Abstract
Background
Patient-reported outcome (PRO) data evaluating the physical and psychosocial impact of cryolipolysis (CoolSculpting) treatment are limited.
Objectives
The aim of this study was to assess, by means of PRO instruments, multidimensional aspects of satisfaction following cryolipolysis treatment of the flanks and abdomen.
Methods
This was a multinational, prospective, single-cohort, interventional study. The primary endpoint was the proportion of participants stating they were “satisfied” or “very satisfied” with treatment at 12 weeks post final treatment. Secondary endpoints included satisfaction categorized by treatment area, total number of treatment cycles, baseline BMI, and fat volume reduction measured by 3-dimensional photography at 12 weeks post final treatment. Exploratory endpoints assessed the physical and psychosocial impacts of treatment. Safety was monitored throughout the study.
Results
Of 112 participants who were treated, 74.1% were female. The mean age and BMI were 42.5 years and 24.9 kg/mg2, respectively. Of the 106 evaluable participants, 89.6% were "satisfied" or "very satisfied" with treatment results. Satisfaction was high regardless of body area(s), total number of treatment cycles, or baseline BMI. Mean [standard deviation] fat volume reduction was 264.8 [411.4] mL. Overall, 90.6% reported "noticeable" or "very noticeable" fat reduction, 89.6% were "likely" or "very likely" to treat additional areas, and 93.4% would recommend cryolipolysis to a friend. Twenty-four (21.4%) participants reported treatment-emergent adverse events; 23 (20.5%) reported these as adverse device effects. No serious device-related or unanticipated adverse effects occurred.
Conclusions
Cryolipolysis (CoolSculpting) for fat reduction of the flanks and/or abdomen was well-tolerated and associated with high levels of satisfaction across multidimensional PROs.
Level of Evidence: 4

Noninvasive fat reduction procedures have become increasingly popular over the last decade. Most procedures can be administered at a wide variety of medical practices and can be completed in relatively short in-office visits with little to no downtime. Many plastic surgeons incorporate noninvasive procedures into their practice not only to expand the continuum of care for their surgical patients but to serve a broader range of patients, including those who are not candidates for surgical procedures. Over the last decade, the safety and effectiveness of cryolipolysis (CoolSculpting, Allergan Aesthetics [Irvine, CA], an AbbVie Company [North Chicago, IL]) as a treatment for subcutaneous fat reduction has been well-established since its FDA clearance for treatment of the flanks and abdomen in 2010 and 2012, respectively.1-5 Cryolipolysis effectively reduces discrete, localized subcutaneous fat with evidence supporting long-term, sustainable results and a high rate of patient satisfaction (>80%).2,6-10
Although assessing any treatment modality’s clinical effectiveness remains essential, the value of treatment outcomes from the patient’s perspective is perhaps the most meaningful. Appropriately developed and evaluated patient-reported outcome (PRO) instruments can provide more sensitive and specific measurements, which are useful in comparative effectiveness research and can contribute to patient consultation and treatment planning.11 Ultimately, PRO data are essential for evaluating the impact of treatment on psychosocial well-being—a primary motive for seeking elective cosmetic procedures.
A literature review of currently available PRO instruments used to measure the psychosocial impact associated with body contouring yielded none applicable in their original format for cryolipolysis due to limitations of items and concepts. Furthermore, although numerous clinical cryolipolysis studies have been conducted over the last decade, the racial/ethnic distribution of the participant population has been dominated by the Caucasian demographic subgroup. Those studies conducted outside North America, including non-Caucasian participant populations, are small and limited in number.2,6,12-14
The objective of this study was to assess the participant-reported value of cryolipolysis treatment of the flanks and abdomen according to a more comprehensive PRO assessment among a broader demographic population at international sites.
METHODS
Design
This study was a prospective, nonrandomized, single-arm, open-label, multinational, postmarketing study conducted to evaluate the effectiveness (as PROs) and safety associated with noninvasive fat reduction treatment of the abdomen, flanks, or both abdomen and flanks with the CoolSculpting system fitted with CoolAdvantage applicators. Each participant’s treatment plan was permitted to consist of up to 24 treatment cycles administered over 2 treatment sessions (scheduled 8 weeks apart) as determined by the investigators. During each session, up to 12 treatment cycles (each lasting up to 45 minutes depending on the applicator used) could be administered. Final follow-up was conducted approximately 12 weeks after the participant’s final treatment, at Week 12 following a single treatment session, or at Week 20 following 2 treatment sessions. Study assessments were conducted at the initial treatment visit (before treatment), at 8 weeks following the first treatment session, and at 12 weeks post final treatment. A clinical assessment of the treated area(s) was conducted at each clinic visit, and safety was assessed throughout the study (Supplemental Figure 1, available online at www.aestheticsurgeryjournal.com).
PRO Instruments
Three separate questionnaires were used to capture PROs: (1) the 4-item Cryolipolysis Satisfaction Questionnaire (CSQ) to assess satisfaction and effectiveness of the procedure with item 1 modified to address treatment-specific areas (ie, abdomen only, flanks only, or abdomen and flanks); (2) the 5-item Cryolipolysis Psychosocial Impact Questionnaire—Midsection (CPIQ-M) to assess the psychosocial impact of treatment related to the appearance of the midsection; and (3) a 3-item Cryolipolysis General Procedure Questionnaire (CGPQ) to assess general procedure-related items (Supplemental Table 1, available online at www.aestheticsurgeryjournal.com). The questionnaires used in this study were created by the study sponsor, administered by study site personnel, and conducted on paper. Questionnaires were semi-anonymous in that study identifiers were used to collate the data, but responses were deidentified by a third party prior to data analysis.
