Abstract
Cardiac output (CO) measurement is an important element of hemodynamic assessment in critically ill children and existing methods are difficult and/or inaccurate. There is insufficient literature regarding CO as measured by noninvasive electrical cardiometry (EC) as a predictor of outcomes in critically ill children. We conducted a retrospective chart review in children <21 years, admitted to our pediatric intensive care unit (PICU) between July 2018 and November 2018 with acute respiratory failure and/or shock and who were monitored with EC (ICON monitor). We collected demographic information, data on CO measurements with EC and with transthoracic echocardiography (TTE), and data on ventilator days, PICU and hospital days, inotrope score, and mortality. We analyzed the data using Chi-square and multiple linear regression analysis. Among 327 recordings of CO as measured by EC in 61 critically ill children, the initial, nadir, and median CO (L/min; median [interquartile range (IQR)]) were 3.4 (1.15, 5.6), 2.39 (0.63, 4.4), and 2.74 (1.03, 5.2), respectively. Low CO as measured with EC did not correlate well with TTE ( p = 0.9). Both nadir and mean CO predicted ventilator days ( p = 0.05 and 0.01, respectively), and nadir CO was correlated with peak inotrope score (correlation coefficient of –0.3). In our cohort of critically ill children with respiratory failure and/or shock, CO measured with EC did not correlate with TTE. Both nadir and median CO measured with EC predicted outcomes in critically ill children.
Keywords: cardiac output, children, electrical cardiometry, outcomes
Introduction
Cardiac output (CO) measurement is one of the essential elements in the hemodynamic assessment and management of critically ill pediatric patients. The accurate diagnosis of low CO state (LCOS) has shown to improve outcomes and decrease mortality in pediatric patients. 1 2
Swan et al introduced pulmonary artery catheterization (PAC) and spawned invasive hemodilution and thermodilution techniques in 1970 that provided a means to monitor CO in the critical care setting. 3 4 5 Despite being the gold standard for CO measurement, the utilization of PAC is controversial due to the difficulty with cannulation in pediatric populations and the high risk of complications. 6 7 Noninvasive modalities have since emerged to address these limitations while still providing CO measurements. Currently, transthoracic echocardiography (TTE) is the primary method used to evaluate hemodynamic status in the critically ill adult population given its accuracy. 8 9 However, the evidence validating TTE in the pediatric intensive care unit (PICU) is scarce, and its utility is limited due to its operator dependence and its inability to provide continuous measurements or detect small fluid changes. 10
Electrical cardiometry (EC) using bioimpedance for hemodynamic monitoring is a newer alternative that addresses these limitations. The ICON monitor (Osypka Medical, Berlin, Germany), one example of EC, passes a low amplitude, high-frequency current through the thorax and measures the resistance from the red blood cells moving from the aorta to calculate flow velocity and orientation. Inputting this data into an algorithm, ICON can isolate the changes in conductivity created by the circulatory system to provide continuous hemodynamic data. 11 12 13 14 Prior studies have demonstrated that measurements of CO obtained with EC are comparable to TTE and PAC for pediatric patients, including patients who have undergone cardiac surgery and who have structural heart disease. 6 15 16 17 18 19 20
However, no study has examined whether CO as measured with EC can predict outcomes of children admitted to the intensive care unit for acute respiratory failure and/or shock. In this study, we sought to explore the association between CO, when measured with ICON with traditional critical care outcomes, such as length of stay, and dependence on ventilator. We also attempted to investigate the correlation of CO measured by ICON and therapeutic intervention, mainly the use of inotrope support. Finally, we attempted to validate the identification of LCOS with EC in our population, using echocardiogram evaluation as a comparison.
Methods
We conducted a retrospective analysis of critically ill children admitted to the PICU at The Children's Hospital of San Antonio from July 2018 to November 2018. We obtained approval from the institutional review board of Baylor College of medicine and feasibility committee of Voelcker Clinical Research Centre.
Inclusion criteria are the following:
All patients admitted to our PICU with respiratory failure and/or shock.
Patients in whom ICON monitor data on fluid and hemodynamic status was available.
Respiratory failure was defined as evidence of hypoxemia and/or hypercarbia needing high flow nasal cannula or noninvasive mechanical ventilator or invasive mechanical ventilator. Shock was defined as patient with tachycardia and/or hypotension requiring fluid and/or inotropes. We collected demographic information, such as age, gender, and ethnicity, from our hospital electronic medical record. The primary diagnoses on admission was categorized into cardiac, respiratory, gastrointestinal, genitourinary, neurological, and “other causes” for those not falling within any of the organ system categories.
