Patients with chronic lymphocytic leukemia (CLL) are at increased risk of developing a second cancer compared with the general population. Several factors may contribute to this increased risk, including the higher age of CLL patients, chronic antigenic stimulation, immune impairment inherently associated with CLL or chemotherapy.1,2
A leukemogenic potential of the purine analog fludarabine has been reported in several studies, especially when combined with other DNA-damaging agents such as cyclophosphamide, since fludarabine inhibits DNA repair and increases the cytotoxic effect of these drugs. An enhancement of DNA damage may thus affect bone marrow (BM) progenitor cells, leading to prolonged myelosuppression and impaired immune surveillance, resulting in an increased risk of therapy-related myeloid neoplasm (t-MN).3–6 Moreover, rituximab, commonly prescribed in CLL, can lead to long-lasting neutropenia, reported to range between 30% and 52% of treated patients.7 Prolonged exposure to rituximab may also induce B-cell depletion, further compromising immune-surveillance, hence facilitating the occurrence of second cancers.
In a previous study, we reported the influence of immunosuppression on the appearance of secondary cancers in patients with various types of B-cell lymphoproliferative disorders after a fludarabine or cyclophosphamide or rituximab (FCR) combination.8
Here we investigated a cohort of CLL patients who received FCR or FC as frontline therapy and developed t-MN. Analysis of their clinical and biological characteristics disclosed that post-treatment prolonged cytopenia stood out as the single risk-factor of t-MN.
Data were collected retrospectively from 20 different centers of the French Innovative Leukaemia Organization (FILO), tracking patients who developed t-MN between August 2008 and March 2018. The study was undertaken in compliance with the principles of the Helsinki declaration and was approved by a local ethics committee.
From this large retrospective series of 3200 CLL patients treated with FCR or FC, 61 t-MN were retrieved (1.9%), including 46 after frontline FC or FCR who were finally retained.
Forty-one (80.9%) patients received FCR and 5 (10.9%) patients received FC regimen.
Patient characteristics are described as Suppl. Table S1. The median number of cycles was 6 (range: 4–6).
Thirty-seven patients (80.4%) developed therapy-related myelodysplastic syndrome (t-MDS) and 9 (19.6%) therapy-related acute myeloid leukemia (t-AML), with more than 30% of BM blasts for 5 of the latter. At that time, cytogenetics revealed an abnormal karyotype in 28 of 30 cases (24/25 t-MDS and 4/5 t-AML). As expected in secondary myeloid malignancies, more than half the cases had poor-risk anomalies: 16 patients had monosomy 5/del(5q) or 7/del(7q) while 14 had a complex karyotype. Consequently, 80% of evaluable patients (N = 20) had a poor or very poor revised international prognostic scoring system (IPSS-R) cytogenetic risk. Moreover, 76% of them (N = 19) had a high or very high risk IPSS-R prognostic score. Molecular information by NGS disclosed TP53 alteration in 5 of 7 tested cases. In 10 patients (21.7%), persistent CLL cells were found upon BM examination at the time of t-MN. The median time between CLL diagnosis and CLL last treatment or t-MN diagnosis was 78.9 (12.6-305.6) and 20.2 [1.3–129.8] months, respectively.
After t-MN diagnosis, 27 patients (58.7%) received 5-azacytidine, 13 (28.3%) best supportive care, 3 (6.5%) chemotherapy, and 2 (4.3%) lenalidomide. One (2.2%) proceeded to allogeneic hematopoietic stem-cell transplantation. According to International Working Group-2006 criteria, the 10.9% of responders reached medullar complete response (6.5%) or partial response (4.3%). At the last follow-up, 40 of 46 patients were died (Suppl. Table S2).
After a median follow-up of 8.5 months (0.03–71.2), median overall survival was 8.97 months (range, 7.3–13.2). In univariate analysis, IPSS-R cytogenetic risk (P < 0.001), IPSS-R prognostic score (P < 0.001), and best supportive care (P = 0.001) were disclosed as poor prognosis factors for t-MN evolution (Suppl. Table S3).
