Background:
Botulinum neurotoxin A (BoNT-A) injection is the most widely performed aesthetic procedure and a first-line therapeutic option for various medical conditions. The potential for BoNT-A immunoresistance and secondary nonresponse related to neutralizing antibody (NAb) formation warrants attention as the range of BoNT-A aesthetic applications continues to expand.
Methods:
An international multidisciplinary panel reviewed published evidence on BoNT-A immunoresistance in aesthetic and therapeutic applications and discussed best practices integrating clinical, ethical, and aesthetic considerations. Consensus statements relating to awareness, assessment, and management of the risk of NAb-related secondary nonresponse in aesthetic practice were developed.
Results:
There was a consensus that, as doses used in aesthetic practice become like those in therapeutics, rates of NAb formation may be expected to increase. However, the true extent of NAb formation in aesthetics is likely underestimated due to limitations of published evidence and variability in treatment patterns of aesthetic patients. Since BoNT-A therapy is often lifelong, practitioners need to recognize immunogenicity as a potential complication that might affect future therapeutic use and strive to minimize modifiable risk factors. The selection and use of a BoNT-A product with the least immunogenic potential from the beginning may thus be advantageous, especially when treatment with high doses is planned.
Conclusions:
In view of current trends in BoNT-A aesthetic use, it is essential for practitioners to conduct thorough clinical assessments, inform patients of treatment risks, and develop BoNT-A treatment plans to minimize immunogenicity. This can help preserve the option of continued or future BoNT-A treatment with satisfactory outcomes.
Takeaways
Question: How can aesthetic practitioners minimize risks of NAb formation and SNR with BoNT-A treatment?
Findings: An international multidisciplinary panel reviewed published evidence on BoNT-A immunoresistance and established a consensus on the need for awareness, assessment, and management of NAb-related SNR risks. The panel advocates for practitioners to recognize the potential impact of immunogenicity on future treatment options and strive to minimize modifiable risk factors.
Meaning: Since BoNT-A therapy is often lifelong, it is important for practitioners to minimize the risk of immunogenicity by using a highly purified BoNT-A and injecting the lowest dose required at appropriate intervals.
INTRODUCTION
Injection of botulinum neurotoxin A (BoNT-A) has remained the most frequently performed aesthetic procedure since 1999. It accounted for one-third of 13.3 million minimally invasive aesthetic procedures performed in 2020 in the United States.1,2 Beyond facial rhytid treatment, the range of BoNT-A aesthetic applications has expanded to include cosmetic treatment of masseteric hypertrophy and, more recently, body contouring.3–7 BoNT-A is also considered a first-line treatment for various therapeutic indications.8,9
BoNT-A, a potent neurotoxin produced by Clostridium botulinum, causes muscle paralysis by blocking synaptic neurotransmission.9,10 Its therapeutic and aesthetic use derives from this ability to selectively weaken or paralyze the injected muscle group.8–11 Since the effects of BoNT-A diminish over time, repeated injections are required to maintain the treatment effect. However, repeated injections of BoNT-A may stimulate antibody formation, including neutralizing antibodies (NAbs) that counteract its biological activity.12,13 With NAb formation, the patient may develop partial or complete nonresponse to further BoNT-A treatment. This immunoresistance potentially has direct and long-term implications for future therapeutic options and should be considered in BoNT-A treatment decisions.
Three BoNT-A formulations [onabotulinumtoxinA (ONA), abobotulinumtoxinA (ABO), and incobotulinumtoxinA (INCO)] are currently approved by the US Food and Drug Administration (FDA) for therapeutic and aesthetic use.11,14 Besides ONA, ABO, and INCO, an increasing number of other formulations are commercially available around the world.15,16
To highlight issues surrounding BoNT-A resistance and propose approaches for best practice, a multidisciplinary panel (Aesthetic Council for Ethical use of Neurotoxin Delivery) comprising 14 experts in aesthetic medicine, dermatology, plastic surgery, neurology, immunology, and bioethics was convened. This article reviews emerging concerns related to increasing BoNT-A use and possible immunoresistance due to NAb formation in the current aesthetic treatment landscape and discusses how BoNT-A treatment decision-making can integrate relevant biological, clinical, ethical, and aesthetic considerations.
Emerging Trends in BoNT-A Resistance: An Overview of Recent Literature
Globally, BoNT-A usage has increased due to growing numbers of patients seeking treatment and expanding off-label applications.6 With this growth, concerns have emerged regarding secondary nonresponse (SNR) to BoNT-A aesthetic treatment, initially highlighted in case reports.17,18 SNR refers to the reduction or absence of therapeutic effects (partial or complete SNR) after initial successful treatments.12,19,20 BoNT-A SNR may be related to NAb formation or other factors, including disease progression, inadequate dosage, incorrect muscle target, or improper injection technique.12 Typical signs of SNR include dose or interval creep, wherein higher BoNT-A doses or shorter injection intervals are required to achieve the desired therapeutic effect. However, such signs may be overlooked, resulting in underrecognition of the issue.
For therapeutic BoNT-A use, rates of NAb formation have been estimated using systematic reviews/meta-analyses (SR/MAs) and clinical studies. The reported range is 0.3%–27.6%, highest for therapeutic applications involving high-dose BoNT-A, especially dystonias (1.3%–27.6%) and spasticity (0.3%–13%) (Table 1).21–24 Reported NAb formation frequency was lowest for patients receiving INCO (0%–1.1%), followed by ONA (0.3%–5.6%) and ABO (0%–13.3%) (Table 2).21,23,25–28 These overall estimates warrant careful interpretation because patients could have previous exposure to BoNT-A formulations other than those addressed in these studies. However, a similar trend is apparent when considering patients who exclusively used specific formulations: the percentages of patients with NAbs were 0%, 0.6%, and 5.3% with exclusive use of INCO, ONA, and ABO, respectively (Table 3).22,23,26,28
Table 1.
Reported NAb Formation Frequency by Therapeutic Indication
Table 2.
Reported NAb Formation Frequency in Therapeutic Indications, by Formulation*
| Formulation | NAb Formation Frequency (%) | No. Publications |
|---|---|---|
| ABO | 0–13.3 | 621,23,25–28 |
| ONA† | 0.9–5.6 | 321,26,28 |
| ONA (new) | 0.3–4.0 | 323,25,27 |
| ONA (old) | 7.2 | 127 |
| INCO‡ | 0–1.1 | 621,23,25–28 |
| Not specified | 1.9–2.5 | 221,27 |
*Estimates in these studies may be associated with either overall use or exclusive use of the BoNT-A formulations studied.
