Abstract
Background
The World Health Organization (WHO) recommends artemisinin‐based combination therapies (ACTs) to treat uncomplicated Plasmodium falciparum malaria. Concerns about artemisinin resistance have led to global initiatives to develop new partner drugs to protect artemisinin derivatives in ACT. Pyronaridine‐artesunate is a novel ACT.
Objectives
To evaluate the efficacy of pyronaridine‐artesunate compared to alternative ACTs for treating people with uncomplicated P falciparum malaria, and to evaluate the safety of pyronaridine‐artesunate and other pyronaridine treatments compared to alternative treatments.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE; Embase; and LILACS. We also searched ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform, and the ISRCTN registry for ongoing or recently completed trials. The date of the last search was 27 October 2021.
Selection criteria
For the efficacy analysis, we included randomized controlled trials (RCTs) of pyronaridine‐artesunate for treating uncomplicated P falciparum malaria. For the safety analysis, we included RCTs that used pyronaridine alone or in combination with any other antimalarials. In addition to these analyses, we conducted a separate systematic review summarizing data on safety from non‐randomized studies (NRS) of any patient receiving pyronaridine (NRS safety review).
Data collection and analysis
Two review authors independently extracted all data and assessed the certainty of the evidence. We meta‐analysed data to calculate risk ratios (RRs) for treatment failures between comparisons, and for safety outcomes between and across comparisons.
Main results
We included 10 relevant RCTs. Seven RCTs were co‐funded by Shin Poong Pharmaceuticals, and three were funded by government agencies.
Efficacy analysis (RCTs)
For the efficacy analysis, we identified five RCTs comprising 5711 participants. This included 4465 participants from 13 sites in Africa, and 1246 participants from five sites in Asia. The analysis included 541 children aged less than five years. Overall, pyronaridine‐artesunate had a polymerase chain reaction (PCR)‐adjusted treatment failure rate of less than 5%. We evaluated pyronaridine‐artesunate versus the following.
• Artemether‐lumefantrine. Pyronaridine artesunate may perform better for PCR‐adjusted failures at day 28 (RR 0.59, 95% confidence interval (CI) 0.26 to 1.31; 4 RCTs, 3068 participants, low‐certainty evidence); for unadjusted failures at day 28 (RR 0.27, 95% CI 0.13 to 0.58; 4 RCTs, 3149 participants, low‐certainty evidence); and for unadjusted failures at day 42 (RR 0.61, 95% CI 0.46 to 0.82; 4 RCTs, 3080 participants, low‐certainty evidence). For PCR‐adjusted failures at day 42, there may be little or no difference between groups (RR 0.86, 95% CI 0.49 to 1.51; 4 RCTs, 2575 participants, low‐certainty evidence).
• Artesunate‐amodiaquine. Pyronaridine artesunate may perform better for PCR‐adjusted failures at day 28 (RR 0.55, 95% CI 0.11 to 2.77; 1 RCT, 1245 participants, low‐certainty evidence); probably performs better for unadjusted failures at day 28 (RR 0.49, 95% CI 0.30 to 0.81; 1 RCT, 1257 participants, moderate‐certainty evidence); may make little or no difference for PCR‐adjusted failures at day 42 (RR 0.98, 95% CI 0.20 to 4.83; 1 RCT, 1091 participants, low‐certainty evidence); and probably makes little or no difference for unadjusted failures at day 42 (RR 0.98, 95% CI 0.78 to 1.23; 1 RCT, 1235 participants, moderate‐certainty evidence).
• Mefloquine plus artesunate. Pyronaridine artesunate may perform better for PCR‐adjusted failures at day 28 (RR 0.37, 95% CI 0.13 to 1.05; 1 RCT, 1117 participants, low‐certainty evidence); probably performs better for unadjusted failures at day 28 (RR 0.36, 95% CI 0.17 to 0.78; 1 RCT, 1120 participants, moderate‐certainty evidence); may make little or no difference for unadjusted failures at day 42 (RR 0.84, 95% CI 0.54 to 1.31; 1 RCT, 1059 participants, low‐certainty evidence); but may lead to higher PCR‐adjusted failures at day 42 (RR 1.80, 95% CI 0.90 to 3.57; 1 RCT, 1037 participants, low‐certainty evidence).
Safety analysis (RCTs)
For the RCT safety analysis, we identified eight RCTs, one of which was delineated by study site, comparing pyronaridine‐artesunate to other antimalarials. Pyronaridine‐artesunate was associated with raised liver enzymes compared to other antimalarials: alanine aminotransferase (ALT) (RR 3.59, 95% CI 1.76 to 7.33; 8 RCTS, 6669 participants, high‐certainty evidence) and aspartate transaminase (AST) (RR 2.22, 95% CI 1.12 to 4.41; 8 RCTs, 6669 participants, moderate‐certainty evidence). No such effect was demonstrated with bilirubin (RR 1.03, 95% CI 0.49 to 2.18; 7 RCTs, 6384 participants, moderate‐certainty evidence). There was one reported case in which raised ALT occurred with raised bilirubin. No study reported severe drug‐induced liver injury. Electrocardiograph (ECG) abnormalities were less common with pyronaridine‐artesunate compared to other antimalarials. We identified no other safety concerns.
NRS safety review
A review on safety in NRS allowed us to increase the population within which safety was assessed. We included seven studies with 9546 participants: five single‐arm observational studies, one cohort event monitoring study, and one dose‐escalation study. All studies provided data on adverse event frequency, with a small number of participants experiencing serious adverse events and adverse effects related to pyronaridine: serious adverse events average 0.37%; drug‐related 9.0%. In two studies reporting elevations in liver enzymes, small percentages of participants (2.4% and 14.1% respectively) experienced increases in either ALT, AST, or bilirubin on day 7; however, these were small increases that returned to normal by day 42.
