Abstract
Objective
To investigate the correlation between tropomyosin (TM) and clinical characteristics of bladder cancer. In addition, the relationship between TM and immune cell infiltration in bladder cancer was further analyzed.
Methods
Based on The Cancer Genome Atlas (TCGA) database, the relationship between TM expression and clinicopathological features in bladder cancer was analyzed. Receiver operating characteristic (ROC) curve was used to evaluate the value of TM as a diagnostic marker for bladder cancer. Univariate and multivariate Cox regression was used to analyze the independent factors affecting the prognosis of patients with bladder cancer. The relationship between TM and immune cell infiltration was analyzed.
Results
ROC curve showed that TPM1, TPM2, and TPM3 had significant diagnostic ability (AUC was 0.845, 0.848, and 0.873, respectively). The high expression of TPM1 and TPM2 is associated with poor overall and disease-specific survival in patients with bladder cancer (P < 0.05). Multivariate Cox analysis showed that age and TPM1 were independent prognostic factors. The expression levels of TPM1, TPM2, TPM3, and TPM4 in low grade bladder cancer were lower than those in high grade bladder cancer (P < 0.05). TPM1 and TPM2 are positively correlated with the infiltration of macrophages and NK cells in bladder cancer. TPM3 is positively associated with Th2. TPM4 is positively correlated with Th1 cells, macrophages, and neutrophils (P < 0.05).
Conclusions
TPM1 and TPM2 are effective markers for the diagnosis of bladder cancer. TPM1 is an independent prognostic factor for bladder cancer. TM is also associated with the infiltration of various immune cells in bladder cancer. TM may have influenced the development of bladder cancer through immune inhibition.
1. Introduction
Bladder cancer is the eleventh most common cancer in the population and the seventh most common cancer in men [1]. Muscle invasive bladder cancer (MIBC) has a high degree of malignancy. Current treatment of bladder cancer is still mainly surgical treatment. At present, targeted therapy and immunotherapy have shown great application prospects in MIBC, but there are still some problems such as low response rate and drug resistance [2].
The human genome contains four tropomyosin (TM) family genes, namely, TPM1, TPM2, TPM3, and TPM4. TM is not only present in muscle cells but also regulates cell viability and differentiation in other cells, and changes in the tropomyosin gene expression will directly affect changes in cell morphology [3]. At present, there are there few reports about the correlation between TM and bladder cancer. TPM1 has been found to be a promising diagnostic and prognostic marker for bladder cancer [4, 5]. The biological mechanism of TM in bladder cancer remains unclear. In addition, some studies have reported that the occurrence and development of bladder cancer is also related to the infiltration of immune cells [6, 7]. However, the association between TM and immune cell residence in bladder cancer has not been reported.
Therefore, we want to further analyze whether other genes in TM family are also a promising marker for bladder cancer diagnosis and prognosis as TPM1 has been reported in literatures. In addition, we want to further analyze the correlation between TM and immune cell infiltration of bladder cancer. To discuss whether TM affects the occurrence and development of bladder cancer through immunological mechanisms, this study will be conducted using appropriate bioinformatics analysis methods based on multiple databases including TCGA.
2. Materials and Methods
2.1. RNAseq Data Download and Data Analysis of Bladder Cancer
Download RNAseq data in HTSEQ-FPKM format (fragments per kilobase per million) from BLCA project in the TCGA database (https://portal.gdc.cancer.gov/). RNAseq data in FPKM format is converted into log2 data. Samples included 414 bladder cancer samples and 19 adjacent normal tissue samples. The significance of expression level was as follows: ns, P ≥ 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. ROC analysis uses the “pROC” package (version 1.17.0.1). The area values under the ROC curve are between 0.5 and 1. The closer AUC is to 1, the better the diagnostic effect is. The abscissa is false positive rate (FPR), and the ordinate is true positive rate (TPR). “Survival” package (version 3.2-10) is used for statistical analysis of survival data, and “SurvMiner” package (version 0.4.9) is used for visualization. Univariate and multivariate Cox regression was used to analyze the independent factors affecting the prognosis of patients with bladder cancer. The prognostic data used in the analysis came from a Cell article [8].
2.2. Correlation Analysis of TM and Immunoinvasion in Bladder Cancer
The single-sample GSEA (SSGSEA) method in the GSVA package (1.34.0 version) of R (3.6.3 version) was used to analyze the infiltration of immune cells in bladder cancer. The markers and classification of 24 kinds of immune cells were obtained from an article of Immunity [9].
2.3. Statistical Methods
All analytical methods were performed using R (V.3.6.3). The visualization of the image was completed using GGplot2 (version 3.3.3) on xiantao academic platform (https://www.xiantao.love/). Wilcoxon rank sum test, chi-square test, Fisher exact test, and logistics regression were used to analyze the correlation between TM and clinical indicators of samples. Spearman correlation analysis was used to analyze the correlation between TM and immune cell infiltration level in bladder cancer. P value less than 0.05 was considered statistically significant.