†This publication did not distinguish between the old and new formulations of ONA.
‡Most patients had previously received ABO and/or ONA.
Table 3.
Reported NAb Formation Frequency in Therapeutic Indications, by Formulation (Exclusive Use*)
| Formulation | Patients with NAbs or Who Were Considered Nonresponders (n) | Total Number of Patients (N) | Percentage of Patients with NAbs or Nonresponders (n/N, %) | No. Publications |
|---|---|---|---|---|
| ABO | 21 | 399 | 5.3 | 323,26,28 |
| ONA | 19 | 2839 | 0.6 | 422,23,26,28 |
| INCO | 0 | 529 | 0 | 323,26,28 |
*Estimates in these studies were associated with exclusive use of the BoNT-A formulations studied.
Although it is recognized that NAb-related SNR may arise in patients receiving high doses of BoNT-A for chronic medical conditions, the extent and clinical relevance of NAb formation in aesthetic treatment has been questioned, citing reasons such as the lower doses typically used in aesthetics.29 There may also be a perception that the issue is not of substantive clinical concern because published reports of SNR in aesthetic settings have been relatively rare. However, this overlooks the potential cumulative effects of BoNT-A doses received over an individual’s lifetime. Several off-label aesthetic BoNT-A applications involve higher amounts than on-label indications. Examples include masseter reduction (approximately 40–80 units of ONA/INCO per session) or calf contouring treatments where ≥100 units are injected per gastrocnemius.5–7,17 High-dose intradermal BoNT-A injections are also increasingly popular, and these are believed to be more immunogenic than intramuscular injections due to high concentrations of dendritic cells (DCs) in the dermis.30,31 Thus, total doses received for aesthetic procedures could easily reach the range used for therapeutic indications.
The real-world extent and implications of NAb formation in aesthetic practice remain unclear. Therefore, we systematically searched the published literature for information on BoNT-A-related NAb formation and SNR in aesthetic indications to complement what is known for therapeutic indications. (See appendix, Supplemental Digital Content 1, which displays the literature search for NAb formation and SNR in aesthetic indications, http://links.lww.com/PRSGO/C78.) We identified 18 relevant publications with data on NAb-related SNR with aesthetic use (Table 4). SR/MAs reported overall rates of NAb formation with aesthetic BoNT-A use ranging from 0.2% to 0.4%, lower than for therapeutic indications.21,22,25 Except for one SR/MA focusing on ONA, these estimates represent various BoNT-A formulations and aesthetic applications.
Table 4.
Identified Publications with Data on BoNT-A NAb-related SNR in Aesthetic Applications
| Publication | Type | Application(s) |
|---|---|---|
| Borodic32 | Case report | Facial lines |
| Borodic33 | Case report | Facial lines |
| Cohen and Scuderi34 | SR | Glabellar lines; crow’s feet |
| Dressler et al19 | Case series | Facial lines (four cases) |
| Fabbri et al21 | SR/MA | Ax: glabellar lines |
| Tx: dystonia, spasticity, urologic conditions, and hyperhidrosis | ||
| Fischer et al35 | Clinical study (interventional) | Facial lines |
| Helmstaedter36 | Clinical study (chart review) | Facial lines |
| Imhof and Kühne37 | Clinical study (interventional) | Glabellar lines |
| Lacroix-Desmazes et al27 | SR | Ax: glabellar lines |
| Tx: dystonia, blepharospasm, spasticity, and urological indications | ||
| Lawrence and Moy38 | Secondary analysis (safety and efficacy) of clinical trial data | Glabellar lines |
| Lee17 | Case report | Masseteric hypertrophy |
| Naumann et al22 | SR/MA | Ax: glabellar lines |
| Tx: dystonia, urologic conditions, spasticity, and hyperhidrosis | ||
| Rahman et al25 | SR/MA | Ax: glabellar lines and crow’s feet |
| Tx: dystonia, urological indications, spasticity, facial hemispasm, blepharospasm, and hyperhidrosis | ||
| Srinoulprasert et al39 | Clinical study (interventional) | Aesthetic indications (various) |
| Stengel and Bee18 | Case report | Glabellar lines |
| Stephan et al40 | Case report | Facial lines |
| Torres et al41 | Case series | Facial rejuvenation (four cases) |
| Wanitphakdeedecha et al42 | Clinical study (interventional) | Aesthetic indications (various) |
Ax, aesthetic indications; Tx, therapeutic indications.
Thirteen cases of NAb-related SNR emerging during aesthetic BoNT-A treatment were identified in case series or case reports, which would have been excluded from SR/MAs (Table 5).17–19,40–41 Across these cases, we noted a pattern of regular repeated treatments (with the same or different formulations) before detection of SNR, usually with clear signs of dose and/or interval creep. In all cases, patients had initially or exclusively received ABO or ONA; three patients were switched to INCO after partial or complete SNR with previous treatments. Duration of therapy before NAb detection varied considerably (2–72 months). Systematic testing was uncommon, and it was unclear precisely when NAb formation first occurred in most cases. These observations illustrate the difficulty of identifying precisely when and how NAbs/SNR arose. Nevertheless, considering only patients treated exclusively with one formulation, no cases of NAb-related SNR have been reported with exclusive INCO aesthetic use. This is consistent with observations for therapeutic indications, even those requiring high BoNT-A doses.23,26,28,35,37
Table 5.