Authors' conclusions
Pyronaridine‐artesunate was efficacious against uncomplicated P falciparum malaria; achieved a PCR‐adjusted treatment failure rate of less than 5% at days 28 and 42; and may be at least as good as, or better than, other marketed ACTs.
Pyronaridine‐artesunate increases the risk of episodes of abnormally raised ALT. The observational data did not signal an excess of clinically important adverse effects.
Plain language summary
Pyronaridine‐artesunate for treating uncomplicated Plasmodium falciparum malaria
What is the aim of this review?
The aim of this Cochrane Review was to find out if the antimalarial drug pyronaridine‐artesunate is effective and safe in treating uncomplicated cases of an important type of malaria (Plasmodium falciparum). We collected and analysed all relevant studies to answer this question and found 10 studies.
Key messages
Pyronaridine‐artesunate is effective in treating uncomplicated P falciparum malaria. Pyronaridine‐artesunate is generally safe, but some people who receive it have blood tests suggesting liver damage. This appears to neither be long‐lasting nor to make people ill.
What was studied in the review?
The World Health Organization (WHO) recommends that malaria be treated with combinations of drugs called artemisinin‐based combination therapies (ACTs). Pyronaridine‐artesunate is a new ACT. New ACTs are needed to treat malaria that has become resistant to currently available ACTs, and to help prevent malaria from becoming more resistant to treatment.
We compared pyronaridine‐artesunate to other ACTs to evaluate its effectiveness against P falciparum malaria, and compared pyronaridine‐artesunate and pyronaridine alone to other drugs to evaluate its safety.
What are the main results of the review?
We included five studies in our analysis of treatment effectiveness. Four studies compared pyronaridine‐artesunate to artemether‐lumefantrine in adults and children of all ages in Africa and Asia. One study compared pyronaridine‐artesunate to artesunate‐amodiaquine in adults and older children in Africa, and one study compared pyronaridine‐artesunate to mefloquine plus artesunate in adults and older children in Africa and Asia. We included another five studies in our analysis of drug safety.
Pyronaridine‐artesunate effectively treated uncomplicated P falciparum malaria, and may be at least as good as or better than existing ACTs (low‐ to moderate‐certainty evidence). Pyronaridine‐artesunate increases the risk of having blood tests that suggest mild liver injury (moderate‐ to high‐certainty evidence). We did not find evidence that any such liver injury was severe or irreversible. We do not know how pyronaridine‐artesunate might affect people who already have liver damage.
We found two trials that exclusively recruited children under 12, with a total of 732 participants. When only including data from these trials, we did not find differences in treatment effectiveness or safety between pyronaridine‐artesunate and artemether‐lumefantrine.
We identified a further seven studies that provided observational data on the safety of pyronaridine. The data from these studies allowed us to increase the population within which safety was assessed. The observational data did not suggest an excess of important adverse effects.
How up‐to‐date is the review?
We searched for studies that had been published up to 27 October 2021.
Summary of findings
Background
Description of the condition
Malaria poses a global health challenge, with an estimated 229 million cases and 409,000 deaths in 2019 (WHO 2020). Plasmodium falciparum is the most important species of malaria, causing 99% of malaria cases in the World Health Organization (WHO) Africa region, and 66% in the South‐East Asia region (WHO 2017).
The WHO defines uncomplicated P falciparum malaria as the presence of asexual P falciparum parasitaemia in the absence of clinical features of severe malaria (WHO 2015). Severe malaria is P falciparum parasitaemia with one or more of: impaired consciousness, prostration, multiple convulsions, acidosis, hypoglycaemia, severe malarial anaemia, renal impairment, jaundice, pulmonary oedema, significant bleeding, shock, raised lactate, or a parasitaemia of greater than 10%. If untreated, uncomplicated malaria can develop into severe malaria.
The WHO has recommended artemisinin‐based combination therapies (ACTs) as first‐line treatment of uncomplicated P falciparum malaria since 2006, recognizing the risk of resistance with monotherapy (WHO 2006). Artemisinin resistance has emerged in South‐East Asia, initially from the Thai‐Cambodian border, and has since become prevalent in Laos, Myanmar, Thailand, and Vietnam (Dondorp 2009; Noedl 2008). The spread of artemisinin resistance could lead to high mortality (Lubell 2014). Global initiatives to contain the spread of artemisinin resistance include the development of new drugs to partner and protect the artemisinin derivatives in ACT (WHO 2011).
Description of the intervention
The WHO currently recommends the following five ACTs for first‐line treatment of malaria (WHO 2020).
Artemether‐lumefantrine
Artesunate‐amodiaquine
Artesunate‐mefloquine
Artesunate‐sulphadoxine‐pyrimethamine
Dihydroartemisinin‐piperaquine
The artemisinin in ACTs rapidly clears parasites from the blood. It also kills some sexual forms of the parasite, and may reduce onward transmission to mosquitoes. The longer‐acting partner drug clears residual infections, and protects against resistance to artemisinin (WHO 2015). Drug combinations with long half‐lives (artesunate‐mefloquine and dihydroartemisinin‐piperaquine) can provide a period of post‐treatment prophylaxis which may last for up to six weeks (Sinclair 2009).
Pyronaridine is a potential partner drug for artesunate. Researchers in China developed pyronaridine during the mid‐1970s, using the nucleus of an earlier antimalarial compound (mepacrine) with an added amodiaquine side‐chain (Fu 1991). Clinicians thereafter used pyronaridine extensively as monotherapy for P falciparum and P vivax infections in China (Chang 1992). Concerns about observed in vitro resistance to pyronaridine led Chinese researchers to use pyronaridine in combinations with sulphadoxine and pyrimethamine, and primaquine (Fu 1991).
