Abstract
The microbial ecology of anaerobic carbon oxidation processes was investigated in Black Sea shelf sediments from mid-shelf with well-oxygenated bottom water to the oxic-anoxic chemocline at the shelf-break. At all stations, organic carbon (Corg) oxidation rates were rapidly attenuated with depth in anoxically incubated sediment. Dissimilatory Mn reduction was the most important terminal electron-accepting process in the active surface layer to a depth of ∼1 cm, while SO42− reduction accounted for the entire Corg oxidation below. Manganese reduction was supported by moderately high Mn oxide concentrations. A contribution from microbial Fe reduction could not be discerned, and the process was not stimulated by addition of ferrihydrite. Manganese reduction resulted in carbonate precipitation, which complicated the quantification of Corg oxidation rates. The relative contribution of Mn reduction to Corg oxidation in the anaerobic incubations was 25 to 73% at the stations with oxic bottom water. In situ, where Mn reduction must compete with oxygen respiration, the contribution of the process will vary in response to fluctuations in bottom water oxygen concentrations. Total bacterial numbers as well as the detection frequency of bacteria with fluorescent in situ hybridization scaled to the mineralization rates. Most-probable-number enumerations yielded up to 105 cells of acetate-oxidizing Mn-reducing bacteria (MnRB) cm−3, while counts of Fe reducers were <102 cm−3. At two stations, organisms affiliated with Arcobacter were the only types identified from 16S rRNA clone libraries from the highest positive MPN dilutions for MnRB. At the third station, a clone type affiliated with Pelobacter was also observed. Our results delineate a niche for dissimilatory Mn-reducing bacteria in sediments with Mn oxide concentrations greater than ∼10 μmol cm−3 and indicate that bacteria that are specialized in Mn reduction, rather than known Mn and Fe reducers, are important in this niche.
The complete oxidation of organic compounds through the dissimilatory reduction of Mn or Fe oxide constitutes the most recently discovered and least explored of the major types of anaerobic respiration in nature (34). Over recent years, bacteria and archaea that carry out these types of metabolism have been found in many different environments, and a large phylogenetic and metabolic diversity is becoming evident (35, 70). Also, a basic understanding of the ecological niches of Mn- and Fe-reducing organisms has emerged. In natural environments, the occurrence of Mn and Fe reduction has been found to depend primarily on the presence of Mn oxide and poorly crystalline Fe oxide, respectively. Thus, when Mn oxide is abundant under anoxic conditions, microbial Mn reduction dominates carbon oxidation over Fe and sulfate reduction (10, 38, 47). When Mn oxides are absent and enough poorly crystalline Fe(III) is available, Fe reduction dominates over sulfate reduction (13, 39, 65). This sequence can be explained through differences between the metabolic types in their efficiency of competition for common substrates (36, 37).
The first determinations of the processes in natural environments have shown that dissimilatory Mn or Fe reduction may, under certain circumstances, dominate carbon oxidation in both marine and freshwater sediments (13, 54). Microbial Fe reduction is an important process in many aquatic sediments. In a recent compilation, Fe reduction contributed 22% on average to anaerobic carbon oxidation in 16 different continental margin sediments, with the rest being primarily coupled to sulfate reduction (65). In contrast, organotrophic microbial Mn reduction has only been identified in two offshore basins, the Panama Basin and the Norwegian Trough, characterized by extremely high Mn oxide contents, where Mn reduction was the most important carbon oxidation pathway (2, 10). In coastal sediments, microbial Mn reduction is generally concluded to be of little significance in carbon oxidation, due to a relatively low abundance and shallow vertical penetration of Mn oxides, although studies of microbial Mn reduction are often hampered by the spatial resolution of available techniques (65). Because the sedimentation of Mn and Fe oxides to most sediments is much smaller than the benthic carbon oxidation rate, an efficient recycling of reduced Mn and Fe must take place when levels of Mn and Fe reduction are significant in carbon oxidation (13). The reoxidation of reduced Mn and Fe is stimulated by bioturbation through particle mixing or pore-water irrigation, and this Mn and Fe cycling ultimately depends on the presence of oxygen in the bottom water (2, 13).
A detailed understanding of the regulation of Mn and Fe reduction in sediments includes knowledge of the quantitatively important Mn- and Fe-reducing bacteria. However, the size and composition of the Mn- and Fe-reducing microbial communities in marine sediments are virtually unknown. With few exceptions, reduction of Mn and Fe is catalyzed by the same cultivated organisms (35), but most of these were enriched on Fe oxide, and the existence of specialized Mn reducers has been poorly investigated. Some sulfate-reducing bacteria which also couple carbon oxidation to Fe oxide reduction could also contribute to benthic Fe reduction (17, 40), although dissimilatory Fe reduction was not observed after addition of poorly crystalline Fe oxide to a sulfate-reducing coastal sediment (15). In this sediment, most-probable-number (MPN) counts furthermore yielded 1,000-fold-fewer Fe-reducing than sulfate-reducing bacteria, and this relationship was suggested to explain why sulfate reduction was not outcompeted by Fe reduction after the Fe oxide addition.
The aim of the present study was to investigate the microbiology of Mn and Fe reduction and the competitive relationships between these processes and sulfate reduction in coastal marine sediments. The investigations were performed on stations along a transect across the Black Sea shelf to the rim of the anoxic basin, where a range of bottom water oxygen concentrations potentially limit the cycling of Mn and Fe to various degrees. Furthermore, the sediments had moderately high Mn concentrations. We determined the rates of Mn, Fe, and sulfate reduction relative to the distribution of Mn and Fe oxides and analyzed the microbiology of Mn and Fe reduction.
MATERIALS AND METHODS
Sites and sampling.
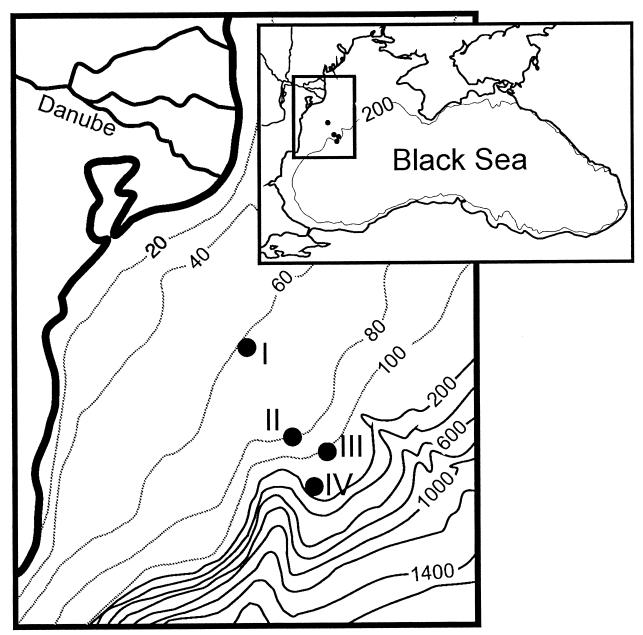
This study was conducted in September 1997 onboard RV Petr Kottsov on four stations on the Romanian Black Sea shelf (Fig. 1). The deepest station was located at the shelf break at a depth which, at the time of sampling, coincided with the oxic-anoxic interface in the water column (Table 1) (A. Weber, W. Riess, F. Wenzhöfer, and B. B. Jørgensen, submitted for publication). At all stations, the sediment was covered by an ∼1-cm-thick layer of small shells of Mytilus galloprovincialis and Modiolus phaseolinus filled in with fine-grained sediment. At stations I to III, the mussels dominated the fauna, while biomass decreased strongly from I to III (W. Riess, U. Luth, and F. Wenzhöfer, unpublished data). At station IV, only hydroides and meiofauna were observed. Below the shell layer, the sediments were fine-grained brown muds with a high shell content and few or no faunal burrows.
FIG. 1.
Maps of the Black Sea and the study area on the Romanian shelf. The numbers indicate water depth (meters).
TABLE 1.