Our unit acquired ICON monitor in May 2018. We used the monitor to capture data on noninvasive assessment of hemodynamic status for patients with respiratory failure and/or shock. Due to availability of a single ICON monitor in our PICU, our PICU team created a protocol of obtaining data on fluid and hemodynamic status in critically ill patients every 6 hours throughout their PICU admission ( Fig. 1 ). Since the device was new to our team, we did not initiate or change any hemodynamic intervention in the patients based on ICON monitor data during the study period. The providers were blinded to the information collected by the monitor to reduce risk of introducing bias into the treatment of these patients. We applied four ICON monitor sensors to the left side of the patient neck and thorax, two of which emitted a low amplitude, high-frequency alternating current, and the remaining sensors measured the amount of current reflected back. Based on the pulsatile red blood cell (RBC) flow through the aorta, the monitor utilized an algorithm to determine the CO, along with other hemodynamic parameters. We captured hemodynamic data in eligible patients using ICON monitor at four regular intervals a day. Since the CO measurements are displayed by EC with the use of an algorithm following 100% signal strength, we obtained all the measurements when 100% signal strength was confirmed. The approach was chosen to best ensure accurate CO measurements in our cohort. We retrieved the hemodynamic data which was stored in ICON monitor during study period. We determined the median CO for each patient during the PICU admission and recorded and indexed initial and nadir COs for each patient. We recorded echocardiographic data obtained for purposes independent to this study and matched to corresponding readings by the ICON monitor within a 6-hour timeframe.
Fig. 1.
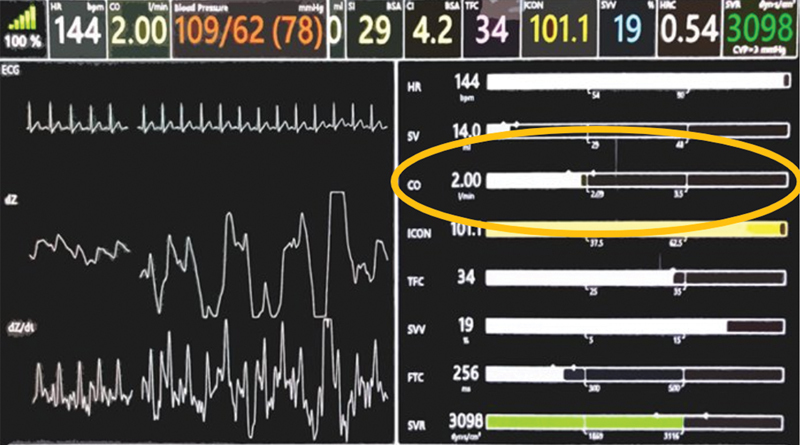
ICON monitor with hemodynamic parameters, especially CO. CO, cardiac output.
Definition of Low Cardiac Output
The ICON monitor/EC provides the normal ranges for CO based on age and size. Any CO below the normal range was defined as “low CO” based on EC. Since the ejection fraction is a calculated number with significant inter- and intraobserver variability and room for significant error, we did not use ejection fraction for analysis. We defined CO using TTE as “depressed ventricular function.” All the echocardiographs are reported by pediatric cardiologists routinely in our institution. And thus, the findings reported by them to define depressed ventricular function were used in our study.
We assessed mortality as the primary outcome and mechanical ventilation (days), length of PICU stay (days), length of hospital stay (days), and inotrope score as secondary outcomes. We defined survival as survival to ICU discharge or transfer. The only exception to these criteria was renal insufficiency, which was defined by the use of any mode of renal replacement therapy rather than dialysis alone. We used data regarding administration of dopamine, dobutamine, and epinephrine to calculate the patient inotrope score. 21 22
We performed multiple linear regression analysis to determine if indices of CO (initial CO, nadir CO, and median CO) predicted primary and secondary outcomes. We chose to obtain the data throughout the PICU course to accurately define the nadir CO during the PICU course. This approach allowed us appropriate logistic regression analysis for assessing initial and nadir CO as predictors of outcomes. Using Chi-square test, we compared CO as measured by ICON with values obtained with echocardiogram. We considered p ≤ 0.05 significant.
