Progressive multifocal leukoencephalopathy (PML) is an uncommon but severe opportunistic infection of the central nervous system, affecting almost exclusively immunocompromised patients and characterized by demyelinating lesions caused by reactivation of the John Cunningham polyomavirus (JCV), a ubiquitous human neurotropic virus present in approximately half of the general population.1 To allow PML to develop, dysfunction of both B and T lymphocyte populations is required. In fact, antiviral surveillance hinges on an adequate CD8+ T-cell activity. However, B lymphocytes play an important role in stimulating T cell–mediated responses through specific cytokine secretion: Th1-type cytokines such as IFN-γ, secreted by B lymphocytes, help to reinvigorate the CD8+ T-cell responses against JCV reactivation. Moreover, intrathecal B cell–mediated antibody response against JCV has shown to prevent the progression of the infection.3
The outcome of HIV-associated PML has improved following the introduction of highly active antiretroviral therapy, and PML occurring after natalizumab treatment for multiple sclerosis can be managed favorably withholding the drug: in those cases, immune suppression can be reverted by removing its cause, thus allowing immune restoration and prevention of JCV replication. Otherwise, patients with PML and an underlying hematologic disease still have a very poor prognosis, as death occurs within 2 months in 90% of patients.1,2 In such situation immunosurveillance seems irreversibly compromised by the underlying disease and immunosuppressive treatments, impairing the control of JCV reactivation. Currently, there are no specific antiviral treatments for PML infection and the only therapeutic strategies available are intended to restore the immune function.2 Recent emphasis has gained the treatment of PML with the immune checkpoint inhibitor pembrolizumab, a monoclonal antibody targeting PD-1, a T-cell surface receptor whose interaction with his ligands PD-L1 and PD-L2 causes T-cell exhaustion.4 By preventing this interaction, pembrolizumab favors the reactivation of T cell–mediated immune response not only against tumors but also against chronic infections: also CD4+ and CD8+ T cells of PML patients overexpress PD-1, which is particularly enriched on JCV-specific CD8+ T lymphocytes.4,5 However, hematological patients treated with pembrolizumab show inconsistent outcomes with pembrolizumab, probably owing to the heterogeneity of the predisposing immune defect, which needs to be addressed by a strategy both aiming to control the hematological disease and relieve the immune suppression, and then boosted by immune checkpoint release.5–7
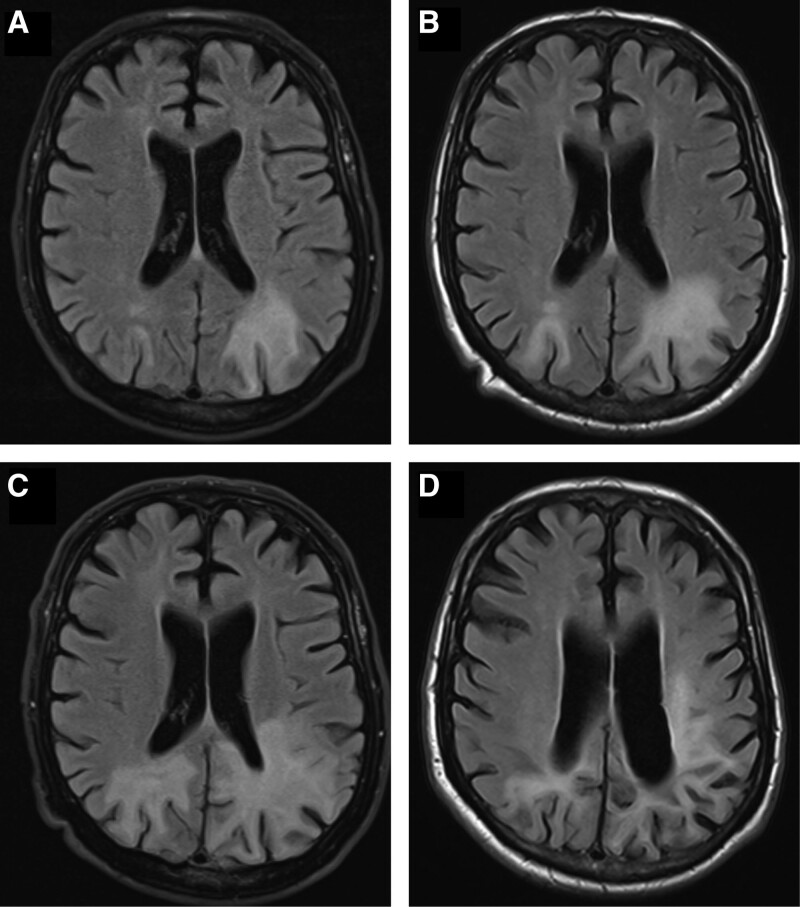
In this case report, we present the favorable outcome of a 63-year-old male patient with chronic lymphocytic leukemia (CLL), treated with a combination of venetoclax and then pembrolizumab after PML onset occurring after several chemoimmunotherapy regimens. Diagnosis of CLL was made in October 2014 (p53 unmutated, IGHV mutated); immediately after treatment with fludarabine-cyclophosphamide-rituximab was administered for 6 courses, reaching a complete response in February 2015. Following early disease progression, second line treatment with bendamustine-rituximab for 6 courses was performed from September 2017 to March 2018; an even earlier relapse was documented in January 2019 with lymphocytosis (34,000/mmc), multiple adenopathies (up to 6 cm), splenomegaly (longest diameter 21 cm, 9 cm below costal margin) and hypercalcemia (2.9 mEq/L). From April 2019 the patient started a third line treatment with ibrutinib (420 mg daily) with very rapid resolution of palpable adenopathies and splenomegaly. However, ibrutinib was stopped after 38 days due to the onset of progressive and severe vision disturbance, at first deemed of ocular origin. Nine days later, the patient underwent a magnetic resonance of the brain, which showed a bilateral subcortical lesion in the parieto-occipital area, more extended to the left side, hyperintense in T2 and FLAIR images, without contrast enhancement, suggestive for PML infection. A first investigation for JCV-DNA in the cerebrospinal fluid with lumbar puncture was negative. Aiming to reduce immunosuppression, ibrutinib was permanently discontinued, but rapid progression of CLL developed, with rapidly enlarging adenopathies (up to 7 cm), splenomegaly (9 cm below costal margin) and hypercalcemia (4.4 mEq/L). On July 11, the patient was therefore started on a fourth-line therapy with Bcl-2 inhibitor venetoclax. Mirtazapine was prescribed by the consultant neurologist and intravenous immunoglobulin supplementation was provided given the severe hypogammaglobulinemia (IgG <400 mg/dL). A second brain MRI, performed 8 weeks after the first one, showed a stability of the subcortical lesion on the left and a slight size increase on the right, suggesting a limited progression. However, during the following