Sites of sediment sampling
| Station | Water depth (m) | Sediment temp (°C) | Bottom water oxygen concn (μM) |
|---|---|---|---|
| I | 62 | 6.6 | 211 |
| II | 77 | 7.2 | 213 |
| III | 100 | 7.6 | 75 |
| IV | 130 | 8 | 0 |
Sediment cores of 9.6 cm in diameter, up to 40 cm long, and visually undisturbed were retrieved with a multiple corer and immediately brought to a cold room at 8°C, where all subsequent handling for pore water analysis and incubations took place. Sediment for microbiological and molecular ecological analyses was subsampled and processed immediately after retrieval of cores.
Sediment incubations.
For the determination of total anaerobic mineralization rates and the relative contributions of manganese, iron, and sulfate reduction, sediment from seven cores was sliced in 0.5- to 2-cm-thick sections to a depth of 10 cm, and parallel sections were pooled, mixed, and loaded into gastight plastic bags, all in an N2-filled glove bag as previously described (67). The bags of sediment were incubated in the dark at 8°C in larger N2-filled bags to ensure anoxia.
The dependence of microbial Fe reduction on the presence of Fe oxide was further investigated at station I, where the sediment from 0.5 to 1.5 cm and 8 to 10 cm was split into two portions, one of which was amended with 40 μmol of Fe cm−3 as 2-line ferrihydrite [Fe(OH)3] from a 280-mmol-liter−1 N2-purged suspension synthesized from FeCl3 (59).
Subsamples for pore water analysis were withdrawn five to six times during 2 weeks. In the glove bag, the sediment was loaded into centrifuge tubes leaving no headspace, and after centrifugation the supernatant was withdrawn and filtered through 0.45-μm-pore-diameter cellulose acetate filters in an anoxic glove bag. A 1.8-ml aliquot was collected for ΣCO2 (total dissolved inorganic carbon) and NH4+ analysis in glass vials with no headspace and stored at 4°C, ∼2 ml were acidified with 6N HCl (1:100 vol) and stored at 4°C for Mn2+, Fe2+, and Ca2+ determination, and an ∼1-ml aliquot for H2S and SO42− analysis was preserved with 50-μl of 2 M Zn acetate. Pore water pH was determined in whole sediment with a glass electrode calibrated with NBS buffers. Sediment for analysis of Mn and Fe in the solid phase was sampled at the beginning of the incubations and stored frozen. Sulfate reduction rates were determined by the radiotracer technique (25) three times during each incubation in subsamples of sediment loaded into cut off glass syringes. Reduced 35S was recovered in a single-step Cr2+ distillation, and sulfate reduction rates were calculated according to reference 22. The initial distribution of NO3− and NO2− at each station was determined in a separate sediment core, which was sectioned under N2 shortly after retrieval. Pore water was extruded as in the incubations, and a 2-ml aliquot for NO3− + NO2− analysis was stored frozen.
Chemical analyses.
Pore water constituents were analyzed by the following procedures: ΣCO2 and NH4+, flow injection analysis with conductivity detection (23); NO3− + NO2−, chemiluminescence detection of NO produced by reduction with V(III) (8); Mn2+ and Ca2+, flame atomic absorption spectrometry; Fe2+, colorimetry with Ferrozine (62, 68); H2S, colorimetry with methylene blue (14); and SO42−, nonsuppressed anion chromatography.
Particulate Mn was quantified through extraction with dithionite-citrate-acetic acid (33). Poorly crystalline Fe(III) was determined by a combination of oxic and anoxic ammonium oxalate extractions (67). The extractions were made in duplicate.
Calculations.
Accumulation rates of pore water constituents were calculated from slopes ± standard errors of linear regression lines of concentrations versus time. Contrary to previous applications of the incubation technique, ΣCO2 concentrations were significantly affected by calcium carbonate precipitation (see Results). Rates of CaCO3 precipitation were calculated from changes in soluble Ca2+, taking into account that these changes are partially compensated for by reversible sorption to particle surfaces (5):
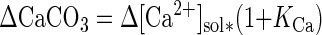 |
1 |
where KCa is the adsorption constant for Ca2+ (KCa = 1.6) (30). Rates of ΣCO2 production corrected for CaCO3 precipitation were calculated as
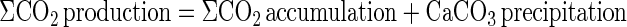 |
2 |
The saturation states of pore waters with respect to rhodocrocite (MnCO3) and siderite (FeCO3) were calculated by using PHREEQ-C with the thermodynamic constants of the PHREEQ database (50). Seawater compositions were calculated to the appropriate bottom water salinities (45) with the measured values of pH and concentrations of Ca2+, ΣCO2, Mn2+, and Fe2+.
Enumeration of Mn and Fe reducers.
At all stations, acetate-oxidizing Mn- and Fe-reducing bacteria were enumerated by the MPN technique with acetate as the sole organic carbon source and either ferrihydrite or vernadite (δMnO2) as an electron acceptor. Sediment from a depth of 1 to 2 cm served as a primary inoculum. The anaerobic, sulfate-free, bicarbonate-buffered marine mineral medium with vitamins and trace elements (19, 71) was supplemented with 10 mM acetate and 40 mmol of either 2-line ferrihydrite or vernadite per liter (46). The MPN tube batteries were inoculated anaerobically in 10-fold dilution steps and incubated at 20°C for 1 year. Positive tubes were recognized from the change in the color of the precipitates from reddish brown to black (Fe) and from dark brown to white (Mn) (34). Results were calculated according to standard procedures (18).
Molecular methods.
The natural microbial populations were analyzed at all stations by 4′,6′-diamidino-2-phenylindole (DAPI) staining and fluorescent in situ hybridization (FISH) in sediment sectioned in 0.5-cm intervals, and the highest positive dilutions from the MPN series of stations I, II, and IV were analyzed by 16S rRNA gene amplification, cloning, and sequencing. Sample manipulation, fixation, and subsequent processing were performed as previously published (55). PCR amplification of the nearly complete 16S rRNA genes was performed with universal primers (24). New sequences were added to an alignment of about 13,000 homologous bacterial 16S rRNA primary structures (42; http://www.mikro.biologie.tu-muenchen.de) by using the aligning tool of the ARB program package (http://www.mikro.biologie.tu-muenchen.de). Distance matrix, maximum-parsimony, and maximum-likelihood methods were applied as implemented in the ARB software package. Phylogenetic trees were constructed by using subsets of data that included complete or almost complete sequences of representative members of Proteobacteria. Topologies were evaluated by using the different approaches to elaborate a consensus tree (41).
RESULTS
ΣCO2 production and carbonate precipitation.
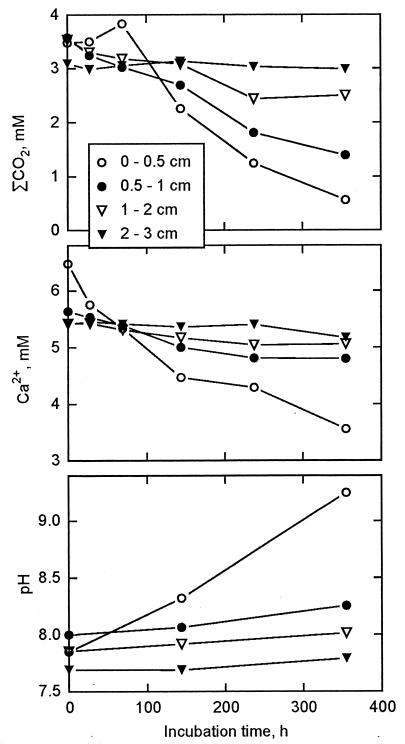
At all four stations, the ΣCO2 concentration typically increased linearly throughout the incubation, and accumulation rates decreased toward zero with sediment depth (data not shown). The exceptions to this were all of the 0- to 0.5-cm intervals as well as the 0.5- to 2-cm intervals at station III, where ΣCO2 concentrations decreased significantly either from the beginning or after an initial rise (Fig. 2). The decreasing ΣCO2 concentrations were accompanied by decreasing concentrations of Ca2+ (Fig. 2), which demonstrated an involvement of CaCO3 precipitation. This precipitation was further associated with marked increases in pH at all stations, while no pH changes were detected in the deeper sediment sections (Fig. 2). Rates of CaCO3 precipitation (equation 1) in the surface sections were of similar magnitude to the initial ΣCO2 accumulation rates. No precipitation was observed below a depth of 2 to 3 cm (data not shown).