3. Results
3.1. TM Can Be Used as an Effective Diagnostic Marker for Bladder Cancer
We analyzed the TM expression in 414 bladder cancer specimens and 19 adjacent normal tissue specimens. The results showed that TPM1, TPM2, and TPM4 were underexpressed in bladder cancer tissues, while TPM3 was overexpressed in bladder cancer tissues (Figures 1(a)–1(d)). ROC curve was used to analyze the accuracy of TM expression level in the diagnosis of bladder cancer. ROC curve showed that TPM1, TPM2, and TPM3 had significant accuracy in the diagnosis of bladder cancer (AUC was 0.845, 0.848, and 0.873, respectively) (Figures 2(a)–2(d)). These results indicated that TPM1, TPM2, and TPM3 could be ideal biomarkers for the diagnosis of bladder cancer.
Figure 1.
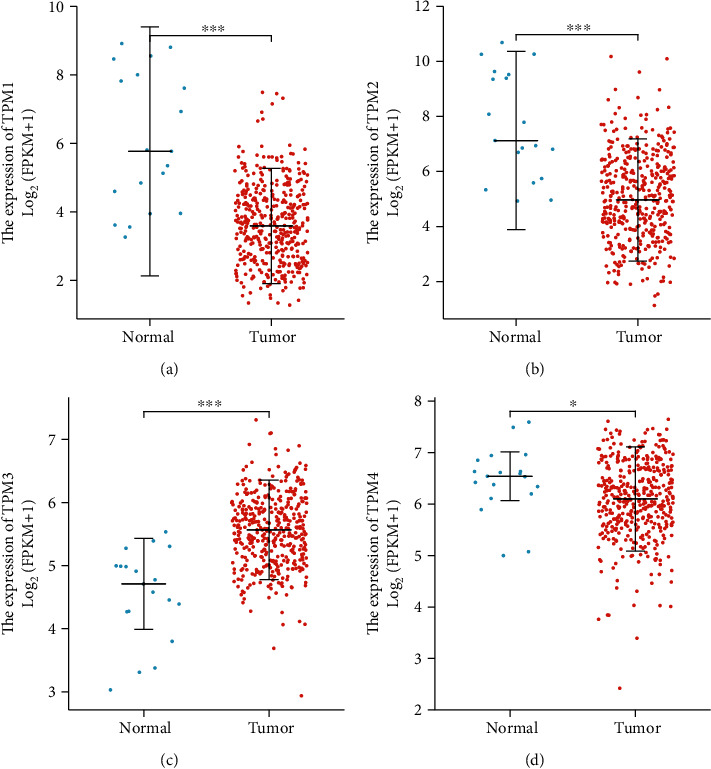
TM expression level in bladder cancer. (a) The expression of TPM1 in bladder cancer tissues and adjacent tissues. (b) The expression of TPM2 in bladder cancer tissues and adjacent tissues. (c) The expression of TPM3 in bladder cancer tissues and adjacent tissues. (d) The expression of TPM4 in bladder cancer tissues and adjacent tissues.
Figure 2.
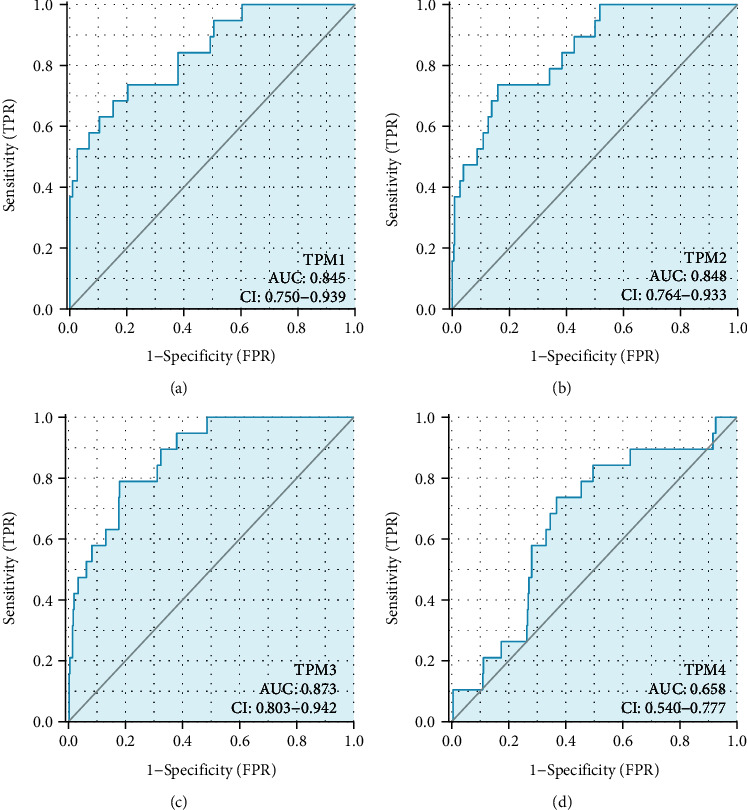
ROC curve analysis of TM prediction of bladder cancer. (a) ROC curve showed the efficacy of TPM1 expression level in distinguishing bladder cancer tissue from nontumor tissue. (b) ROC curve showed the efficacy of TPM2 expression level in distinguishing bladder cancer tissue from nontumor tissue. (c) ROC curve showed the efficacy of TPM3 expression level in distinguishing bladder cancer tissue from nontumor tissue. (d) ROC curve showed the efficacy of TPM4 expression level in distinguishing bladder cancer tissue from nontumor tissue.
