If we have learned anything as a biomedical research community in the past 2 years because of the COVID-19 pandemic, it is that there are major unaddressed challenges and inefficiencies in how research is traditionally conducted and disseminated. This ‘inefficiency’ in biomedical research has been documented for decades1 but the pandemic has reinvigorated discussion, and provided vivid examples of unnecessary duplication, irresponsible dissemination of flawed studies, and poor study design.2 At the start of the pandemic, the global community responded swiftly to minimize waste in COVID-19 research. The Wellcome Trust initiated a statement on sharing research data and findings related to the COVID-19 outbreak.3 This statement committed to open science practices including immediate open access publishing, use of preprints, and data sharing. More than 150 diverse stakeholders globally signed the statement. The consequence has been a greater provision of publicly available information, increased transparency, and rapid access to new COVID-19 information. Even though implementation of these commitments has not been without challenge, likely exacerbated by the rushed nature of their execution, the adoption of actions to open the biomedical research ecosystem in this way, even if incremental rather than truly widespread, is unprecedented. The ability for the research community to share the genetic sequence of the SARS-CoV-2, and the identification of new variants such as omicron, are testaments of the benefit of rapid information sharing for fast-tracking health solutions.
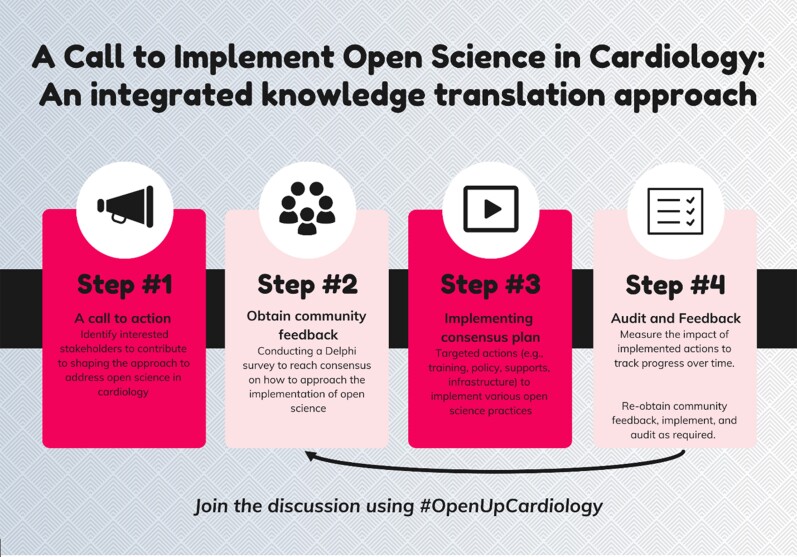
Figure 1.
Proposed approach to create a cardiovascular research community road map related to open science.
In the longer term, we need to ask ourselves what the biomedical community can do to make sustained and widespread changes to ensure that research outputs are rigorous, reproducible, disseminated in a timely fashion, transparent, and publicly accessible. These practices should be central tenants of the research and research dissemination process in all areas of biomedicine and not just temporary actions to address a pandemic. Given the interaction between COVID-19 infection and cardiovascular disease,4,5 some of the cardiology community has been directly impacted by COVID-19 research mandates related to increased openness and transparency. Unfortunately, the community is not broadly prepared to comply with these mandates. Consider data sharing: despite calls over several years to implement data sharing,6,7 and the invaluable insights that could be gained from pooled publicly available datasets, limited data sharing occurs in cardiology. A recent analysis of more than 200 randomly selected articles in cardiology journals found that almost none shared data (96.6%) or analysis scripts (98.7%) openly.8 To reach the goal of open data in cardiology, many challenges need to be addressed, including the creation of data management and sharing policies. Where such policies already exist, they are often not implemented or monitored. As the saying goes, ‘what gets measured, gets done’. One must also consider how to balance open sharing of data with patient consent and privacy, and academic acknowledgement of contributions. Patients have expressed support for data sharing.9 We believe that cardiovascular researchers have not had the tradition, role models, or external requirement to share data. Yet as stewards of patient data, they have an ethical obligation to make data as open as possible to provide access, stimulate discovery, and promote research integrity. We acknowledge that this viewpoint conflicts with the widespread academic reward system that stresses a publish or perish mantra and promotes protectionist thinking about data and intellectual property. Indeed, researchers who aggregate openly available data and publish findings are often labelled as ‘data parasites’ rather than applauded for their distinct methodological influence on innovation and knowledge growth. The time and effort taken to collect individual patient data can be enormous and without proper systems to acknowledge this and to reward researchers for their openness and transparency, behaviour change is unlikely. Finally, there are technical and practical consideration and risks associated with data sharing including the potential for data to be re-identified. Data sharing is unlikely to be successful, even among researchers eager to comply, if standardized training and support are not available. The FAIR Principles10 provide guidance on data management yet we suspect few cardiology researchers are familiar with these.