Summary of BoNT-A NAb Formation and Secondary Nonresponse Reported in Aesthetic Cases
| No. | Publication | Age | Sex | Condition | Treatments | Intervention | Results | Duration of Treatment before NAb Detection |
|---|---|---|---|---|---|---|---|---|
| 1 | Borodic32 | 48 | F | Facial lines | 1–14 | ONA | Cycles 1–14: response lasted for 3–4 mo | Unclear when NAb test was done. Duration (first to last treatment): 72 mo |
| >14 | ONA | |||||||
| Cycle >14: no response | ||||||||
| 2 | Borodic33 | 44 | F | Facial lines | 1–14 | ONA 30–50 U | Cycle 15: no response, no effect on forced frown | Unclear when NAb test was done. Duration (first to last treatment): 60+ mo |
| 15 | ONA 100 U | |||||||
| 3 | Dressler et al19 | 53 | F | Facial lines | 1–10 | ABO 10–180 MU | Cycles 1–5: normal response | NAb detected (7.0 mU/mL) at cycle 10 |
| Cycles 6–9: PSNR | ||||||||
| Cycle 10: CSNR | ||||||||
| 4 | Dressler, 201019 | 46 | F | Facial lines | 1–3 | ONA 80 MU | Cycle 1: normal response | NAb detected (2.7 mU/ml) at cycle 6 |
| 4 | ||||||||
| 5–6 | BoNT–B | Cycle 2: PSNR | ||||||
| 7–9 | ||||||||
| ONA 40–136 MU | Cycle 3: CSNR | |||||||
| Cycles 5–6: CSNR | ||||||||
| BoNT–B | ||||||||
| 5 | Dressler et al19 | 51 | F | Facial lines | 1–9 | ABO 30 MU | Cycles 1–11: normal response | NAb detected (1.0 mU/mL) at cycle 12, and (>10.0 mU/mL) at cycle 13 |
| 10–13 | ONA 30 MU | Cycle 12: PSNR | ||||||
| Cycle 13: CSNR | ||||||||
| 6 | Dressler et al19 | 45 | F | Facial lines | 1–6 | ABO 25–105 MU | Cycle 3: PSNR | NAb detected (>10.0 mU/ml) at cycle 7 |
| 7 | INCO 33 MU | Cycle 5: CSNR | ||||||
| 7 | Lee17 | 20 | F | Masseteric hypertrophy | 1–6 | ONA 180 U | Cycles 1–3: response lasted for 4–5 mo | >18 mo |
| 7 | ABO 180 U | |||||||
| Cycles 4–5: response lasted for 1.5 mo | ||||||||
| Cycles 6–7: no response | ||||||||
| 8 | Stengel and Bee18 | 41 | F | Glabellar lines | 1–5 | ABO | Cycles 1–2: response lasted for 4–8 mo | 72 mo |
| 6–8 | ONA 9–28 U | |||||||
| 9–11 | INCO 20–44 U | Cycles 3–11: response lasted for 3–4 wk | ||||||
| 9 | Stephan et al40 | 51 | F | Facial lines | 1–3 | ONA, ABO | Cycles 1–3: partial response (<2 mo), required high-dose booster injections | NAb testing was not available |
| NR | ONA 75 U | |||||||
| NR | ||||||||
| INCO | ||||||||
| Cycle >3: partial response with even shorter duration of efficacy | ||||||||
| 10 | Torres et al41 | 55 | F | Facial rejuvenation | 1 | ONA 33 U | Cycle 1: no response | 2 mo |
| 2 | ABO 80 SU | Cycle 2: mild response lasting 3 mo | ||||||
| 11 | Torres et al41 | 54 | F | Facial rejuvenation | 1–8 | ABO 25–180 U | Cycles 1–7: normal response | Unclear when NAb test was done. |
| 9 | ||||||||
| 10 | ONA 70 U | |||||||
| Cycle 8: loss of efficacy | ||||||||
| 11 | ABO 120 U | |||||||
| INCO 69 U | Cycle 9: little treatment effect | |||||||
| Cycle 10: no effect after 4 wk | ||||||||
| Cycle 11: no effect after 2 wk | ||||||||
| 12 | Torres et al41 | 43 | F | Facial rejuvenation | 1–6 | ABO 100–260 U | Initial response lasted for 6–8 mo, decreased to 3 mo at later treatments | Unclear when NAb test was done. Duration (first to last treatment): 96 mo |
| 13 | Torres et al41 | 38 | M | Facial rejuvenation | 1–3 | ABO 120–250 U | Cycle 3: CSNR | Unclear when NAb test was done. Duration (first to last treatment): 36 mo |
CSNR, complete secondary nonresponse; F, female; M, male; MU, mouse unit; NR, not reported; PSNR, partial secondary nonresponse; SU, speywood unit; U, unit.
We note certain caveats in interpreting these estimates. First, the summary estimates reported in SR/MAs are based on data aggregated from randomized controlled trials and observational studies, and are limited by the heterogeneity of study designs and measured outcomes. Such estimates are convenient for overall description but may obscure meaningful variation due to differences in the design and intent of studies. For example, one SR/MA noted differences between studies that were or were not primarily designed to detect NAbs.25 Second, all the published aesthetic studies on NAb formation and SNR evaluated only approved indications, such as glabellar lines,21,22,25,43 whereas a large proportion of real-world BoNT-A use includes off-label applications involving higher BoNT-A doses. Third, follow-up periods were relatively short, ranging from 4 to 16 months, whereas it is known that NAbs typically develop over a more extended period of years.24 For a complete view of NAb formation and SNR in clinical practice, one should consider the full range of published literature, including evidence from case reports and case studies. The frequency of BoNT-A NAb formation and SNR in real-world aesthetic practice may be higher than published estimates suggest. Although the reported frequency of NAb-related SNR appears relatively low for aesthetic versus therapeutic use of BoNT-A, the issue warrants further attention because of current aesthetic treatment trends and the potential implications of immunoresistance on access to future therapeutic options.
BoNT-A NAb Formation and Resistance: An Immunological Perspective
Although the true extent of NAb-related SNR in real-world practice remains unclear, the underlying biological process (how the immune system assesses and responds to the presence of BoNT-A or other biologic products) is well understood. This understanding can guide practitioners in evaluating the risk of immunogenicity and taking measures to manage that risk.