FIG. 2.
Changes in the pore water constituents: total dissolved inorganic carbon (ΣCO2), calcium, and pH during anoxic incubation of the four upper depth intervals at station III.
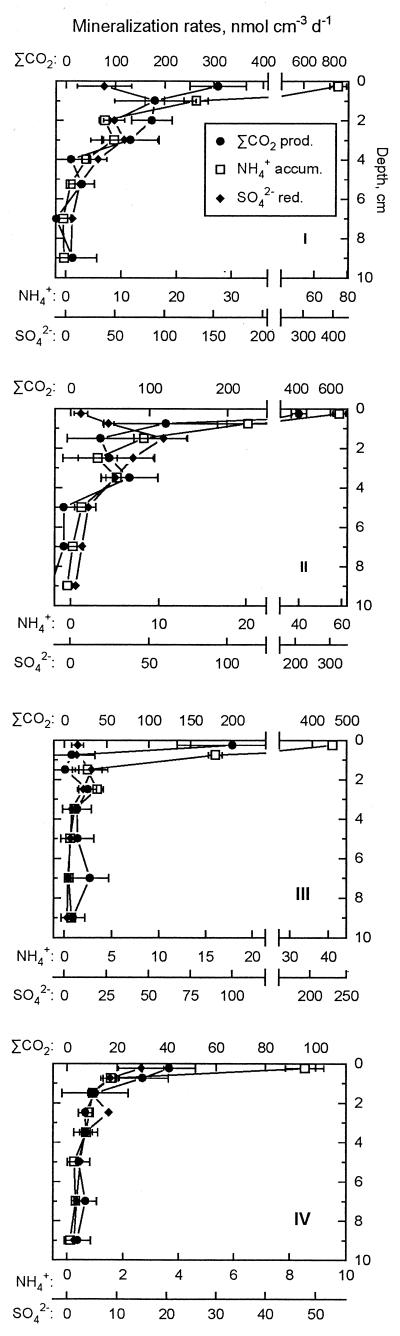
When CaCO3 precipitation was included in the calculations of ΣCO2 production (equation 2), the highest production rates were observed in the 0- to 0.5-cm interval at all stations and decreased 10-fold from station I to station IV (Fig. 3). Rates also decreased strongly with depth in the sediment, and in the deeper sections, ΣCO2 production was hardly detectable.
FIG. 3.
Vertical profiles of organic matter mineralization rates in Black Sea sediments from station I (top) to station IV (bottom). Circles, ΣCO2 production; squares, NH4+ accumulation; diamonds, sulfate reduction. Error bars indicate standard errors from linear regressions (ΣCO2 and NH4+) and standard deviations of triplicate sulfate reduction rates. Note different scales for different processes. At each station, the three scales are plotted at the ratio 11.2:1:5.6 for ΣCO2 production/NH4+ accumulation/sulfate reduction, corresponding to the inferred stoichiometric ratio of these processes during sulfate reduction (see text for details).
In addition to CaCO3 precipitation, ΣCO2 may also have been affected by the precipitation of Mn carbonate, because pore waters were generally strongly supersaturated with rhodocrocite (MnCO3), with ion activity products exceeding the solubility constant more than fivefold to a depth of 2 cm at stations I to III. However, because Mn2+ was produced simultaneously by Mn reduction (see below), a correction of ΣCO2 production similar to the one made for CaCO3 was not possible (see also Discussion). Iron carbonate precipitation was not of general importance, because siderite (FeCO3) supersaturation only developed toward the end of the incubation at station I (0 to 2 cm) and station II (0.5 to 2 cm) (data not shown).
NH4+ accumulation.
Similar to ΣCO2 production, the mineralization of organic nitrogen resulted in maxima of NH4+ accumulation in the pore water at 0- to 0.5-cm depth at all sites with rates decreasing rapidly and approaching zero below 4 cm (Fig. 3). The rates decreased offshore, with an ∼10-fold difference between maximum rates at stations I and IV, similar to the difference in ΣCO2 production.
Nitrate reduction.
Nitrate and nitrite were not important as electron acceptors during the incubations, because only small peaks of NO2− + NO3− were initially located at 0 to 0.5 cm, with concentrations of 7, 11, 8, and 4 μM at stations I through IV. Below 0.5 cm, initial concentrations were at a background level of ≤2 μM.
Mn and Fe reduction.
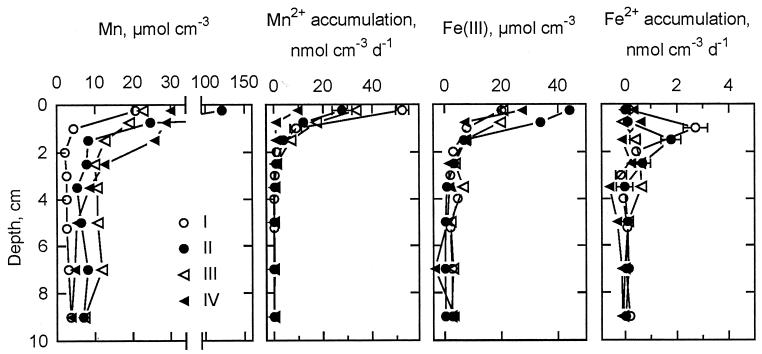
Extractable Mn was present in high concentrations (≥25 μmol cm−3) at 0 to 0.5 cm at all stations (Fig. 4). Manganese concentrations decreased with depth and reached stable levels below 1 to 2 cm, suggesting the depletion of reactive Mn oxides around this depth. At stations I to III, this depth distribution of reactive Mn oxide was further supported by the parallel distribution of accumulation rates of soluble Mn2+ (Fig. 4). At station IV, however, Mn2+ accumulation was restricted to a 0- to 0.5-cm depth, and since results concerning Fe and sulfate reduction at this site were also consistent with a depletion of reactive Mn below this interval, we interpret the elevated concentrations of extractable Mn at 0.5 to 2 cm as nonreactive Mn, possibly an authigenic Mn(II) phase. The maximum Mn2+ accumulation rates decreased offshore, but at all stations, rates corresponded to the accumulation of ≥250 μM Mn2+.
FIG. 4.
Depth distributions at stations I to IV of (left to right) extractable Mn, Mn2+ accumulation rates during anoxic incubations, poorly crystalline Fe(III), and Fe2+ accumulation rates. Concentrations are means of duplicate determinations, and error bars indicate standard errors of rates.
Concentrations of poorly crystalline Fe(III) were similar to those of Mn at the surface (excluding a particularly high Mn content at station II), but Fe(III) penetrated deeper into the sediment and first reached low background concentrations at a depth of ∼3 cm (Fig. 4). In contrast to the rapid accumulation of Mn2+, soluble Fe2+ concentrations generally increased by ≤10 μM during the 2 weeks, with small peaks of accumulation located below the peak of Mn2+ accumulation at each station (Fig. 4). As was the case for Mn2+, the rates were highest at station I.
Sulfate reduction rates.
At stations I to III, sulfate reduction rates increased from the surface to a maximum at a depth of 1 to 2 cm, below which rates decreased asymptotically toward zero (Fig. 3). At station IV, the rate was highest in the surface 0 to 0.5 cm. Both maximum and integrated rates decreased approximately fivefold offshore along the transect.
Bottom water sulfate concentrations were 14 to 17 mM and the decrease in pore-water concentration over the upper 10 cm at all sites was ≤2 mM. At all stations and depths, the initial concentration of H2S was <1 μM, but slight accumulations to ≤10 μM were observed below a depth of 3 cm at all stations during the first 2 weeks of incubation, with the highest rates at station IV (data not shown).
Assuming an overall stoichiometry of 2 mol of organic carbon to 1 mol of sulfate for carbon oxidation, with sulfate as the terminal electron acceptor (67), there was a good agreement between sulfate reduction-based and measured ΣCO2 production rates below ∼1 cm of depth at all stations, implying that all ΣCO2 production there could be attributed to sulfate reduction. In contrast, the measured ΣCO2 production at the surface significantly exceeded that calculated from sulfate reduction rates at stations I to III, while at station IV, only a small excess ΣCO2 production was indicated.