Participants
Eligible participants were male or female aged ≥22 years and ≤65 years with a BMI of 18.5 to 30.0 kg/m2 who had visible fat on the flanks and/or abdomen, no weight fluctuations exceeding 4.5 kg (or 5% of body weight) in the preceding month, and who agreed to maintain weight (within 5% of baseline weight) with no changes in diet/exercise routine during the study. Key exclusion criteria were: a history of cryoglobulinemia, cold agglutinin disease, or paroxysmal cold hemoglobinuria; a history of invasive fat reduction of the intended treatment area; noninvasive body contouring procedure in the intended treatment area within the past 12 months; subcutaneous injections to the intended treatment area within the past 6 months; recent surgery, scar tissue, or hernias as well as impaired skin sensation, or skin conditions including open or infected wounds in the intended treatment area; any active implanted device or drug-delivery system within the intended treatment area; a known bleeding disorder or concomitant use of blood thinners (or any medication) associated with an increased risk of bruising, neuropathic disorders, or sensitivities to cold; were pregnant or intending to become pregnant or were lactating or had been lactating in the past 6 to 9 months. This study was conducted between July 2019 and February 2020, was carried out in compliance with good clinical practice and received IRB and ethics committee approval at each study site (Health Research Authority, Manchester, UK; Belberry Limited, Eastwood, Australia; Parkway Hospitals Singapore Pte Ltd, Singapore; Advarra Canada, Aurora, Canada). Participants provided written informed consent for the use and analysis of their data. This trial is registered at clinicaltrials.gov (NCT#03909100).
Effectiveness Assessments
Primary Effectiveness
The primary endpoint was the proportion of participants stating they were “satisfied” or “very satisfied” with the overall results in the treated areas (CSQ item 1) at 12 weeks post final treatment.
Secondary Effectiveness
Secondary analyses included categorization of the primary endpoint data by: (1) treated area(s), (2) total number of treatment cycles received, and (3) baseline BMI category (18.5 to <25.0 kg/m2 and 25.0 to ≤30.0 kg/m2). Change in fat volume (mL) was quantified by 3-dimensional (3D) image analysis (3D LifeViz Body system, Quantificare SA, Sophia Antipolis, France).15 Briefly, a 360° stitched image consisting of 8 frames of the entire midsection (abdomen and flank areas) was captured at 8 weeks post first treatment and 12 weeks post final treatment and compared against the pretreatment image.
Exploratory Assessments
The perception of treatment effectiveness was assessed by the proportion of participants achieving: at least “noticeable” fat reduction in the treated area (CSQ item 2); at least “a little improvement” in the fit of clothing over the treated areas (CSQ item 3); and reporting the overall effect of the procedure was at least “about what I had expected” (CSQ item 4) at 12 weeks post final treatment. Change in psychosocial impact was assessed by the mean change in the 5-item CPIQ-M scores from baseline to 12 weeks post final treatment, including items related to self-consciousness (item 1), happiness (item 2), anxiety (item 3), bothersomeness (item 4), and avoidance of social situations (item 5). The difference in the CPIQ-M total score was calculated as the sum of items 1, 3, 4, and 5, plus the reverse score of item 2, and transformed on a numerical point scale (ranging from 0 = no impact to 100 = highest impact). The general treatment procedure experience was measured with the 3-item CGPQ, which evaluated the proportion of participants who were: at least “comfortable” (CGPQ item 1) during the procedure; at least “likely” to consider additional treatments on a different part of the body (CGPQ item 2); and willing to recommend the treatment procedure to a friend (CGPQ item 3).
Safety Assessments
Adverse events (AEs), treatment-emergent adverse events (TEAEs), adverse device effects (ADEs), and procedure-related AEs were monitored and documented throughout the study period.
Analysis
Descriptive statistical methods were used for effectiveness parameters, and 95% CIs for percentages were calculated by the normal approximation method. For CPIQ-M total scores, the 95% CIs and P values for mean change from baseline were calculated using a paired t test as exploratory analyses.
RESULTS
Participant Demographics
Participants were recruited from 7 sites across 4 countries (Australia, Canada, Singapore, and the United Kingdom). Of 120 participants enrolled, 112 received at least 1 treatment cycle (safety population), and 105 completed the study (1 discontinued but still met the evaluable criteria). Therefore, 106 out of 112 (94.6%) participants who were treated made up the evaluable population for primary endpoint analysis (defined as having at least 1 treatment procedure and responded to CSQ item 1). Among the enrolled population, there were 15 study discontinuations characterized as withdrawal by participant (n = 4); technical problems (n = 4); protocol deviation (n = 2); lost to follow-up (n = 1); or other (n = 4) (ie, could not return in time for exit visit within visit schedule). The majority of all participants were Asian (61/112, 54.5%), predominantly of Chinese descent (51/61, 83.6%), female (83/112, 74.1%), with an average age of 42.5 years (range, 22-62 years), a mean BMI of 24.9 kg/m2 (range, 19.0-30.0 kg/m2), and a Fitzpatrick skin type of III/IV (59.8%), which also reflected the majority number of participants of Asian descent enrolled in the study (Table 1).
Table 1.