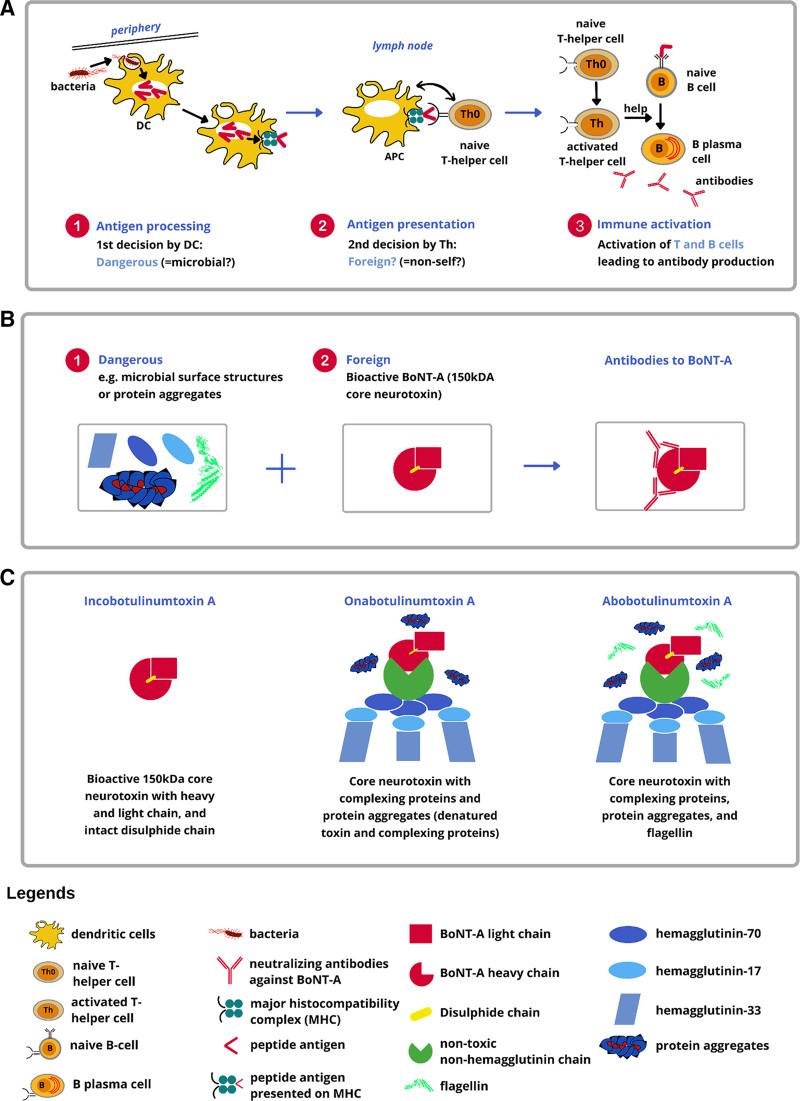
Immune system activation by BoNT-A (or any other antigen) is controlled via two key decision points (Fig. 1A, B). Both are necessary to stimulate classical T-helper cell-dependent antibody production. The first decision is made by DCs, which determine whether an antigen is potentially dangerous. Toll-like receptors (TLRs) on DCs recognize characteristic microbial cell surface features (eg, flagellin) as “danger signals.” This triggers phagocytosis of the microbe or other “dangerous” particles by the DCs, which migrate to lymph nodes to act as antigen-presenting cells (APCs). APCs process the microbe or other “dangerous” particles and present the “dangerous” peptide antigens to naive T-helper cells while providing costimulatory signals to trigger clonal expansion of antigen-specific T-cells. The second decision involves antigen-specific T-cells that recognize presented peptide antigens as “foreign.” Fully activated antigen-specific T-helper cells expand clonally and then support antigen-specific B-cell activation as well as their clonal expansion, finally producing antibodies against the original antigen. These two decisions are strictly hierarchical since naive T-helper cells always require peptide-antigen presentation by a fully activated DC.
Fig. 1.
BoNT-A treatment from the immunological perspective. A, Dangerous + foreign? Two key decisions controlling the immune response to biologics. The first decision involves DCs that determine whether or not a particle (eg, a microbe) is likely to be “dangerous.” DCs can recognize microbial surface molecules (eg, flagellin) as “danger signals.” Upon recognition of microbial danger signals, DCs will be activated and phagocytose the particle bearing the danger signal. Subsequently, these activated DCs migrate to lymph nodes and become professional APCs. The second decision involves naive T-helper cells that determine whether a particle is self or foreign. Upon encountering foreign antigen peptides presented by APCs along with co-stimulatory signals, naive T-helper cells become activated and undergo clonal expansion, leading to activation and clonal expansion of antigen-specific B cells. These mature into plasma cells that produce antibodies specific to the antigen that triggered the immune response. B, Development of BoNT-A neutralizing antibodies. C, Composition of FDA-approved BoNT-A formulations. Figure credit: Michael Martin.
The physiological BoNT-A supramolecular complex produced in nature by C. botulinum comprises the core 150-kDa neurotoxin and various neurotoxin-associated proteins (NAPs), including hemagglutinins (HAs) and non-HAs.44 Of the FDA-approved formulations, ONA and ABO are known to include NAPs and/or other unnecessary bacterial proteins, whereas INCO contains only the core 150-kDa neurotoxin10,45,46 (Fig. 1C). If a highly purified BoNT-A formulation is injected, peptides derived from the BoNT-A core neurotoxin subunits could be identified as “foreign” by naive T-helper cells.47 However, the pure bioactive 150-kDa core neurotoxin lacks the concomitant “danger signals” required to fully activate DCs to become APCs. Without these signals, the first decision-maker (the DCs) would not register BoNT-A core neurotoxin subunits as “dangerous,” escaping the immune cascade.46 In contrast, NAPs, such as HA-33, and other bacterial contaminants, particularly flagellin, inactive/denatured toxin, and clostridial DNA, can trigger an immune response.11,13,46 HA-33 is reported to be an immune response stimulator,48 whereas flagellin and clostridial DNA are adjuvants that bind readily to TLR5 and TLR9 on DCs, respectively, activating the immune cascade.49,50 In the context of BoNT-A treatment, NAPs have no therapeutic role and merely enhance the immunogenicity of the injected product.10,11,45,46,51
It is, thus, clear that antigen-specific immune activation by BoNT-A is not determined by the indication (therapeutic versus aesthetic) for which it is administered. Instead, factors that could influence the risk of NAb formation with a given BoNT-A formulation include its purity, dose administered, and the number and interval between injections. These factors are generally modifiable within a BoNT-A treatment plan. As noted, the purity of each formulation and the presence of potential adjuvants are product-specific features. High doses or repeated injections increase the exposure to potentially “dangerous and foreign” material. Thus, a risk-based approach, such as that outlined by the FDA and European Medicines Agency (EMA),52,53 seems eminently applicable to evaluate and manage the risk of immunogenicity associated with therapeutic protein products when used for aesthetics.
Navigating the Complex Landscape of BoNT-A Treatment Decision-making
Advances in BoNT-A treatment have engendered new aesthetic enhancement possibilities and challenges for everyday practice, particularly in terms of decision-making. For example, practitioners must carefully consider the patient’s treatment history, which could be potentially complex, including extensive prior BoNT-A treatment for multiple indications from different practices, and weigh the implications of specific treatment choices throughout a patient’s medical history. Considering the risk of immunoresistance, we suggest that it is clinically prudent to minimize the risk of NAb formation to facilitate continued clinical response over time.