Sulfate reduction and ammonium accumulation rates were also closely correlated below 1 cm of depth at all sites (r2 = 0.87) with a ratio of sulfate reduction to ammonium accumulation for all stations of 5.6 ± 0.4:1, which corresponds to a ratio of carbon oxidation to ammonium accumulation of (2·5.6=) 11.2:1.
Addition of ferrihydrite.
In the sediment from depths of both 0.5 to 1.5 and 8 to 10 cm at station I amended with ferrihydrite, soluble Fe2+ accumulated at rates two to three times higher than in the unamended controls, while H2S remained undetectable. However, addition of ferrihydrite had no significant effect on either sulfate reduction rates, ΣCO2 production, or ammonium accumulation (Student's t test; P > 0.05) (Table 2).
TABLE 2.
Effects of ferrihydrite amendments on mineralization rates at station I
| Process | Treatment | Mineralization rate (nmol cm−3 day−1) at deptha:
|
|
|---|---|---|---|
| 0.5–1.5 cm | 8–10 cm | ||
| ΣCO2 production | Unamended | 179 ± 20 | 14 ± 49 |
| + Ferrihydrite | 200 ± 67 | 71 ± 24 | |
| NH4+ accumulation | Unamended | 24 ± 2 | −0.3 ± 0.2 |
| + Ferrihydrite | 26 ± 4 | −0.2 ± 0.6 | |
| Sulfate reduction | Unamended | 97 ± 23 | 5.2 ± 0.9 |
| + Ferrihydrite | 109 ± 42 | 3.4 ± 0.3 | |
Values are means ± standard errors.
Bacterial counts.
At all four stations, total bacterial counts were highest at the surface and decreased exponentially with depth, and cell numbers reached ∼10% of the surface value at 10 cm (data not shown; see profile from station II in reference 55). Cell numbers decreased slightly offshore, with maxima of 3.6 × 109, 2.0 × 109, 1.7 × 109, and 0.9 × 109 cells cm−3 at stations I through IV. The 1- to 2-cm layers used for MPN enumerations held numbers close to these maxima: 2.2 × 109, 1.5 × 109, 1.5 × 109, and 0.5 × 109 cells cm−3 at stations I through IV. Detection rates as well as single-cell signal intensities with FISH using the general probe for bacteria EUB338 (4) were very low in all sediments. The rates were ≤20% of total cell counts at stations I to III and ≤4% at station IV in the layer where MPN counts were performed. At all stations, the fraction of total cells that were detected with FISH decreased with depth, and the decreases through the vertical profile were parallel to the decreases in ΣCO2 production and NH4+ accumulation rates (see also reference 55).
The MPN counts resulted in extremely low numbers of Fe-reducing acetate oxidizers, since Fe reduction only occurred at the lowest dilution (Table 3). In contrast, the MPNs of Mn reducers were up to 1.1 × 105 cells cm−3 with this maximum at station II and the minimum at station IV (Table 3). The complete reduction of 40 mmol MnO2 liter−1 in the MPN tubes indicated that the oxidation of all of the 10 mM acetate added was coupled to Mn reduction.
TABLE 3.
MPN counts of acetate-oxidizing, Mn- and Fe-reducing bacteria in Black Sea sediments
| Station | Mn reducers
|
Iron reducers
|
||
|---|---|---|---|---|
| MPN (cells cm−3) | 95% Confidence interval | MPN (cells cm−3) | 95% Confidence interval | |
| I | 2.1 × 103 | 350–4.7 × 103 | 23 | 4–120 |
| II | 1.1 × 105 | 1.5 × 104–4.8 × 105 | 23 | 4–120 |
| III | 1.1 × 103 | 150–4.8 × 103 | 23 | 4–120 |
| IV | 460 | 71–2.4 × 103 | 23 | 4–120 |
Molecular screening.
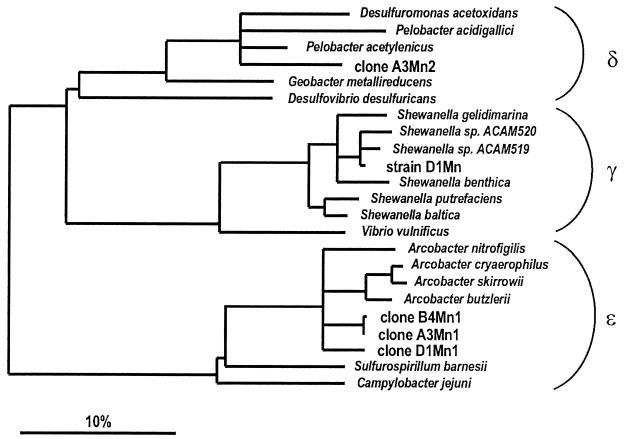
From the highest positive MPN dilutions with MnO2 at stations I, II, and IV, rRNA genes were amplified and cloned after 3 months of incubation. Fifteen clones from each library were screened with ARDRA (53) to distinguish different restriction patterns that were then sequenced. This resulted in two clone types from station I (A3Mn1 and A3Mn2) and only one clone type each from stations II and IV (B4Mn1 and D1Mn1). The 16S rRNA phylogenetic reconstruction showed that the clones A3Mn1, B4Mn1, and D1Mn1 were affiliated with the branch that comprises the genus Arcobacter in the ɛ-subclass of Proteobacteria (Fig. 5). Two clone types, A3Mn1 and B4Mn1, were nearly identical. The second most abundant organism from station I was affiliated with Desulfuromonas acetoxidans and Pelobacter species.
FIG. 5.
16S rRNA-based tree reflecting the phylogenetic relationships of the clone sequences and a selection of sequences belonging to different subclasses of Proteobacteria. The tree is based on the results of a maximum parsimony analysis including complete or almost complete 16S rRNA sequences from representative bacteria of a phylogenetic branch. The topology of the tree was evaluated and corrected according to the results of distance matrix, maximum parsimony, and maximum likelihood analyses of various data sets. Branching patterns within each subclass were also evaluated by using a 50% conservation filter for the members of their corresponding subclass (41). Multifurcations indicate topologies that could not be unambiguously resolved. The bar indicates 10% estimated sequence divergence. EMBL accession numbers of the new sequences are as follows: A3Mn2, AJ271656; D1Mn, AJ271657; B4Mn1, AJ271653; A3Mn1, AJ271655; and D1Mn1, AJ271654.
Attempts to continue the cultures from the highest positive dilutions with acetate were unsuccessful. Subcultivation of the MPN culture from the lowest dilution at station IV on lactate and δMnO2 resulted in the enrichment of an organism, strain D1Mn, which was isolated and identified as belonging to the genus Shewanella. Phylogenetic analysis of the rRNA sequence showed that its closest known relatives were Shewanella species isolated from Antarctic sea ice (Fig. 5).
DISCUSSION
No method is currently available for a direct quantification of organotrophic microbial Mn and Fe reduction rates in natural environments such as marine sediments, where reduced inorganic species will compete with organic matter as reductants of the metal oxides (66). Instead, our approach was to identify zones in the sediment where carbon oxidation rates exceeded the carbon oxidation coupled to bacterial sulfate reduction and to assign the excess carbon oxidation to alternative dissimilatory processes according to the zonation of the electron acceptors O2, NO3−, and Mn and Fe oxides. The principles of this method are discussed by Thamdrup and Canfield (66).
Carbon oxidation and sulfate reduction.
A clear divergence of total and sulfate-based ΣCO2 production rates demonstrated the importance of electron acceptors other than sulfate in the surface layers at stations I to III, whereas deeper in the sediment, and at all depths at station IV, contributions from other oxidants could not be discerned by this approach (Fig. 3). The complete dominance of sulfate reduction below depths of 1 to 2 cm at all stations was also supported by the stable background levels of metal oxides attained around a depth of 2 cm (Fig. 4). Accumulation of H2S in the deeper sections during the incubations further documented the absence of easily reducible metal oxides, since such oxides would rapidly scavenge H2S to submicromolar concentrations (12). Sulfate was also the single most important terminal electron acceptor when mineralization was stimulated by the addition of labile organic matter to sediment from a depth of 1 to 6 cm at station II (55).