A public‐private partnership including the Medicines for Malaria Venture (MMV) and Shin Poong Pharmaceuticals Inc developed pyronaridine‐artesunate in combination from 2002 onwards (MMV 2002), with its first national registration in 2011, with the Korean Food and Drug Administration. For uncomplicated malaria, the treatment is taken once daily for three days. Treatment is provided as tablets for adults and children over 20 kg, or in granules for children and infants between 5 kg and 20 kg.
How the intervention might work
The mode of action of pyronaridine is unclear, with several possible mechanisms (Croft 2012). Pyronaridine has been shown to have potent in vitro activity versus P falciparum (Basco 1992; Chang 1992; Childs 1988; Pradines 1998; Ringwald 1999), even in strains with resistance to other antimalarials, including chloroquine, cycloguanil, amodiaquine, and sulfadoxine‐pyrimethamine (Chavalitshewinkoon‐Petmitr 2000; Kurth 2009; Price 2010). In vitro studies also indicate synergy between pyronaridine and artesunate versus parasites that are resistant to either agent (Peters 1997; Vivas 2008).
Why it is important to do this review
In the absence of resistance, ACTs are effective drugs. However, with emerging resistance to the above currently recommended ACTs, it is necessary to identify new drug combinations with equivalent efficacy. This review is an update of a Cochrane Review first published in 2007 (Unnikrishnan 2007), and subsequently updated in 2014, Bukirwa 2014, and 2019 (Pryce 2019).
The latest update, Pryce 2019, concluded that pyronaridine‐artesunate was efficacious against uncomplicated P falciparum malaria, achieving a polymerase chain reaction (PCR)‐adjusted treatment failure rate of less than 5% at days 28 and 42, and may be at least as good as, or better than, other marketed ACTs. It also concluded that pyronaridine‐artesunate causes an increased risk of abnormal levels of the liver enzyme alanine aminotransferase (ALT). This was a safety signal that warranted further investigation.
Subsequently, the WHO Advisory Committee on Safety of Medicinal Products conducted an independent expert review, and advised that safety restrictions on the use of pyronaridine‐artesunate were unjustified (WHO 2019). As guidelines in this area are evolving, we conducted an update of this review to ensure that it reflects the latest available evidence from randomized controlled trials (RCTs) on the efficacy and safety of pyronaridine‐artesunate.
NRS safety review
Non‐randomized studies (NRS) may provide additional evidence regarding the safety of pyronaridine‐artesunate, particularly when used to treat individuals who are commonly excluded from RCTs, such as those with pre‐existing medical conditions. We therefore conducted an additional separate review on the safety of pyronaridine‐artesunate using evidence from NRS only (NRS safety review). The NRS safety review is described in full detail in Appendix 1.
Objectives
To evaluate the efficacy of pyronaridine‐artesunate compared to alternative ACTs for treating people with uncomplicated P falciparum malaria, and to evaluate the safety of pyronaridine‐artesunate and other pyronaridine treatments compared to alternative treatments.
Methods
Criteria for considering studies for this review
Types of studies
RCTs
Types of participants
Adults and children with uncomplicated P falciparum malaria, as confirmed by either microscopy or rapid diagnostic tests.
For the safety analysis, we extended the inclusion criteria to adults and children with all‐cause malaria.
Types of interventions
Intervention
Pyronaridine‐artesunate
Control
WHO‐recommended ACTs for treating malaria
For the safety analysis, we extended the inclusion criteria to all RCTs comparing pyronaridine alone or in combination with any other antimalarial.
Types of outcome measures
Primary outcomes
Total treatment failure at day 28 (PCR‐adjusted and unadjusted)
Total treatment failure at day 42 (PCR‐adjusted and unadjusted)
We do not report 'adequate clinical and parasitological response', as this is defined in terms of absence of failure and therefore represents duplication of the above outcomes.
Secondary outcomes
Efficacy
-
Early treatment failure (WHO 2009):
danger signs or severe malaria on day 1, 2, or 3, in the presence of parasitaemia;
parasitaemia on day 2 higher than on day 0, irrespective of axillary temperature;
parasitaemia on day 3 with axillary temperature ≥ 37.5 °C;
parasitaemia on day 3 ≥ 25% of count on day 0.
Safety
Serious adverse events (leading to death, requiring hospitalization or prolongation of existing hospitalization, are life‐threatening, or result in persistent or significant disability or incapacity)
Adverse events leading to withdrawal from treatment (discontinuation of trial drug or withdrawal from trial)
Elevated liver function tests
Other adverse events
Search methods for identification of studies
We sought to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
The review authors and the Cochrane Infectious Diseases Group (CIDG) Information Specialist, Vittoria Lutje (VL), attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress). VL searched the following databases up to 27 October 2021 using the search terms and strategy described in Appendix 2: the Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL; Issue 10 of 12, 2021), published in the Cochrane Library; MEDLINE (PubMed, from 1966); Embase (Ovid, from 1947); and LILACS (Latin American and Caribbean Health Science Information database) (BIREME, from 1982). We also searched ClinicalTrials.gov (clinicaltrials.gov), the WHO International Clinical Trial Registry Platform (ICTRP; www.who.int/ictrp/search/en), and the ISRCTN registry (www.isrctn.com/) for ongoing or recently completed trials using 'pyronaridine', 'pyramax', and 'malaria' as search terms.
Searching other resources
Reference lists
We checked the reference lists of all trials identified by the above methods.
Contacting organizations and experts
We did not formally contact experts for this update.