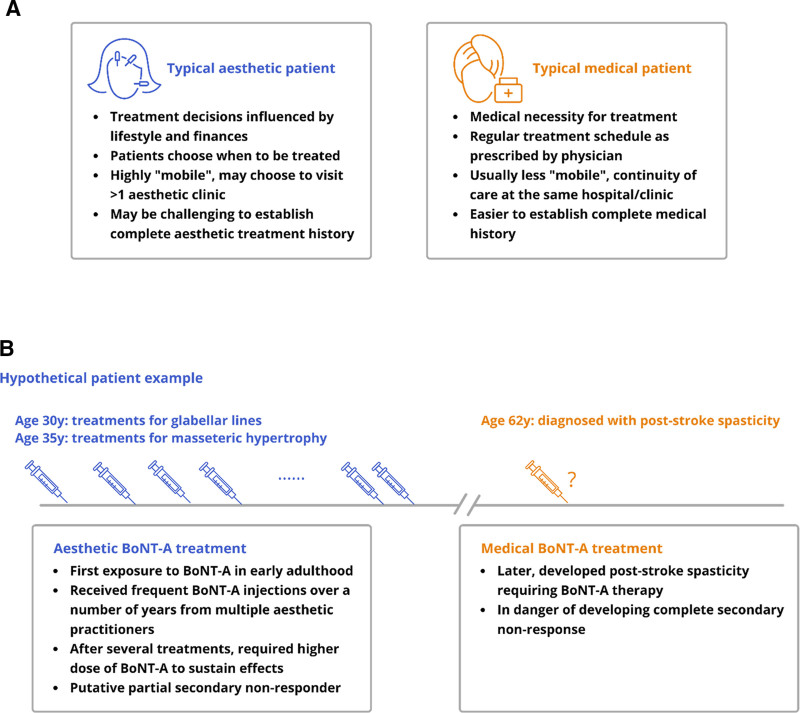
Since aesthetic and medical treatments are associated with distinct contexts and expectations, aesthetic patients’ clinical course and behaviors may be expected to differ from therapeutic patients (Fig. 2A). BoNT-A use in both aesthetic and therapeutic contexts becomes “complicated” in cases where injection patterns in one setting have clinical implications within the other. For example, extensive aesthetic treatment with high BoNT-A doses, along with frequent retreatments, results in greater exposure to potentially immunogenic material, and could, thus, increase the risk of developing NAb-related SNR. As illustrated in the hypothetical example (Fig. 2B), this potentially leads to suboptimal outcomes if this patient later develops a chronic medical condition that requires BoNT-A treatment. Furthermore, the younger a patient is when beginning aesthetic BoNT-A treatment and the more extensive the use of BoNT-A, the greater the possible lifetime exposure and risk of developing NAb-related SNR. Such cases may have medicolegal implications, especially if risks such as NAb-related SNR were not thoroughly discussed with the patient before treatment. A better understanding of patients’ awareness, attitudes, and motivations in relation to their BoNT-A treatment choices is warranted. This could help practitioners to more effectively communicate and work with their patients to manage the risk of BoNT-A resistance.
Fig. 2.
Patient journey. A, Patient archetypes in aesthetic vs medical practice. B, Hypothetical case example illustrating the potential implications of aesthetic BoNT-A treatment patterns for later therapeutic use in a patient.
The ethical principles of medicine underpin therapeutic and aesthetic practice alike. Accordingly, it is often suggested that patient safety and empowerment in decision-making are of prime importance in good aesthetic practice. However, few published guidelines deal with ethics in aesthetic practice. The topic is usually covered only briefly within general guidance for aesthetic practitioners. Nevertheless, the applicability of core medical ethics principles is a recurring theme across the literature,54 including respect for patient autonomy and obtaining informed consent, comprehensive assessment of expectations within and from clinical encounters (eg, aesthetic enhancement and improved quality of life), and empathic and truthful communication of possible risks and outcomes.55
Recognizing the strong influence of patient preference and choice in aesthetic medicine, we suggest that a collaborative patient-centered approach offers a better chance of achieving safe and satisfactory outcomes. In our view, a patient-centered approach in aesthetic practice encompasses not only consideration of patients’ individual preferences and circumstances but also individualized assessment, patient education, and informed decision-making following adequate discussion of possible risks and outcomes.
There are strong clinical and ethical reasons for making thorough pretreatment assessments and informed discussions of risk/benefit integral to the aesthetic consultation process. A comprehensive treatment history (including the BoNT-A products used, number of previous injections, doses, indications, and injection intervals) would help practitioners evaluate and mitigate risks. However, with greater patient choice and “mobility” in aesthetic practice, it may prove challenging to construct a complete history and assess all relevant risk factors (Fig. 2A). Information on concurrent medical conditions or treatment may be highly relevant but may not often be solicited or volunteered. Nevertheless, it is essential to recognize clinical signs of BoNT-A resistance, know the appropriate diagnostic tests to perform, and make informed decisions on options for management.
Consensus on BoNT-A Resistance and Implications for Aesthetic Practice
The panel discussed the above issues surrounding BoNT-A resistance and achieved consensus on a set of recommendations (Table 6). This was achieved through a blinded voting process, in which panel members indicated their position on each statement (agree/disagree). The results were categorized as strong consensus (>95% agreement); consensus (>75%–95% agreement); majority consent (>50%–75% agreement); no majority consent (≤50% agreement).
Table 6.
Consensus Statements
| Statements | % Agreement* | Consensus* |
|---|---|---|
| The true extent of antibody-induced SNR in aesthetic practice is likely to be underestimated/underreported in the medical literature | 100 | Strong consensus |
| Clinicians should refer to published literature beyond SRMAs (including single-arm studies and case reports) for real-world evidence and a more complete picture of NAb formation in clinical practice | 100 | Strong consensus |
| A typical aesthetic patient’s treatment journey, follow-up behavior, and treatment patterns are distinct from that of a medical patient | 100 | Strong consensus |
| The aforementioned differences further contribute to the underreporting or missed diagnosis of BoNT-A resistance | 93 | Consensus |
| Although the frequency of antibody-induced SNR for BoNT-A is low compared with other therapeutic protein products, it is a real problem that warrants further attention as the clinical applications of BoNT-A continue to expand | 100 | Strong consensus |
| As the doses used in aesthetic practice become similar to those in therapeutics owing to the rise in off-label applications, a corresponding increase in the rate of NAb formation can be expected | 100 | Strong consensus |
| The first step in preventing NAb formation against BoNT-A is for aesthetic practitioners to acknowledge that immunogenicity is a potential complication that might affect future therapeutic use | 100 | Strong consensus |
| The nature of antigen and the presence of adjuvants are modifiable risk factors for immunogenicity that are directly influenced by an injector’s choice of BoNT-A formulation | 93 | Consensus |
| Aesthetic practitioners are obliged to make treatment decisions in accordance with the key pillars of medical ethics and should strive to minimize modifiable risk factors | 100 | Strong consensus |
| As BoNT-A therapy is often lifelong, the risk of immunogenicity should be a key consideration in treatment decisions regarding BoNT-A formulation | 100 | Strong consensus |
| Using a highly purified BoNT-A formulation with the lowest immunogenic risk to minimize the risk of NAb formation is a prudent clinical decision | 100 | Strong consensus |
| Where efficacy and safety are comparable, a BoNT-A formulation that is less likely to cause antibody-induced SNR should be considered as a first-line therapy | 100 | Strong consensus |
| The FDA and EMA recommendations on assessing and mitigating adverse immunologically related responses associated with therapeutic protein products are equally applicable to BoNT-A use in aesthetics | 93 | Consensus |
| There is a need to raise public awareness on the risk of immunogenicity associated with BoNT-A therapy via patient education programs supported by health authorities and professional societies | 100 | Strong consensus |
*Cutoffs are as follows: strong, more than 95% agreement; consensus, more than 75%–95% agreement; majority consent, more than 50%–75% agreement; no majority consent, less than 50% agreement.
