Factors Associated with Health-Related Quality of Life Among Colorectal Cancer Survivors. Am J Prev Med. NIH Public Access; 2015;49:S518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yousuf Zafar S Financial Toxicity of Cancer Care: It’s Time to Intervene. J Natl Cancer Inst. 2016;108:djv370. [DOI] [PubMed] [Google Scholar]
- 45.National Cancer Institute. Financial Toxicity and Cancer Treatment. 2019. https://www.cancer.gov/about-cancer/managing-care/track-care-costs/financial-toxicity-hp-pdq#_155_toc. Accessed 2019 Jul 31.
- 46.Shankaran V, Leahy T, Steelquist J, Watabayashi K, Linden H, Ramsey S, et al. Pilot Feasibility Study of an Oncology Financial Navigation Program. J Oncol Pract. 2018;14:e122–9. [DOI] [PubMed] [Google Scholar]
- 47.Sedrak MS, Myers JS, Small DS, Nachamkin I, Ziemba JB, Murray D, et al. Effect of a Price Transparency Intervention in the Electronic Health Record on Clinician Ordering of Inpatient Laboratory Tests. JAMA Intern Med. 2017;177:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kline RM, Bazell C, Smith E, Schumacher H, Rajkumar R, Conway PH. Centers for Medicare and Medicaid Services: Using an Episode-Based Payment Model to Improve Oncology Care. J Oncol Pract. 2015;11:114–6. [DOI] [PubMed] [Google Scholar]
- 49.Nipp RD, Lee H, Gorton E, Lichtenstein M, Kuchukhidze S, Park E, et al. Addressing the Financial Burden of Cancer Clinical Trial Participation: Longitudinal Effects of an Equity Intervention. Oncologist. AlphaMed Press; 2019;theoncologist.2019–0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.PDQ Adult Treatment Editorial Board PATE. Financial Toxicity (Financial Distress) and Cancer Treatment (PDQ®): Patient Version. PDQ Cancer Inf. Summ National Cancer Institute; (US: ); 2002. [PubMed] [Google Scholar]
- 51.Carpentier MY, Tiro JA, Savas LS, Kay L, Melhado TV, Coan SP, et al. A feasibility study. 2014;7:155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millar MM, Kinney AY, Camp NJ, Cannon-Albright LA, Hashibe M, Penson DF, et al. Predictors of Response Outcomes for Research Recruitment through a Central Cancer Registry: Evidence from 17 Recruitment Efforts for Population-Based Studies. Am J Epidemiol. 2019;188:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 31:233–40. [DOI] [PubMed] [Google Scholar]


