Abstract
Objective:
To evaluate glycemic outcomes in the Wireless Innovation for Seniors with Diabetes Mellitus (WISDM) randomized clinical trial (RCT) participants during an observational extension phase.
Research Design and Methods:
WISDM RCT was a 26-week RCT comparing continuous glucose monitoring (CGM) with blood glucose monitoring (BGM) in 203 adults aged ≥60 years with type 1 diabetes. Of the 198 participants who completed the RCT, 100 (98%) CGM group participants continued CGM (CGM-CGM cohort) and 94 (98%) BGM group participants initiated CGM (BGM-CGM cohort) for an additional 26 weeks.
Results:
CGM was used a median of >90% of the time at 52 weeks in both cohorts. In the CGM-CGM cohort, median time <70 mg/dL decreased from 5.0% at baseline to 2.6% at 26 weeks and remained stable with a median of 2.8% at 52 weeks (P < 0.001 baseline to 52 weeks). Participants spent more time in range 70–180 mg/dL (TIR) (mean 56% vs. 64%; P < 0.001) and had lower hemoglobin A1c (HbA1c) (mean 7.6% [59 mmol/mol] vs. 7.4% [57 mmol/mol]; P = 0.01) from baseline to 52 weeks. In BGM-CGM, from 26 to 52 weeks median time <70 mg/dL decreased from 3.9% to 1.9% (P < 0.001), TIR increased from 56% to 60% (P = 0.006) and HbA1c decreased from 7.5% (58 mmol/mol) to 7.3% (57 mmol/mol) (P = 0.025). In BGM-CGM, a severe hypoglycemic event was reported for nine participants while using BGM during the RCT and for two participants during the extension phase with CGM (P = 0.02).
Conclusions:
CGM use reduced hypoglycemia without increasing hyperglycemia in older adults with type 1 diabetes. These data provide further evidence for fully integrating CGM into clinical practice. Clinicaltrials.gov (NCT03240432)
Keywords: Older adults, CGM use, Hypoglycemia, Hyperglycemia
Introduction
Older adults with type 1 diabetes, particularly those with longstanding diabetes, are prone to hypoglycemia and hypoglycemia unawareness.1,2 The Wireless Innovation for Seniors with Diabetes Mellitus (WISDM) study was conducted to assess whether real-time continuous glucose monitoring (CGM) was effective in reducing hypoglycemia among older adults with type 1 diabetes. Results of the 6-month randomized clinical trial (RCT) showed significant reductions in hypoglycemia and improvement in overall glycemic control among participants using CGM compared to a group using standard blood glucose monitoring (BGM).3
Following the 6-month RCT, participants completed a 6-month observational extension phase with less intensive follow-up during which the CGM group participants continued CGM (CGM-CGM) use and the BGM group crossed over to use CGM. The objectives of the extension study were to determine if glycemic improvements observed during the 26-week RCT could be sustained over a longer period in the CGM group and whether the results of the RCT could be replicated in the BGM group even with reduced study contact.
This report summarizes glycemic outcomes for both groups over a 52-week period.
Methods
This study was conducted at 22 endocrinology practices in the United States. The protocol and informed consent forms were approved by Institutional Review Boards. The protocol is available at https://public.jaeb.org/datasets
The RCT included 203 participants aged ≥60 years with type 1 diabetes who had not used real-time CGM in the 3 months before enrollment and a hemoglobin A1c (HbA1c) <10.0% (<86 mmol/mol). Participants were randomly assigned 1:1 to use of CGM (Dexcom G5; Dexcom, San Diego, CA, USA) or to use the study BGM without CGM for 6 months. The RCT was completed by 102 of 103 participants in the CGM group and 96 of 100 participants in the BGM group.
Following the 26-week RCT, all 198 of the participants completing the RCT entered an extension phase and were followed for an additional 26 weeks for a total of 52 weeks. In this phase, the RCT BGM group initiated using CGM (referred to as BGM-CGM) and the RCT CGM group continued CGM use (CGM-CGM).
During the RCT phase, all participants had clinic visits at 4, 8, 16, and 26 weeks. In addition, the BGM group was seen in clinic at weeks 7, 15, and 25 for placement of a masked Dexcom G4 Platinum Professional CGM (same algorithm as Dexcom G5) which was worn for 1 week at each time period to collect data during the randomized phase. During the extension phase, study contact was reduced to clinic visits at 39 and 52 weeks. During both phases, participants had a clinic visit ∼2 weeks after initiating CGM for additional training. General guidelines were provided to participants about using CGM. Additional instructions were provided on using CGM trend arrows to adjust insulin dosing based on guidelines specific to a vulnerable older adult population. Insulin dose changes were made at investigator discretion.
Reportable adverse events included severe hypoglycemia (defined as an event that required assistance from another person due to altered consciousness), hyperglycemia resulting in treatment at a health care facility or that involved diabetic ketoacidosis (DKA) (as defined by the Diabetes Control and Complications Trial),4 device-related events with potential effect on participant safety, falls, fractures, emergency room visits, and all serious adverse events regardless of causality.
Glycemic outcomes included percent of time in hypoglycemia (<70 and <54 mg/dL), proportion of participants meeting clinical targets for hypoglycemia,5 rate of CGM measured hypoglycemic episodes per week (defined as 15 consecutive minutes with a CGM glucose value <54 mg/dL with the end of the hypoglycemic episode defined as a minimum of 15 consecutive minutes with a CGM glucose concentration >70 mg/dL),6 percent of time in target range 70–180 mg/dL, proportion of participants meeting clinical targets for time in range,5 mean glucose, coefficient of variation, percent of time in hyperglycemia (>180, >250, and >300 mg/dL), HbA1c, and percent of participants meeting HbA1c target <7.0% (53 mmol/mol) and <7.5% (58 mmol/mol). Additional analyses of glycemic outcomes included stratification by insulin delivery method for all outcomes and by daytime versus nighttime for prespecified CGM metrics.
For both cohorts, the CGM-measured outcomes were calculated at baseline using the masked data collected during the screening phase. For the CGM-CGM cohort, CGM outcomes were calculated at the 8, 16, 26, 39, and 52-week visits by pooling data from 4 weeks prior. For the BGM-CGM cohort, CGM outcomes were calculated at the 8, 16, and 26-week visits using data from the masked CGM and at the 39- and 52-week visits by pooling real-time CGM data from 4 weeks prior.