Participant Demographics and Baseline Characteristics (Safety Population)
| Parameter | All participants (N = 112) | Chinese participants (N = 51) |
|---|---|---|
| Gender, n (%) | ||
| Female | 83 (74.1) | 36 (70.6) |
| Male | 29 (25.9) | 15 (29.4) |
| Age (years) | 42.5 [9.86] | 43.5 [9.19] |
| Range | (22-62) | (29-62) |
| Weight (kg) | 68.9 [11.80] | 66.1 [11.67] |
| Range | (46.0-107.0) | (46-95) |
| BMI (kg/m2) | 24.9 [2.78] | 24.6 [2.89] |
| Range | (19.0-30.0) | (19.0-30.0) |
| 18.5 to <25.0, n (%) | 55 (49.1) | 27 (52.9) |
| 25.0-30.0, n (%) | 57 (50.9) | 24 (47.1) |
| Race/ethnicity, n (%) | ||
| Caucasian | 48 (42.9) | 0 (0.0) |
| Black/African American | 1 (0.9) | 0 (0.0) |
| Asian—Chinese | 51 (45.5) | 51 (100) |
| Asian—Japanese | 0 (0.0) | 0 (0.0) |
| Asian—Korean | 1 (0.9) | 0 (0.0) |
| Asian—Other | 9 (8.0) | 0 (0.0) |
| Arab/Middle Eastern | 1 (0.9) | 0 (0.0) |
| Other | 1 (0.9) | 0 (0.0) |
| Fitzpatrick skin type, n (%) | ||
| I, II | 35 (31.3) | 1 (2.0) |
| III, IV | 67 (59.8) | 50 (98.0) |
| V, VI | 10 (8.9) | 0 (0.0) |
Values are n (%) or mean [standard deviation].
Treatment Populations and Treatment Characteristics
Among the evaluable population (n = 106), 101 participants received treatment to the abdomen, 101 received treatment to the flanks, and 96 received treatment to both the abdomen and flanks. Overall, 102 of 106 (96.2%) participants had treatment plans completed over 2 treatment sessions, with 51 of 106 (48.1%) participants receiving 24 total treatment cycles in their treatment plan (the maximum number allowed), of whom 39 of 51 (76.5%) were Chinese participants, making up 36.8% of the evaluable population. Other commonly used treatment plans comprised 16 treatment cycles (12.3%) and 12 treatment cycles (9.4%). Within the evaluable population, 82 of 106 (77.4%) had some form of simultaneous multi-CoolSculpting treatment as part of their treatment plan comprising 7 to 24 treatment cycles. These simultaneous treatments varied from participant to participant but typically included simultaneous treatment of both areas, ie, flanks or abdomen, in the same treatment session. The average duration of exposure for all treatment areas (upper abdomen, lower abdomen, right flank, and left flank), including retreatment due to applicator dislodgement or other interruptions, equated to approximately 19 treatment cycles for a complete treatment plan (based upon 35- or 45-minute treatment cycles).
Effectiveness Endpoints
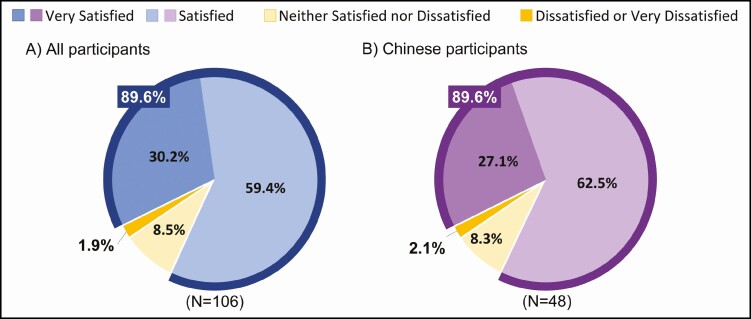
For the primary analysis, at 12 weeks post final treatment, 95 of 106 participants (89.6%; 95% CI, 83.8-95.4) were "satisfied" or "very satisfied" with the fat reduction procedure on the treated area(s) (Figure 1A). Within the subgroup of Chinese participants, 43 of 48 (89.6%; 95% CI, 80.9%-98.2%) were also "satisfied" or "very satisfied" (Figure 1B).
Figure 1.
Satisfaction with overall treatment procedure measured by the Cryolipolysis Satisfaction Questionnaire (CSQ item 1) at 12 weeks post final treatment.
When the primary analysis was categorized by treatment area, the total number of treatment cycles, and baseline BMI, the proportion of participants "satisfied" or "very satisfied" ranged from 87.1% to 93.1% across treatment areas, 80.0% to 92.3% among the most common number of treatment cycles received, and 88.5% to 90.7% among the baseline BMIs; these proportions were consistent among Chinese participants (Table 2). Although numerically different, there was no statistical difference between these groups.
Table 2.
Primary Analysis Data Categorized by Treated Area, Total Treatment Cycles, and BMI
| Category | All participants | Chinese participants | ||
|---|---|---|---|---|
| Satisfied or very satisfied/participants assessed (N = 106) | Satisfied or very satisfied/participants assessed (N = 48) | |||
| n/N1 (%) | 95% CI | n/N1 (%) | 95% CI | |
| Treatment area | ||||
| Abdomen | 88/101 (87.1) | 80.6-93.7 | 41/48 (85.4) | 75.4-95.4 |
| Flanks | 94/101 (93.1) | 88.1-98.0 | 46/48 (95.8) | 90.2-100.0 |
| Abdomen and flanks | 86/96 (89.6) | 83.5-95.7 | 43/48 (89.6) | 80.9-98.2 |
| Most common treatment cycle regimens | ||||
| 24 | 47/51 (92.2) | 84.8-99.5 | 35/39 (89.7) | 80.2-99.3 |
| 16 | 12/13 (92.3) | 77.8-100.0 | 5/6 (83.3) | 53.5-100.0 |
| 12 | 8/10 (80.0) | 55.2-100.0 | 2/2 (100.0) | 100.0-100.0 |
| Baseline BMI (kg/m2) | ||||
| 18.5 to < 25.0 | 46/52 (88.5) | 79.8-97.1 | 23/26 (88.5) | 76.2-100.0 |
| 25.0 to <30.0 | 49/54 (90.7) | 83.0-98.5 | 20/22 (90.9) | 78.9-100.0 |
All participants: at Week 12 for participants who received 1 treatment session and Week 20 for those who received 2 treatment sessions. At each treatment session, a participant could have received a maximum of 12 cycles. n = number of participants within a subcategory; N1 = number of participants with assessments for the corresponding category and represents the denominator for percentage values.