Data collection and analysis
Selection of studies
For this review update, Melissa Taylor (MT) and Rebecca Thomas (RT) independently screened the results of the search update to identify potentially relevant trials, obtaining the full‐text reports of those trials deemed potentially relevant. MT and RT used a standard eligibility form to assess the studies newly identified in this update. There were no disagreements. We documented the excluded trials and the reasons for their exclusion in the Characteristics of excluded studies table. We prepared a PRISMA diagram to summarize the identification, screening, and inclusion of studies in this review (Moher 2009).
Data extraction and management
Unadjusted total failure rate: day 28, day 42
We extracted the following data, and summed the data, to form the numerator.
Early treatment failure.
Late clinical failure.
Late parasitological failure.
We aimed to extract the following data, and subtract the data from the number of participants randomized, to form the denominator.
Those found not to have fulfilled the inclusion criteria after randomization.
Those voluntarily withdrawing consent.
Those lost to follow‐up.
Those violating protocol, including (but not limited to) missed or vomited doses, those failing to complete treatment, and those taking additional antimalarials.
PCR‐adjusted total failure rate: day 28, day 42
We aimed to extract the following data, and sum the data, to form the numerator.
Early treatment failure due to PCR‐confirmed recrudescence.
Late clinical failure due to PCR‐confirmed recrudescence.
Late parasitological failure due to PCR‐confirmed recrudescence.
We aimed to extract the following data, and subtract the data from the number of participants randomized, to form the denominator.
Those with indeterminate PCR results.
Those with missing PCR results.
Those with PCR‐confirmed new infections.
Those found not to have fulfilled the inclusion criteria after randomization.
Those voluntarily withdrawing consent.
Those lost to follow‐up.
Those violating protocol, including (but not limited to) missed or vomited doses, those failing to complete treatment, and those taking additional antimalarials.
This approach is based on standard WHO definitions (WHO 2003; WHO 2009).
Adverse events data
For adverse events, we extracted the number of people experiencing the events in each study as the numerator. In contrast to the efficacy analysis, we extracted the number of people who received at least one dose of the study drug as the denominator. Recognizing that studies often use different terminology to describe adverse events, we referenced the Medical Dictionary for Regulatory Activities (MedDRA) to find the preferred term (MedDRA 2016), and grouped adverse events according to MedDRA's "High Level Term" descriptors.
Assessment of risk of bias in included studies
Two review authors assessed risk of bias of the included studies using the Cochrane tool for assessing risk of bias (Higgins 2011), assigning a judgement of low, high, or unclear risk for each domain, and recording these judgements in risk of bias tables and a summary risk of bias graph.
We assessed the following domains for efficacy.
Sequence generation
Allocation concealment
Blinding of participants, trial personnel, and outcome assessors
Incomplete outcome data
Selective reporting
Other sources of bias
For adverse events, we assessed the two following domains, which we selected based on Cochrane and PRISMA recommendations (Loke 2007; Zorzela 2016).
Adverse event detection
Incomplete reporting of adverse events
For this update, the two new records identified corresponded to trials already included in the previous version of this review, therefore we did not conduct any additional risk of bias assessments.
Examples of risk of bias assessment decisions are provided in Appendix 3.
Measures of treatment effect
We extracted data from each included trial to calculate risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data, and have presented all measures with the corresponding 95% confidence interval (CI).
Unit of analysis issues
We did not encounter any unit of analysis issues.
Dealing with missing data
In the event of missing or unclear data, we contacted the trial authors for clarification or to provide additional information. It was not always possible to extract each data item required to itemize the denominator for treatment failures, particularly where study authors reported amalgamations of the denominator component. Where this was the case, we kept clear records of inferences made to inform the denominator data.
Assessment of heterogeneity
We visually inspected the forest plots for overlapping CIs as an indicator of clinical heterogeneity. We also took into account Chi2 and I2 tests of heterogeneity. We considered a Chi2 test P < 0.1 or an I2 statistic > 75%, or both, as indicative of substantial heterogeneity. If we judged there to be substantial heterogeneity, we did not pool results in a meta‐analysis, instead presenting a narrative synthesis of the findings.
Assessment of reporting biases
There were too few trials to examine funnel plot asymmetry for evidence of small‐trial effects or publication bias.
Data synthesis
We analysed data using Review Manager 5 (Review Manager 2020). For the primary analysis, we stratified by comparator ACT. We performed meta‐analysis where appropriate after investigation of heterogeneity. In the first instance, we used a fixed‐effect model. Where we found evidence of heterogeneity, we used a random‐effects model, and applied this consistently across similar outcomes.
We deemed it inappropriate to combine continuous data for the outcomes of parasite clearance, fever clearance and gametocyte carriage due to heterogeneity in the measurements of these outcomes.
Subgroup analysis and investigation of heterogeneity
We intended to explore causes of heterogeneity using subgroup analysis of age, country, and geographic region. There were too few trials to perform these subgroup analyses; however, to explore the applicability of the evidence to child populations, we presented the findings from a subset of trials that exclusively recruited paediatric participants.
Sensitivity analysis
We planned to conduct a series of sensitivity analyses, as detailed in Appendix 4. Our aim was to restore the integrity of the randomization process by adding excluded groups back into the analysis in a stepwise fashion. However, as we were unable to reliably extract data pertinent to the missing or indeterminate PCR values, we did not conduct these sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach (Schünemann 2013), in relation to the following criteria.
Study design
Risk of bias
Inconsistency
Indirectness
Imprecision
Other considerations (including publication bias)
We used GRADEpro GDT software to create summary of findings tables for each comparison evaluated in the review (GRADEpro GDT). We included our primary outcomes and adverse event outcomes, and used the tables to guide our conclusions.
NRS safety review
Full methods for the NRS safety review are detailed in Appendix 1.
Criteria for considering studies for this review
Types of studies
Prospective cohort studies including single‐arm and comparative studies, retrospective cohort studies of individual case series, and case reports.