HbA1c was measured by central laboratory at randomization 16, 26, 39, and 52 weeks at the University of Minnesota using the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer. Participant-reported outcomes assessed at baseline, 26, and 52 weeks included general quality of life (PROMIS Global Health Short Form; National Institute of Health Toolbox [NIHTB] Emotion Battery), hypoglycemia awareness (Clarke Survey7), hypoglycemia fear (Hypoglycemia Fear Survey-II−Worry scale8), and diabetes distress (T1D Diabetes Distress Scale9). Descriptions of these outcomes, scoring, and clinically relevant change (where known) are described in Supplementary Table S3a.
Statistical analysis
A paired t-test, signed rank test, or McNemar's test, as appropriate, was used to evaluate the change in each outcome between baseline and 52 weeks in the CGM-CGM cohort and between 26 and 52 weeks in the BGM-CGM cohort. To assess sustained hypoglycemia reduction in the CGM-CGM group, the CGM measured hypoglycemia outcomes were compared between 26 and 52 weeks. In the BGM-CGM cohort, the incidence of severe hypoglycemia events during the RCT phase was compared with the extension phase. P-values are two-sided and have been adjusted for multiple comparisons to control the false discovery rate using the adaptive Benjamini-Hochberg procedure.10 No adjustment was done for the analysis of severe hypoglycemic events.
All analyses were conducted based on randomized treatment group assignment and on available cases only, including those who completed the 52-week visit. Baseline refers to prerandomization measurements. Analyses were conducted with SAS software version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
The 52 weeks of follow-up was completed by 194 of the 198 participants who completed the RCT; 100 of 102 (98%) in the CGM-CGM cohort, and 94 of 96 (98%) in the BGM-CGM cohort. The age range of the 194 participants was 60 to 87 years, 52% were female, 93% were non-Hispanic white, and 54% used an insulin pump. Characteristics at the start of the extension phase (26 weeks) for the two cohorts are shown in Supplementary Table S1.
Glycemic outcomes in CGM-CGM cohort
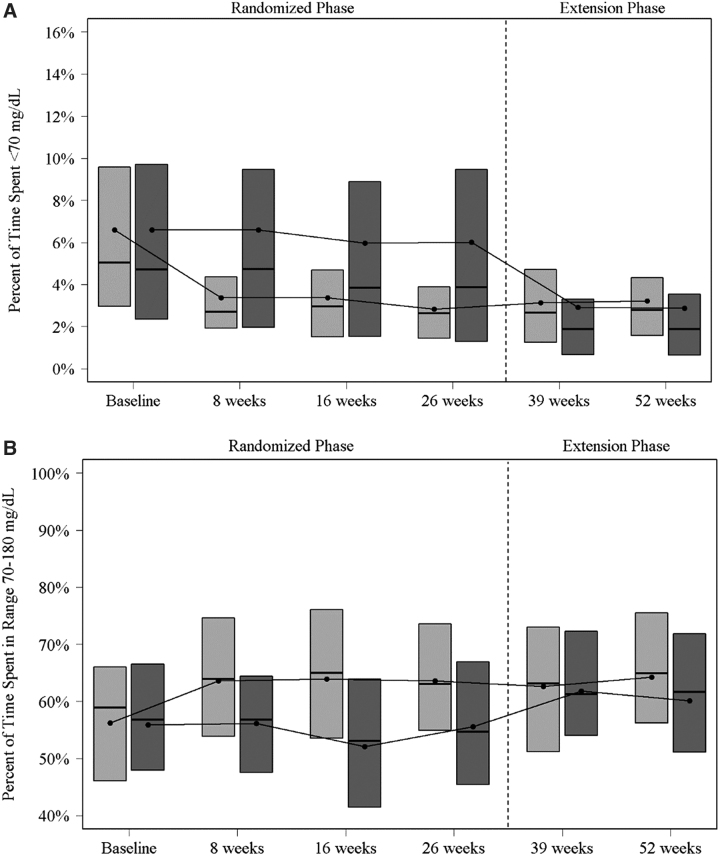
Among the 100 participants in the CGM-CGM cohort, median time <70 mg/dL decreased from 5.0% (73 min/day) at baseline (prerandomization) to 2.6% (38 min/day) at 26 weeks and remained stable with a median of 2.8% (40 min/day) at 52 weeks (P < 0.001 baseline to 52 weeks, P = 0.45 26 to 52 weeks) (Table 1). The proportion of participants with percent time <70 mg/dL <4% and <1% was 34 (34%) and 8 (8%) at baseline, respectively, and 67 (71%) and 16 (17%) at 52 weeks, respectively. A significant reduction in percent of time <70 mg/dL was reached by the 8-week visit and was sustained through the end of the 52-week study (Fig. 1A).
Table 1.