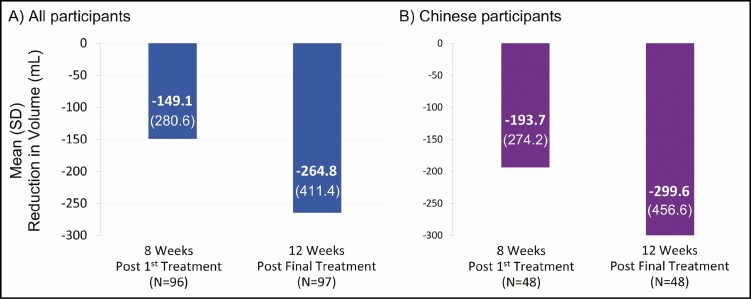
Among all participants, at 8 weeks post first treatment and 12 weeks post final treatment, the mean [standard deviation, SD] fat volume reduction was quantified as 149.1 [280.6] mL and 264.8 [411.4] mL, respectively (Figure 2A). The reduction was comparable among Chinese participants, with values of 193.7 [274.2] mL and 299.6 [456.6] mL at the respective time points (Figure 2B).
Figure 2.
Mean [standard deviation] reduction in fat volume throughout the study.
Exploratory Analyses
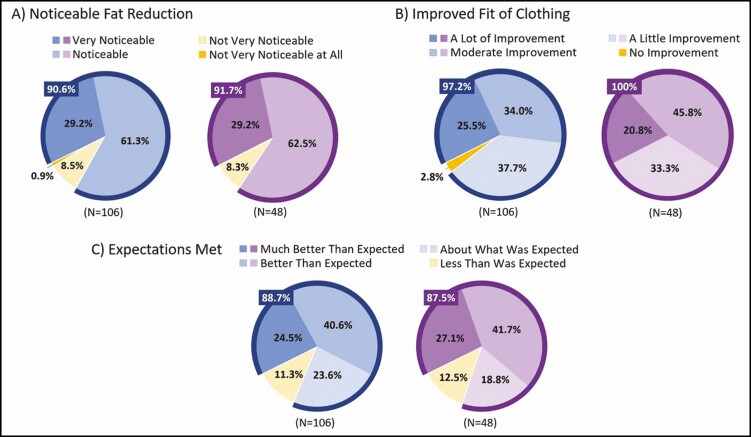
At 12 weeks post final treatment, the perception of treatment effectiveness showed that 90.6% (95% CI, 85.0%-96.1%) of all participants achieved "noticeable" or "very noticeable" fat reduction (Figure 3A), 97.2% (95% CI, 94.0%-100.0%) reported at least "a little improvement" (or better) in the fit of clothes over the treated areas (Figure 3B), and 88.7% (95% CI, 82.6%-94.7%) felt treatment results were "about what was expected" (or better) (Figure 3C). Similarly, among Chinese participants, 91.7% (95% CI, 83.8%-99.5%) achieved noticeable or very noticeable fat reduction, 100.0% (95% CI, 100.0%-100.0%) reported at least a little improvement (or better), and 87.5% (95% CI, 78.1%-96.9%) felt treatment results were "about what was expected" (or better).
Figure 3.
Perception of treatment effect measured by the Cryolipolysis Satisfaction Questionnaire (CSQ items 2, 3, and 4) at 12 weeks post final treatment. All participants are shown in blue charts, and Chinese participants are shown in purple charts. Note: 9 out of 106 participants were excluded from 3-dimensional imaging analysis due to nonassessable data.
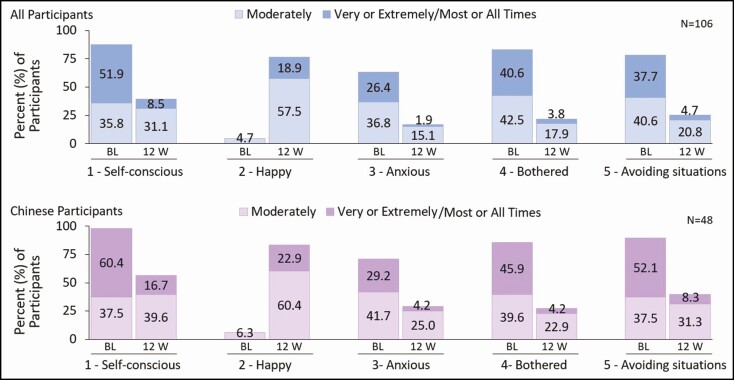
At 12 weeks post final treatment, the change in psychosocial impact related to midsection demonstrated improvement across all items, corresponding to a decrease in moderate-to-extreme affirmative responses to feeling: self-conscious (1), anxious (3), bothered (4), and avoiding situations (5), and an increase to feeling happy about appearance (2) (Figure 4). The total mean [SD] impact score (transformed sum of all items) improved by 30.7 [19.8] points (P < 0.0001) (from 58.3 to 27.6 points) among all participants and by 30.0 [18.1] points (P < 0.0001) (from 63.3 to 33.2 points) among the Chinese subgroup.
Figure 4.
Change from baseline in psychosocial impact as assessed by the 5-item Cryolipolysis Psychological Impact Questionnaire—Midsection (CPIQ-M) at 12 weeks post final treatment. Data presented show moderate-to-extreme affirmative responses to each CPIQ-M questionnaire item. BL, baseline; 12 W, 12 weeks post final treatment.