months, the neurological symptoms worsened, with the onset of aphasia, ideomotor apraxia, severe right hemiparesis, and amaurosis. A third brain MRI in November showed an expansion of the alteration of the temporal lobes bilaterally. A second lumbar puncture resulted positive for JCV reactivation in the cerebrospinal fluid (3085 copies/mL). Given the worsening clinical status notwithstanding an excellent response of the CLL, the possibility of a further treatment for PML was discussed. Thus, the patient was treated from December 2019 to February 2020 with 3 doses of pembrolizumab (2 mg/kg monthly) and remained clinically stable during the treatment. Cerebrospinal fluid re-evaluation in April 2020 showed a negativization of JCV-DNA. A brain MRI 4 months after last pembrolizumab dose confirmed that, not only PML evolution was halted, but subcortical lesions in the bilateral temporo-occipito-parietal area had slightly shrunk (Figure 1). The patient underwent motor and speech rehabilitation. About 2 years after pembrolizumab administration, the vision loss is stable and motor aphasia has fairly improved. The patient is continuing venetoclax treatment with an ongoing complete response with a resolution of lymphocytosis, lymphadenopathies, and splenomegaly.
Figure 1.
Radiological progression by MRI of PML-associated brain changes from June 2019 (before starting venetoclax, A) to end of July 2019 (8 weeks after venetoclax start, B) and November 2019 (5 months after venetoclax start). Reduction of PML-associated alterations in May 2020 (4 months after pembrolizumab start).
Treatment of hematological malignancies with chemoimmunotherapy is known to cause immunosuppression. Sometimes, the combined effects of the haematological neoplasm, that is, CLL, whose hallmark is a dysfunctional immune environment, coupled with the profound and prolonged immune depression caused by purine analogues may result in an apparently irreversible abatement of the adaptive immune system.8 Such situation represents an ideal condition for the development of PML, where both the B and T compartments of adaptive immunity have to be compromised to be permissive for the infection. In CLL, both fludarabine and bendamustine have shown to promote a prolonged CD4+ lymphopenia, increasing the risk of opportunistic infections.9 B-lymphocyte depletion, selectively mediated by rituximab, cause a lack of stimulatory signals on T-cell population, already severely compromised by previous chemotherapy and the underlying haematological disease. Moreover, there is a synergy between rituximab and fludarabine or bendamustine in promoting the onset of PML.10 The inhibition of B-cell receptor (BCR) caused by ibrutinib is another risk factor for the onset of PML. Defective humoral immune responses and their reduced influence on T cell–mediated activity, ensuing from BTK inhibition, contribute to JCV reactivation.11 For these reasons, it is advised to reduce or halt immunosuppressive agents for PML arising in the context of a secondary iatrogenic immunodeficiency. In our patient, we decided to stop ibrutinib and to substitute it with venetoclax, which has a different antileukemic activity, i.e., antiapoptotic. Since this measure did not result in significant improvements, we evaluated further therapeutic strategies for PML. Interleukin-7 (IL-7) is an essential cytokine for T-cell proliferation and functionality; preliminary data suggest that this agent can counteract PML reactivation, restoring the integrity of the immune system.12 However, administration of IL-7 is not recommended in patients with CLL, considering its potential role as a growth factor for the leukemic cells.13
Recently, new therapeutic approaches have been developed, aiming to restore the compromised immune system of patients with PML. For example, transfer of JCV-specific T cells expanded from autologous or allogenic T lymphocytes stimulated with JCV antigen peptides, has been proposed and demonstrated to effectively counteract JCV reactivation in preliminary studies.14 However, adoptive T-cell therapy is not easily available and wasn’t deemed feasible in our case because of timing and logistic considerations. Lastly, use of checkpoint inhibitors has been suggested for PML since CD4+ and CD8+ T lymphocytes of PML patients exhibit a high expression of PD-1 (even higher in JCV-specific CD8+ T lymphocytes). Low lymphocyte count at baseline, particularly an absence or an insufficient number of JCV-specific CD8+ T cells before anti-PD-1 therapy, may represent an important predictor of treatment failure.15 In our patient, we can assume that discontinuation of BTK inhibitor and substitution with a Bcl-2 inhibitor could not halt alone JCV progression; however, a combined strategy of reduced B-lymphocyte suppression by ibrutinib substitution and enhancement of T-cell functionality through reversion of T-cell exhaustion with pembrolizumab achieved success in a patient with an extremely poor prognosis.