Results
A total of 327 recordings of CO in 61 critically ill children using the ICON monitor was available for retrospective analysis. The median (IQR) age of the population sample was 48 months (9, 132). There was an equivalent representation of both genders with 35/61 (57%) of patients being females. The most witnessed ethnicity was Hispanic (54%) followed by Caucasian (26%) and African American (10%). The most common underlying organ system dysfunction upon admission were neurologic, respiratory, and cardiac ( Table 1 ). Though the results suggest that 26% of patients had neurologic etiology, all the patients had respiratory failure and/or shock. For example, patients who presented with status epilepticus and respiratory failure and those who presented with meningoencephalitis had respiratory failure and/or shock.
Table 1. Median and percentiles for demographics and outcomes.
| Patient characteristics | Median (IQR)/% |
|---|---|
| Age (mo) | 48 (9, 132) |
| Gender (male:female) | (26:35) |
| Ethnicity | |
| Hispanic | 54 |
| White | 26 |
| African American | 10 |
| Asian | 2 |
| Others | 8 |
| Underlying etiology | |
| Neurological | 26 |
| Respiratory | 25 |
| Cardiac | 23 |
| Endocrinological | 8 |
| Gastrointestinal | 5 |
| Genitourinary | 3 |
| Others | 10 |
| Primary outcomes | |
| Mortality | 3 |
| Secondary outcomes | |
| Length of PICU stay in days | 5 (3, 10) |
| Length of hospital stay in days | 7 (3.5, 13) |
| Ventilator days | 0 (0, 4.5) |
Abbreviations: IQR, interquartile range; PICU, pediatric intensive care unit.
The initial and nadir CO (median [IQR]) recorded with ICON monitor for each of the 61 patients were 3.4 L/min (1.15, 5.6) and 2.39 L/min (0.63, 4.4), respectively ( Table 2 ). The median CO (median [IQR]) of all patients, calculated using the median CO values for each individual patient throughout their hospital course, was 2.74 L/min (0.63, 4.4; Table 3 ). The corresponding echocardiographic data regarding the systolic function was available in 41 of 61 (70%) patients. Findings of low cardiac function on echo was seen in 21 of 41(48%) of the patients.
Table 2. Median of hemodynamic parameters versus outcomes.
| Variable | Median (IQR) | Length of PICU stay ( p -Value) | Length of hospital stay ( p -Value) | Days on ventilator ( p -Value) |
|---|---|---|---|---|
| Cardiac output (L/min) initial | 3.4 (1.15, 5.6) | 0.26 | 0.13 | 0.06 |
| Cardiac output (L/min) nadir | 2.39 (0.63, 4.4) | 0.58 | 0.33 | 0.05 |
| Cardiac output (L/min) median | 2.74 (1.02, 5.2) | 0.25 | 0.07 | 0.01 |
| Inotrope score initial | 0 (0, 3.75) | 0.95 | 0.71 | 0.01 |
| Inotrope score peak | 0 (0, 3.75) | 0.77 | 0.37 | 0.22 |
| Inotrope score median | 0 (0, 2.75) | 0.70 | 0.77 | 0.30 |
Abbreviations: IQR, interquartile range; PICU pediatric intensive care unit.
Table 3. Two-by-two table for Chi-square test for analysis of CO on TTE and CO measured using electrical cardiography (ICON).
| ICON TTE → ↓ |
Low CO | Normal CO | Total |
|---|---|---|---|
| Low CO | 12 | 13 | 25 |
| Normal CO | 8 | 16 | |
| Total | 20 | 21 | 41 |
Abbreviations: CO, cardiac output; TTE, transthoracic echocardiography.
The median (IQR) length of PICU and hospital stay in days and median (IQR) ventilator days were 5 (3, 10), 7 (3.5, 13), and 0 (0, 4.5), respectively. Two of 61(3%) of the patients did not survive to ICU discharge. Nine of 61(15%) patients had abnormal creatinine values suggestive of acute renal failure. A total of 25 of 61 (41%) patients were on inotropes at any point during their PICU course. Of these 25 patients, the (median [IQR]) peak inotrope score was 5 (2.5, 10).