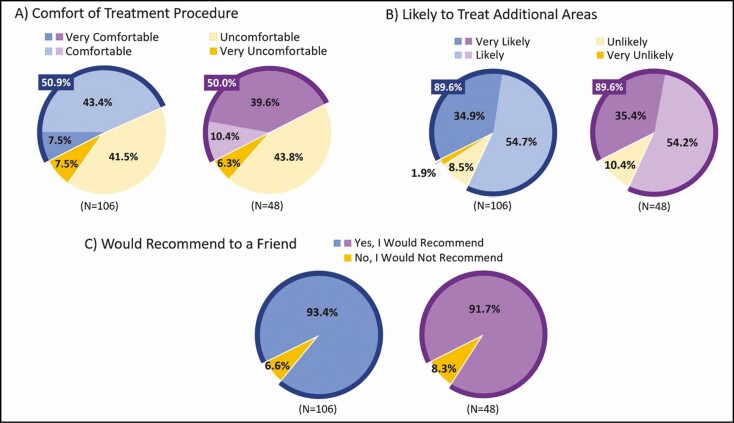
At 12 weeks post final treatment, the general procedure experience assessment indicated 50.9% of all participants felt the procedure was "comfortable" or "very comfortable" (Figure 5A), 89.6% were "likely "or "very likely" to have other areas treated (Figure 5B), and 93.4% of all participants would recommend the procedure to a friend (Figure 5C). Among Chinese participants, 50.0% felt the procedure was "comfortable" or "very comfortable", 89.6% were "likely" or "very likely" to have other areas treated, and 91.7% would recommend the procedure to a friend.
Figure 5.
Treatment procedure experience measured by the Cryolipolysis General Procedure Questionnaire (CGPQ items 1, 2, and 3) at 12 weeks post final treatment. All participants are shown in blue charts, and Chinese participants are shown in purple charts.
Safety
A total of 65 TEAEs were reported in 24 of 112 (21.4%) treated participants, which included 16 of 112 (14.3%) participants reporting TEAEs in treatment session 1 and 11 of 112 (10.3%) in treatment session 2 (Table 3). Of these 24 participants, 23 participants reported TEAEs as ADEs or procedure-related AEs. The most common TEAEs (>2% of participants) were pruritus (10/112, 8.9%), medical device discomfort (7/112, 6.3%), and nausea (3/112, 2.7%). The majority of participants who reported the TEAEs reported them as mild (19/24, 79.2%) or moderate (4/24, 16.7%) in severity. However, 1 participant experienced severe medical device discomfort during the second treatment session and subsequently discontinued. One other participant discontinued the study due to a serious AE (spontaneous miscarriage reported 10 weeks post final treatment), which was not considered device- or procedure-related. For those participants with mild AEs, 6 had applicators adjusted or removed at the time of the AE, with all but 1 AE resolving by the time the study completed. The participant with “ongoing” mild treatment site discoloration at the time of study completion was prescribed tretinoin as well as hydrocortisone for associated erythema and swelling. In terms of concomitant analgesia medications, paracetamol was prescribed in 6 participants, and ibuprofen or codeine phosphate were prescribed in 1 participant each, respectively. Of those participants with moderate AEs, all AEs resolved with no further action taken. There were no unanticipated AEs. It should be noted that TEAEs were predominantly reported by participants who received >16 treatment cycles (16/24, 66.7%).
Table 3.
Incidence of TEAEs and SAEs
| All participants | Chinese participants | |||
|---|---|---|---|---|
| System, organ, class preferred term | Treatment session 1 (N = 112) | Treatment session 2 (N = 107) | Treatment session 1 (N = 51) | Treatment session 2 (N = 50) |
| Participants with at least 1 TEAE | 16 (14.3) | 11 (10.3) | 12 (23.5) | 5 (10.0) |
| Mild | 14 (12.5) | 8 (7.5) | 11 (21.6) | 4 (8.0) |
| Moderate | 2 (1.8) | 2 (1.9) | 1 (2.0) | 1 (2.0) |
| Severe | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal disorders | 1 (0.9) | 3 (2.8) | 1 (2.0) | 2 (4.0) |
| Abdominal tenderness (mild) | 1 (0.9) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Nausea (all mild) | 0 (0.0) | 3 (2.8) | 0 (0.0) | 2 (4.0) |
| General disorders/administration site conditions | 3 (2.7) | 5 (4.7) | 1 (2.0) | 1 (2.0) |
| Medical device discomfort (6 mild, 1 severea) | 2 (1.8) | 5 (4.7) | 0 (0.0) | 1 (2.0) |
| Medical device site discoloration (mild) | 1 (0.9) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Medical device site erythema (mild) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (2.0) |
| Injury, poisoning, and procedural complications | 1 (0.9) | 2 (1.9) | 0 (0.0) | 1 (2.0) |
| Cold burn (moderate) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Postprocedural swelling (1 mild, 1 moderate) | 1 (0.9) | 1 (0.9) | 0 (0.0) | 1 (2.0) |
| Musculoskeletal/connective tissue disorders | 1 (0.9) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Muscle tightness (mild) | 1 (0.9) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Nervous system disorders | 2 (1.8) | 1 (0.9) | 1 (2.0) | 1 (2.0) |
| Dizziness (mild) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (2.0) |
| Paresthesia (1 mild, 1 moderate) | 2 (1.8) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Skin and subcutaneous tissue disorders | 10 (8.9) | 2 (1.9) | 9 (17.6) | 1 (2.0) |
| Panniculitis (moderate) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Pruritus (all mild) | 10 (8.9) | 1 (0.9) | 9 (17.6) | 1 (2.0) |
| Skin hyperpigmentation (mild) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 0 (0.0) |
| Participants with at least 1 SAEb | ||||
| Pregnancy, puerperium, and perinatal conditions | 0 (0.0) | 1 (1.3) | 0 (0.0) | 1 (2.9) |
| Spontaneous abortion (miscarriage, moderate) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1 (2.0) |
Values are n (%). SAE, serious adverse event; TEAE, treatment-emergent adverse event.
aSevere medical device discomfort was experienced by 1 participant who received 5 treatment cycles. AE dictionary: MedDRA Version 22.0 (MedDRA, Herndon, VA). Participants are counted only once within each category. TEAEs that occurred on or after the date of treatment session 1 and before the date of treatment session 2 were counted for treatment session 1; TEAEs that occurred on or after the date of treatment session 2 were counted for treatment session 2. Total column includes TEAEs in both treatment sessions.
bFor gender-specific TEAEs, percentages are relative to the number of participants of the appropriate gender.