The excess ΣCO2 production in the surface sections of stations I to III corresponded to rates of 0.9 to 2.4 mmol m−2 day−1 or 33 to 81% of the carbon oxidation coupled to sulfate reduction (Table 4). However, the determination of ΣCO2 production rates was complicated by carbonate precipitation, and although ΣCO2 production rates were corrected for the precipitation of CaCO3, the strong supersaturation indicated that precipitation of MnCO3 was also likely taking place. As will be discussed below, the carbonate precipitation was driven by the reduction of Mn and possibly Fe. It was therefore not possible to make corrections for the precipitation of carbonate with Mn2+ or Fe2+, such as for Ca2+, and the ΣCO2 production rates in the zones of Mn and Fe reduction are therefore minimum estimates of carbon oxidation rates.
TABLE 4.
Rates of carbon oxidation coupled to sulfate reduction and reduction of other electron acceptors during anoxic incubations of Black Sea sedimentsa
| Station | Rate of C oxidation
|
Interval (cm)c | ||||
|---|---|---|---|---|---|---|
| Sulfate (mmol of C m−2 day−1) | Other electron acceptor
|
|||||
| ΣCO2 production
|
NH4+ accumulation
|
|||||
| mmol of C m−2 day−1 | %b | mmol of C m−2 day−1 | %b | |||
| I | 5.68 ± 0.57 | 1.90 ± 1.05 | 25 | 4.41 ± 0.98 | 44 | 0–2.5 |
| II | 3.70 ± 0.22 | 2.39 ± 0.46 | 39 | 4.11 ± 0.26 | 53 | 0–1 |
| III | 1.10 ± 0.12 | 0.89 ± 0.36 | 45 | 3.04 ± 0.06 | 73 | 0–1 |
| IV | 0.81 ± 0.03 | 0.12 ± 0.09 | 13 | 0.34 ± 0.20 | 29 | 0–2 |
Rates are expressed as means ± standard deviations for 0- to 10-cm sediment depth (see text for details of calculation).
Percentage of total carbon oxidation during incubations.
Depth interval used for calculation of non-sulfate-based carbon oxidation rates.
Rates of ammonium accumulation provide an alternative estimate of the mineralization rates of organic matter in the zone of carbonate precipitation (13). In incubations similar to ours, with sediments free of carbonate precipitation, good correlations have been found between rates of carbon mineralization and NH4+ accumulation (13, 26, 27, 67). In the subsurface layers of the Black Sea sediments, where sulfate reduction was the only significant respiratory pathway, there was also a close correlation between rates of sulfate reduction and NH4+ accumulation with a ratio of sulfate-based carbon oxidation to NH4+ accumulation of 11.2:1 for all stations, which is in good agreement with such ratios observed in other sediments with low activity (13, 26, 27, 67). The sulfate reduction rates are preferred for the determination of an NH4+/ΣCO2 production ratio over a direct correlation of NH4+ and ΣCO2 production rates because of the smaller coefficients of variance associated with sulfate reduction than with ΣCO2 production determinations (Fig. 3). In Fig. 3, the scales for ΣCO2 production and NH4+ accumulation are plotted at a ratio of 11.2:1. Thus, ΣCO2 production rates estimated from this ratio and NH4+ accumulation rates can be found by projection of the data points for NH4+ accumulation onto the ΣCO2 production axis. The NH4+-based estimates confirm the small contribution of sulfate reduction to mineralization in the upper sections at all stations. Furthermore, the rates of ΣCO2 production calculated from NH4+ data are up to threefold higher than the measured rates of ΣCO2 production near the sediment surface (Table 4), which suggests that a large part of the produced ΣCO2 precipitated immediately with Mn2+ (or Fe2+ at stations I and II) and implies a greater importance of electron acceptors other than sulfate than calculated from the direct determinations of ΣCO2 production (Table 4). The NH4+-based rates can be considered as upper limits of the actual rates. This is because the steep increases in mineralization rates toward the surface could be associated with a moderate decrease in ΣCO2/NH4+ production ratios (27, 28, 67).
Mn and Fe reduction.
The depth distribution of electron acceptors implied that the non-sulfate-based carbon oxidation in the incubations was coupled to the reduction of Mn or Fe oxides. The increases in pH with time, which correlated with the rates of excess carbon oxidation, were also consistent with Mn or Fe reduction as terminal electron-accepting processes, whereas only small pH changes are expected during sulfate reduction (11):
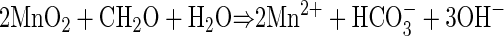 |
3 |
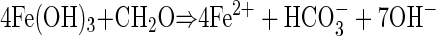 |
4 |
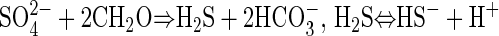 |
5 |
Mn reduction was directly evidenced by the rapid accumulation of soluble Mn2+, the rates and depth distribution of which correlated well with the rates of excess carbon oxidation (Fig. 3 and 4). In contrast, changes in soluble Fe2+ concentrations gave no strong indications of Fe reduction. Although zones of microbial Mn and Fe reduction in sediments are often indicated by the accumulation of Mn2+ and Fe2+, the pore water accumulation rates are typically lower than the gross production rates of Mn(II) and Fe(II) by an order of magnitude or more due to adsorption and/or precipitation (9, 13, 60). The ratio of the rates of excess ΣCO2 production (NH4+-based) to Mn2+ accumulation in the present study (∼1:15; compare Fig. 3 and 4) was consistent with the range of sorption coefficients determined for Mn-rich sediment (13). Thus, it is feasible that the excess carbon oxidation was coupled to Mn reduction.
Little is known about the modes of Fe(II) sorption in sediments, and Fe reduction cannot be excluded based on the low rates of Fe2+ accumulation. Furthermore, Fe reduction in the presence of Mn oxides is masked by rapid abiotic reoxidation (38, 47, 51). Two other lines of evidence suggest, however, that dissimilatory microbial Fe reduction did not play a significant role in carbon oxidation in the Black Sea sediments: (i) MPN counts showed very low numbers of Fe-reducing bacteria (FeRB) at the depth in the sediment where the process could be important (Table 3), and (ii) addition of ferrihydrite had no discernible effect on the pathways of carbon oxidation (Table 2).
The MPN counts of FeRB (∼10 cm−3; Table 3) were low both compared to total bacterial counts, which were similar to counts in other fine-grained continental marine sediments (∼109 cm−3) (57, 58), to the counts of Mn-reducing bacteria (103 to 105 cm−3) in the same sediment, and to the relatively few other MPN enumerations of acetate-utilizing FeRB reported from other marine sediments (103 to 107 cm−3) (15, 19). Thus, although viable counts are likely to underestimate the size of the total FeRB community, the relative abundances imply poor growth conditions for FeRB in the Black Sea sediments.
In the marine sediments investigated so far, the contribution of dissimilatory Fe reduction to carbon oxidation has been found to be limited by Fe(III) at in situ concentrations of poorly crystalline Fe(III) below 30 μmol Fe(III) cm−3 (65). Thus, below a depth of 0.5 to 1 cm in the Black Sea sediments, dissimilatory Fe reduction is predicted to be strongly inhibited by the low Fe(III) concentrations (Fig. 4). The competitive relationship between microbial Fe and sulfate reduction is presumed to function through concentrations of important common substrates such as acetate and H2, where Fe reduction may deplete these concentrations below the threshold of utilization by sulfate reduction when sufficient Fe(III) is available (36, 37). Hence, if a significant population of FeRB were present, addition of ferrihydrite would be expected to relieve any inhibition of Fe reduction and allow this process to outcompete sulfate reduction, as previously demonstrated with estuarine and freshwater sediments (1, 37). The lack of response to the ferrihydrite additions therefore further supports that FeRB were not important. An analogous conclusion based on similar results has been reached for a marine sediment from San Diego Bay (15).
