Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) and carries a great deal of diversity. Although nearly 60% of patients can be cured with standard rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, 35% relapse (80% relapse within the 1st 18 mo), and 10%–15% can reveal primary refractory DLBCL. While the typical salvage is high-dose therapy followed by autologous stem cell transplantation (HDT-ASCT), patients with early failure of chemoimmunotherapy do very poorly as shown in the SCHOLAR-1 trial with an overall response rate (ORR) of 26%, complete response (CR) of 7%, and median overall survival (OS) of less than 6 months. In addition, a significant proportion of patients are ineligible for HDT-ASCT, illustrating the need for alternative therapies.1
Utilizing small molecule drugs to inhibit multiple pathways has become of interest in patients with lymphoma. B-cell lymphoma 2 (BCL-2) is an anti-apoptotic protein that, when overexpressed, leads to dysregulated cancer cell proliferation and survival. BCL-2 is the most common aberrant gene in germinal center B-cell (GCB) DLBCL.2 Venetoclax, a second generation BCL-2 inhibitor, has shown efficacy as monotherapy in patients with relapsed/refractory (R/R) NHL, resulting in a median progression-free survival (PFS) of 5.4 months (95% confidence interval, 3.5-9.4 mo).3 Bruton’s tyrosine kinase (BTK) is an enzyme downstream of the B-cell receptor that is involved in the regulation of B-cell signaling and differentiation of B cells.4 Ibrutinib, a selective small-molecule inhibitor of BTK, has been tested alone and in combination with rituximab showing an ORR of 41.6% and 72% and CRR of 15.2% and 47.5%, respectively, in patients with R/R DLBCL.5 Preclinical studies have suggested synergy when venetoclax and ibrutinib are given concomitantly, which may be the result of ibrutinib-mediated BTK inhibition leading to enhanced mitochondrial BCL-2 dependence and consequently enabling enhanced killing by venetoclax.6
These previous studies provided the rationale for this phase 1b study, where we investigated the safety, tolerability, and potential activity of triplet therapy with venetoclax, ibrutinib, and rituximab in patients with R/R DLBCL.
Patients ≥18 years were included in the study if they had histologically or cytologically confirmed DLBCL, R/R disease after ≥1 prior line of therapy, and Eastern Cooperative Oncology Group ≤2 (see Suppl. Methods for a full list of inclusion and exclusion criteria). The trial was conducted under the International Conference on Harmonization Good Clinical Practice guidelines and according to the Declaration of Helsinki. This study was approved by the Institutional Review Board at John Theurer Cancer Center. The maximum planned sample size was 30 participants; only 9 patients were enrolled. The primary objective was to determine the maximum tolerated dose (MTD) of venetoclax when given in combination with ibrutinib and rituximab. Secondary endpoints included the incidence of adverse effects, ORR, PFS, and OS.
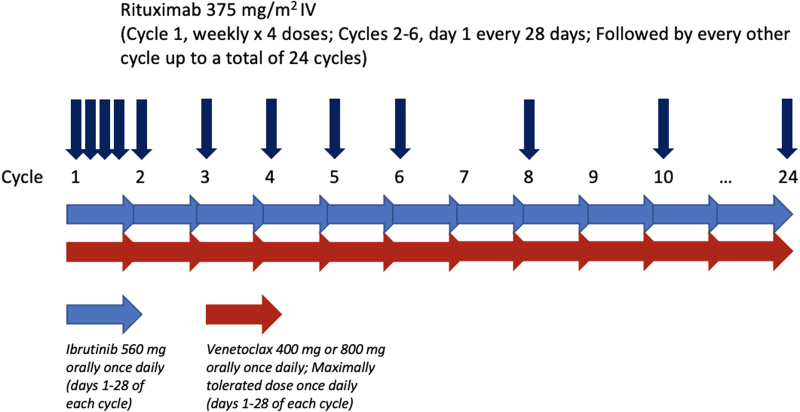
The trial was conducted as a standard 3 + 3 dose-escalation design of venetoclax, with expansion of the study population at the protocol-defined MTD of 800 mg (Suppl. Methods for MTD). Patients were assigned to receive venetoclax at 400 mg or 800 mg orally daily on days 1 to 28 (Suppl. Table S1). Ibrutinib was administered at a dose of 560 mg orally daily on days 1 to 28 of each cycle. Rituximab 375 mg/m2 was administered weekly for 4 doses during cycle 1, followed by monthly on day 1 for cycles 2 through 6, then every other cycle for up to a total of 24 cycles (Figure 1).
Figure 1.
Treatment schema.