Discussion
This was the first international CoolSculpting study to enroll participants across 4 countries generating data on participant-reported insights and safety from a more geographically diverse population. This was also the first known study to assess participant satisfaction as a primary endpoint for noninvasive fat reduction using cryolipolysis by administering 3 questionnaires (CSQ, CPIQ-M, and CGPQ) designed to capture PROs more reflective of cryolipolysis treatment of the midsection. The findings showed that consistently high levels of satisfaction were achieved across a range of PRO domains among a diverse population consisting of 42.9% Caucasian and 54.5% Asian participants (83.6% of whom were of Chinese descent).
For the primary analysis, 89.6% of all participants were "satisfied" or "very satisfied" with their overall treatment results, with similarly high satisfaction rates among the subgroup of Chinese participants (89.6%) (Figure 1). Among those satisfied or very satisfied participants, the level was consistently high (≥80.0%) independent of body areas treated, the total number of treatment cycles received, and BMI category (Table 2). In comparison with previous studies reporting a 63.9% weighted average participant satisfaction with the use of the legacy parallel-plate applicators for abdomen/flanks, this study demonstrated significant improvement in PROs with the use of CoolAdvantage applicators.16-19
There was a progressive reduction in fat volume quantified by 3D imaging analysis measured as a mean [SD] of 149.1 [280.61] mL at 8 weeks and 264.8 [411.36] mL at 12 weeks post final treatment (Figure 2). Upon further review, this reduction was observed in treatment plans utilizing a higher number of cycles. For those participants receiving 24 treatment cycles, a mean reduction of 334 [455.13] mL was quantified 12 weeks post final treatment. In comparison, a mean reduction of 259.7 [311.19] mL and 176.6 [433.77] mL was quantified in those participants who had 16 or 12 treatment cycles in their treatment plan, respectively. Overall, significant variance in fat volume reduction was noted. Numerous contributing factors may have included a procedural difference in standard image capture across the 7 different study sites (ie, subject posture and/or clothing, camera operator use) as well as identification and isolation of the area of interest (AOI). The latter was a contributing factor in the inability to assess the image data of at least 9 participants and may represent an inherent limitation in assessing a large surface area such as the midsection (abdomen and flanks) of the body. In a small single-site split-body study (N = 11), which also utilized 3D imaging to assess subcutaneous fat volume reduction of the flank following cryolipolysis, an absolute mean difference of 39.6 mL (P < 0.0001) between treated (56.2 [25.6] mL) and untreated (16.6 [17.6] mL) flank at 2 months following a single treatment cycle was quantified.8 Notably, the volume loss observed in the untreated flank was attributed to subject weight fluctuations and/or limitations of the precision of the software in calculating volume change in the AOI. These insights may corroborate the observations in our much larger study and highlight the need for further refinement of 3D image analysis as a consistent and reliable tool to assess volume changes occurring in the midsection of the body. Representative pre- and posttreatment photos show visual improvements in body contour corresponding with treatment details and 3D volume change (Figure 6, Supplemental Figure 2, and Videos 1 and 2, available online at www.aestheticsurgeryjournal.com; videos reproduced with permission from AbbVie, Chicago, IL).
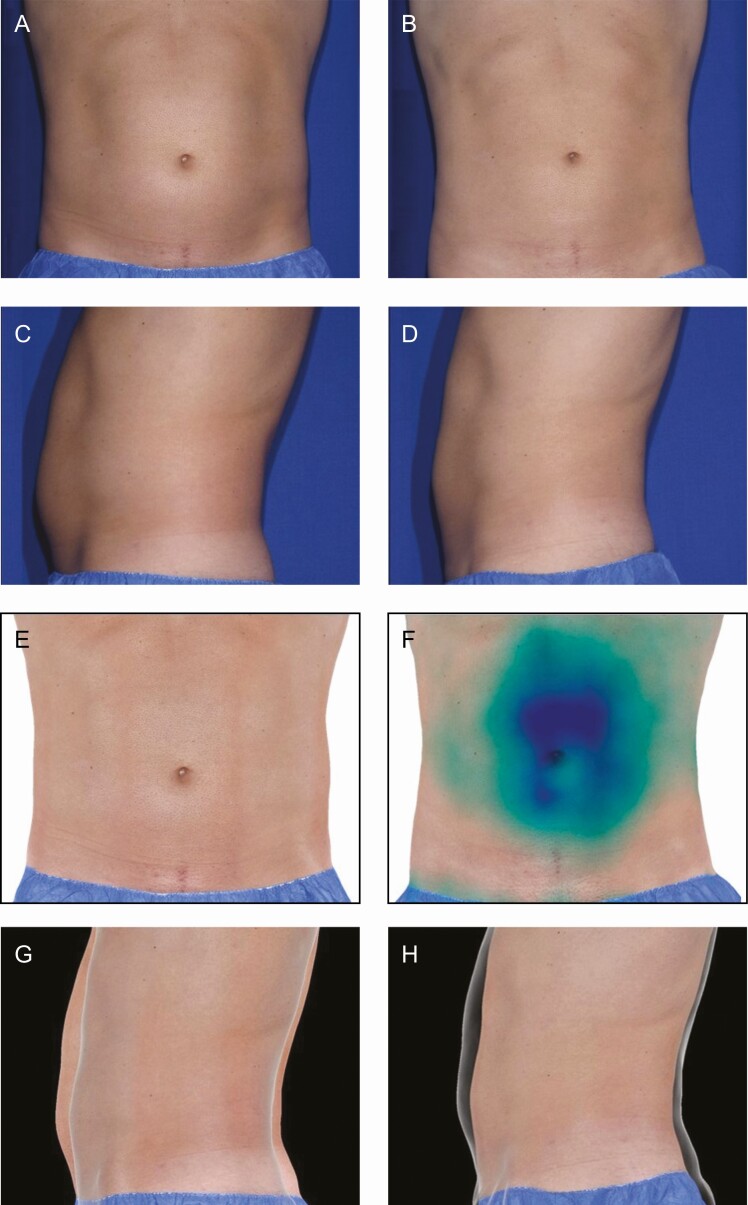
Figure 6.