Although, CO did not predict hospital and PICU length of stay, the nadir and median CO predicted ventilator days ( p = 0.05 and 0.01, respectively). The proportion of patients with LCOS who died was not significant ( p = 0.35). There was no significant relationship between low CO as measured by ICON and corresponding findings of low cardiac function on echocardiography ( p = 0.9). Nadir CO had a moderate, inverse correlation with inotrope score ( r = − 0.3). It was also shown that initial, peak, or median inotrope scores were not predictors of length of stay in PICU and hospital, but the initial inotrope score was associated with ventilator days ( p < 0.01).
Discussion
Our study suggests that CO as measured by EC is associated with outcomes in critically ill children with shock and/or respiratory failure. To our knowledge, this is the first study which has assessed CO as measured by noninvasive EC for outcomes in critically ill children. EC, a noninvasive method of CO monitoring, provides an appealing alternative to standard methods such as PAC with thermodilution and TTE. The risks of cannulation in the pediatric population and the high risk of complications limit PAC, while previous studies supporting TTE as a means of monitoring CO mainly examined adult population or single-center and selected pediatric populations. 6 7 23
EC has not been sufficiently studied in critically ill children; however, data confirming its validity relative to TTE and PAC are predominantly based on studies examining children with structural heart disease or who have undergone cardiac surgery. 15 Given that ICON utilizes resistance of blood flow in its algorithm to measure CO, which can be impacted by disease processes like shock, significant concerns of its utility in a general critically ill population exist that we attempted to address with this study. 11 15
Our retrospective single-center analysis is the only existing study that addresses the predictive value of CO as measured with EC for patients admitted to the PICU in respiratory failure and/or shock. In our cohort, the nadir and median CO predicted days on mechanical ventilation. These relationships, which had not been established prior to this study, validate the importance of monitoring CO in the PICU setting and suggest a clinical utility of EC for diagnostic purposes. CO did not predict length of stay, both in the PICU and in the hospital, which is consistent with existing literature. 24 Given that CO predicted mechanical ventilation days, our data suggest that monitoring CO may be more useful for stabilization and resuscitation efforts. Nadir CO was inversely correlated with inotrope score, a finding that has not been previously established. This finding portends the utility of CO monitoring with EC to guide therapeutic interventions, especially the use of inotropes. Regarding the primary outcome, our sample size was not big enough to detect significant changes in mortality given its overall low incidence in our study cohort.
Our study also explores the use of TTE to identify LCOS in critically ill pediatric patients. Previous studies supporting TTE as a mean of monitoring CO mainly examine adult populations. 23 The selected few studies that examine pediatric populations are primarily single-center analyses or involve specific subgroups, such as patients undergoing cardiac surgery. 23 In our cohort, we found that the identification of low CO state did not correlate in the 41 patients who had both EC and TTE measurements available. The lack of correlation between TTE and EC in the identification of low CO state supports current literature which is inconclusive for correlation between the two techniques for determination of CO. 9 17 18 19 20 23 25 26 27 28 29 Future studies, ideally prospective, are necessary to examine whether EC or TTE is better suited to identify LCOS.
There is a growing interest in using noninvasive, objective tools, such as EC, to provide a more objective and more frequent assessment of CO. Prior studies have demonstrated the validity and reliability of EC, and our study offers evidence that EC provides clinical utility for patients in the PICU with respiratory failure and/or shock. Further studies are needed to better understand the role of EC in the PICU. The predictive nature of CO measurements on patient outcomes for pediatric populations that our study demonstrated needs to be validated. Additionally, prospective studies are needed examining whether therapeutic interventions, such as inotrope usage, guided by CO values obtained by EC can impact patient outcomes.
Limitations
Our study had several limitations. It was a retrospective chart review of a single-center population, thus limiting its application to other populations. Second, we did not use correlation coefficient or Bland–Altman analysis for the comparison of CO as measured by ICON and TTE. CO measurement by EC can be affected by variety of factors, such as pleural effusions, pulmonary edema, arrhythmias, electrical interference, internal or external pacemakers, or movement, but as per the manufacturer ensuring a signal strength of 100% before downloading the data helps eliminate these disturbing factors. In our study, we confirmed 100% signal strength before capturing the hemodynamic data and, therefore, we believe that our CO data using EC are accurate. Also, 30% of our cohort did not have corresponding echocardiographs.