3.2. TM Can Be Used as Effective Markers to Predict OS and Disease-Specific Survival in Patients with Bladder Cancer
The high expression of TPM1 and TPM2 was associated with poor overall and disease-specific survival in bladder cancer patients (P < 0.05). There was no significant correlation between the TPM3 and TPM4 expression and overall survival and disease-specific survival in patients with bladder cancer (Figures 3(a)–3(h)). Univariate analysis showed that age, TPM1, and TPM2 were prognostic factors for bladder cancer (P < 0.05) (Table 1). Multivariate Cox analysis showed that age and TPM1 were independent prognostic factors (P < 0.05) (Table 1).
Figure 3.
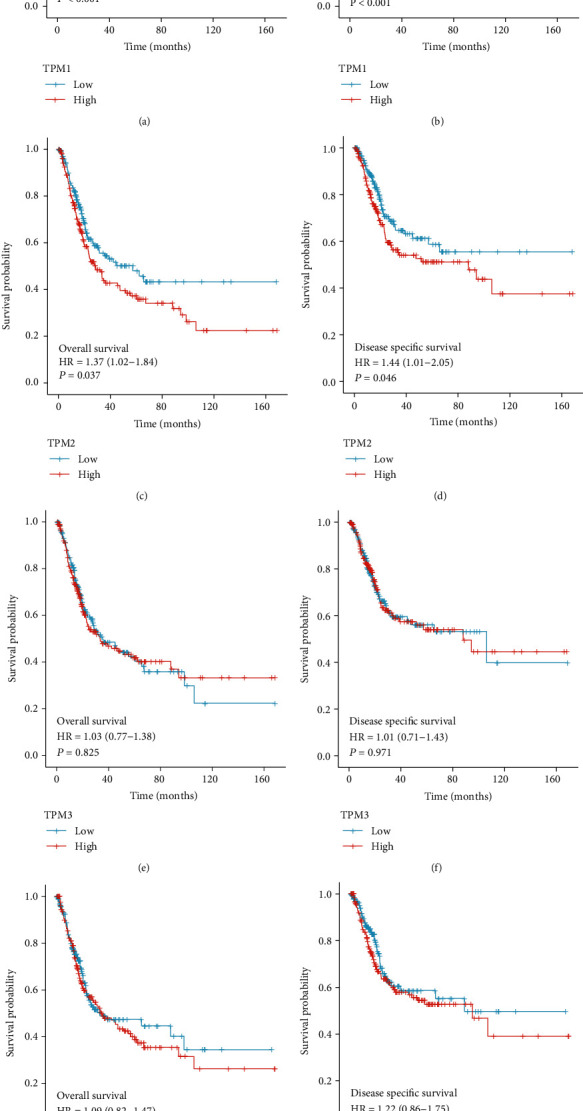
Relationship between TM expression and overall and disease-specific survival in patients with bladder cancer. (a) Relationship between TPM1 expression and overall survival in patients with bladder cancer. (b) Relationship between TPM1 expression and disease-specific survival in patients with bladder cancer. (c) Relationship between TPM2 expression and disease-specific survival in patients with bladder cancer. (d) Relationship between TPM2 expression and disease-specific survival in patients with bladder cancer. (e) Relationship between TPM3 expression and overall survival in patients with bladder cancer. (f) Relationship between TPM3 expression and disease-specific survival in patients with bladder cancer. (g) Relationship between TPM4 expression and disease-specific survival in patients with bladder cancer. (h) Relationship between TPM4 expression and disease-specific survival in patients with bladder cancer.
Table 1.