Two-dimensional and 3D images showing results for a 36-year-old White Caucasian male participant (BMI, 24.6 kg/m2) with Fitzpatrick skin phototype III at (A, C, E, G) baseline and (B, D, F, H) 12 weeks post final treatment visit. The participant received 24 total treatment cycles over 2 sessions: 8 abdomen (2 upper and 6 lower), and 8 each flank. The participant achieved a total 3D volume change of –967 mL from baseline and was “very satisfied” with the overall results of the treatment. The 3D images illustrate volume difference by an “elevation color map” in which smaller negative volumes are light blue and larger negative volumes are dark blue. The silhouette images illustrate contour change by overlay of the posttreatment transparency shadow on top of the pretreatment image. 3D, 3-dimensional. Reproduced with permission from AbbVie (Chicago, IL).
Ultimately, the perception of fat reduction from the participant’s perspective revealed that 90.6% achieved "noticeable" or "very noticeable" fat reduction, whereas nearly all (97.2%) noticed an improvement in the fit of their clothing (ranging from "a little" to "a lot"), and 88.7% felt that the treatment results met or exceeded their expectations (Figure 3). Aligned with these perceptions, an improvement in every item on the psychosocial impact questionnaire was observed (decrease in moderate/extreme affirmative response). Further, the proportion of participants reporting they were "very" or "extremely happy" with their appearance was >70% after treatment compared with 6.3% at baseline (Figure 4). Among the most positive impacts was the decrease from 78.4% to 25.5% in the affirmative response to avoiding certain places or situations for all participants and from 89.6% to 39.6% for Chinese participants.
Because most treatment plans included treatment of both the flanks and abdomen (96/106, 90.6%) and nearly all included ≥12 treatment cycles (96/106, 90.6%), the CGPQ was important to assess how well treatment plans were tolerated and whether treatment discomfort corresponded with a lack of perceived treatment value. Although almost half of the participants reported they were either "uncomfortable" (41.5%) or "very uncomfortable" (7.5%), a high proportion (89.6%) were still "likely" (or "very likely") to consider treating a different body area; furthermore, 93.4% would consider recommending the treatment to a friend (Figure 5).
Two participants were "dissatisfied" or "very dissatisfied" with their overall treatment results; one of these participants received 12 treatment cycles, and the other received 24. Both found the procedure "uncomfortable" or "very uncomfortable," and neither achieved a measurable fat reduction by 3D imaging or noticed any significant improvement in appearance at 12 weeks post final treatment. Had the sharing of pre- and posttreatment images with those participants been permitted (as is typical in clinical practice to demonstrate improvement), it may have improved the perceived effectiveness in those 2 cases. However, further investigation is needed to assess why there was no measurable change or perceived improvement after receiving a comprehensive treatment plan.
From a safety perspective, cryolipolysis treatment plans comprising up to 24 treatment cycles were shown to be well-tolerated among the entire safety population. The majority (95.8%) of TEAEs were mild or moderate in severity. However, 2 participants discontinued the study prematurely: 1 following a spontaneous miscarriage (reported as unrelated to the study procedure by the investigator), and 1 following severe discomfort during a second treatment session. The majority of the TEAEs were reported in participants receiving ≥16 treatment cycles.
Some apparent limitations in this study include the lack of randomization and controls in the study population, possible limitations inherent to the 3D imaging method used to assess the midsection, and the lack of additional measurements (independent blinded review of before-and-after photography, caliper/tape measure, or ultrasound) to corroborate or contradict 3D image findings and participant satisfaction reported outcomes. With a limited number of studies utilizing 3D imaging to assess subcutaneous fat reduction of the abdomen and flanks, more studies are needed to establish the utility of this technology in this context. Another potential limitation of this study is the small number of participants (n = 13; 11.6%) who were identified as being non-White or non-Chinese. It is difficult to make meaningful statistical comparisons across the much smaller demographic subgroups, and future studies enrolling more diverse participants globally is warranted. Finally, an important consideration for the interpretation of the PRO data in this study is that there was no participant cost associated with receiving the treatment, which has the inherent potential to bias questionnaire responses.
Conclusions
This international study reports a high level of participant satisfaction in participants receiving cryolipolysis for their abdomen and/or flanks. Effectiveness measured as PROs and safety endpoints were consistently comparable across different ethnic subgroups regardless of the number of treatment cycles in the treatment plan, treated body area, or BMI category.
Supplemental Material
This article contains supplemental material located online at www.aestheticsurgeryjournal.com.