In conclusion, clinicians should be aware of rare diagnoses with potential life-threatening consequences which arise in the context of severe combined immunodeficiency resulting from sequential use of multiple chemotherapeutic treatments and targeted agents. We have reported the first case of PML arising in CLL successfully treated with venetoclax (after ibrutinib discontinuation) and pembrolizumab, where withdrawal of suppression of humoral immunity combined with restoration of cellular immunity with checkpoint inhibitors allowed to regain control of a chronic viral infection, avoiding a likely fatal prognosis.
ACKNOWLEDGMENTS
We thank Dr. Paola Cinque and Dr. Simonetta Gerevini for fruitful discussion about management of this case.
AUTHOR CONTRIBUTIONS
JO and PL designed research, performed research, analyzed data, and wrote the article. SV, GS, and PC contributed data. MG, GP, and AC reviewed the article. RF supervised the study.
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pavlovic D, Patera AC, Nyberg F, et al. Progressive Multifocal Leukeoncephalopathy Consortium . Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8:255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neil EC, DeAngelis LM. Progressive multifocal leukoencephalopathy and hematologic malignancies: a single cancer center retrospective review. Blood Adv. 2017;1:2041–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durali D, de Goër de Herve MG, Gasnault J, et al. B cells and progressive multifocal leukoencephalopathy: search for the missing link. Front Immunol. 2015;6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koralnik IJ. Can immune checkpoint inhibitors keep JC virus in check? N Engl J Med. 2019;380:1667–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortese I, Muranski P, Enose-Akahata Y, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. 2019;380:1597–1605. [DOI] [PubMed] [Google Scholar]
- 6.Du Pasquier RA. Pembrolizumab as a treatment for PML? Waiting for Godot. Neurol Neuroimmunol Neuroinflamm. 2019;6:e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes A, Wellings T, Walsh O, et al. Progressive multifocal leukoencephalopathy associated with a lymphoproliferative disorder treated with pembrolizumab. J Neurovirol. 2020;26:961–963. [DOI] [PubMed] [Google Scholar]
- 8.Moreno C, Muñoz C, Terol MJ, et al. Restoration of the immune function as a complementary strategy to treat chronic lymphocytic leukemia effectively. J Exp Clin Cancer Res. 2021;40:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaiolla R, Hartley S, Beech A, et al. Extended follow-up of CD4+ T cell recovery kinetics in a large cohort of patients with B-cell lymphoproliferative disease treated with rituximab-bendamustine. Hematol Oncol. 2021;39:137–140. [DOI] [PubMed] [Google Scholar]
- 10.Focosi D, Tuccori M, Maggi F. Progressive multifocal leukoencephalopathy and anti-CD20 monoclonal antibodies: What do we know after 20 years of rituximab. Rev Med Virol. 2019;29:e2077. [DOI] [PubMed] [Google Scholar]
- 11.Raisch DW, Rafi JA, Chen C, et al. Detection of cases of progressive multifocal leukoencephalopathy associated with new biologicals and targeted cancer therapies from the FDA’s adverse event reporting system. Expert Opin Drug Saf. 2016;15:1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miskin DP, Chalkias SG, Dang X, et al. Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurol Neuroimmunol Neuroinflamm. 2016;3:e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasson SC, Smith S, Seddiki N, et al. IL-7 receptor is expressed on adult pre-B-cell acute lymphoblastic leukemia and other B-cell derived neoplasms and correlates with expression of proliferation and survival markers. Cytokine. 2010;50:58–68. [DOI] [PubMed] [Google Scholar]
- 14.Berzero G, Basso S, Stoppini L, et al. Adoptive transfer of JC virus-specific T lymphocytes for the treatment of progressive multifocal leukoencephalopathy. Ann Neurol. 2021;89:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlitzki M, Schneider-Hohendorf T, Rolfes L, et al. Ineffective treatment of PML with pembrolizumab: Exhausted memory T-cell subsets as a clue? Neurol Neuroimmunol Neuroinflamm. 2019;6:e627. [DOI] [PMC free article] [PubMed] [Google Scholar]