Univariable and multivariable analysis of OS in patients with bladder cancer.
| Characteristics | Total (N) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age | 413 | ||||
| < =70 | 233 | Reference | |||
| >70 | 180 | 1.421 (1.063-1.901) | 0.018 | 1.469 (1.096-1.970) | 0.010 |
| Gender | 413 | ||||
| Female | 109 | Reference | |||
| Male | 304 | 0.849 (0.616-1.169) | 0.316 | ||
| Pathologic stage | 411 | ||||
| Stage I | 4 | Reference | |||
| Stage II, stage III, and stage IV | 407 | 9132093.375 (0.000-Inf) | 0.993 | ||
| TPM1 | 413 | ||||
| Low | 207 | Reference | |||
| High | 206 | 1.718 (1.273-2.318) | <0.001 | 1.807 (1.254-2.602) | 0.001 |
| TPM2 | 413 | ||||
| Low | 207 | Reference | |||
| High | 206 | 1.368 (1.019-1.836) | 0.037 | 0.947 (0.661-1.355) | 0.764 |
| TPM3 | 413 | ||||
| Low | 207 | Reference | |||
| High | 206 | 1.033 (0.772-1.382) | 0.825 | ||
| TPM4 | 413 | ||||
| Low | 207 | Reference | |||
| High | 206 | 1.094 (0.816-1.468) | 0.547 | ||
3.3. Correlation between TM and Clinical Indicators of Patients
We further used logistics regression to analyze the correlation between TM and clinical indicators of samples. The results showed that the expression level of TPM1 was affected by N stage, gender, and histologic grade (P <0.05) (Table 2). The expression level of TPM2 was affected by N stage, age and histologic grade (P < 0.05) (Table 3). The expression level of TPM3 is affected by histologic grade (P < 0.05) (Table 4). The expression level of TPM4 has not been affected by clinical indicators (Table 5). The expression levels of TPM1, TPM2, TPM3, and TPM4 in low grade bladder cancer were lower than those in high grade bladder cancer (P < 0.05) (Figures 4(a)–4(d)).
Table 2.
Logistics regression of single gene TPM1.
| Characteristics | Total (N) | Odds ratio (OR) | P value |
|---|---|---|---|
| T stage (T2, T3, and T4 vs. T1) | 380 | 4.287 (0.627-84.290) | 0.195 |
| N stage (N1, N2, and N3 vs. N0) | 370 | 2.611 (1.683-4.094) | <0.001 |
| M stage (M1 vs. M0) | 213 | 2.901 (0.848-11.381) | 0.098 |
| Pathologic stage (stage II, stage III, and stage IV vs. stage I) | 412 | 3.059 (0.388-62.130) | 0.335 |
| Gender (male vs. female) | 414 | 0.479 (0.304-0.748) | 0.001 |
| Age (>70 vs. <=70) | 414 | 1.040 (0.705-1.535) | 0.843 |
| Histologic grade (high grade vs. low grade) | 411 | 22.162 (4.552-399.669) | 0.003 |
Table 3.
Logistics regression of single gene TPM2.
| Characteristics | Total (N) | Odds ratio (OR) | P value |
|---|---|---|---|
| T stage (T2, T3, and T4 vs. T1) | 380 | 4.523 (0.661-88.920) | 0.179 |
| N stage (N1, N2, and N3 vs. N0) | 370 | 2.204 (1.425-3.439) | <0.001 |
| M stage (M1 vs. M0) | 213 | 3.092 (0.903-12.136) | 0.079 |
| Pathologic stage (stage II, stage III, and stage IV vs. stage I) | 412 | 46226230.510 (0.000-NA) | 0.993 |
| Gender (male vs. female) | 414 | 0.723 (0.464-1.120) | 0.148 |
| Age (>70 vs. <=70) | 414 | 1.484 (1.005-2.196) | 0.048 |
| Histologic grade (high grade vs. low grade) | 411 | 10.419 (2.972-65.963) | 0.002 |
Table 4.
Logistics regression of single gene TPM3.
| Characteristics | Total (N) | Odds ratio (OR) | P value |
|---|---|---|---|
| T stage (T2, T3, and T4 vs. T1) | 380 | 3.979 (0.582-78.223) | 0.219 |
| N stage (N1, N2, and N3 vs. N0) | 370 | 1.262 (0.824-1.937) | 0.285 |
| M stage (M1 vs. M0) | 213 | 0.657 (0.168-2.243) | 0.512 |
| Pathologic stage (stage II, stage III, and stage IV vs. stage I) | 412 | 45997389.201 (0.000-NA) | 0.993 |
| Gender (male vs. female) | 414 | 0.975 (0.629-1.512) | 0.911 |
| Age (>70 vs. <=70) | 414 | 1.000 (0.678-1.475) | 1.000 |
| Histologic grade (high grade vs. low grade) | 411 | 10.313 (2.941-65.289) | 0.002 |
Table 5.