Acknowledgments
The authors thank the Quantificare (Cumming, GA) team for their support in acquiring and analyzing the 3D images.
Funding
This research was supported by Allergan Aesthetics (Irvine, CA), an AbbVie Company (North Chicago, IL). Writing and editorial assistance was provided by Dr Erika von Grote, an employee of AbbVie Inc. Employees of AbbVie Inc. participated in the research, interpretation of data, review of the manuscript, and the decision to submit for publication.
Disclosures
All authors met the ICMJE authorship criteria. Neither honoraria nor any other form of compensation was provided for authorship. Dr Tan serves as a consultant, advisory board member, and investigator for Allergan Aesthetics (Irvine, CA), an AbbVie company (North Chicago, IL). Dr Snell serves as an investigator for Allergan Aesthetics. Dr Braun serves as a consultant, advisory board member, and investigator for Allergan Aesthetics. Dr Mohan serves as an investigator for Allergan Aesthetics. Drs Jo, Patel, and Zheng are employees of AbbVie, Inc., and may own stock/stock options in the company. Drs Manson Brown and Hickling are employees of Allergan Aesthetics and may own stock/stock options in the company. AbbVie is committed to responsible data sharing regarding the clinical trials the company sponsors. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researcher who is engaging in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. Coleman SR, Sachdeva K, Egbert BM, Preciado J, Allison J. Clinical effectiveness of noninvasive cryolipolysis and its effects on peripheral nerves. Aesthet Plast Surg. 2009;33:482-488. doi: 10.1007/s00266-008-9286-8 [DOI] [PubMed] [Google Scholar]
- 2. Dierickx CC, Mazer JM, Sand M, Koenig S, Arigon V. Safety, tolerance, and patient satisfaction with noninvasive cryolipolysis. Dermatol Surg. 2013;39(8):1209-1216. [DOI] [PubMed] [Google Scholar]
- 3. Stevens WG, Pietrzak LK, Spring MA. Broad overview of a clinical and commercial experience with CoolSculpting. Aesthet Surg J. 2013;33(6):835-846. [DOI] [PubMed] [Google Scholar]
- 4. Bernstein EF, Bloom JD, Basilavecchio LD, Plugis JM. Non-invasive fat reduction of the flanks using a new cryolipolysis applicator and overlapping, two-cycle treatments. Lasers Surg Med. 2014;46(10):731-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein KB, Bachelor EP, Becker EV, Bowes LE. Multiple same day cryolipolysis treatments for the reduction of subcutaneous fat are safe and do not affect serum lipid levels or liver function tests. Lasers Surg Med. 2017;49(7):640-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shek SY, Chan NP, Chan HH. Non-invasive cryolipolysis for body contouring in Chinese—a first commercial experience. Lasers Surg Med. 2012;44(2):125-130. [DOI] [PubMed] [Google Scholar]
- 7. Bernstein EF. Longitudinal evaluation of cryolipolysis efficacy: two case studies. J Cosmet Dermatol. 2013;12(2):149-152. [DOI] [PubMed] [Google Scholar]
- 8. Garibyan L, Sipprell WH 3rd, Jalian HR, Sakamoto FH, Avram M, Anderson RR. Three-dimensional volumetric quantification of fat loss following cryolipolysis. Lasers Surg Med. 2014;46(2):75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kennedy J, Verne S, Griffith R, Falto-Aizpurua L, Nouri K. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29(9):1679-1688. [DOI] [PubMed] [Google Scholar]
- 10. Bernstein EF. Long-term efficacy follow-up on two cryolipolysis case studies: 6 and 9 years post-treatment. J Cosmet Dermatol. 2016;15(4):561-564. [DOI] [PubMed] [Google Scholar]
- 11. Patrick DL, Burke LB, Powers JH, et al. . Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10 Suppl 2:S125-S137. [DOI] [PubMed] [Google Scholar]
- 12. Wanitphakdeedecha R, Sathaworawong A, Manuskiatti W. The efficacy of cryolipolysis treatment on arms and inner thighs. Lasers Med Sci. 2015;30(8):2165-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suh DH, Park JH, Jung HK, Lee SJ, Kim HJ, Ryu HJ. Cryolipolysis for submental fat reduction in Asians. J Cosmet Laser Ther. 2018;20(1):24-27. [DOI] [PubMed] [Google Scholar]
- 14. Oh CH, Shim JS, Bae KI, Chang JH. Clinical application of cryolipolysis in Asian patients for subcutaneous fat reduction and body contouring. Arch Plast Surg. 2020;47(1):62-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bonan P, Verdelli A. Combined microwaves and fractional microablative CO2 laser treatment for postpartum abdominal laxity. J Cosmet Dermatol. 2021;20(1):124-131. [DOI] [PubMed] [Google Scholar]
- 16. Kilmer SL, Burns AJ, Zelickson BD. Safety and efficacy of cryolipolysis for non-invasive reduction of submental fat. Lasers Surg Med. 2016;48(1):3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernstein EF, Bloom JD. Safety and efficacy of bilateral submental cryolipolysis with quantified 3-dimensional imaging of fat reduction and skin tightening. JAMA Facial Plast Surg. 2017;19(5):350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leal Silva H, Carmona Hernandez E, Grijalva Vazquez M, Leal Delgado S, Perez Blanco A. Noninvasive submental fat reduction using colder cryolipolysis. J Cosmet Dermatol. 2017;16(4):460-465. [DOI] [PubMed] [Google Scholar]
- 19. Rivers JK, Ulmer M, Vestvik B, Santos S. A customized approach for arm fat reduction using cryolipolysis. Lasers Surg Med. 2018;50(7):732-737. doi: 10.1002/lsm.22811 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.