Ten patients were screened for this study, and 9 were enrolled between September 2017 and January 2019. One patient was screened but did not meet criteria to receive study treatment. Baseline characteristics of patients are listed in Suppl. Table S2. Of note, 5 patients (56%) had double expressor (DE) phenotype, of which 2 had concurrent double-hit (DH) and DE disease. Seven of 9 patients (78%) had a history of primary refractory disease, defined as persistent or progression of disease within 6 months of initial therapy. All patients (9/9) discontinued therapy because of either disease progression (n = 6, 67%), withdrawal of consent (n = 1, 11%), noncompliance with study (n = 1, 11%), or adverse effects (n = 1, 11%).
Four patients received venetoclax 400 mg in combination with ibrutinib and rituximab. Of these patients, one experienced grade 3 neutropenia persisting for greater than 3 weeks and was de-escalated from venetoclax 400 mg to venetoclax 100 mg after cycle 4 (Suppl. Table S3). Five patients received venetoclax 800 mg in combination with ibrutinib and rituximab; no dose-limiting toxicities (DLTs) occurred in this cohort. Therefore, venetoclax 800 mg daily was established as the MTD. The number of cycles patients received at the respective dose levels are shown in Suppl. Table S4.
All patients (9/9) experienced treatment-related toxicities (Table 1). Two patients experienced grade 3 hematological toxicities: 1 patient developed grade 3 neutropenia that resolved with growth factor support after cycle 1, and 1 patient experienced grade 3 anemia after cycle 3. No adverse events above grade 3 were observed. The most common side effects were hypokalemia (33%), hypomagnesemia (33%), nausea (33%), diarrhea (22%), vomiting (22%), and neutropenia (22%).
Table 1.
Adverse Events and Best Overall Response
| Adverse Events | Grade 1–2 | Grade 3 |
|---|---|---|
| ALP elevation | 1 (11) | 0 (0) |
| Abdominal pain | 0 (0) | 1 (11) |
| Anemia | 1 (11) | 1 (11) |
| AST elevation | 0 (0) | 1 (11) |
| Brain abscess | 0 (0) | 1 (11) |
| Clostridioides difficile | 1 (11) | 0 (0) |
| Dizziness | 1 (11) | 0 (0) |
| Diarrhea | 2 (22) | 0 (0) |
| Fatigue | 1 (11) | 0 (0) |
| GERD | 1 (11) | 0 (0) |
| Hematuria | 1 (11) | 0 (0) |
| Hemorrhoidal bleed | 0 (0) | 1 (11) |
| Headaches | 1 (11) | 0 (0) |
| Hip pain | 0 (0) | 1 (11) |
| Hypercalcemia | 2 (2) | 1 (11) |
| Hypokalemia | 3 (33) | 1 (11) |
| Hypomagnesemia | 3 (33) | 0 (0) |
| Hyponatremia | 2 (22) | 0 (0) |
| Laboratory TLS | 0 (0) | 0 (0) |
| Lower extremity edema | 1 (11) | 0 (0) |
| LDH elevation | 1 (11) | 0 (0) |
| Nausea | 3 (33) | 0 (0) |
| Neutropenia | 2 (22) | 1 (11)a |
| Pleural effusion | 1 (11) | 0 (0) |
| Rhinorrhea | 1 (11) | 0 (0) |
| Sinusitis | 0 (0) | 1 (11) |
| Syncope | 0 (0) | 1 (11) |
| Seasonal allergies | 1 (11) | 0 (0) |
| Urinary incontinence | 1 (11) | 0 (0) |
| Urinary tract infections | 1 (11) | 0 (0) |
| Vaginal bleed | 1 (11) | 0 (0) |
| Vomiting | 2 (22) | 0 (0) |
| Weakness | 0 (0) | 1 (11) |
| Best overall response | Patients, N = 9, n (%) | |
| Progressive disease | 5 (56) | |
| Stable disease | 3 (33) | |
| Partial response | 0 (0) | |
| Complete response | 1 (11) | |
aOne patient experienced a dose-limiting toxicity of grade 3 neutropenia persisting for 3 weeks with venetoclax 400 mg orally daily
ALP = alkaline phosphatase; AST = aspartate aminotransferase; GERD = gastrointestinal reflux disease; LDH = lactate dehydrogenase; TLS = tumor lysis syndrome.
Of the 9 patients who received treatment, the best ORR (CR +partial response [PR]) was 11% and the clinical benefit rate (CR + PR + stable disease [SD] ≥8 wk) was 44% (Table 1). For the patient with a CR, the duration of response on study was 10 months, and then the patient developed a brain abscess that was treated with oxacillin, metronidazole, and voriconazole, lasted for 9 months, and was caused by Staphylococcus lugdunensis. This patient continues to be in remission and is off all treatment regimens. For the overall cohort, the median PFS was 2 months, and the median OS was 8 months.