Logistics regression of single gene TPM4.
| Characteristics | Total (N) | Odds ratio (OR) | P value |
|---|---|---|---|
| T stage (T2, T3, and T4 vs. T1) | 380 | 65249103.465 (0.000-NA) | 0.993 |
| N stage (N1, N2, and N3 vs. N0) | 370 | 1.157 (0.755-1.776) | 0.503 |
| M stage (M1 vs. M0) | 213 | 1.058 (0.296-3.623) | 0.928 |
| Pathologic stage (stage II, stage III, and stage IV vs. stage I) | 412 | 46226232.235 (0.000-NA) | 0.993 |
| Gender (male vs. female) | 414 | 0.760 (0.488-1.177) | 0.220 |
| Age (>70 vs. <=70) | 414 | 1.000 (0.678-1.475) | 1.000 |
| Histologic grade (high grade vs. low grade) | 411 | 2.084 (0.848-5.601) | 0.121 |
Figure 4.
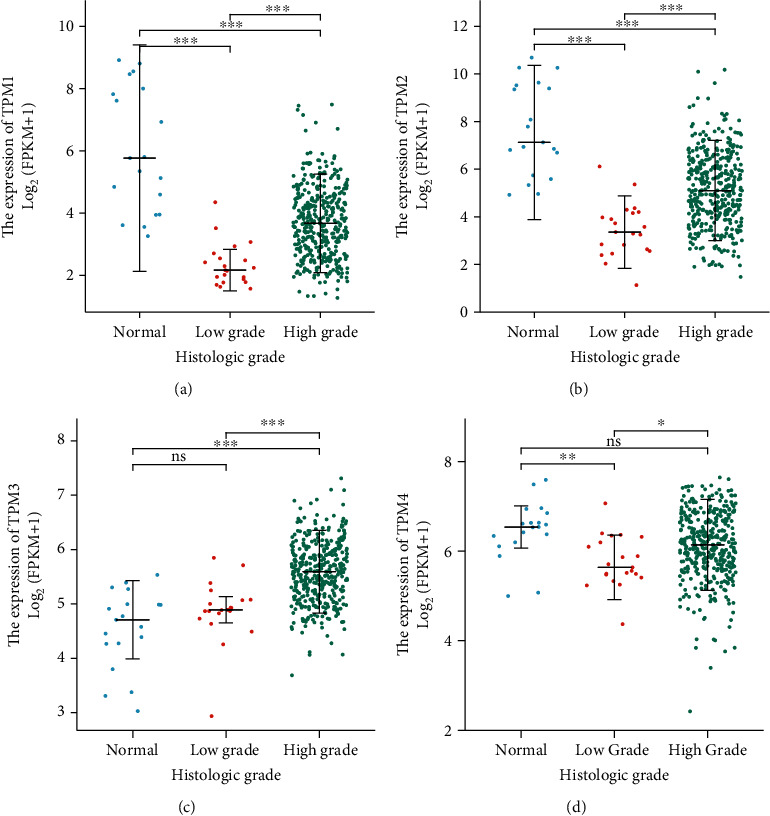
Relationship between TM expression and histological grade of bladder cancer. (a) Relationship between TPM1 expression and histological grade of bladder cancer. (b) Relationship between TPM2 expression and histological grade of bladder cancer. (c) Relationship between TPM3 expression and histological grade of bladder cancer. (d) Relationship between TPM4 expression and histological grade of bladder cancer.
3.4. Relationship between TM Expression and Immune Cell Infiltration in Bladder Cancer
SSGSEA was used to further analyze the relationship between TM expression and immune cell invasion in bladder cancer. The results showed that TPM1 and TPM2 were positively correlated with macrophage and NK cell infiltration in bladder cancer. TPM3 was positively correlated with Th2. TPM4 was positively correlated with Th1 cells, macrophages, and neutrophils (P < 0.05) (Figures 5(a)–5(d) and 6(a)–6(h)).
Figure 5.