Types of participants
Adults and children, including both healthy participants, pregnant women, and participants with co‐existing disease (HIV, tuberculosis, or liver disease).
Types of interventions
Pyronaridine‐artesunate (PY‐AS), pyronaridine in combination with other antimalarial, or pyronaridine monotherapy.
Primary outcomes
Clinically important effects; abnormal liver enzyme tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin; other evidence of liver failure.
Search methods for identification of studies
For the NRS safety review, the search strategy was updated to include terms specific to safety and adverse events and performed across the databases described for the main review.
Data collection and analysis
Full details of the methods used for the data collection and analysis are provided in Appendix 1. Briefly, two review authors (TF and MT) independently screened all titles and abstracts for inclusion. We assessed risk of bias of the included studies using methods adapted from the 2017 Cochrane Review 'Mefloquine for preventing malaria during travel to endemic areas' (Tickell‐Painter 2017). We extracted data on the frequency of participants experiencing adverse events, including: serious adverse events; life‐threatening adverse events; adverse events leading to discontinuation of the study drug; and the frequency of participants experiencing increases in liver enzyme levels. We attempted to contact study authors when sufficient detail was lacking. Finally, we summarized the proportion of participants experiencing adverse events in each included study.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies sections.
Results of the search
The database search (current to 27 October 2021) identified a total of 52 database records and 21 trial registry records. Contact with authors of a relevant registered trial led to the identification of one additional published record. Two review authors (JP and PH) independently screened all titles for the previous version of this review (date of search 8 May 2018). For this update, MT and RT independently screened all titles published thereafter.
In total, 36 potentially relevant full‐text records were identified through title and abstract screening. After further assessment, 11 records were excluded (see Characteristics of excluded studies table). Utlimately, 23 records relating to 10 studies were identified and included in qualitative and quantitative synthesis (see Characteristics of included studies); two ongoing trials were also identified. Of these 23 records, two records were newly identified for this review update (Compaore 2020; Soulama 2019). Both records relate to the previously included trial Sagara 2018.
The Sagara 2018 trial compared different drug combinations at different sites, but presented the results in an aggregated analysis, in which the numbers of outcome events were summarized across sites. We were concerned that this presented an unpredictable bias, therefore we contacted the trial authors to obtain data disaggregated to site level for the efficacy and raised liver enzyme outcomes. As a result, we were able to present the data from individual sites separately in the meta‐analysis. However, we did not have disaggregated data for the outcomes of serious adverse events or adverse events leading to withdrawal of treatment, and have therefore presented these outcomes in narrative form. Further details of the comparisons examined and the number of participants are provided in the Characteristics of included studies tables for each site.
The search results are illustrated in a PRISMA flow diagram (Figure 1).
1.
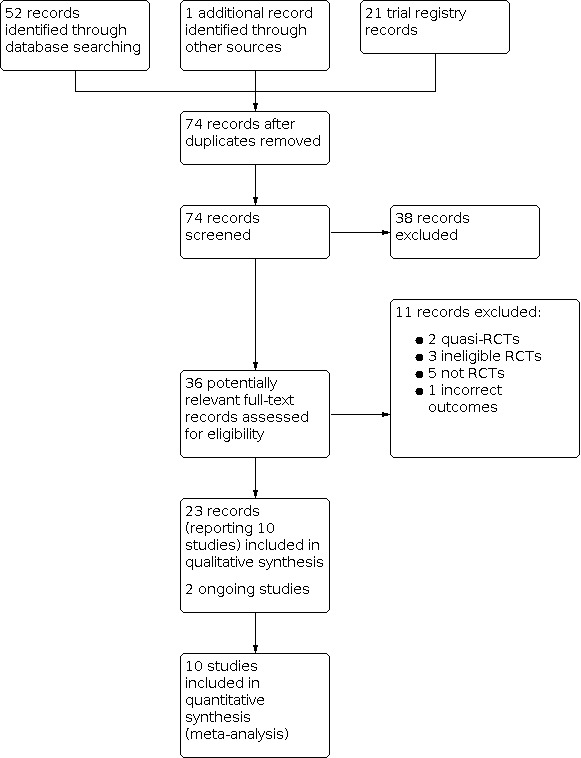
Study flow diagram.
Included studies
Studies meeting the inclusion criteria for efficacy outcomes
Five studies met the inclusion criteria for efficacy outcomes (see Characteristics of included studies) (Kayentao 2012; Roth 2018a; Rueangweerayut 2012; Sagara 2018; Tshefu 2010). The length of follow‐up was 42 days for each study.
Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine
Four RCTs evaluated this comparison (Kayentao 2012; Roth 2018a; Sagara 2018; Tshefu 2010).
Sample sizes ranged from 197 participants in Roth 2018a to 1344 participants in Sagara 2018, yielding a total number of 3415 for inclusion in quantitative synthesis. Two studies were multicentred in Africa and Asia (Kayentao 2012; Tshefu 2010); one was multicentred in Africa (Sagara 2018); and one was a single‐centre study in Africa (Roth 2018a). None of the studies described the P falciparum resistance profile to currently available antimalarials. In total, 3128 (94%) participants were recruited in Africa, and 213 (6%) participants were recruited in Asia.
Two studies included adults and children (Sagara 2018; Tshefu 2010), and two studies included children only (Kayentao 2012; Roth 2018a). In total, 541 (16%) participants were aged less than five years. All studies included both male and female participants. In total, 1568 (47%) participants were female.
All studies used three‐day regimens of pyronaridine‐artesunate, with the dose adjusted according to weight. There were minimal differences in dose by weight. The two paediatric trials used granule formulation.
All studies reported 'adequate clinical and parasitological response' rate) at day 28 and day 42, PCR‐adjusted and unadjusted. All studies also reported parasite clearance time (defined as first dose to aparasitaemia) and fever clearance time (defined as first dose to apyrexia).
Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine
One RCT evaluated this comparison, which was conducted in multiple centres in West Africa (Sagara 2018). The P falciparum resistance profile to currently available antimalarials was not described. In total, 1317 participants randomized to this comparison received at least one study treatment. Of these, 477 (36%) participants were aged less than five years, and 658 (50%) participants were female.
Both pyronaridine‐artesunate and artesunate‐amodiaquine were administered once daily for three days at doses according to bodyweight.
Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate
A single trial evaluated this comparison (Rueangweerayut 2012).
The sample size was 1271 participants. Most participants (1033, 81.3%) were from Asia (Cambodia, India, Thailand, and Vietnam), with a smaller number (238, 18.7%) from Africa (Burkina Faso, Ivory Coast, and Tanzania). The trial authors described malaria endemicity as high in most sites. In Cambodia, significantly extended parasite clearance times (for both treatment arms) were suggestive of in vivo resistance to artemisinin. Resistance in the other sites or to other antimalarials was not described. The trial planned to recruit participants aged between three and 60 years; the youngest participant was five years old.
Both pyronaridine‐artesunate and mefloquine plus artesunate were administered once daily for three days. The trial did not use a fixed‐dose combination of mefloquine and artesunate. The mefloquine dose ranged from 6.2 mg/kg to 12.5 mg/kg, and the artemether dose ranged from 2.2 mg/kg to 5.0 mg/kg.
Studies meeting the inclusion criteria for safety outcomes
In addition to the five studies meeting the inclusion criteria for efficacy, we included five further studies that met the inclusion criteria for safety outcomes. Two studies had a follow‐up period of 14 days (Ringwald 1996; Ringwald 1998); two had a follow‐up of 42 days (Poravuth 2011; Shin 2011); and one study had a follow‐up of one year (Nelwan 2015).
We included three of these studies in a meta‐analysis pertaining to serious adverse events and liver function tests (Nelwan 2015; Poravuth 2011; Shin 2011), in addition to the studies included in the efficacy analysis. These three studies contributed a further 666 participants to the meta‐analysis, and included participants with Plasmodium vivax malaria recruited from sites in Asia. One study included only adult male soldiers (Nelwan 2015). No participants were aged less than five years. Two studies excluded individuals with existing hepatic impairment (Nelwan 2015; Poravuth 2011). Further details of the inclusion and exclusion criteria are provided in the Characteristics of included studies tables.
The Nelwan 2015 study compared pyronaridine‐artesunate versus artesunate alone or dihydroartemisinin‐piperaquine. The other two studies were based on the same protocol (Poravuth 2011; Shin 2011), and compared pyronaridine‐artesunate versus chloroquine.
We included two further studies (Ringwald 1996; Ringwald 1998), contributing an additional 184 participants and comparing pyronaridine monotherapy to chloroquine, in the analysis of other adverse events.
We encountered heterogeneity in the threshold at which elevated liver function tests were deemed by study authors to be significant (see Table 6). It should be noted that these thresholds do not necessarily correspond with internationally accepted definitions of drug‐induced liver injury (summarized in Appendix 5) (Aithal 2011).
2. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin increase grading.
| Trial | ALT increased (grade 3 and above) | AST increased (grade 3 and above) | Blood bilirubin increased |
| Kayentao 2012 | 10 × ULN | 10 × ULN | 3 × ULN |
|
Poravuth 2011 Rueangweerayut 2012 Sagara 2018 Tshefu 2010 |
5 × ULN | 5 × ULN | 2.5 × ULN |
|
Roth 2018a Shin 2011 |
3 × ULN | 3 × ULN | ‐ |
| Nelwan 2015 | 3 × ULN if associated with bilirubin > 2 × ULN | ‐ | ‐ |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; ULN: upper limit of normal.
As is common in clinical trials, patients with known or suspected pre‐existing liver dysfunction were excluded. Concomitant paracetamol administration was allowed in at least two of the trial protocols (Poravuth 2011; Sagara 2018); the remaining trials do not record whether concomitant paracetamol was allowed or the extent to which it was used.
Excluded studies
We excluded 11 records after full‐text assessment (see Characteristics of excluded studies). Two were quasi‐RCTs; three were RCTs that were not relevant to this review; five were not RCTs; and one study did not report our outcomes of interest.
An additional five studies have been added to the excluded studies list as they are not RCTs, but these studies are included in the non‐randomized studies analysis of safety (NRS (safety)). Two previously identified excluded studies are also included in this analysis (Appendix 1).
NRS safety review
Full details of the results of the NRS safety review search and the characteristics of included studies are provided in Appendix 1. Briefly, we identified 374 records from database searches, of which 266 records remained after removal of duplicates. After title and abstract and full‐text screening, seven studies met our inclusion criteria, two of which were studies excluded in a previous version of this review as they were not RCTs (Leang 2016; Ramharter 2008). The studies included in the NRS safety review were five single‐arm observational studies with a total of 1007 participants; one cohort event monitoring study with 7746 participants; and one dose escalation study with 59 participants. Six studies included adults and children as participants, and one study included children only. All studies reported adverse event frequency; five reported serious adverse events; and two reported adverse effects related specifically to pyronaridine. Two studies reported elevations in liver function tests throughout the study as an indicator of liver injury, and two studies did not exclude participants with evidence of liver disease at baseline, allowing safety to be assessed in a representative population.
Risk of bias in included studies
See Figure 2.
2.