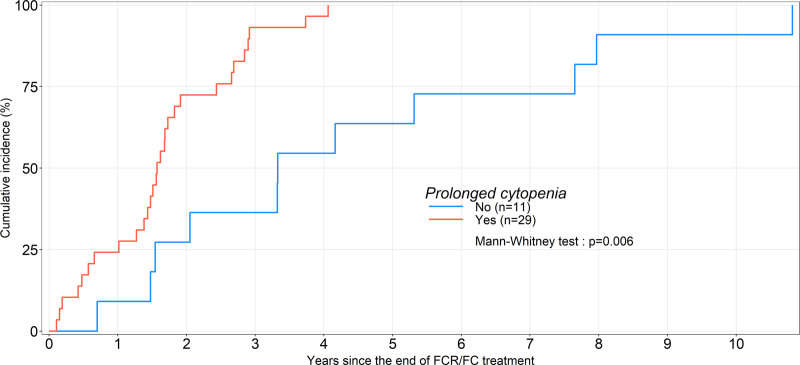
To investigate for risk factors indicative of evolution towards t-MN, patient characteristics at the time of FC or FCR treatment were investigated. Besides age, gender, cytogenetics or fluorescence in situ hybridization (FISH), hypogammaglobulinemia, FC versus FCR treatment, and number of cycles, post-therapy cytopenia was more closely examined. Prolonged (PC), long-lasting (LC), and very long-lasting (VLC) cytopenias were defined as the persistence of grade 2–4 neutropenia or thrombocytopenia or and anemia respectively for >4, >26, and 52 weeks after the end of CLL treatment, all other etiologies having been excluded. PC was recorded in 29 of 40 evaluable patients (72.5%), LC in 14 of 33 (42.4%), and VLC in 12 of 33 (36.4%), respectively. Neutropenia was observed in 17 of 40 patients (42.5%), thrombocytopenia in 24 of 40 (60%), anemia in 22 of 40 (55%), and pancytopenia in 12 of 40 (30.0%). granulocyte colony stimulating factor was administered to 17 (42.5%) evaluable patients. Only 7 patients with PC (24.1%) had recovered normal blood counts at t-MN diagnosis (Suppl. Table S4). The median time between last CLL treatment and t-MN diagnosis was 39.9 (8.4–129.8), 39.9 (19.4–34.7), and 17.4 months (1.3–48.7), in patients without PC, with resolved PC and with nonresolved PC (P = 0.002), respectively. It was globally of 18.9 (1.3–48.8) months in patients with PC (P = 0.006, Figure 1). Only PC was significantly associated with a higher t-MN incidence (P < 0.001; Table 1).
Figure 1.
Time to therapy-related myeloid neoplasms in patients with CLL who received FCR or FC as frontline single therapy. CLL = chronic lymphocytic leukemia; FCR or FC = fludarabine or cyclophosphamide or rituximab.
Table 1.
Univariate Analysis on Incidence of TRMN
| Variable | Reference Level | Class Level | HR (95% CI) | P Value |
|---|---|---|---|---|
| Age | Continuous variable | −0.06 (−0.93 to 0.80) | 0.886 | |
| Gender | f | m | 4.30 (−13.12 to 21.72) | 0.631 |
| Karyotype | Normal | Other | −7.37 (−33.47 to 18.73) | 0.583 |
| Complex | −19.15 (−46.22 to 7.92) | 0.173 | ||
| Diploid | −7.90 (−43.71 to 27.91) | 0.668 | ||
| FISH high risk | No | Yes | −1.05 (−12.36 to 10.25) | 0.856 |
| Hypogammaglobulinemia | No | Yes | 9.56 (−9.45 to 28.58) | 0.332 |
| Persistence of cytopenia | No | Yes resolved | −25.17 (−46.49 to −3.85) | 0.026 |
| Yes nonresolved | −35.05 (−51.33 to −18.76) | <0.001 | ||
| Yes | −32.66 (−48.28 to −17.04) | <0.001 | ||
| Long last cytopenia | No | Yes | −10.84 (−30.18 to 8.49) | 0.280 |
| Type of frontline therapy | FC | FCR | 5.61 (−20.64 to 31.86) | 0.671 |
| Number of FCR cycles | <6 | 6 | 3.21 (−12.67 to 19.48) | 0.714 |
CI = confidence interval; f = female; FC = fludarabine or cyclophosphamide; FCR = fludarabine or cyclophosphamide or rituximab; HR = hazard ratio; m = male; TRMN = therapy-related myeloid neoplasms.