Manganese-reducing bacteria (MnRB) were counted in much higher numbers than FeRB, and there was a positive correlation between the rates of non-sulfate-based carbon oxidation and the numbers of MnRB. Furthermore, Mn oxide concentrations were as high as those of poorly crystalline Fe(III). Based on these results as well as the geochemical evidence for Mn reduction and the lack of biogeochemical and microbiological evidence for dissimilatory Fe reduction discussed above, we conclude that dissimilatory Mn reduction was responsible for the non-sulfate-based carbon oxidation during the incubations.
Ecological significance of Mn reduction.
Based on a comparison of sulfate reduction rates measured in situ at the sea floor and in sediment cores onboard the ship during the cruise at the same stations as investigated here, it was concluded that the recovery of the sediment did not substantially affect sulfate reduction rates (Weber et al., submitted). Sulfate reduction rates in our incubations were quite similar to the rates determined in intact cores both in terms of maxima and depth distributions. We therefore expect that the total carbon mineralization rates from our incubations also provide good estimates of the in situ rates.
The contributions from dissimilatory Mn reduction (Table 4) were estimated from anoxic conditions. This corresponded to the conditions in situ at station IV, where the bottom water was anoxic at the time of sampling, and, consequently, the 13 to 29% contribution from dissimilatory Mn reduction estimated there should reflect the partitioning in situ. At the other stations with oxic bottom water, rates of dissimilatory Mn reduction in situ would have been smaller than in the incubations due to competition with organotrophic oxygen respiration. Due to the high shell content, it was not possible to directly determine the oxygen penetration depth in the sediments, but oxygen penetration depths (zmax) can be estimated from the diffusive oxygen uptakes (DOU) of the sediments as determined from the oxygen microgradients just above the sediment surface (Weber et al., submitted) according to reference 6:
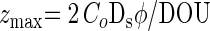 |
6 |
where CO is the concentration at the sediment surface, Ds is the sediment diffusion coefficient of O2, and φ is porosity (see also reference 52). Estimates of zmax from DOU for stations I, II, and III, respectively, are 1.5, 4.6, and 3.3 mm. Hence, oxygen is predicted to be present in situ in only a part of the depth intervals where non-sulfate-based carbon oxidation occurred. The competitive relationship between Mn and oxygen respiration is not well explored, and we can therefore not further constrain the relative importances of the two processes in situ.
The turnover of Mn oxide in coastal sediments is relatively fast, and maintenance of Mn reduction requires efficient recycling of Mn2+ by reoxidation with oxygen (3, 68). Along the transect, carbon oxidation rates and bottom water oxygen concentrations varied in parallel (Tables 1 and 4), and it appears that a balance in Mn oxide demand and reoxidation potential leads to roughly similar relative contributions of Mn reduction at all sites. Significant Mn reduction cannot be maintained at station IV in the absence of oxygen. However, ∼25-m fluctuations in the depth of the chemocline were observed during the two-week cruise (B. B. Jørgensen and A. Weber, personal communication), which would allow oxygen to reach the sediment at station IV periodically. Fluctuating bottom water oxygen concentrations at the outer shelf stations may thus cause large temporal variations in the rates of microbial Mn reduction.
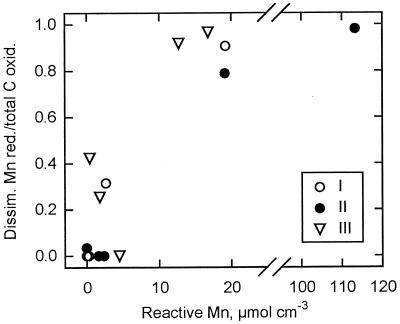
Significant contributions from dissimilatory Mn reduction to benthic carbon oxidation have only been demonstrated in two earlier cases (2, 10). Both of these were from well-ventilated offshore basins with extreme concentrations of Mn oxides (≥100 μmol cm−3), i.e., environments much different from the Black Sea sediments. In our incubations, the relative contribution of dissimilatory Mn reduction to carbon oxidation depended on the concentration of reactive Mn, with strong inhibition of sulfate reduction at concentrations above ∼10 μmol cm−3 (Fig. 6). The reactive Mn concentrations in Fig. 6 were calculated by subtraction of the low unreactive background concentrations measured at depths of 4 to 10 cm at each site (3) (Fig. 4). Station IV was excluded from these calculations because of the high concentrations of nonreactive Mn measured at 0.5 to 2 cm. A similar relationship to that in Fig. 6 has been observed and discussed for the competition between iron and sulfate reduction in marine sediments (65).
FIG. 6.
The relative contribution of dissimilatory Mn reduction to carbon oxidation as a function of the reactive Mn concentration in sediment from individual depth intervals from stations I to III in the Black Sea. Reactive Mn was calculated by subtraction of the average background concentration at a 4- to 10-cm depth from each station (see text for references).
Coastal marine sediments have typical maximum concentrations of reactive Mn of <10 μmol cm−3 restricted to the upper millimeters, and the relationship in Fig. 6 predicts that dissimilatory Mn reduction is of little importance in such sediments, thus supporting previous conclusions (3, 13, 56, 67, 68). Higher Mn concentrations, as found in the Black Sea, are observed in sediments in coastal troughs and bordering low-oxygen basins (49, 63), and consequently, dissimilatory Mn reduction may be of importance there. Although dissimilatory Mn reduction is not of general importance in marine sediments, the present results indicate that the process may be found in considerably wider areas than previously recognized.
Mn- and Fe-reducing bacteria.
Most known dissimilatory Fe-reducing bacteria also reduce Mn oxide when tested (35, 65), and likewise, most organisms isolated as dissimilatory Mn reducers also grow with Fe(III) as the electron acceptor (7, 20, 29). It was therefore a surprise to find much higher numbers of MnRB than FeRB in otherwise identical MPN series. Because the cultures could not be continued, we have no definite proof that the MnRB could not utilize Fe(III), but they must reduce Mn oxide much more efficiently than ferrihydrite. This suggests that Mn reduction is not necessarily conveyed by Fe reducers and that organisms that specialized in Mn reduction could play a relevant role in environments with abundant Mn oxide. The clone-type A3Mn2 from station I was affiliated with the Desulfuromonas-Pelobacter cluster which contains numerous Fe-reducing bacterial species (16, 32). However, several of the species most closely related to the clone-type A3Mn2 from station I have been found to reduce only soluble Fe(III) complexes and not ferrihydrite (21, 32). The Mn-reducing capabilities of these organisms have not been reported.
The only Mn reducer that we could isolate was affiliated with the Shewanella branch of the γ-subclass of Proteobacteria. This strain, D1Mn, was obtained by subcultivation on lactate of an inoculum from the same MPN tube from which the clone library of station IV was established. However, no clones with identical or similar sequence were obtained in this library. High numbers of Shewanella cells have been found in the chemocline of the central Black Sea (48); however, in spite of its isolation, we do not have any clue about Shewanella's environmental relevance as an Mn reducer in the sediment.
The exclusive recovery of Arcobacter-related organisms in the clone libraries from the highest positive dilutions of the MnRB MPN series at stations II and IV and their presence at station I are strong indications that these bacteria conducted the oxidation of acetate with Mn oxide in those series and that they were quantitatively significant in the Black Sea sediments. Dissimilatory Mn or Fe reduction has not been demonstrated for species of Arcobacter that are known as microaerophiles and nitrate reducers (64, 69). The only Mn- and Fe-reducing organism from the ɛ-subclass of Proteobacteria yet in culture is Sulfurospirillum barnesii (29, 61). Thus, Arcobacter-related organisms may represent an ecologically significant new group of dissimilatory Mn-reducing bacteria. Arcobacters are mainly known as potential pathogens isolated from humans and livestock (69), but strains have also been isolated from salt marsh sediment and a hypersaline microbial mat (43, 44, 64). In further support of their ecological significance, species of Arcobacter have recently been detected by FISH in a marine tidal flat sediment, where they were most abundant (>107 cm−3) at depths of 0.5 to 2 cm (31). Their ecological role was not identified, but their concentration in the upper part of the anoxic sediment is in agreement with the depth distribution expected for an organism that grows by the reduction of Mn oxides.