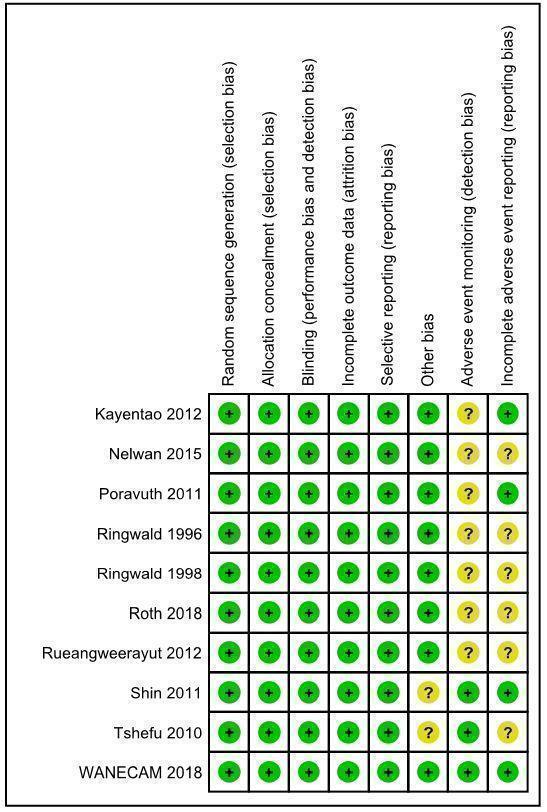
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven studies reported the use of computer‐generated allocation sequences (Kayentao 2012; Poravuth 2011; Roth 2018a; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). The Nelwan 2015 study reported "statistician block‐allocated treatment". Two studies reported block randomization, but it is unclear how the blocks were generated (Ringwald 1996; Ringwald 1998). We judged random sequence generation as at low risk of bias across studies.
Five studies concealed allocation using sealed, opaque envelopes (Nelwan 2015; Poravuth 2011; Roth 2018a; Sagara 2018; Shin 2011). Three studies concealed allocation using individually numbered treatment packs (Kayentao 2012; Rueangweerayut 2012; Tshefu 2010). Two studies reported central randomization in correspondence with the previous authors of this review (Ringwald 1996; Ringwald 1998). We judged allocation concealment as at low risk of bias across studies.
Blinding
Five studies reported that participants were blinded to treatment allocation (Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018a; Tshefu 2010). Six studies reported that the investigators performing clinical assessments were blinded to treatment allocation (Kayentao 2012; Poravuth 2011; Roth 2018a; Rueangweerayut 2012; Shin 2011; Tshefu 2010). Eight studies reported that the investigators performing parasitological assessments were blinded to treatment allocation (Kayentao 2012; Nelwan 2015; Poravuth 2011; Roth 2018a; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010).
Notwithstanding the different degrees to which studies were blinded, we judged there to be a low risk of performance bias and detection bias in relation to the outcomes assessed.
Incomplete outcome data
All of the included trials reported attrition with details of all randomized participants. Our analysis focused on evaluable participants. We did not have concerns that there was differential loss to follow‐up between interventions.
Selective reporting
We located trial registration documents for eight studies (Kayentao 2012; Nelwan 2015; Poravuth 2011; Roth 2018a; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). These appeared to be free of selective reporting based on comparison of registration documents and trial protocols, where available. Though trial registration documents were not available for the remaining two studies (Ringwald 1996; Ringwald 1998), we also considered them to be at low risk of reporting bias, as all expected outcomes were reported.
Other potential sources of bias
Seven of the 10 included studies were funded by the public‐private partnership of Medicines for Malaria Venture and Shin Poong Pharmaceuticals (Kayentao 2012; Nelwan 2015; Poravuth 2011; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). The Medicines for Malaria Venture or Shin Poong Pharmaceuticals, or both, employed study authors in six of these studies (Kayentao 2012; Poravuth 2011; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). We considered this to pose a low risk of bias, as all authors took responsibility for reporting accuracy, apart from one study (Shin 2011), where the lead authors were Shin Poong Pharmaceuticals employees.
Of the remaining three studies not funded by Medicines for Malaria Venture and Shin Poong Pharmaceuticals, one assessed pyronaridine‐artesunate (Roth 2018a), and the other two assessed pyronaridine monotherapy (Ringwald 1996; Ringwald 1998); these last two studies did not contribute to the main analyses.
We considered one study to have unclear risk of other bias in relation to bioavailability of lumefantrine (Tshefu 2010).
Adverse event monitoring (detection bias)
Seven studies provided unclear descriptions, definitions, or schedules for adverse advent monitoring, and were therefore deemed to be at unclear risk of detection bias for adverse events (Kayentao 2012; Nelwan 2015; Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018a; Rueangweerayut 2012). We deemed the remaining studies to be at low risk of detection bias for adverse events (Sagara 2018; Shin 2011; Tshefu 2010).