This is the first phase 1b dose-finding study of venetoclax in combination with ibrutinib and rituximab in patients with R/R DLBCL. No DLTs were observed at the per-protocol MTD of venetoclax 800 mg daily, and toxicity profiling was consistent with what had previously been observed in prior studies.7–10 In our study, there were 2 incidences (22%) of grade 3 hematological toxicities, specifically neutropenia and anemia. In other studies that have combined targeted agents in lymphoma patients, an increased incidence in hematologic adverse events was also observed.9,10
Patient numbers in this study were small due to a rapidly evolving landscape with emerging novel chemoimmunotherapies offered at the time, including chimeric antigen receptor T-cell therapies. Many patients enrolled were primary refractory to dose-intensive chemotherapy, indicating the aggressiveness and chemotherapy-resistant biology of the lymphoma. The best ORR reflected this, with 1 patient (11%) in CR and 3 patients (33%) with SD beyond 8 weeks. Overall, the median PFS for the entire cohort was 2 months and median OS was 8 months.
Of interest, 1 patient achieved a CR and continued to be in CR at 3 years of follow-up (censored). This patient had non-GCB, non-DH, DE (wild-type BCL-2 and C-MYC positive by immunohistochemistry) lymphoma. Prior to entering the study, the patient was treated with rituximab and hyper-cyclophosphamide, vincristine, doxorubicin, dexamethasone and achieved a complete remission with this regimen. The patient had relapsed disease within 1 year, which persisted even with salvage chemotherapy, and was subsequently started on this protocol. In this study, this patient achieved a complete remission after 12 cycles of venetoclax but ultimately discontinued the protocol due to the development of a brain abscess thought to be related to study drug.
There is growing interest in the use of triplet therapy in patients with R/R DLBCL, especially in molecular high-risk subsets of patients. The results of the CAVALLI trial showed that venetoclax combined with chemoimmunotherapy may improve ORR and CRR in patients with aggressive subtypes of DLBCL. Of note, 7 of 8 patients (88%) who were DE of BCL-2 and C-MYC achieved a CR.11 The results of the ALLIANCE trial show venetoclax in combination withrituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin had an ORR of 96.7% in patients with DH or DE lymphomas.10 Combining all 4 agents was the rationale of the venetoclax, ibrutinib, prednisone, obinutuzumab, and lenalidomide clinical trial, and this study has shown early encouraging results including an ORR >70% and a CR rate >50%.12,13
This study has several limitations, as it was a single-center study and included a small patient population. Patients included in this study were heavily pretreated, and many patients had a history of primary refractory disease. At the time of this study, the concept of triplet therapy in R/R DLBCL was rather new, which was in part the cause of a longer process and design to implement this study than usual.
In conclusion, this phase 1b study showed the combination of venetoclax, ibrutinib, and rituximab was feasible and safe, with no new safety signals. Modest activity was seen in this study reflecting a heavily pretreated, primary refractory population. One patient with DE lymphoma (wild-type BCL-2 and C-MYC) achieved a CR at 3 years of follow-up (censored). Further studies should explore this regimen in the setting of R/R DLBCL with molecular high-risk features and in combination with other targeted therapies.
ACKNOWLEDGMENTS
The team thanks all of the patients involved in this study and their families. We thank Janssen Scientific Affairs, LLC for providing the treatment and funding for this study. We acknowledge Dr Adele Lubell for her thorough expertise with medical writing.
AUTHOR CONTRIBUTIONS
AI and AG involved in conception and design. JZ involved in administrative support. AI, TF, LAL, and AG involved in provision of study materials or patients. AP, SG, JZ, and MG involved in collection and assembly of data. AP, AI, ADP, SG, and AG involved in data analysis and interpretation. All authors involved in article writing and final approval of article.