Our results provide the first evidence that microbial Mn reduction may be important in carbon oxidation in shelf sediments. The relative contribution of Mn reduction to carbon oxidation depends on the Mn oxide concentration, which may thus be used as an indicator of sites where the process is potentially significant. Due to the rapid turnover and shallow extension of Mn oxides, reduction rates are likely to vary temporally, e.g., in response to changes in bottom water oxygen concentrations. Large temporal and spatial variability is a general feature of the coastal Mn cycle (3, 68).
The integration of microbiological and biogeochemical approaches proved very useful for investigating the ecology of microbial Mn reduction, and the finding that Mn reduction was catalyzed by bacteria which were not previously known as Mn reducers emphasizes the need for a specific search for Mn-reducing microorganisms in the environment.
ACKNOWLEDGMENTS
We are grateful to Swantje Fleischer for skillful technical assistance and to Jan Kuever, Ingrid Kunze, and Karsten Zengler for help with MPN enumerations. We thank Andreas Weber and Bo Barker Jørgensen for excellent planning and coordination of the Black Sea cruise and the master, crew, and scientific party on R/V Petr Kottsov for a highly productive cruise.
This study was supported by the Max Planck Society and by the Danish Research Foundation through the Danish Center for Earth System Science.
REFERENCES
- 1.Achtnich C, Bak F, Conrad R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil. Biol Fertil Soils. 1995;19:65–72. [Google Scholar]
- 2.Aller R C. Bioturbation and manganese cycling in hemipelagic sediments. Phil Trans R Soc Lond. 1990;A331:51–58. [Google Scholar]
- 3.Aller R C. The sedimentary Mn cycle in Long Island Sound: its role as intermediate oxidant and the influence of bioturbation, O2 and Corg flux on diagenetic reaction balance. J Mar Res. 1994;52:259–295. [Google Scholar]
- 4.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berner R A. Early diagenesis. Princeton, N.J: Princeton University Press; 1980. [Google Scholar]
- 6.Bouldin D R. Models for describing the diffusion of oxygen and other mobile constituents across the mud-water interface. J Ecol. 1968;556:77–87. [Google Scholar]
- 7.Bowman J P, McCammon S A, Nichols D S, Skerratt J H, Rea S M, Nichols P D, McMeekin T A. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int J Syst Bacteriol. 1997;47:1040–1047. doi: 10.1099/00207713-47-4-1040. [DOI] [PubMed] [Google Scholar]
- 8.Braman R S, Hendrix S A. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium(III) reduction with chemiluminescence detection. Anal Chem. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- 9.Canfield D E. Organic matter oxidation in marine sediments. In: Wollast R, Mackenzie F T, Chou L, editors. Interactions of C, N, P, and S biogeochemical cycles and global change. Berlin, Germany: Springer; 1993. pp. 333–363. [Google Scholar]
- 10.Canfield D E, Jørgensen B B, Fossing H, Glud R, Gundersen J, Ramsing N B, Thamdrup B, Hansen J W, Nielsen L P, Hall P O J. Pathways of organic carbon oxidation in three continental margin sediments. Mar Geol. 1993;113:27–40. doi: 10.1016/0025-3227(93)90147-n. [DOI] [PubMed] [Google Scholar]
- 11.Canfield D E, Raiswell R. Carbonate precipitation and dissolution, its relevance to fossil preservation. In: Allison P A, Briggs D E G, editors. Taphonomy: releasing the data locked in the fossil record. New York, N.Y: Plenum Press; 1991. pp. 411–453. [Google Scholar]
- 12.Canfield D E, Raiswell R, Bottrell S. The reactivity of sedimentary iron minerals toward sulfide. Am J Sci. 1992;292:659–683. [Google Scholar]
- 13.Canfield D E, Thamdrup B, Hansen J W. The anaerobic degradation of organic matter in Danish coastal sediments: Fe reduction, Mn reduction and sulfate reduction. Geochim Cosmochim Acta. 1993;57:2563–2570. doi: 10.1016/0016-7037(93)90340-3. [DOI] [PubMed] [Google Scholar]
- 14.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 15.Coates J D, Anderson R T, Woodward J C, Phillips E J P, Lovley D R. Anaerobic hydrocarbon degradation in petroleum-contaminated harbor sediments under sulfate-reducing and artificially imposed iron-reducing conditions. Environ Sci Technol. 1996;30:2784–2789. [Google Scholar]
- 16.Coates J D, Lonergan D J, Philips E J P, Jenter H, Lovley D R. Desulfuromonas palmitatis sp. nov., a marine dissimilatory Fe(III) reducer. Arch Microbiol. 1995;164:406–413. [PubMed] [Google Scholar]
- 17.Coleman M L, Hedrick D B, Lovley D R, White D C, Pye K. Reduction of Fe(III) in sediments by sulfate-reducing bacteria. Nature. 1993;361:436–438. [Google Scholar]
- 18.de Man J C. The probability of most probable numbers. Eur J Appl Microbiol. 1975;1:67–78. [Google Scholar]
- 19.Detmers J. Dissimilatorisch-Fe(III)-reduzierende Bakterien—eine mikrobiologisch-ökologische Untersuchung. M.S. thesis. Bremen, Germany: University of Bremen; 1997. [Google Scholar]
- 20.Ehrlich H L. Electron transfer from acetate to the surface of MnO2 particles by a marine bacterium. J Ind Microbiol. 1993;12:121–128. [Google Scholar]
- 21.Finster K, Coates J D, Liesack W, Pfennig N. Desulfuromonas thiophila sp. nov., a new obligately sulfur-reducing bacterium from anoxic freshwater sediment. Int J Syst Bacteriol. 1997;47:754–758. doi: 10.1099/00207713-47-3-754. [DOI] [PubMed] [Google Scholar]
- 22.Fossing H, Jøorgensen B B. Measurement of bacterial sulfate reduction in sediments: evaluation of a single-step chromium reduction method. Biogeochemistry. 1989;8:223–245. [Google Scholar]
- 23.Hall P O J, Aller R C. Rapid small-volume flow injection analysis for ΣCO2 and NH4+ in marine and freshwaters. Limnol Oceanogr. 1992;37:1113–1119. [Google Scholar]
- 24.Harms G, Zengler K, Rabus R, Aekersberg F, Minz D, Rosselló-Móra R, Widdel F. Anaerobic oxidation of o-xylene, m-xylene, and homologous alkylbenzenes by new types of sulfate-reducing bacteria. Appl Environ Microbiol. 1999;65:999–1004. doi: 10.1128/aem.65.3.999-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jøorgensen B B. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. I. Measurement with radiotracer techniques. Geomicrobiol J. 1978;1:11–27. [Google Scholar]
- 26.Kostka J E, Canfield D E, Thamdrup B. Rates and pathways of carbon oxidation in permanently cold Arctic sediments. Mar Ecol Prog Ser. 1999;180:7–21. [Google Scholar]
- 27.Kristensen E, Devol A H, Hartnett H E. Organic matter diagenesis in sediments on the continental shelf and slope of the Eastern Tropical and temperate North Pacific. Cont Shelf Res. 1999;19:1331–1351. [Google Scholar]
- 28.Kristensen E, Hansen K. Decay of plant detritus in organic-poor marine sediment: production rates and stoichiometry of dissolved C and N compounds. J Mar Res. 1995;53:675–702. [Google Scholar]
- 29.Laverman A M, Blum J S, Schaefer J K, Phillips E J P, Lovley D R, Oremland R S. Growth of strain SES-3 with arsenate and other diverse electron acceptors. Appl Environ Microbiol. 1995;61:3556–3561. doi: 10.1128/aem.61.10.3556-3561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y-H, Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta. 1974;38:703–714. [Google Scholar]
- 31.Llobet-Brossa E, Rosselló-Mora R, Amann R. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl Environ Microbiol. 1998;64:2691–2696. doi: 10.1128/aem.64.7.2691-2696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonergan D J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord C J., III . The chemistry and cycling of iron, manganese, and sulfur in salt marsh sediments. Ph.D. thesis. Newark: University of Delaware; 1980. [Google Scholar]