Glycemic Outcomes in CGM-CGM Cohort
| Baseline | 26 Weeks | 52 Weeks | P-value baseline versus 52 Weeksa | |
|---|---|---|---|---|
| CGM-measured outcomes | ||||
| N | 100 | 96 | 94 | |
| Hours of data | ||||
| Mean ± SD | 353 ± 74 | 604 ± 97 | 592 ± 111 | |
| Median (IQR) | 324 (309, 388) | 640 (608, 652) | 638 (597, 653) | |
| Hypoglycemiab | ||||
| % Time <70 mg/dL | <0.001 | |||
| Mean ± SD | 6.6 ± 4.9 | 2.8 ± 1.9 | 3.2 ± 2.4 | |
| Median (IQR) | 5.0 (3.0, 9.6) | 2.6 (1.5, 3.9) | 2.8 (1.6, 4.3) | |
| % Time <54 mg/dL | <0.001 | |||
| Mean ± SD | 2.5 ± 2.4 | 0.6 ± 0.7 | 0.7 ± 1.1 | |
| Median (IQR) | 1.9 (0.9, 3.5) | 0.4 (0.2, 0.8) | 0.4 (0.1, 0.9) | |
| Rate of hypoglycemic episodes per weekc | <0.001 | |||
| Mean ± SD | 3.0 ± 2.4 | 1.3 ± 1.4 | 1.3 ± 1.3 | |
| Median (IQR) | 2.6 (1.5, 3.8) | 0.9 (0.3, 1.7) | 0.9 (0.3, 2.0) | |
| Glucose control | ||||
| % Time in range 70–180 mg/dL | <0.001 | |||
| Mean ± SD | 56 ± 14 | 64 ± 14 | 64 ± 15 | |
| Median (IQR) | 59 (46, 66) | 63 (55, 74) | 65 (56, 76) | |
| Mean glucose (mg/dL) | 0.05 | |||
| Mean ± SD | 167 ± 29 | 163 ± 22 | 161 ± 26 | |
| Median (IQR) | 162 (149, 183) | 162 (145, 176) | 160 (145, 174) | |
| Coefficient of variation (%) | <0.001 | |||
| Mean ± SD | 41 ± 6 | 36 ± 5 | 36 ± 5 | |
| Median (IQR) | 41 (37, 46) | 36 (33, 39) | 36 (33, 39) | |
| Hyperglycemia | ||||
| % Time >180 mg/dL | 0.002 | |||
| Mean ± SD | 37 ± 16 | 34 ± 14 | 33 ± 16 | |
| Median (IQR) | 34 (27, 49) | 35 (23, 42) | 32 (21, 41) | |
| % Time >250 mg/dL | <0.001 | |||
| Mean ± SD | 14 ± 11 | 10 ± 8 | 10 ± 9 | |
| Median (IQR) | 10 (6, 20) | 8 (3, 14) | 7 (3, 12) | |
| % Time >300 mg/dL | <0.001 | |||
| Mean ± SD | 6.0 ± 6.5 | 3.6 ± 4.2 | 3.7 ± 5.2 | |
| Median (IQR) | 3.7 (1.5, 8.3) | 2.2 (0.6, 5.2) | 2.1 (0.5, 4.1) | |
| Meeting targets, n (%) | ||||
| % Time <70 mg/dL <4% | 34 (34) | 72 (75) | 67 (71) | <0.001 |
| % Time <70 mg/dL <1% | 8 (8) | 16 (17) | 16 (17) | 0.009 |
| % Time <54 mg/dL <1% | 30 (30) | 78 (81) | 72 (77) | <0.001 |
| % Time in range 70–180 mg/dL >70% | 13 (13) | 34 (35) | 34 (36) | <0.001 |
| % Time in range 70–180 mg/dL >50% | 70 (70) | 79 (82) | 80 (85) | <0.001 |
| HbA1c | ||||
| N | 100 | 99 | 99 | |
| HbA1c % [mmol/mol] | 0.010 | |||
| Mean ± SD | 7.6 ± 0.9 [59 ± 10] | 7.2 ± 0.9 [56 ± 10] | 7.4 ± 1.0 [57 ± 10] | |
| Median (IQR) | 7.4 (6.9, 8.1) [57 (52, 65)] | 7.2 (6.6, 7.7) [55 (49, 61)] | 7.2 (6.7, 7.9) [55 (50, 63)] | |
| HbA1c <7.0%, n (%) | 28 (28) | 40 (40) | 35 (35) | 0.16 |
| HbA1c <7.5%, n (%) | 51 (51) | 64 (65) | 55 (56) | 0.37 |
P-value from a paired t-test, signed rank test, or McNemar's test, as appropriate. Only includes participants who had values at both time points. P-values are adjusted for multiple comparisons to control the false discovery rate.
The P-values for 26 weeks versus 52 weeks for the hypoglycemia metrics were P = 0.45 for % time <70 mg/dL, P = 0.77 for % time <54 mg/dL, and P = 0.79 for rate of hypoglycemic episodes per week.
A CGM-measured hypoglycemic event was defined as 15 consecutive minutes with a sensor glucose value <54 mg/dL. The end of the hypoglycemic event was defined as a minimum of 15 consecutive minutes with a sensor glucose concentration >70 mg/dL.6
CGM, continuous glucose monitoring; HbA1c, hemoglobin A1c; IQR, interquartile range; SD, standard deviation.
FIG. 1.
(A) Boxplots of percent of time spent below 70 mg/dL by visit. Light gray represents CGM-CGM group. Dark gray represents BGM-CGM group. The top and bottom of the boxes denote the 25th and 75th percentile, the line represents the median and the dot the mean. (B) Boxplots of Percent of time spent in range 70–180 mg/dL by Visit. Light gray represents CGM-CGM group. Dark gray represents BGM-CGM group. The top and bottom of the boxes denote the 25th and 75th percentile, the line represents the median and the dot the mean. BGM, blood glucose monitoring; CGM, continuous glucose monitoring; BGM-CGM, BGM group participants initiated CGM; CGM-CGM, CGM group participants continued CGM.
Mean percent time in range 70–180 mg/dL (TIR) improved over the 52 weeks of follow-up in the CGM-CGM cohort from 56% (13 h/day) at baseline to 64% (15 h/day) at both 26 and 52 weeks (P < 0.001 baseline to 52 weeks) (Table 1 and Fig. 1B). Significant favorable reductions from baseline to 52 weeks were observed for all other CGM hypoglycemia metrics, glycemic variability, and all other hyperglycemia metrics, with the exception of mean glucose (Table 1).
When stratified by insulin delivery method (insulin pump, multiple daily injections), the improvement in CGM metrics from baseline within each subgroup mimicked the overall results for the CGM-CGM cohort (Table 3).
Table 3.