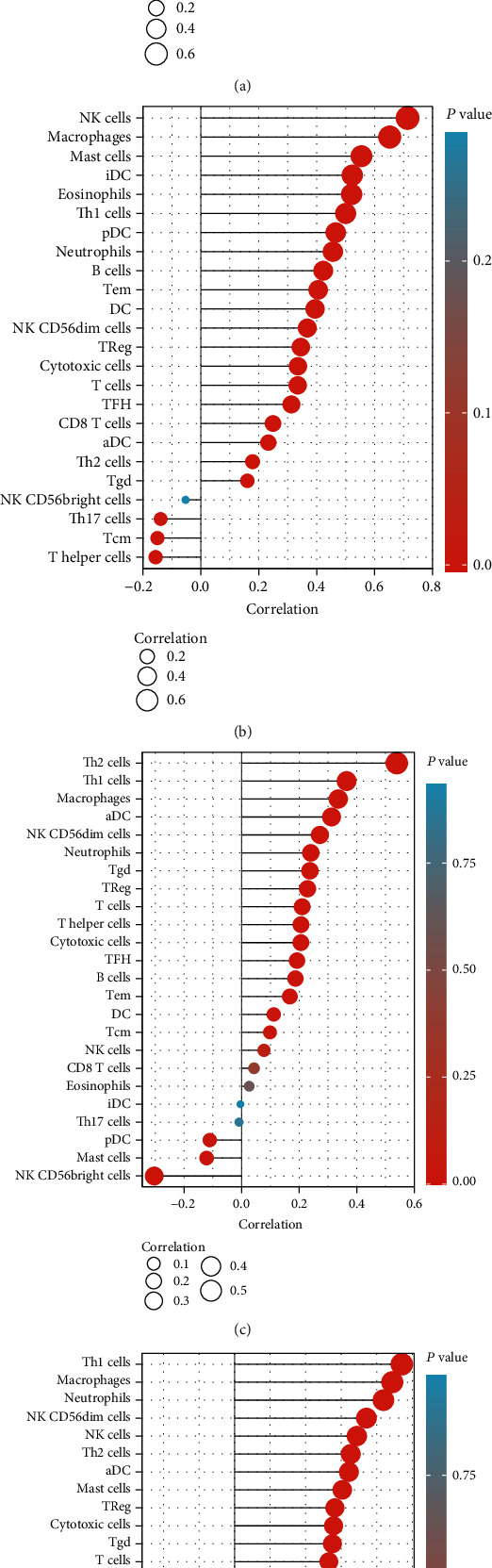
Lollipop plot shows the correlation between TM expression and immune cell infiltration in bladder cancer. (a) Correlation between TPM1 expression and immune cell infiltration in bladder cancer. (b) Correlation between TPM2 expression and immune cell infiltration in bladder cancer. (c) Correlation between TPM3 expression and immune cell infiltration in bladder cancer. (d) Correlation between TPM4 expression and immune cell infiltration in bladder cancer.
Figure 6.
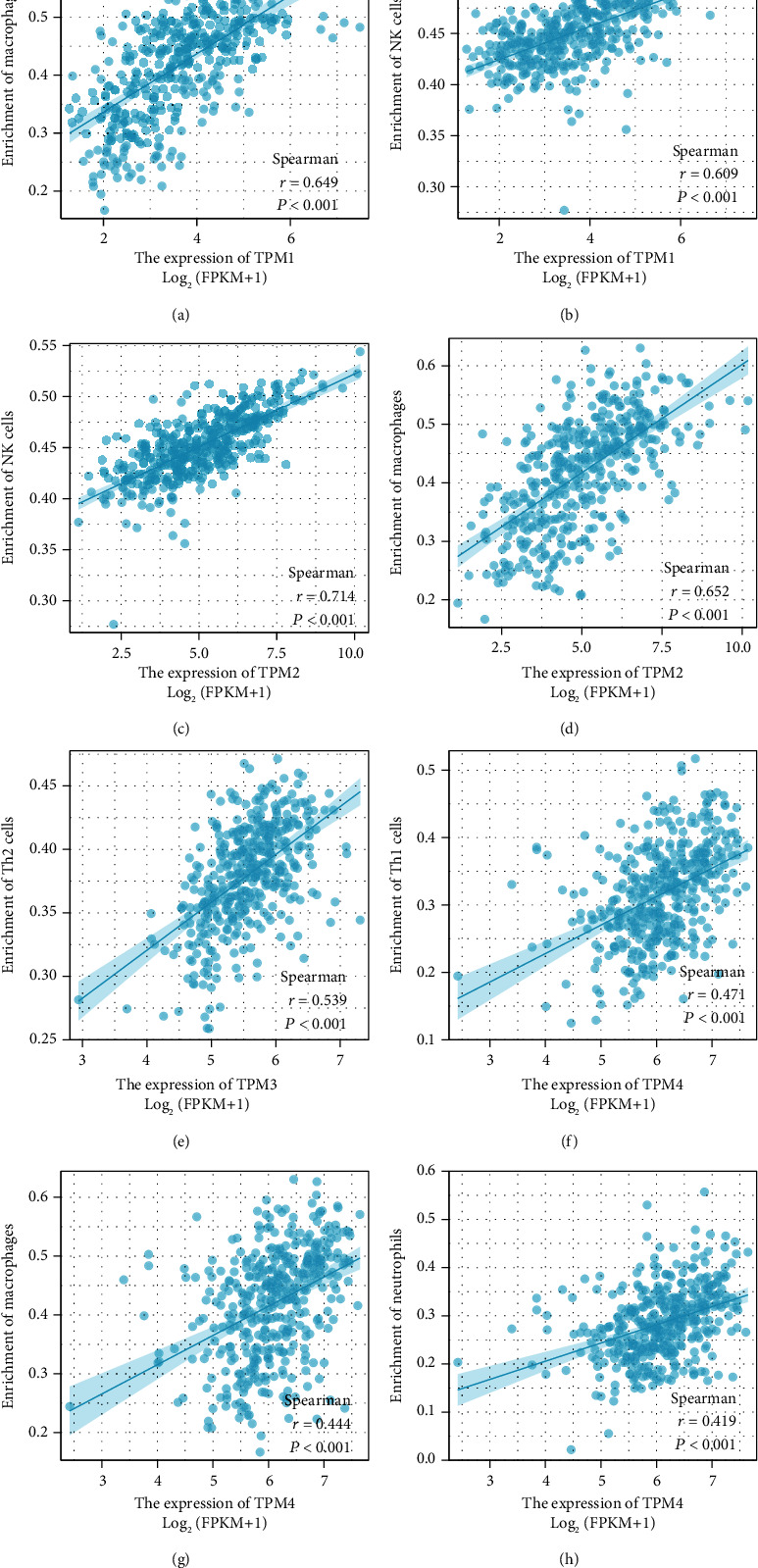
Scatter plot shows the correlation between TM expression and immune cell infiltration in bladder cancer. (a) Scatter plot shows the correlation between TPM1 expression and macrophage infiltration in bladder cancer. (b) Scatter plot shows the correlation between TPM1 expression and NK cell infiltration in bladder cancer. (c) Scatter plot shows the correlation between TPM2 expression and NK cell infiltration in bladder cancer. (d) Scatter plot shows the correlation between TPM2 expression and macrophage infiltration in bladder cancer. (e) Scatter plot shows the correlation between TPM3 expression and Th2 invasion in bladder cancer. (f) Scatter plot shows the correlation between TPM4 expression and Th1 invasion in bladder cancer. (g) Scatter plot shows the correlation between TPM4 expression and macrophage infiltration in bladder cancer. (h) Scatter plots show the correlation between TPM4 expression and neutrophil infiltration in bladder cancer.
4. Discussion
Tropomyosin, a member of the actin binding protein family, was originally thought to be a myofibrillary structural protein involved in the contractile activity of skeletal and cardiac muscles [10]. Although TM has been studied for a long time, further studies have shown that TM is not only present in skeletal muscle and cardiac muscle cells but also expressed in almost all cells [3]. The TM family genes consists of four genes: TPM1, TPM2, TPM3, and TPM4. At present, most of the researches on TM family genes only focus on one or two tropomyosin genes and lack of systematic studies. Studies have shown that TPM1 is a very important tumor suppressor, and its expression level is low in many solid tumors [11]. At present, there are few reports about the correlation between TM and bladder cancer, especially the correlation between TM and immune cell infiltration of bladder cancer remains