Data sharing in cardiovascular research is used above to illustrate the potential challenges involved in implementing open science. Developing and adopting a range of open science practices including open code, open access, preprints, study registration, and reporting guideline compliance is necessary to move the research system from its current ‘closed’ standard. Each open science practice will present unique implementation challenges but resource and training requirements, as well as changes to the need to re-evaluate the academic rewards system, are likely to be relevant considerations across all practices.
A unified roadmap for how to achieve openness and transparency has not yet been constructed—an effective approach will require breaking down silos among diverse stakeholders in the cardiovascular research community including clinical and preclinical researchers, funders, journal publishers, professional societies, industry, academic institutions, and patients.
Here we provide a call to action to start these organized discussions within the cardiovascular research community. Our goal is to clarify which open science practices we ought to prioritize and how to develop a strategic plan and the related training, supports, and resources needed for successful implementation. We envision four steps to move this forward. First, we share this call to action. Then, working with a core leadership team we facilitate a Delphi survey to obtain community feedback. A Delphi is a survey technique that involves controlled iterative surveying of experts to reach consensus on controversial issues. By adopting this standardized survey approach, and through integrated knowledge translation, where knowledge users are engaged from the conception of the work to its translation, we anticipate that the outputs of our efforts will best resonate with the community. Then, we can implement and monitor the plan. The path to implementing openness and transparency in cardiology is unlikely to be a linear one and we recognize that for some open science practices implementation may be incremental rather than disruptive. Cardiovascular research can forge the way within medicine and share lessons learned with the broader biomedical community. If you are interested in joining in on our efforts, please contact us or share your thoughts using the hashtag #OpenUpCardiology.
Disclosures
K.D.C. is on the Steering Committee of DORA (Declaration On Research Assessment)—this is a volunteer and unpaid role.
Conflicts of interest: K.D.C. and P.P.L. declare no conflicts of interest.
Contributor Information
Kelly D Cobey, University of Ottawa Heart Institute, University of Ottawa, Ottawa, ON, Canada; School of Epidemiology and Public Health, University of Ottawa, Ottawa, ON, Canada.
Peter P Liu, University of Ottawa Heart Institute, University of Ottawa, Ottawa, ON, Canada; Department of Medicine and Cellular and Molecular Medicine, University of Ottawa, Ottawa, ON, Canada.
References
- 1. Altman DG. The scandal of poor medical research. BMJ 1994;308:283–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glasziou PP, Sanders S, Hoffmann T. Waste in covid-19 research. BMJ 2020;369:m1847. [DOI] [PubMed] [Google Scholar]
- 3. Wellcome Trust. Sharing research data and findings relevant to the novel coronavirus (COVID-19) outbreak. https://wellcome.ac.uk/coronavirus-covid-19/open-data (31 January 2020)
- 4. Bonow RO, O’Gara PT, Yancy CW. Cardiology and COVID-19. JAMA 2020;324:1131–1132. [DOI] [PubMed] [Google Scholar]
- 5. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19. Circulation 2020;142:68–78. [DOI] [PubMed] [Google Scholar]
- 6. Ross JS, Krumholz HM. Ushering in a new era of open science through data sharing: the wall must come down. JAMA 2013;309:1355–1356. [DOI] [PubMed] [Google Scholar]
- 7. Bauchner H, Golub RM, Fontanarosa PB. Data sharing: an ethical and scientific imperative. JAMA 2016;315:1238–1240. [DOI] [PubMed] [Google Scholar]
- 8. Anderson JM, Wright B, Rauh S, Tritz D, Horn J, Parker I, et al. Evaluation of indicators supporting reproducibility and transparency within cardiology literature. Heart 2021;107:120–126. [DOI] [PubMed] [Google Scholar]
- 9. Mello MM, Lueou V, Goodman SN. Clinical trial participants’ views of the risks and benefits of data sharing. N Engl J Med 2018;378:2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton Ge, Axton M, Baak A, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data 2016;3:160018. [DOI] [PMC free article] [PubMed] [Google Scholar]