Continuous Glucose Monitoring Metrics by Cohort and Insulin Delivery Method
| |
CGM-CGM |
BGM-CGM |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
26 Weeks |
52 Weeks |
P-value baseline versus 52 Weeksa |
Baseline |
26 Weeks |
52 Weeks |
P-value 26 weeks versus 52 Weeksa |
|
| Insulin pump | N = 56 | N = 53 | N = 51 | N = 46 | N = 44 | N = 43 | ||
| Hours of data | ||||||||
| Mean ± SD | 362 ± 71 | 624 ± 60 | 590 ± 128 | 353 ± 68 | 155 ± 35 | 591 ± 115 | ||
| Median (Q1, Q3) | 326 (314, 396) | 640 (624, 653) | 642 (605, 656) | 326 (310, 409) | 153 (144, 156) | 633 (596, 651) | ||
| Hypoglycemia | ||||||||
| % Time <70 mg/dL | <0.001 | <0.001 | ||||||
| Mean ± SD | 5.8 ± 4.4 | 2.5 ± 1.7 | 2.4 ± 1.5 | 6.8 ± 5.2 | 7.3 ± 6.7 | 2.9 ± 3.1 | ||
| Median (Q1, Q3) | 4.6 (2.8, 9.0) | 2.1 (1.1, 3.3) | 2.1 (1.1, 3.9) | 5.4 (2.4, 9.3) | 5.0 (2.0, 12.4) | 1.9 (0.6, 3.9) | ||
| % Time <54 mg/dL | <0.001 | <0.001 | ||||||
| Mean ± SD | 2.0 ± 2.0 | 0.5 ± 0.5 | 0.4 ± 0.4 | 2.7 ± 2.6 | 2.9 ± 3.6 | 0.8 ± 1.3 | ||
| Median (Q1, Q3) | 1.2 (0.9, 2.9) | 0.3 (0.1, 0.5) | 0.3 (0.1, 0.6) | 1.9 (0.5, 3.8) | 1.2 (0.3, 5.1) | 0.3 (0.1, 1.0) | ||
| Glucose control | ||||||||
| % Time in range 70–180 mg/dL | <0.001 | 0.06 | ||||||
| Mean ± SD | 58 ± 13 | 64 ± 13 | 65 ± 14 | 55 ± 13 | 55 ± 14 | 59 ± 14 | ||
| Median (Q1, Q3) | 60 (47, 67) | 63 (57, 73) | 66 (56, 74) | 56 (48, 64) | 53 (45, 66) | 59 (50, 69) | ||
| Mean glucose | 0.23 | 0.83 | ||||||
| Mean ± SD | 165 ± 27 | 163 ± 21 | 163 ± 23 | 170 ± 30 | 167 ± 29 | 169 ± 25 | ||
| Median (Q1, Q3) | 163 (147, 185) | 163 (148, 173) | 163 (147, 175) | 169 (145, 190) | 165 (144, 181) | 169 (152, 185) | ||
| Coefficient of variation | <0.001 | <0.001 | ||||||
| Mean ± SD | 40 ± 5 | 35 ± 4 | 35 ± 5 | 43 ± 6 | 42 ± 9 | 36 ± 6 | ||
| Median (Q1, Q3) | 39 (37, 44) | 36 (33, 39) | 35 (32, 38) | 42 (39, 46) | 41 (35, 47) | 37 (32, 41) | ||
| Hyperglycemia | ||||||||
| % Time >180 mg/dL | 0.016 | 0.78 | ||||||
| Mean ± SD | 36 ± 15 | 33 ± 13 | 33 ± 14 | 39 ± 16 | 38 ± 16 | 39 ± 16 | ||
| Median (Q1, Q3) | 35 (25, 50) | 34 (24, 42) | 33 (23, 42) | 37 (24, 48) | 38 (25, 47) | 36 (27, 48) | ||
| % Time >250 mg/dL | <0.001 | 0.020 | ||||||
| Mean ± SD | 13 ± 9 | 9 ± 8 | 9 ± 9 | 16 ± 11 | 15 ± 10 | 12 ± 9 | ||
| Median (Q1, Q3) | 10 (5, 19) | 8 (3, 13) | 7 (4, 12) | 14 (7, 22) | 13 (8, 20) | 9 (6, 16) | ||
| Multiple daily injections | N = 42 | N = 41 | N = 42 | N = 44 | N = 42 | N = 42 | ||
| Hours of data | ||||||||
| Mean ± SD | 342 ± 78 | 584 ± 122 | 593 ± 89 | 350 ± 73 | 145 ± 43 | 600 ± 100 | ||
| Median (Q1, Q3) | 324 (302, 370) | 640 (582, 651) | 629 (594, 647) | 329 (310, 386) | 146 (125, 155) | 631 (600, 653) | ||
| Hypoglycemia | ||||||||
| % Time <70 mg/dL | <0.001 | <0.001 | ||||||
| Mean ± SD | 7.7 ± 5.5 | 3.2 ± 1.9 | 4.1 ± 2.9 | 6.7 ± 5.4 | 5.0 ± 4.6 | 3.0 ± 3.4 | ||
| Median (Q1, Q3) | 6.4 (3.6, 11.3) | 3.3 (1.9, 4.4) | 3.6 (1.9, 5.9) | 4.9 (2.5, 10.2) | 3.6 (1.1, 7.7) | 1.7 (0.4, 3.4) | ||
| % Time <54 mg/dL | <0.001 | <0.001 | ||||||
| Mean ± SD | 3.2 ± 2.8 | 0.8 ± 0.8 | 1.1 ± 1.5 | 2.5 ± 2.6 | 1.9 ± 2.1 | 0.6 ± 1.1 | ||
| Median (Q1, Q3) | 2.3 (0.9, 5.0) | 0.6 (0.3, 1.0) | 0.6 (0.3, 1.4) | 1.2 (0.4, 4.5) | 1.0 (0.1, 3.3) | 0.1 (0.0, 0.7) | ||
| Glucose control | ||||||||
| % Time in range 70–180 mg/dL | <0.001 | 0.06 | ||||||
| Mean ± SD | 54 ± 14 | 63 ± 15 | 64 ± 17 | 59 ± 15 | 58 ± 16 | 63 ± 16 | ||
| Median (Q1, Q3) | 58 (46, 62) | 62 (52, 74) | 65 (56, 78) | 60 (49, 69) | 57 (51, 70) | 65 (56, 75) | ||
| Mean glucose | 0.14 | 0.67 | ||||||
| Mean ± SD | 167 ± 32 | 162 ± 24 | 159 ± 29 | 163 ± 31 | 167 ± 28 | 165 ± 32 | ||
| Median (Q1, Q3) | 160 (150, 178) | 162 (144, 180) | 158 (139, 174) | 156 (140, 180) | 162 (146, 183) | 158 (145, 176) | ||
| Coefficient of variation | <0.001 | 0.005 | ||||||
| Mean ± SD | 42 ± 7 | 36 ± 5 | 37 ± 5 | 41 ± 7 | 38 ± 8 | 35 ± 5 | ||
| Median (Q1, Q3) | 43 (38, 46) | 36 (34, 40) | 37 (34, 40) | 41 (35, 46) | 39 (32, 43) | 35 (31, 38) | ||
| Hyperglycemia | ||||||||
| % Time >180 mg/dL | 0.019 | 0.59 | ||||||
| Mean ± SD | 38 ± 17 | 33 ± 16 | 32 ± 17 | 35 ± 17 | 37 ± 16 | 34 ± 17 | ||
| Median (Q1, Q3) | 34 (28, 46) | 35 (22, 46) | 30 (18, 41) | 31 (23, 47) | 35 (26, 45) | 32 (21, 43) | ||
| % Time >250 mg/dL | <0.001 | 0.45 | ||||||
| Mean ± SD | 15 ± 13 | 10 ± 9 | 10 ± 11 | 13 ± 12 | 12 ± 11 | 11 ± 13 | ||
| Median (Q1, Q3) | 11 (6, 21) | 8 (3, 15) | 7 (2, 12) | 8 (4, 17) | 9 (4, 19) | 7 (3, 12) | ||
P-value from a paired t-test or signed rank test, as appropriate. Only includes participants who had values at both time points. P-values are adjusted for multiple comparisons to control the false discovery rate.