With advances in technology, there is increasing use of noninvasive respiratory support such as high-flow nasal cannula (HFNC) and noninvasive ventilator (NIV). More and more critically ill children with respiratory failure and/or shock are being supported with noninvasive modalities. In our cohort, 21 of 56 children were supported on HFNC or NIV and therefore, the median (IQR) ventilator days were 0 (0, 4.5). Though ventilator days are a standard outcome measure used in critical care literature, in this cohort, a more appropriate measure may instead have been ventilator free days. Mortality could affect ventilator days and therefore ventilator-free days might be a better outcome measure in critically ill children. Since ventilator days are a standard outcome used in critically ill children and since there were only 2 of 61 (3%) patients who died in our study, we believe that mortality did not affect the ventilator days.
Conclusion
In our cohort of critically ill children with respiratory failure and/or shock, nadir and median CO as measured by EC showed an association with morbidity. Low CO as measured by EC was inversely correlated with inotrope score. There was no significant relationship between low CO as measured by ICON and corresponding findings of low cardiac function on echocardiography.
Funding Statement
Funding None.
Conflict of Interest None declared.
Contribution to the Field
Cardiac output (CO) measurement is an important element of hemodynamic assessment in critically ill children, with existing methods being difficult and/or inaccurate. Electrical cardiometry (EC) has the capability of measuring CO noninvasively. We believe that our study is the first study in the literature to demonstrate a relationship between CO measured with EC and outcomes.
References
- 1.Massé L, Antonacci M.Low cardiac output syndrome: identification and management Crit Care Nurs Clin North Am 20051704375–383., x [DOI] [PubMed] [Google Scholar]
- 2.Chandler H K, Kirsch R. Management of the Low Cardiac output Syndrome following surgery for congenital heart disease. Curr Cardiol Rev. 2016;12(02):107–111. doi: 10.2174/1573403X12666151119164647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swan H J, Ganz W, Forrester J, Marcus H, Diamond G, Chonette D. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(09):447–451. doi: 10.1056/NEJM197008272830902. [DOI] [PubMed] [Google Scholar]
- 4.Mehta Y, Arora D. Newer methods of cardiac output monitoring. World J Cardiol. 2014;6(09):1022–1029. doi: 10.4330/wjc.v6.i9.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangkum L, Liu G L, Yu L, Yan H, Kaye A D, Liu H. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth. 2016;30(03):461–480. doi: 10.1007/s00540-016-2154-9. [DOI] [PubMed] [Google Scholar]
- 6.Narula J, Chauhan S, Ramakrishnan S, Gupta S K. Electrical cardiometry: a reliable solution to cardiac output estimation in children with structural heart disease. J Cardiothorac Vasc Anesth. 2017;31(03):912–917. doi: 10.1053/j.jvca.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Critical Care Clinical Trials Group . Sandham J D, Hull R D, Brant R F. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(01):5–14. doi: 10.1056/NEJMoa021108. [DOI] [PubMed] [Google Scholar]
- 8.Peeters Y, Bernards J, Mekeirele M, Hoffmann B, De Raes M, Malbrain M L. Hemodynamic monitoring: to calibrate or not to calibrate? Part 1--calibrated techniques. Anaesthesiol Intensive Ther. 2015;47(05):487–500. doi: 10.5603/AIT.a2015.0073. [DOI] [PubMed] [Google Scholar]
- 9.Mercado P, Maizel J, Beyls C. Transthoracic echocardiography: an accurate and precise method for estimating cardiac output in the critically ill patient. Crit Care. 2017;21(01):136. doi: 10.1186/s13054-017-1737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jozwiak M, Mercado P, Teboul J L. What is the lowest change in cardiac output that transthoracic echocardiography can detect? Crit Care. 2019;23(01):116. doi: 10.1186/s13054-019-2413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noninvasive Cardiac Output Electrical Cardiometry Available at: www.osypkamed.com/sites/default/files/public_resources/C2_C3_Brochure_EN_2014_A4_online_0.pdf. Accessed October 4, 2020