unclear. Therefore, based on the TCGA database, bioinformatics analysis method was adopted to comprehensively analyze the correlation between TM and bladder cancer. The great potential of TM in diagnosing and predicting bladder cancer is expected to be explored. In addition, this study also analyzed the correlation between TM and the presence of immune cells in bladder cancer, hoping to provide more research evidence for the further study of the immunological mechanism of bladder cancer.
Our study showed that TPM1, TPM2, and TPM4 were underexpressed in bladder cancer tissues. The above results are consistent with current literature reports [12, 13]. It has been reported that TPM1 and TPM2 are highly expressed in normal urothelial tissues, but the expression of TPM1 and TPM2 is decreased in the early stage of bladder cancer, which may be a marker event of the occurrence and development of bladder cancer [12]. Cell experiments have confirmed that TPM1 inhibits the proliferation of bladder cancer cells and promotes cell apoptosis [5]. Therefore, the low expression of TPM1, TPM2, and TPM4 in the tumor tissues of bladder cancer may be beneficial to the rapid proliferation of bladder cancer cells, indicating the development of bladder cancer. In addition, ROC curve indicated that TPM1, TPM2, and TPM3 had significant accuracy in the diagnosis of bladder cancer. Therefore, TPM1, TPM2, and TPM3 are expected to be new markers for the diagnosis of bladder cancer and will play a potentially great value in clinical conversion application.
We further explored the application value of TM in predicting the prognosis of bladder cancer. The high expression of TPM1 and TPM2 is associated with poor overall and disease-specific survival in bladder cancer patients. Multivariate Cox analysis showed that age and TPM1 were independent prognostic factors. In conclusion, TPM1 can be used as an effective marker to predict the survival and prognosis of bladder cancer patients.
When we analyzed the correlation between TM and clinical indicators of bladder cancer, we found that the expression of TM was affected by the histological grade of bladder cancer. The expression levels of TPM1, TPM2, TPM3, and TPM4 in low-grade bladder cancer were lower than those in high-grade bladder cancer. This may explain why the high expression of TM has a worse prognosis when we discuss the relationship between TM and prognosis.