SRMA, systematic reviews/meta-analyses.
All panel members agreed that the true extent of BoNT-A antibody-induced SNR in aesthetic applications is likely underestimated within the published literature. They noted that conclusions of SR/MAs are based on data aggregated across studies that may miss clinically relevant observations concerning individual-level data. There was a strong consensus that practitioners should refer to the full range of clinical evidence for a complete picture of antibody-induced SNR and its implications for their practice. There was also consensus that the variability of a typical aesthetic patient’s treatment journey may contribute to missed diagnoses or underreporting of BoNT-A resistance.
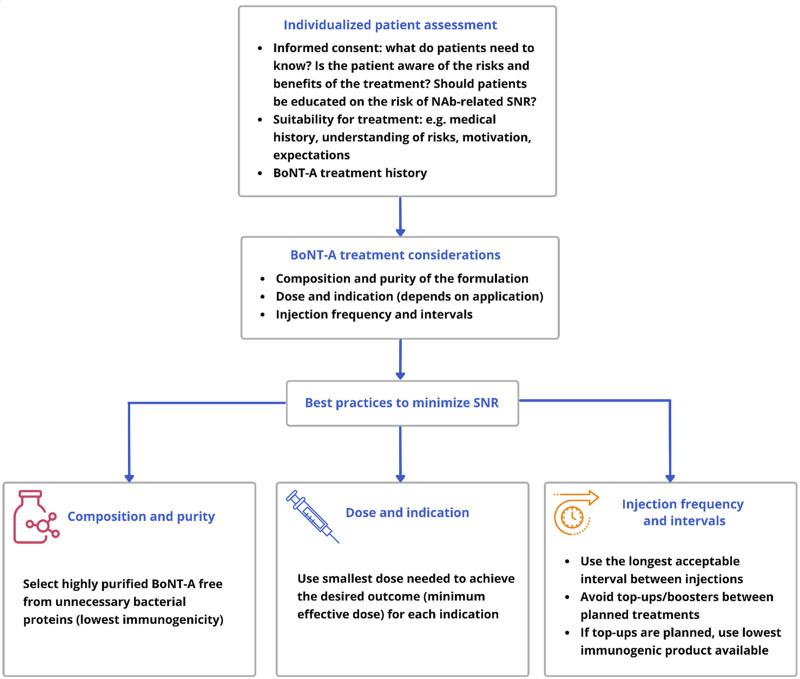
There was a strong consensus that BoNT-A resistance is a problem that warrants attention. The panel noted that, with expanding off-label applications and doses used in aesthetics becoming more like those in therapeutics, increased rates of NAb formation could be expected. In light of these trends, all panelists agreed that the first step toward preventing BoNT-A NAb formation is for practitioners to acknowledge immunogenicity as a potential complication that might affect future treatment options. Recognizing that BoNT-A therapy is often lifelong, there was a strong consensus that immunogenicity should be considered when making BoNT-A-related treatment decisions. All panelists agreed that using a highly purified BoNT-A formulation with the lowest immunogenicity to minimize the risk of NAb formation may be a prudent clinical decision. Where efficacy and safety are comparable, a lower immunogenicity formulation may offer advantages for further treatment, even though increasing the dose and/or reducing treatment intervals can compensate for partial SNR in some patients. These views are summarized in Figure 3 and are consistent with observations suggesting that using a highly purified BoNT-A formulation and administering the lowest acceptable dose at appropriate intervals may help limit the development of immunoresistance.11–13,46
Fig. 3.
Key treatment considerations for BoNT-A use in aesthetics.
To minimize adverse immunologically related responses, the FDA and EMA have provided recommendations on immunogenicity assessment and risk-based management with therapeutic biologics.52,53 The panel concluded that these recommendations are also applicable to aesthetic BoNT-A use. In addition, the panelists discussed BoNT-A-specific advisories on NAb formation by the Korean FDA for patients, physicians, and manufacturers as examples of how regulatory bodies could provide leadership in promoting prudent use in aesthetics.56–58 These Korean Food and Drug Administration advisories provide patient education on risk factors and physician guidance on prevention strategies. Furthermore, manufacturers were recommended to conduct clinical trials assessing the immunological impact of repeated administration for at least 1 year.
As in therapeutic decision-making, the panelists concurred that treatment decisions in aesthetics should be aligned with core medical ethics principles, alongside the relevant clinical and aesthetic considerations. Given the diverse applications of BoNT-A and an increasingly complex aesthetic treatment landscape, practitioners should strive to recognize and minimize modifiable risk factors for future adverse outcomes. Finally, there was strong consensus on the need to raise public awareness of the risk of immunogenicity associated with BoNT-A therapy, as the issue can only be fully addressed with the understanding and cooperation of patients. However, the panel members acknowledged that existing resources for clinicians might be overly technical for use in patient education. Consumer advisories in lay language, such as those issued by the Korean Food and Drug Administration, may be more helpful for highlighting the issue.
CONCLUSIONS
With millions of aesthetic BoNT-A treatments performed worldwide, especially off-label applications involving higher doses than traditional on-label indications, more practitioners may expect to encounter possible cases of NAb-related SNR. They will need to make appropriate clinical assessments and design/adjust treatment plans accordingly. A collaborative patient-centered approach and informed decision-making may offer a better chance of achieving safe and satisfactory treatment outcomes. We advocate individualized assessment and thorough discussion of BoNT-A treatment issues and risks, including immunogenicity, with patients from the outset. It may be clinically prudent to minimize immunogenic risk to preserve the option of continued or future BoNT-A treatment. The selection and use of a BoNT-A product with the highest purity and lowest immunogenicity from the beginning may be advantageous, especially when treatment with high doses is planned. We believe that this view is aligned with relevant clinical, ethical, and aesthetic considerations, and with recommendations in the therapeutic space for risk-based management of adverse immunological responses related to biologic drugs, including BoNT-A.