BGM, blood glucose monitoring; BGM-CGM, BGM group participants initiated CGM; CGM-CGM, CGM group participants continued CGM.
Daytime (6 am to 12 midnight) median time <70 mg/dL was 4.9% (53 min/day) at baseline (prerandomization) versus 2.8% (31 min/day) at 52 weeks, and nighttime (12 midnight to 6 am) was 5.7% (20 min/night) versus 2.0% (7 min/night), respectively (Supplementary Table S2). A significant reduction in time <54 mg/dL and a significant improvement in TIR also were observed for both daytime and nighttime hours (Supplementary Table S2).
Mean HbA1c was 7.6% ± 0.9% (59 ± 10 mmol/mol) at baseline, 7.2% ± 0.9% (56 ± 10 mmol/mol) at 26 weeks, and 7.4% ± 1.0% (57 ± 10 mmol/mol) at 52 weeks in the CGM-CGM cohort (P = 0.01 for baseline vs. 52 weeks) (Table 1).
Glycemic outcomes in BGM-CGM cohort
Among the 94 participants in the BGM-CGM cohort, median time <70 mg/dL decreased from 3.9% (56 min/day) at 26 weeks (before real-time CGM initiation) to 1.9% (27 min/day) at 52 weeks (P < 0.001; 26–52 weeks) (Table 2). The proportion of participants with percent time <70 mg/dL <4% and <1% was 46 (51%) and 17 (19%) at 26 weeks, respectively, and 70 (80%) and 29 (33%) at 52 weeks, respectively. A reduction in time <70 mg/dL following initiation of CGM was observed at 39 weeks and sustained through 52 weeks (Fig. 1A). An improvement in the mean TIR also was observed in the BGM-CGM cohort from 56% (13 h/day) at 26 weeks to 60% (14 h/day) at 52 weeks (P = 0.006) (Table 2 and Fig. 1B). Significant reductions from 26 weeks were observed in all CGM hypoglycemia metrics, glycemic variability, and two of the additional hyperglycemia metrics (Table 2).
Table 2.
Glycemic Outcomes in BGM-CGM Cohort
| Baseline | 26 Weeks | 52 Weeks | P-value 26 weeks versus 52 weeksa | |
|---|---|---|---|---|
| CGM-measured outcomes | ||||
| N | 94 | 90 | 88 | |
| Hours of data | ||||
| Mean ± SD | 351 ± 69 | 150 ± 38 | 592 ± 109 | |
| Median (IQR) | 327 (310, 400) | 152 (138, 156) | 633 (593, 652) | |
| Hypoglycemia | ||||
| % Time <70 mg/dL | <0.001 | |||
| Mean ± SD | 6.6 ± 5.2 | 6.0 ± 5.8 | 2.9 ± 3.2 | |
| Median (IQR) | 4.7 (2.4, 9.7) | 3.9 (1.3, 9.5) | 1.9 (0.6, 3.5) | |
| % Time <54 mg/dL | <0.001 | |||
| Mean ± SD | 2.5 ± 2.5 | 2.3 ± 2.9 | 0.7 ± 1.2 | |
| Median (IQR) | 1.5 (0.5, 4.1) | 1.1 (0.2, 3.4) | 0.2 (0.0, 0.8) | |
| Rate of hypoglycemic episodes per weekb | <0.001 | |||
| Mean ± SD | 3.0 ± 2.7 | 2.7 ± 2.8 | 1.2 ± 1.7 | |
| Median (IQR) | 2.1 (0.9, 4.1) | 1.8 (0.0, 4.3) | 0.5 (0.0, 1.7) | |
| Glucose control | ||||
| % Time in range 70–180 mg/dL | 0.006 | |||
| Mean ± SD | 56 ± 15 | 56 ± 15 | 60 ± 16 | |
| Median (IQR) | 57 (48, 67) | 55 (45, 67) | 62 (51, 72) | |
| Mean glucose (mg/dL) | 0.69 | |||
| Mean ± SD | 168 ± 31 | 169 ± 30 | 168 ± 29 | |
| Median (IQR) | 164 (143, 183) | 164 (146, 185) | 165 (149, 182) | |
| Coefficient of variation (%) | <0.001 | |||
| Mean ± SD | 42 ± 7 | 40 ± 8 | 36 ± 5 | |
| Median (IQR) | 41 (38, 46) | 40 (34, 45) | 36 (32, 39) | |
| Hyperglycemia | ||||
| % Time >180 mg/dL | 0.32 | |||
| Mean ± SD | 37 ± 17 | 38 ± 17 | 37 ± 17 | |
| Median (IQR) | 35 (23, 49) | 37 (26, 47) | 35 (25, 48) | |
| % Time >250 mg/dL | 0.022 | |||
| Mean ± SD | 15 ± 12 | 14 ± 12 | 12 ± 11 | |
| Median (IQR) | 13 (6, 21) | 12 (5, 21) | 8 (4, 15) | |
| % Time >300 mg/dL | 0.016 | |||
| Mean ± SD | 6.7 ± 7.0 | 6.0 ± 7.1 | 4.3 ± 6.5 | |
| Median (IQR) | 4.2 (1.3, 9.7) | 3.8 (1.1, 8.0) | 2.1 (0.9, 5.2) | |
| Meeting targets, n (%) | ||||
| % Time <70 mg/dL <4% | 39 (41) | 46 (51) | 70 (80) | <0.001 |
| % Time <70 mg/dL <1% | 8 (9) | 17 (19) | 29 (33) | <0.001 |
| % Time <54 mg/dL <1% | 39 (41) | 42 (47) | 72 (82) | <0.001 |
| % Time in range 70–180 mg/dL >70% | 15 (16) | 16 (18) | 25 (28) | 0.004 |
| % Time in range 70–180 mg/dL >50% | 63 (67) | 62 (69) | 68 (77) | 0.007 |
| HbA1c | ||||
| N | 94 | 94 | 93 | |
| HbA1c % [mmol/mol] | 0.025 | |||
| Mean ± SD | 7.5 ± 0.8 [58 ± 9] | 7.5 ± 0.8 [58 ± 9] | 7.3 ± 0.8 [57 ± 9] | |
| Median (IQR) | 7.4 (6.8, 8.0) [57 (51, 64)] | 7.3 (6.8, 8.1) [56 (51, 65)] | 7.3 (6.8, 7.8) [56 (51, 62)] | |
| HbA1c <7.0%, n (%) | 30 (32) | 29 (31) | 29 (31) | 0.81 |
| HbA1c <7.5%, n (%) | 48 (51) | 53 (56) | 55 (59) | 0.51 |
P-value from a paired t-test, signed rank test, or McNemar's test, as appropriate. Only includes participants who had values at both time points. P-values are adjusted for multiple comparisons to control the false discovery rate.