DISCLOSURES
LAL received consultancy, honoraria, and/or speakers bureau from Kite/Gilead Sciences, Seattle Genetics, Celgene, Janssen, Pharmacyclics, Beigene, Abbvie, AstraZeneca, TG Therapeutics, Epizyme, Merck, and Karyopharm. She is with advisory board from Kite/Gilead Sciences, Seattle Genetics, ADV, Janssen, Pharmacyclics, Beigene, Abbvie, AstraZeneca, TG Therapeutics, and Epizyme. AG received consultancy, honoraria, and/or speakers bureau from Pharmacyclics, Janssen, Celgene, Novartis, Hoffman la Roche, Novartis, Celgene, Kite, Pharmacyclics/Abbvie, Elsevier’s Practice Update Oncology, Clinical Advances in Hematology & Oncology, Medscape, Physicians Education Resource, LLC, Rosewell Park, OncLive Peer Exchange, Michael J Hennessey Associates, Inc., BMS, Xcenda, MorphoSys, and AstraZeneca. He received research funding from Janssen, Acerta, Celgene, Constellation, Genentech, Hoffman la Roche, Infinity Pharmaceuticals, MorphoSys, Karyopharm, Kite, Pharmacyclics, AstraZeneca, Verastem, Seattle Genetics, and BMS. He is with advisory board from Janssen, Vincerx pharma, Janssen, Kite Pharma, Elsevier’s Practice Update Oncology, Gilead, Abbvie, Pharmacyclics, BMS, AstraZeneca, and Alloplex. He is with board of directors from COTA, Genomix Testing Cooperative, and Resilience. He received stock from COTA, Genomic Testing Cooperative, Alloplex, and Resilience. TF received consultancy, honoraria, and/or speakers bureau from Abbvie, ADC Therapeutics, BMS, Celgene, Janssen, Pharmacyclics, Seattle Genetics, and Takeda. She is with advisory board from Abbvie, ADC Therapeutics, Daiichi Sankyo, Genmab, Karyopharm, KITE, Kyowa Kirin, and Morphosys. AI received consultancy, honoraria, and/or speaker bureau from TG Therapeutics and AstraZeneca. He is with advisory board from Secura Bio. MG received consultancy, honoraria, and/or speakers bureau from Celularity and Guardant360. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This trial is funded by Janssen Scientific Affairs, LLC who provided free of charge study drug for this study.
Supplementary Material
Footnotes
ClinicalTrials.gov Identifier: NCT03136497.
Supplemental digital content is available for this article.
REFERENCES
- 1.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuetz J, Johnson NA, Morin RD, et al. BCL2 mutations in diffuse large B-cell lymphoma. Leukemia. 2012;26:1383–1390. [DOI] [PubMed] [Google Scholar]
- 3.Davids MS, Roberts AW, Kenkre VP, et al. Long-term follow-up of patients with relapsed or refractory non-Hodgkin lymphoma treated with venetoclax in a phase I, first-in-human study. Clin Cancer Res. 2021;27:4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiestner A. Targeting B-cell receptor signaling for anticancer therapy: the Bruton’s tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31:128–130. [DOI] [PubMed] [Google Scholar]
- 5.Hou K. Efficacy and safety of ibrutinib in diffuse large B-cell lymphoma: a single-arm meta-analysis. Crit Rev Oncol Hematol. 2020;152:103010. [DOI] [PubMed] [Google Scholar]
- 6.Deng J, Isik E, Fernandes SM, et al. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia. 2017;31:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelenetz AD, Salles G, Mason KD, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133:1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–2236. [DOI] [PubMed] [Google Scholar]
- 9.Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15:1019–1026. [DOI] [PubMed] [Google Scholar]
- 10.Rutherford SC, Abramson JS, Bartlett NL, et al. Venetoclax with dose-adjusted EPOCH-R as initial therapy for patients with aggressive B-cell lymphoma: a single-arm, multicentre, phase 1 study. Lancet Haematol. 2021;8:e818–e827. [DOI] [PubMed] [Google Scholar]
- 11.Morschhauser F, Feugier P, Flinn IW, et al. Venetoclax plus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) improves outcomes in BCL2-positive first-line diffuse large B-cell lymphoma (DLBCL): first safety, efficacy and biomarker analyses from the phase II CAVALLI study. Presented at the 2018 ASH Annual Meeting; December 3, 2018; San Diego, CA: Abstract #782. [Google Scholar]
- 12.ClinicalTrials.gov. Venetoclax, Ibrutinib, Prednisone, Obinutuzumab, and Revlimid (ViPOR) in Relapsed/Refractory B-cell Lymphoma. Bethesda, MD: National Library of Medicine (US); 2021. Available at: http://clinicaltrials.gov/ct2/show/NCT03223610. Accessed December 1, 2021. [Google Scholar]
- 13.Melani C, Lakhotia R, Pittaluga S, et al. Phase 1b/2 study of Vipor (Venetoclax, Ibrutinib, Prednisone, Obinutuzumab, and Lenalidomide) in relapsed/refractory B-cell lymphoma: safety, efficacy and molecular analysis. Oral Presentation at the American Society of Hematology Meeting; December 7, 2020; New Orleans, Louisiana. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.