ACKNOWLEDGMENT
Medical writing and editorial support were provided by Tech Observer Asia Pacific Pte Ltd and funded by Merz Aesthetics.
Supplementary Material
Footnotes
All authors contributed equally to this work.
Published online 20 June 2022.
This article draws on material presented and discussed during a virtual meeting organized by Merz Aesthetics Asia Pacific.
Disclosure: Dr. Ho serves as a consultant for Merz Aesthetics. Dr. Albrecht reports grants and/or personal fees and/or nonfinancial support from Novartis, Biogen, Merz, Teva, Ipsen, Allergan, Celgene/Bristol Meyers Squibb, Janssen Cilag, Roche, Merck, and Sanofi Genzyme outside of the submitted work. Ms. Corduff is a speaker and clinical investigator for Merz Aesthetics. Prof. Martin served as an ad-hoc consultant and speaker for Merz. Dr. Tseng served as a speaker, trainer, and advisory board member for Merz Aesthetics, AbbVie, Bausch & Lomb, Cynosure, Galderma, and Solta. Dr. Vachiramon serves as a speaker for Merz Aesthetics, LG Chem, Leo Pharma, Beiersdorf, and L’Oreal, and as an advisory board member for Merz Aesthetics, AbbVie, and L’Oreal. Dr. Won currently receives grants from BMI Korea, Huons, Inibio, and Medytox. Dr. Yu serves as a key opinion leader for Merz Aesthetic Philippines. Dr. Dingley is/was a speaker and advisor for and has received funding from Merz Aesthetics, Galderma, and Allergan. All authors received honoraria from Merz Aesthetics for participation in the expert panel and article preparation/development. The other authors have no financial interest to declare.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.The Aesthetic Society. Aesthetic Plastic Surgery National Databank Statistics 2019. The Aesthetic Society. 2019. [Google Scholar]
- 2.American Society of Plastic Surgeons. Plastic Surgery Statistics Report 2020. American Society of Plastic Surgeons. 2020. [Google Scholar]
- 3.Dorizas A, Krueger N, Sadick NS. Aesthetic uses of the botulinum toxin. Dermatol Clin. 2014;32:23–36. [DOI] [PubMed] [Google Scholar]
- 4.Flynn TC. Advances in the use of botulinum neurotoxins in facial esthetics. J Cosmet Dermatol. 2012;11:42–50. [DOI] [PubMed] [Google Scholar]
- 5.Ahn BK, Kim YS, Kim HJ, et al. Consensus recommendations on the aesthetic usage of botulinum toxin type A in Asians. Dermatol Surg. 2013;39:1843–1860. [DOI] [PubMed] [Google Scholar]
- 6.Sundaram H, Huang PH, Hsu NJ, et al. ; Pan-Asian Aesthetics Toxin Consensus Group. Aesthetic applications of botulinum toxin A in Asians: an international, multidisciplinary, Pan-Asian consensus. Plast Reconstr Surg Glob Open. 2016;4:e872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng J, Chung HJ, Friedland M, et al. Botulinum toxin injections for leg contouring in East Asians. Dermatol Surg. 2020;46(suppl 1):S62–S70. [DOI] [PubMed] [Google Scholar]
- 8.Dressler D. Clinical applications of botulinum toxin. Curr Opin Microbiol. 2012;15:325–336. [DOI] [PubMed] [Google Scholar]
- 9.Jankovic J. Botulinum toxin: state of the art. Mov Disord. 2017;32:1131–1138. [DOI] [PubMed] [Google Scholar]
- 10.Samizadeh S, De Boulle K. Botulinum neurotoxin formulations: overcoming the confusion. Clin Cosmet Investig Dermatol. 2018;11:273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. 2015;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellows S, Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins (Basel). 2019;11:E491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr WW, Jain N, Sublett JW. Immunogenicity of botulinum toxin formulations: potential therapeutic implications. Adv Ther. 2021;38:5046–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel LL, Ostrem JL, Bledsoe IO. FDA approvals and consensus guidelines for botulinum toxins in the treatment of dystonia. Toxins (Basel). 2020;12:E332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frevert J, Ahn KY, Park MY, et al. Comparison of botulinum neurotoxin type a formulations in Asia. Clin Cosmet Investig Dermatol. 2018;11:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker TJ, Dayan SH. Comparison and overview of currently available neurotoxins. J Clin Aesthet Dermatol. 2014;7:31–39. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SK. Antibody-induced failure of botulinum toxin type a therapy in a patient with masseteric hypertrophy. Dermatol Surg. 2007;33(1 Spec No.):S105–S110. [DOI] [PubMed] [Google Scholar]
- 18.Stengel G, Bee EK. Antibody-induced secondary treatment failure in a patient treated with botulinum toxin type a for glabellar frown lines. Clin Interv Aging. 2011;6:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dressler D, Wohlfahrt K, Meyer-Rogge E, et al. Antibody-induced failure of botulinum toxin a therapy in cosmetic indications. Dermatol Surg. 2010;36(suppl 4):2182–2187. [DOI] [PubMed] [Google Scholar]
- 20.Göschel H, Wohlfarth K, Frevert J, et al. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies–therapeutic consequences. Exp Neurol. 1997;147:96–102. [DOI] [PubMed] [Google Scholar]
- 21.Fabbri M, Leodori G, Fernandes RM, et al. Neutralizing antibody and botulinum toxin therapy: a systematic review and meta-analysis. Neurotox Res. 2016;29:105–117. [DOI] [PubMed] [Google Scholar]
- 22.Naumann M, Carruthers A, Carruthers J, et al. Meta-analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX®) across multiple indications. Mov Disord. 2010;25:2211–2218. [DOI] [PubMed] [Google Scholar]
- 23.Walter U, Mühlenhoff C, Benecke R, et al. Frequency and risk factors of antibody-induced secondary failure of botulinum neurotoxin therapy. Neurology. 2020;94:e2109–e2120. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht P, Jansen A, Lee JI, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. 2019;92:e48–e54. [DOI] [PubMed] [Google Scholar]
- 25.Rahman E, Alhitmi HK, Mosahebi A. Immunogenicity to botulinum toxin type A: a systematic review with meta-analysis across therapeutic indications. Aesthet Surg J. 2022;42:106–120. [DOI] [PubMed] [Google Scholar]
- 26.Mathevon L, Declemy A, Laffont I, et al. Immunogenicity induced by botulinum toxin injections for limb spasticity: a systematic review. Ann Phys Rehabil Med. 2019;62:241–251. [DOI] [PubMed] [Google Scholar]
- 27.Lacroix-Desmazes S, Mouly S, Popoff M.-R, et al. Systematic analysis of botulinum neurotoxin type A immunogenicity in clinical studies. Basal Ganglia. 2017;9:12–17. [Google Scholar]