A CGM-measured hypoglycemic event was defined as 15 consecutive minutes with a sensor glucose value <54 mg/dL. The end of the hypoglycemic event was defined as a minimum of 15 consecutive minutes with a sensor glucose concentration >70 mg/dL.6
When stratified by insulin delivery method, significant reductions in time <70 mg/dL and time <54 mg/dL were observed for both insulin pump and multiple daily injection users; improvement in TIR and hyperglycemic measures were not observed within insulin delivery method strata with the exception of time >250 mg/dL among pump users (Table 3).
Daytime (6 am to 12 midnight) median time <70 mg/dL was 3.5% (38 min/day) at 26 weeks versus 1.9% (20 min/day) at 52 weeks, and nighttime (12 midnight to 6 am) was 3.5% (13 min/night) versus 1.2% (4 min/night), respectively (Supplementary Table S2). A significant reduction in time <54 mg/dL also was observed for both daytime and nighttime hours; improvement in TIR was observed during daytime but not nighttime (Supplementary Table S2).
Mean HbA1c was reduced from 7.5% (58 mmol/mol) at 26 weeks to 7.3% (57 mmol/mol) at 52 weeks in the BGM-CGM cohort (P = 0.03) (Table 2).
Patient-reported outcomes/questionnaires
For the BGM-CGM cohort, no significant differences were observed between 26 weeks (before CGM initiation) and 52 weeks for any of the participant-reported questionnaires, including measures of hypoglycemia awareness, diabetes specific quality of life (hypoglycemia fear, diabetes distress, glucose monitoring satisfaction) and general quality of life (Supplementary Table S3b). A small improvement in hypoglycemia fear and glucose monitoring satisfaction was observed in the CGM-CGM cohort from baseline to 52 weeks.
Sensor use and insulin dosing
The median percentage of time CGM was being used in the CGM-CGM cohort was consistent over the 52-week period with a median use of 96% of time in the 4 weeks before 8-week visit and 95% of time in the 4 weeks before the 52-week visit. Similar CGM use was observed during the extension phase for the BGM-CGM cohort (94% in the 4 weeks before 52-week visit). CGM use is reported in Supplementary Table S4.
At 52 weeks 37% of CGM-CGM cohort and 34% of the BGM-CGM cohort were using the Dexcom Mobile app to view their glucose values in addition to or instead of the CGM receiver. Of note 26% and 31%, respectively, reported not using the mobile app because they did not have a compatible smartphone. Only 11 (12%) and 9 (10%) in the CGM-CGM and BGM-CGM cohorts, respectively, were sharing their glucose values with another person using the Dexcom Share and Follow apps. By the end of the study, most participants were using CGM to dose insulin without BGM fingerstick confirmation (95% in the CGM-CGM cohort and 89% in the BGM-CGM cohort) (Supplementary Table S4).
Insulin dosing remained relatively stable during the study. Total daily insulin per kilogram of body weight and number of bolus injections at baseline, 26 and 52 weeks are shown in Supplementary Table S5.
Safety outcomes
In the BGM-CGM cohort, a severe hypoglycemic event involving impaired cognition requiring the assistance of another person was reported for nine participants during the RCT phase where BGM was being used and for two participants during the extension phase following initiation of CGM (one of the two participants also had an event during the RCT phase) (P = 0.02). In the CGM-CGM cohort, a severe hypoglycemic event was reported for one participant during the RCT phase and for four additional participants during the extension phase. One participant in the CGM-CGM cohort experienced DKA during the RCT phase; no other DKA events were reported. Additional adverse events are reported in Supplementary Table S6.
Discussion
The results of this observational extension of the WISDM trial, which further evaluated CGM use among older adults with type 1 diabetes, support the findings of the preceding RCT that CGM is effective in reducing hypoglycemia in this population. The extension phase included two cohorts. In the cohort that used CGM during both the RCT and extension phase, improvements in hypoglycemia and TIR were maintained during the 52 weeks of follow-up. It is noteworthy that a significant reduction in time <70 mg/dL was reached during the first 4 weeks and was sustained through 52 weeks, suggesting that CGM can provide immediate and sustained benefit.
The cohort using BGM during the 26-week RCT phase experienced a similar benefit with delayed initiation of CGM during the 26-week extension phase, including reductions in CGM measured hypoglycemia, improvement in TIR, and a significant reduction in severe hypoglycemic events from nine participants with an event during the 26-week period when BGM was used to two participants with an event during the 26-week period when CGM was used. These improvements occurred despite this cohort having fewer study contacts after CGM initiation, and further validate the clinical significance of the reduction in severe hypoglycemic events observed between the CGM and BGM group in the RCT.
For both cohorts, the benefit of CGM on hypoglycemia reduction was observed among participants using insulin pumps as well as participants using multiple daily injections of insulin. This finding is of interest since CGM will remain an important standalone device for injection users unable or unwilling to switch to an insulin pump, whereas insulin pump users may be much more likely to switch to using more advanced technology involving automation of insulin delivery.
Most participants, 71% and 80% in the CGM-CGM and BGM-CGM cohorts, respectively, achieved the recommended target for the general population of less than 4% of time spent with glucose levels below 70 mg/dL at 52 weeks. However, only 17% and 33% achieved the stricter target of <1% of time spent with glucose levels <70 mg/dL recommended for older adults due to the increased risk of hypoglycemia unawareness in the older adult population.5 In this study population, severe hypoglycemic events occurred across the spectrum of hypoglycemia awareness, with about 60% of events occurring among participants who were classified as unaware at baseline.