- 34.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovley D R, Coates J D, Saffarini D A, Lonergan D J. Dissimilatory iron reduction. In: Winkelmann G, Carrano C J, editors. Transition metals in microbial metabolism. Amsterdam, The Netherlands: Harwood Academic Publishers; 1997. pp. 187–215. [Google Scholar]
- 36.Lovley D R, Goodwin S. Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim Cosmochim Acta. 1988;52:2993–3003. [Google Scholar]
- 37.Lovley D R, Phillips E J P. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987;53:2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lovley D R, Phillips E J P. Manganese inhibition of microbial iron reduction in anaerobic sediments. Geomicrobiol J. 1988;6:145–155. [Google Scholar]
- 39.Lovley D R, Phillips E J P. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986;51:683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lovley D R, Roden E E, Phillips E J P, Woodward J C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar Geol. 1993;113:41–53. [Google Scholar]
- 41.Ludwig W, Strunk O, Klugbauer S, Klugbauer N, Weizenegger M, Neumaier J, Bachleitner M, Schleifer K-H. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis. 1998;19:554–568. doi: 10.1002/elps.1150190416. [DOI] [PubMed] [Google Scholar]
- 42.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Li B, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClung C R, Patriquin D G. Isolation of a nitrogen-fixing Campylobacter species from the roots of Spartina alterniflora Loisel. Can J Microbiol. 1980;26:881–886. doi: 10.1139/m80-153. [DOI] [PubMed] [Google Scholar]
- 44.McClung C R, Patriquin D G, Davis R E. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. Int J Syst Bacteriol. 1983;33:605–612. [Google Scholar]
- 45.Millero F J. Chemical oceanography. 2nd ed. Boca Raton, Fla: CRC Press; 1996. [Google Scholar]
- 46.Murray J W. The surface chemistry of hydrous manganese dioxide. J Colloid Interface Sci. 1974;46:357–371. [Google Scholar]
- 47.Myers C R, Nealson K H. Microbial reduction of manganese oxides: interactions with iron and sulfur. Geochim Cosmochim Acta. 1988;52:2727–2732. [Google Scholar]
- 48.Nealson K H, Myers C R, Wimpee B B. Isolation and identification of manganese-reducing bacteria and estimates of microbial Mn(IV)-reducing potential in the Black Sea. Deep-Sea Res. 1991;38:S907–S920. [Google Scholar]
- 49.Overnell J, Harvey S M, Parkes R J. A biogeochemical comparison of sea loch sediments. Manganese and iron contents, sulphate reduction and oxygen uptake rates. Oceanol Acta. 1996;19:41–55. [Google Scholar]
- 50.Parkhurst D L. Users guide to PHREEQC - a computer program for speciation, reaction-path, advective transport, and inverse geochemical calculations. Water-resources investigations report 95-4227. U.S. Lakewood, Colo: Geological Survey; 1995. [Google Scholar]
- 51.Postma D. Concentrations of Mn and separation from Fe in sediments. 1. Kinetics and stoichiometry of the reaction between birnessite and dissolved Fe(II) at 10°C. Geochim Cosmochim Acta. 1985;49:1023–1033. [Google Scholar]
- 52.Rasmussen H, Jørgensen B B. Microelectrode studies of seasonal oxygen uptake in a coastal sediment: role of molecular diffusion. Mar Ecol Progr Ser. 1992;81:289–303. [Google Scholar]
- 53.Ravenschlag K, Sahm K, Pernthaler J, Amann R. High bacterial diversity in permanently cold marine sediments. Appl Environ Microbiol. 1999;65:3982–3989. doi: 10.1128/aem.65.9.3982-3989.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roden E E, Wetzel R G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr. 1996;41:1733–1748. [Google Scholar]
- 55.Rosselló-Móra R, Thamdrup B, Schäfer H, Weller R, Amann R. The response of the microbial community of marine sediments to organic carbon input under anaerobic conditions. Syst Appl Microbiol. 1999;22:237–248. doi: 10.1016/S0723-2020(99)80071-X. [DOI] [PubMed] [Google Scholar]
- 56.Rysgaard S, Thamdrup B, Risgaard-Petersen N, Fossing H, Berg P, Christensen P B, Dalsgaard T. Seasonal carbon and nutrient mineralization in a high-Arctic coastal marine sediment, Young Sound, Northeast Greenland. Mar Ecol Progr Ser. 1998;175:261–276. [Google Scholar]
- 57.Sahm K, Berninger U-G. Abundance, vertical distribution, and community structure of benthic prokaryotes from permanently cold marine sediments (Svalbard, Arctic Ocean) Mar Ecol Progr Ser. 1998;165:71–80. [Google Scholar]
- 58.Schmidt J L, Deming J W, Jumars P A, Keil R G. Constancy of bacterial abundance in surficial marine sediments. Limnol Oceanogr. 1998;53:976–982. [Google Scholar]
- 59.Schwertmann U, Cornell R M. Iron oxides in the laboratory. Weinheim, Germany: VCH; 1991. [Google Scholar]
- 60.Sørensen J. Reduction of ferric iron in anaerobic, marine sediment and interaction with reduction of nitrate and sulfate. Appl Environ Microbiol. 1982;43:319–324. doi: 10.1128/aem.43.2.319-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoltz J F, Ellis D J, Switzer Blum J, Ahmann D, Lovley D R, Oremland R S. Sulfurospirillum sp. nov. and Sulfurospirillum arsenophilum sp. nov., new members of the Sulfurospirillum clade of the epsilon Proteobacteria. Int J Syst Bacteriol. 1999;49:1177–1180. doi: 10.1099/00207713-49-3-1177. [DOI] [PubMed] [Google Scholar]
- 62.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 63.Sundby B, Silverberg N. Pathways of manganese in an open estuarine system. Geochim Cosmochim Acta. 1981;45:293–307. [Google Scholar]
- 64.Teske A, Sigalevich P, Cohen Y, Muyzer G. Molecular identification of bacteria from a coculture by denaturing gradient gel electrophoresis of 16S ribosomal DNA fragments as a tool for isolation in pure cultures. Appl Environ Microbiol. 1996;62:4210–4215. doi: 10.1128/aem.62.11.4210-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thamdrup B. Microbial manganese and iron reduction in aquatic sediments. Adv Microb Ecol. 2000;16:41–84. [Google Scholar]
- 66.Thamdrup B, Canfield D E. Benthic respiration in aquatic sediments. In: Sala O, Mooney H, Jackson R, Howarth R, editors. Methods in ecosystem science. New York, N.Y: Springer; 2000. pp. 86–103. [Google Scholar]
- 67.Thamdrup B, Canfield D E. Pathways of carbon oxidation in continental margin sediments off central Chile. Limnol Oceanogr. 1996;41:1629–1650. doi: 10.4319/lo.1996.41.8.1629. [DOI] [PubMed] [Google Scholar]
- 68.Thamdrup B, Fossing H, Jørgensen B B. Manganese, iron, and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim Cosmochim Acta. 1994;58:5115–5129. [Google Scholar]
- 69.Vandamme P, Vancanneyt M, Pot B, Mels L, Hoste B, Dewettinck D, Vlaes L, Van Den Borre C, Higgins R, Hommez J, Kersters K, Butzler J-P, Goossens H. Polyphasic taxonomic studies of the emended genus Arcobacter with Arcobacter comb. nov. and Arcobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. Int J Syst Bacteriol. 1992;42:344–356. doi: 10.1099/00207713-42-3-344. [DOI] [PubMed] [Google Scholar]
- 70.Vargas M, Kashefi K, Blunt-Harris E L, Lovley D R. Microbiological evidence for Fe(III) reduction on early Earth. Nature. 1998;395:65–67. doi: 10.1038/25720. [DOI] [PubMed] [Google Scholar]
- 71.Widdel F, Bak F. Gram-negative mesophilic sulfate-reducing bacteria. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The Prokaryotes. 2nd ed. IV. New York, N.Y: Springer; 1991. pp. 3352–3378. [Google Scholar]