- 12.Moshkovitz Y, Kaluski E, Milo O, Vered Z, Cotter G. Recent developments in cardiac output determination by bioimpedance: comparison with invasive cardiac output and potential cardiovascular applications. Curr Opin Cardiol. 2004;19(03):229–237. doi: 10.1097/00001573-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Summers R L, Shoemaker W C, Peacock W F, Ander D S, Coleman T G. Bench to bedside: electrophysiologic and clinical principles of noninvasive hemodynamic monitoring using impedance cardiography. Acad Emerg Med. 2003;10(06):669–680. doi: 10.1111/j.1553-2712.2003.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 14.Marik P E, Baram M. Noninvasive hemodynamic monitoring in the intensive care unit. Crit Care Clin. 2007;23(03):383–400. doi: 10.1016/j.ccc.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Teboul J L, Saugel B, Cecconi M. Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med. 2016;42(09):1350–1359. doi: 10.1007/s00134-016-4375-7. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida A, Kaji T, Yamada H.Measurement of hemodynamics immediately after vaginal delivery in healthy pregnant women by electrical cardiometry J Med Invest 201966(1.2):75–80. [DOI] [PubMed] [Google Scholar]
- 17.Rauch R, Welisch E, Lansdell N. Non-invasive measurement of cardiac output in obese children and adolescents: comparison of electrical cardiometry and transthoracic Doppler echocardiography. J Clin Monit Comput. 2013;27(06):187–193. doi: 10.1007/s10877-012-9412-7. [DOI] [PubMed] [Google Scholar]
- 18.Noori S, Drabu B, Soleymani S, Seri I. Continuous non-invasive cardiac output measurements in the neonate by electrical velocimetry: a comparison with echocardiography. Arch Dis Child Fetal Neonatal Ed. 2012;97(05):F340–F343. doi: 10.1136/fetalneonatal-2011-301090. [DOI] [PubMed] [Google Scholar]
- 19.Grollmuss O, Gonzalez P. Non-invasive cardiac output measurement in low and very low birth weight infants: a method comparison. Front Pediatr. 2014;2(16):16. doi: 10.3389/fped.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grollmuss O, Demontoux S, Capderou A, Serraf A, Belli E. Electrical velocimetry as a tool for measuring cardiac output in small infants after heart surgery. Intensive Care Med. 2012;38(06):1032–1039. doi: 10.1007/s00134-012-2530-3. [DOI] [PubMed] [Google Scholar]
- 21.Wernovsky G, Wypij D, Jonas R A. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92(08):2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 22.Kumar M, Sharma R, Sethi S K. Vasoactive Inotrope Score as a tool for clinical care in children post cardiac surgery. Indian J Crit Care Med. 2014;18(10):653–658. doi: 10.4103/0972-5229.142174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang Y, Shi J, Hua Z, Xu J. Cardiac output measurements via echocardiography versus thermodilution: a systematic review and meta-analysis. PLoS One. 2019;14(10):e0222105. doi: 10.1371/journal.pone.0222105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang N N, Wang D Y, Li F, Xie W G. [Clinical significance of pulse contour cardiac output monitoring technology in guiding fluid replacement during shock stage of extensive burn] (in Chinese) Zhonghua Shao Shang Za Zhi. 2019;35(06):434–440. doi: 10.3760/cma.j.issn.1009-2587.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Aslan N, Yildizdas D, Horoz O O. Comparison of cardiac output and cardiac index values measured by critical care echocardiography with the values measured by pulse index continuous cardiac output (PiCCO) in the pediatric intensive care unit:a preliminary study. Ital J Pediatr. 2020;46(01):47. doi: 10.1186/s13052-020-0803-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wurzer P, Branski L K, Jeschke M G. Transpulmonary thermodilution versus transthoracic echocardiography for cardiac output measurements in severely burned children. Shock. 2016;46(03):249–253. doi: 10.1097/SHK.0000000000000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wyk L, Smith J, Lawrenson J, de Boode W P. Agreement of cardiac output measurements between bioreactance and transthoracic echocardiography in preterm infants during the transitional phase: a single-centre, prospective study. Neonatology. 2020;117:271–278. doi: 10.1159/000506203. [DOI] [PubMed] [Google Scholar]
- 28.Boet A, Jourdain G, Demontoux S, De Luca D. Stroke volume and cardiac output evaluation by electrical cardiometry: accuracy and reference nomograms in hemodynamically stable preterm neonates. J Perinatol. 2016;36(09):748–752. doi: 10.1038/jp.2016.65. [DOI] [PubMed] [Google Scholar]
- 29.Hsu K H, Wu T W, Wu I H. Electrical cardiometry to monitor cardiac output in preterm infants with patent ductus arteriosus: a comparison with echocardiography. Neonatology. 2017;112(03):231–237. doi: 10.1159/000475774. [DOI] [PubMed] [Google Scholar]