Use of CGM was consistently high during the follow-up with no reduction in CGM use over the 52-week period in the CGM-CGM cohort. Similarly, high use was observed for the BGM-CGM cohort with delayed initiation of CGM. Surprisingly only around 10% of participants shared their glucose data with a significant other, a feature that might have particular benefit for this vulnerable age group.
This study did not enroll participants with significant cognitive impairment who may benefit most from sharing glucose values. It is possible that functionally independent community-dwelling older adults with long-standing type 1 diabetes are reluctant to involve a significant other in the management of their diabetes. Of note, in the CGM-CGM cohort, the willingness to use CGM to inform insulin dosing decisions without confirmatory blood glucose values increased over time, with 84% of participants commonly relying on CGM at 8 weeks to 95% at 52 weeks.
Despite improvements in various measures of hypoglycemia and glycemic control and the high degree of CGM use, there were minimal within group differences in patient-reported outcomes apart from a small improvement in hypoglycemia fear and glucose monitoring satisfaction in the CGM-CGM cohort after 52 weeks of CGM use. One possible explanation is that the baseline scores on these measures left little room for change, indicating already good adjustment to diabetes. Alternatively, improvement in diabetes-related patient-reported outcomes secondary to CGM use may occur over a longer time period than glycemic improvement.
Insulin requirement also remained relatively consistent across the 52-week study, suggesting the timing of insulin delivery rather than the total daily dose contributes toward the improvement in glycemic outcomes with CGM use.
There are no other published studies of this size and length evaluating CGM in an older adult population. The 12-month findings were consistent with results from a 24-month cohort study of 441 adults with type 1 diabetes, which saw significant improvements in glucose control and reductions in hypoglycemia in those using CGM and insulin pump therapy.11
The study intervention did not involve more advanced technology such as automated insulin delivery systems, which are now commercially available, nor were CGM alarm settings strictly applied beyond general education. Two recent small studies12,13 assessing hybrid closed loop in older adults found significant improvement in the hybrid closed-loop group compared with sensor-augmented pump therapy, suggesting further benefit beyond what was observed with CGM in the WISDM study may be observed among pump users with the addition of automated insulin delivery. A larger trial assessing the effect of automated insulin delivery on glycemic outcomes and patient reported outcomes in the older adult population is needed.
The present study demonstrated that CGM can be used successfully by older adults with type 1 diabetes. However, there are many barriers to CGM access not addressed by this study. Medicare historically now covers nonadjunctive CGM for therapeutic use, but there have been several restrictions,14 including requirement of BGM testing at least four times per day before coverage approval and requirement of office visit every 6 months; of note, these restrictions were revised effectively on July 18, 2021 to remove the 4 BGM per day testing minimum.15
The strengths of this study include its multicenter design (22 sites), high rate of retention and adherence with 96% of randomized participants completing the 52 weeks of follow-up and >90% adhering to CGM throughout, and inclusion of enough participants using insulin pump and multiple daily injections to allow for subgroup analysis. However, there are some limitations. Participants came from a more advantaged socioeconomic background and had higher functional status than what may be observed in the general population of older adults with type 1 diabetes. The utility of CGM in older adults with lower functional status and/or cognitive impairment is currently unknown and needs further study.
This study used the Dexcom G5 sensor, which has since been replaced with a newer generation G6 sensor that includes an automatic insertion feature. The G6 automatic inserter may allow for easier use by patients who have problems with dexterity. Also, the G5 sensor required twice daily calibrations with blood glucose measurements, whereas this is no longer required with the G6 factory calibrated sensor. CGM usage might be expected to be even greater with the G6 sensor than what was found using the G5 sensor due to its ease of insertion and no required calibrations. Compared to G5 use, G6 use has been associated with favorable reductions in hypoglycemia, possible due to the added urgent low soon alert, and higher utilization rates.16
This extension study has further demonstrated that CGM can be used to effectively reduce hypoglycemia without increasing hyperglycemia among older adults with type 1 diabetes over a prolonged period of time. The benefits of CGM observed in this study combined with recently improved Medicare coverage for therapeutic CGM should serve to increase adoption of CGM as standard of care in all adult populations, particularly the vulnerable population of older adults with type 1 diabetes at an increased risk of hypoglycemia.
Supplementary Material
Contributor Information
Collaborators: for the WISDM Study Group
Authors' Contributions
K.M.M. researched data, wrote/edited the article. L.G.K. wrote/edited the article and performed statistical analyses. M.R.R., A.J.A., G.A., L.A., A.B., B.W.B., A.C., N.S.C., G.G., R.G., I.B.H., L.K., D.K., Y.C.K., C.J.L., J.B.M., G.O.M., A.L.P., L.H.P., A.P.-T., R.P.-B., M.S., V.N.S., M.J.T., F.V., A.V., R.S.W., L.Y., and R.P. researched data, contributed to discussion, and reviewed/edited the article.
Author Disclosure Statement
K.M.M. reports nonfinancial support of supplies provided from Dexcom. L.G.K. has nothing to disclose. M.R.R. reports grants from Xeris Pharmaceuticals, personal fees from Semma Therapeutics, and personal fees from Sernova Corporation. A.J.A. reports grants from Dexcom and personal fees from Medtronic. G.A. reports grants from Novo Nordisk, grants from Dexcom, grants from Astra Zeneca, grants from Eli Lilly, grants from Insulet, personal fees from Dexcom, and personal fees from Insulet. LA has nothing to disclose.
A.B. reports grants and other from Sanofi, grants and other from Astra Zeneca, grants and other from Eli Lilly, grants from United BioSource Corporation, grants from Abbott Diabetes Care, grants from Abbvie, grants from Insulet Corporation, grants from Dexcom, grants from Teijin America, Inc., grants from Bayer Healthcare Pharmaceuticals, Inc., grants from Boehringer Ingelheim, grants from Bristol-Meyers Squibb Research and Development, grants from Gan & Lee Pharmaceuticals, grants from JAEB Center for Health Research, grants from KOWA Research Institute, Inc., grants from Medtronic MiniMed, grants from Mylan GmbH, grants from Novo Nordisk, and grants from Theracos Sub LLC outside the submitted work.