- 28.Samadzadeh S, Ürer B, Brauns R, et al. Clinical implications of difference in antigenicity of different botulinum neurotoxin type A preparations: clinical take-home messages from our research pool and literature. Toxins (Basel). 2020;12:E499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dover JS, Monheit G, Greener M, et al. Botulinum toxin in aesthetic medicine: myths and realities. Dermatol Surg. 2018;44:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wanitphakdeedecha R, Yan C, Apinuntham C, et al. Intradermal micro-dosing of abobotulinumtoxinA for face-lifting: how long does it last? Dermatol Ther (Heidelb). 2020;10:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JY, Cho SI, Hur K, et al. Intradermal microdroplet injection of diluted incobotulinumtoxin-A for sebum control, face lifting, and pore size improvement. J Drugs Dermatol. 2021;20:49–54. [DOI] [PubMed] [Google Scholar]
- 32.Borodic G. Immunologic resistance after repeated botulinum toxin type a injections for facial rhytides. Ophthalmic Plast Reconstr Surg. 2006;22:239–240. [DOI] [PubMed] [Google Scholar]
- 33.Borodic G. Botulinum toxin, immunologic considerations with long-term repeated use, with emphasis on cosmetic applications. Facial Plast Surg Clin North Am. 2007;15:11–16, v. [DOI] [PubMed] [Google Scholar]
- 34.Cohen JL, Scuderi N. Safety and patient satisfaction of abobotulinumtoxinA for aesthetic use: a systematic review. Aesthet Surg J. 2017;37(suppl_1):S32–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer T, Sattler G, Prager W, et al. Safety, tolerability, and efficacy of repeat-dose injections of incobotulinumtoxinA in the treatment of upper facial lines: results from a prospective, open-label, phase III study. J Drugs Dermatol. 2020;19:461–469. [PubMed] [Google Scholar]
- 36.Helmstaedter V, Wittekindt C, Huttenbrink KB, et al. Safety and efficacy of botulinum toxin therapy in otorhinolaryngology: experience from 1,000 treatments. Laryngoscope. 2008;118:790–796. [DOI] [PubMed] [Google Scholar]
- 37.Imhof M, Kühne U. A phase III study of incobotulinumtoxinA in the treatment of glabellar frown lines. J Clin Aesthet Dermatol. 2011;4:28–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence I, Moy R. An evaluation of neutralizing antibody induction during treatment of glabellar lines with a new US formulation of botulinum neurotoxin type A. Aesthet Surg J. 2009;29(suppl 6):S66–S71. [DOI] [PubMed] [Google Scholar]
- 39.Srinoulprasert Y, Kantaviro W, Nokdhes YN, et al. Development of inhibition ELISA to detect antibody-induced failure of botulinum toxin a therapy in cosmetic indications. J Immunol Methods. 2019;473:112635. [DOI] [PubMed] [Google Scholar]
- 40.Stephan F, Habre M, Tomb R. Clinical resistance to three types of botulinum toxin type A in aesthetic medicine. J Cosmet Dermatol. 2014;13:346–348. [DOI] [PubMed] [Google Scholar]
- 41.Torres S, Hamilton M, Sanches E, et al. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: five case reports. Clin Cosmet Investig Dermatol. 2014;7:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wanitphakdeedecha R, Kantaviro W, Suphatsathienkul P, et al. Association between secondary botulinum toxin A treatment failure in cosmetic indication and anti-complexing protein antibody production. Dermatol Ther (Heidelb). 2020;10:707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carruthers JD, Lowe NJ, Menter MA, et al. ; Botox Glabellar Lines II Study Group. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112:1089–1098. [DOI] [PubMed] [Google Scholar]
- 44.Inoue K, Fujinaga Y, Watanabe T, et al. Molecular composition of clostridium botulinum type A progenitor toxins. Infect Immun. 1996;64:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frevert J. Content of botulinum neurotoxin in Botox®/Vistabel®, Dysport®/Azzalure®, and Xeomin®/Bocouture®. Drugs R D. 2010;10:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JY, Sunga O, Wanitphakdeedecha R, et al. Neurotoxin impurities: a review of threats to efficacy. Plast Reconstr Surg Glob Open. 2020;8:e2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oshima M, Deitiker PR, Jankovic J, et al. Human T-cell responses to botulinum neurotoxin: proliferative responses in vitro of lymphocytes from botulinum neurotoxin a-treated movement disorder patients. J Neuroimmunol. 2011;237:66–72. [DOI] [PubMed] [Google Scholar]
- 48.Bryant AM, Cai S, Singh BR. Comparative immunochemical characteristics of botulinum neurotoxin type A and its associated proteins. Toxicon. 2013;72:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Means TK, Hayashi F, Smith KD, et al. The toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. [DOI] [PubMed] [Google Scholar]
- 50.Yoon SI, Kurnasov O, Natarajan V, et al. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335:859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Sun Y, Yang W, et al. Type A botulinum neurotoxin complex proteins differentially modulate host response of neuronal cells. Toxicon. 2014;82:52–60. [DOI] [PubMed] [Google Scholar]
- 52.FDA. Guidance for Industry - Immunogenicity Assessments for Therapeutic Protein Products. Available at https://www.fda.gov/media/85017/download. Accessed October 15, 2021.
- 53.EMA. Guideline on Immunogenicity Assessment of Therapeutic Proteins. Available at https://www.ema.europa.eu/en/immunogenicity-assessment-biotechnology-derived-therapeutic-proteins. Accessed October 15, 2021.
- 54.Gillon R. Medical ethics: four principles plus attention to scope. BMJ. 1994;309:184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mousavi SR. The ethics of aesthetic surgery. J Cutan Aesthet Surg. 2010;3:38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korean Food and Drug Administration (KFDA). Information on safe use of botulinum toxin (for HCPs) (in Korean). KFDA. 2017:1-8. [Google Scholar]
- 57.Korean Food and Drug Administration (KFDA). Information on safe use of botulinum toxin (for Consumers) (in Korean). KFDA. 2017:1-4. [Google Scholar]
- 58.Korean Food and Drug Administration (KFDA). Considerations for review of Botulinum toxin preparations (for Manufacturers) (in Korean). KFDA. 2020:1-40. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.