To confirm these results, a validation cohort of 384 patients evaluable for post-therapy cytopenia among 542 treated with FCR in the FILO or CLL-SA 2007 trial between December 2007 and February 20149 was analyzed. Seven t-MN were observed (1.8%), after PC for 5 patients (71%) and after LC for 1 (14%). Among patients who did not develop t-MN, there were 30% of PC and 14% of LC (N = 54). These data confirm a significantly increased incidence of PC prior to t-MN (P = 0.029). PC was also the only significant predictive variable in this cohort (P = 0.048), whereas age, gender, cytogenetics or FISH, hypogammaglobulinemia, and number of FCR cycles were no significant.
Additionally, analysis was performed of published data of the CLL8 trial. This disclosed prolonged grade 3/4 neutropenia in 67 (16.8%) and 34 (8.8%) patients treated with FCR or FC, respectively. Thirteen patients developed t-MN (1.5% and 1.8%, respectively), after prolonged neutropenia, lasting 12 months or more in 6 of 13 (46.2%).
Overall, this study, which describes the characteristics of t-MN in CLL patients treated with FC or FCR, shows that the occurrence of myeloid malignancies is significantly associated with prolonged post-treatment cytopenia. This was confirmed in 3 different retrospective cohorts. The role of fludarabine and cyclophosphamide and the neutropenic effect of rituximab mentioned above can be suspected. Yet, another feature enters the picture, which is the advanced age of these patients, likely to also present clonal hematopoiesis of undetermined potential (CHIP) and thus molecular alterations liable to favor the development of t-MN.
Although recent progress in the treatment of CLL, with increasing chemo-free options, will hopefully decrease the risk of cytopenia and hence of developing t-MN, we found interesting to examine what may lead to this rather rare complication. Post-therapy cytopenia will probably be less frequent in upcoming trials. Yet, in real life, some patients are still liable to receive the established FC or FCR regimen. These data indicate that in such settings, close attention to prolonged cytopenia is deserved.
ACKNOWLEDGMENTS
The authors appreciate the contributions of the many physicians and data managers who made this analysis possible, as well as the contributions made by the patients themselves.
AUTHOR CONTRIBUTIONS
KL and KM designed the study, all authors except ABdeM, MCB, and FD enrolled patients, ABdeM collected the data and performed statistical analysis. KL, MCB, and DG wrote the paper. All authors read and approved the final version of the manuscript for submission.
DISCLOSURES
DG received honoraria from Janssen, Abbvie and Astra Zeneca, and research grant from Beigene outside the submitted work. J-BM received honoraria from Abbvie, Jazz Pharmaceuticals and Astellas outside the submitted work. Outside this study, KL received Grants from Novartis, Takeda, Jansen, Abbvie, and personal fees from Novartis, Takeda, Abbvie, Iqone, Astra Zeneca, and Beigene. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126:573–581. [DOI] [PubMed] [Google Scholar]
- 2.Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Neuberg D, Flinn IW, et al. Incidence of therapy-related myeloid neoplasia afte or initial therapy for chronic lymphocytic leukemia or with fludarabine-cyclophosphamide versus fludarabine: long-term follow-up of US Intergroup Study E2997. Blood. 2011;118:3525–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamini O, Jain P, Trinh L, et al. Second cancers in patients with chronic lymphocytic leukemia who received frontline fludarabine, cyclophosphamide and rituximab therapy: distribution and clinical outcomes. Leuk Lymphoma. 2015;56:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Tang G, Medeiros LJ, et al. Therapy-related myeloid neoplasms following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Mod Pathol. 2012;25:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. [DOI] [PubMed] [Google Scholar]
- 8.Denizon N, Baugier de Materre A, Alani M, et al. Significant impact of immunosuppression on the incidence of secondary malignancies following fludarabine, cyclophosphamide, and rituximab (FCR) treatment in patients with indolent B-cell neoplasms. Leuk Lymphoma. 2018;59:2711–2714. [DOI] [PubMed] [Google Scholar]
- 9.Dartigeas C, Van Den Neste E, Léger J, et al. Rituximab maintenance versus observation following abbreviated induction with chemoimmunotherapy in elderly patients with previously untreated chronic lymphocytic leukaemia (CLL 2007 SA): an open-label, randomised phase 3 study. Lancet Haematol. 2018;5:e82–e94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.