B.W.B. reports grants from the employer from Abbott Diabetes Care, Bio-Provention, DexCom, Diasome, Insulet, Janssen, JAEB Center for Health Research, Eli Lilly, Medtronic, Novo Nordisk, Sanvita, Senseonics, Sanofi, Viacyte, and Xeris. B.W.B. has received personal fees for consulting and speaking from Astra Zeneca, Big Foot, Companion Medical, Eli Lilly, MannKind, Medronic, Novo Nordisk, Sanofi, Senseonics, Xeris and Zealand.
A.C. reports grants and other from Novo Nordisk, grants and other from Medtronic, grants and other from Insulet, grants and other from Sanofi, grants from Dexcom, grants and other from Abbott, other from Senseonics, grants and other from Eli Lilly, and grants from UnitedHealth outside the submitted work; in addition, A.C. has a patent to U.S. Provisional Patent Application Serial No. 62/443,004 pending. N.S.C. reports personal fees from Eli Lilly, Inc. G.G. has nothing to disclose. R.G. has nothing to disclose. I.B.H. receives research funding from Medtronic Diabetes, Insulet, and Beta Bionics, and reports consulting from Abbott Diabetes Care and Bigfoot. L.K. reports contract with Lifescan, affiliation with Voluntis. D.K. reports grants and personal fees from Dexcom outside the submitted work. Y.C.K. reports product support from Dexcom and Roche Diabetes and consulting with Novo.
C.J.L. reports grant support from Abbott Diabetes, and grant support and nonfinancial support from Dexcom outside the submitted work, grant support from Insulet, and personal fess from Eli Lilly, Inc. J.B.M. reports personal fees and nonfinancial support from Bayer, personal fees and nonfinancial support from Boehringer Ingelheim, personal fees from Lilly, personal fees from Mannkind, personal fees from Novo Nordisk, personal fees from Salix, and grants and personal fees from Dexcom, grants from Medtronic, grants from NIH, and grants from Leona Helmsley Trust. G.O.M. reports grant support from Abbott Diabetes, and grant support and nonfinancial support from Dexcom outside the submitted work, and grant support from Insulet.
A.P. reports personal fees from Medscape, grants from Dexcom and devices for research received from Abbott Diabetes Care, personal fees from Abbot Diabetes Care, personal fees from MannKind, personal fees from NovoNordisk, personal fees from Eli Lilly, personal fees from Merck, personal fees from Zealand; and Stock options: Omada Health and Teladoc. L.H.P. has nothing to disclose. A.P.T. reports nonfinancial support from Dexcom. R.P.B. reports grants from Dexcom outside the submitted work. M.S. reports personal fees from Lilly. V.N.S. reports receiving research support through University of Colorado from Eli-Lilly, NovoNordisk, Sanofi, Dexcom, vTv therapeutics, Mylan GmbH, and Insulet. V.N.S. also served on advisory board of Sanofi and Medscape LLC. M.J.T. has nothing to disclose. F.V. reports a grant from Tolerion, Inc.
A.V. has nothing to disclose. R.S.W. reports grants from Insulet Corporation, grants from Tolerion, Inc., grants from Eli Lilly and Co, grants from Medtronic, grants from Diasome Pharmaceuticals, and grants from Boehringer Ingelheim. L.A.Y. reports grants from Dexcom, vTv therapeutics, NovoNordisk, Boehringer Ingelheim, Bayer, Sanofi US, Johnson and Johnson, Eli Lilly and Lexicon. R.P. reports grants from Hanmi Pharmaceutical Co.; grants from Janssen; consulting fees from Merck; grants, speaker fees and consulting fees from Novo Nordisk; consulting fees from Pfizer; grants from Poxel SA; grants and consulting fees from Sanofi; consulting fees from Scohia Pharma, Inc.; consulting fees from Sun Pharmaceutical Industries. Fees and honoraria for Dr. Richard Pratley's services were paid for directly to AdventHealth, a nonprofit organization.
Funding Information
Study funded by JDRF and the Leona M. and Harry B. Helmsley Charitable Trust; grant provided to Jaeb Center for Health Research and paid to each of the investigator's institutions. Public Health Service Research Grant UL1TR001878 supports the Center for Human Phenomic Science at the University of Pennsylvania. Nonfinancial support: Dexcom, Inc., provided study CGM devices and sensors.
Supplementary Material
References
- 1. Chow L, Seaquist ER: How significant is severe hypoglycemia in older adults with diabetes? Diabetes Care 2020;43:512–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lipska KJ, Ross JS, Wang Y, et al. : National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med 2014;174:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pratley RE, Kanapka LG, Rickels MR, et al. ; Wireless innovation for seniors with Diabetes Mellitus Study Group: Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. The Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 5. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danne T, Nimri R, Battelino T, et al. : International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clarke WL, Cox DJ, Gonder-Frederick LA, et al. : Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522. [DOI] [PubMed] [Google Scholar]
- 8. Cox DJ, Irvine A, Gonder-Frederick L, et al. : Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621. [DOI] [PubMed] [Google Scholar]
- 9. Polonsky WH, Fisher L, Hessler D, et al. : Development of a new measure for assessing insulin delivery device satisfaction in patients with type 1 and type 2 diabetes. Diabetes Technol Ther 2015;17:773–779. [DOI] [PubMed] [Google Scholar]
- 10. Benjamini Y, Hochberg Y: On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;25:60–83. [Google Scholar]
- 11. Charleer S, De Block C, Nobels F, et al. : Sustained impact of real-time continuous glucose monitoring in adults with type 1 diabetes on insulin pump therapy: results after the 24-Month RESCUE Study. Diabetes Care 2020;43:3016–3023. [DOI] [PubMed] [Google Scholar]
- 12. McAuley SA, Trawley S, Vogrin S, et al. : Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care 2022;45:381–390. [DOI] [PubMed] [Google Scholar]
- 13. Toschi E, Atakov-Castillo A, Slyne C, et al. : Closed-loop insulin therapy in older adults with type 1 diabetes: real-world data. Diabetes Technol Ther 2022;24:140–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Centers for Medicare & Medicaid Services: Medicare National Coverage Determinations Manual. Baltimore, MD: U.S. Centers for Medicare and Medicaid, 2020. [Google Scholar]
- 15. Local Coverage Determination: Glucose Monitors [online], 2021. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=33822&ver=31 (accessed November 4, 2021).
- 16. van der Linden J, Welsh JB, Walker TC: Sustainable use of a real-time continuous glucose monitoring system from 2018 to 2020. Diabetes Technol Ther 2021;323:508–511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.