A current study of bladder cancer has shown that muscle-invasive bladder cancer has a different pattern of immune cell infiltration than normal tissue, and a model based on the difference in immune cell infiltration may offer promising predictive value [7]. Current studies have suggested that the presence of CD8+ T cells in bladder cancer is a favorable prognostic factor, while the increased expression of PD-L1 and the presence of tumor-related macrophages are a negative prognostic factor [14]. In this study, we focused on whether the TM play a role in the infiltration of immune cells in bladder cancer. We found that TPM1 and TPM2 were positively correlated with macrophage and NK cell infiltration in bladder cancer. TPM3 was positively correlated with Th2. TPM4 was positively correlated with Th1 cells, macrophages, and neutrophils. We have previously observed that TPM1, TPM2, and TPM4 are low expressed in bladder cancer, and therefore, the infiltration of NK cell, macrophages, neutrophils, and Th1 may be reduced accordingly in bladder cancer. Therefore, we infer that the low expression of TPM1, TPM2, and TPM4 is a mechanism of immunosuppression in bladder cancer. Therefore, TM is expected to be used as a predictor of immune cell infiltration in bladder cancer and a potential therapeutic target.
In conclusion, TPM1 and TPM2 are effective markers for the diagnosis of bladder cancer. TPM1 is an independent prognostic factor for bladder cancer. TM is also associated with the infiltration of various immune cells in bladder cancer. TM may have influenced the development of bladder cancer through immune inhibition.
Acknowledgments
This research is funded by the following research projects: (1) Youth Research Foundation of Guangdong Medical University, Project No. GDMUQ2021035.
Data Availability
We did not create any data, and all the results we analyzed in our paper were based data from public database. So, we do not have any data that could be uploaded to a repository.
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Conception was contributed by Yunkun Yan and Jianchang Li. Interpretation or analysis of data was contributed by Yunkun Yan and Sining Li. Preparation of the manuscript was contributed by Yunkun Yan. Revision for important intellectual content was contributed by Mushi Ye and Zhuo Li. Supervision was contributed by Yunkun Yan and Jianchang Li. These authors contributed equally: Yunkun Yan and Jianchang Li.
References
- 1.Rozanec J. J., Secin F. P. Epidemiology, etiology and prevention of bladder cancer. Archivos Españoles de Urología . 2020;73(10):872–878. [PubMed] [Google Scholar]
- 2.Peng M., Xiao D., Bu Y., et al. Novel combination therapies for the treatment of bladder cancer. Frontiers in Oncology . 2021;10:p. 539527. doi: 10.3389/fonc.2020.539527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunning P., O'Neill G., Hardeman E. Tropomyosin-based regulation of the actin cytoskeleton in time and space. Physiological Reviews . 2008;88(1):1–35. doi: 10.1152/physrev.00001.2007. [DOI] [PubMed] [Google Scholar]
- 4.Hu J., Zhou L., Song Z., et al. The identification of new biomarkers for bladder cancer: a study based on TCGA and GEO datasets. Journal of Cellular Physiology . 2019;234(9):15607–15618. doi: 10.1002/jcp.28208. [DOI] [PubMed] [Google Scholar]
- 5.Liu G., Zhao X., Zhou J., Cheng X., Ye Z., Ji Z. Long non-coding RNA MEG3 suppresses the development of bladder urothelial carcinoma by regulating miR-96 and TPM1. Cancer Biology & Therapy . 2018;19(11):1039–1056. doi: 10.1080/15384047.2018.1480279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Z., Zhu K., Liu Q., et al. Profiles of immune infiltration in bladder cancer and its clinical significance: an integrative genomic analysis. International Journal of Medical Sciences . 2020;17(6):762–772. doi: 10.7150/ijms.42151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Y. L., Wu Z. S., Lu H. M., et al. Prognostic significance of tumor-infiltrating immune cells in muscle-invasive bladder cancer. American Journal of Translational Research . 2020;12(10):6524–6536. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Lichtenberg T., Hoadley K. A., et al. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell . 2018;173(2):400–416.e11. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bindea G., Mlecnik B., Tosolini M., et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity . 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. The Biochemical Journal . 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khori V., Amani Shalamzari S., Isanejad A., et al. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. European Journal of Pharmacology . 2015;765:179–187. doi: 10.1016/j.ejphar.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Pawlak G., McGarvey T. W., Nguyen T. B., et al. Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. International Journal of Cancer . 2004;110(3):368–373. doi: 10.1002/ijc.20151. [DOI] [PubMed] [Google Scholar]
- 13.Chen R., Feng C., Xu Y. Cyclin-dependent kinase-associated protein Cks2 is associated with bladder cancer progression. The Journal of International Medical Research . 2011;39(2):533–540. doi: 10.1177/147323001103900222. [DOI] [PubMed] [Google Scholar]
- 14.van Wilpe S., Gerretsen E., van der Heijden A. G., de Vries I., Gerritsen W. R., Mehra N. Prognostic and predictive value of tumor-infiltrating immune cells in urothelial cancer of the bladder. Cancers . 2020;12(9):p. 2692. doi: 10.3390/cancers12092692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We did not create any data, and all the results we analyzed in our paper were based data from public database. So, we do not have any data that could be uploaded to a repository.