Incomplete adverse event reporting (reporting bias)
We identified unclear reporting of adverse events in five studies, with differences in reporting numbers or thresholds; we deemed these studies to be at unclear risk of reporting bias for adverse events (Nelwan 2015; Ringwald 1996; Ringwald 1998; Roth 2018a; Rueangweerayut 2012). We judged the remaining studies to be at low risk of reporting bias for adverse events (Kayentao 2012; Poravuth 2011; Sagara 2018; Shin 2011; Tshefu 2010).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Pyronaridine‐artesunate (PY‐AS) compared to artemether‐lumefantrine (AL) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to artemether‐lumefantrine (AL) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: artemether‐lumefantrine (AL) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with AL | Risk with PY‐AS | |||||
| Total failure: day 28 (PCR‐adjusted) | 15 per 1000 | 9 per 1000 (4 to 19) | RR 0.59 (0.26 to 1.31) | 3068 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b,c Due to indirectness and imprecision |
Compared to AL, PY‐AS may result in fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 23 per 1000 | 20 per 1000 (12 to 35) | RR 0.86 (0.49 to 1.51) | 2575 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
There may be little or no difference between PY‐AS and AL in PCR‐adjusted failures at day 42. |
| Total failure: day 28 (unadjusted) | 126 per 1000 | 34 per 1000 (16 to 73) | RR 0.27 (0.13 to 0.58) | 3149 (4 RCTs) | ⊕⊕⊝⊝
LOWa,d,e Due to indirectness and inconsistency |
Compared to AL, PY‐AS may result in fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 254 per 1000 | 155 per 1000 (117 to 208) | RR 0.61 (0.46 to 0.82) | 3080 (4 RCTs) | ⊕⊕⊝⊝
LOWa,d,e Due to indirectness and inconsistency |
Compared to AL, PY‐AS may result in fewer unadjusted failures at day 42. |
| Serious adverse events (42 days) | 3 per 1000 | 5 per 1000 (1 to 19) | RR 1.16 (0.30 to 4.50) | 2004 (3 RCTs) | ⊕⊕⊝⊝
LOWf Due to imprecision |
We do not know if there is a difference in serious adverse events between PY‐AS and AL. |
| First treatment, abnormal ALT increase (42 days) | 3 per 1000 | 10 per 1000 (4 to 25) | RR 3.34 (1.33 to 8.39) | 3415 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
Compared to AL, PY‐AS may result in higher events of abnormal ALT increase. (Aggregate analysis indicates this estimate may be accurate.) |
| First treatment, abnormal AST increase (42 days) | 3 per 1000 | 9 per 1000 (4 to 24) | RR 3.12 (1.23 to 7.94) | 3415 (4 RCTs) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
Compared to AL, PY‐AS may result in higher events of abnormal AST increase. |
| First treatment, abnormal bilirubin increase (42 days) | 6 per 1000 | 5 per 1000 (2 to 12) | RR 0.82 (0.33 to 2.04) | 3130 (3 RCTs) | ⊕⊕⊝⊝
LOWa,g Due to indirectness and imprecision |
We do not know if there is a difference between PY‐AS and AL in bilirubin. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AL: artemether‐lumefantrine;ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for serious indirectness: the trials included adults and children and had sites in Africa and Asia. However, across the trials, only 115 children and 0 adults were randomized to pyronaridine‐artesunate in Asia. Further adequately powered studies in adults and children in Asia would be needed for this result to be fully applicable. bDowngraded by one level for serious imprecision: the CIs are wide and include both almost no effect and clinically significant effect. cCertainty of the evidence differs from the 2014 review version due to the identification of additional data. The previous review reported no substantial difference between PY‐AS and AL for this outcome and therefore did not downgrade for imprecision. In this update, we have reported a reduced rate in the PY‐AS arm. Because we concluded that there may be a difference, we necessarily downgraded for the imprecision. dCertainty of the evidence differs from the 2014 review version due to the identification of additional data; the introduction of more data increased the heterogeneity between the included trials. eDowngraded by one level for serious inconsistency: there was quantitative heterogeneity between studies. fDowngraded by two levels for very serious imprecision: the low numbers of events recorded in the studies are insufficient to confidently estimate the effect size. gDowngraded by one level for serious imprecision: the CI includes both no effect and clinically significant effect.
Summary of findings 2. Pyronaridine‐artesunate (PY‐AS) compared to artesunate‐amodiaquine (AS‐AQ) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to artesunate‐amodiaquine (AS‐AQ) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: artesunate‐amodiaquine (AS‐AQ) | ||||||
| Outcomesa | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with AS‐AQ | Risk with PY‐AS | |||||
| Total failure: day 28 (PCR‐adjusted) | 8 per 1000 | 4 per 1000 (1 to 22) | RR 0.55 (0.11 to 2.77) | 1245 (1 RCT) | ⊕⊕⊝⊝
LOWb,c Due to indirectness and imprecision |
Compared to AS‐AQ, PY‐AS may result in fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 6 per 1000 | 5 per 1000 (1 to 27) | RR 0.98 (0.20 to 4.83) | 1091 (1 RCT) | ⊕⊕⊝⊝
LOWb,d Due to indirectness and imprecision |
There may be little or no difference between PY‐AS and AS‐AQ in PCR‐adjusted failures at day 42. |
| Total failure: day 28 (unadjusted) | 75 per 1000 | 37 per 1000 (22 to 61) | RR 0.49 (0.30 to 0.81) | 1257 (1 RCT) | ⊕⊕⊕⊝
MODERATEb Due to indirectness |
Compared to AS‐AQ, PY‐AS probably results in fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 195 per 1000 | 192 per 1000 (152 to 240) | RR 0.98 (0.78 to 1.23) | 1235 (1 RCT) | ⊕⊕⊕⊝
MODERATEb Due to indirectness |
There is probably little or no difference between PY‐AS and AS‐AQ in unadjusted failures at day 42. |
| First treatment, abnormal ALT increase (42 days) | 1 per 1000 | 1 per1000 (0 to 7) | RR 1.41 (0.28 to 7.09) | 1317 (1 RCT) | ⊕⊕⊝⊝
LOWb,e Due to indirectness and imprecision |
Compared to AS‐AQ, PY‐AS may result in higher events of abnormal ALT increase. (Aggregate analysis indicates this estimate may be accurate.) |
| First treatment, abnormal AST increase (42 days) | 4 per 1000 | 2 per 1000 (0 to 8) | RR 0.40 (0.08 to 2.07) | 1317 (1 RCT) | ⊕⊝⊝⊝
VERY LOWb,f Due to indirectness and imprecision |
We do not know if there is a difference between PY‐AS and AS‐AQ in AST. |
| First treatment, abnormal bilirubin increase (42 days) | 1 per 1000 | 1 per 1000 (0 to 16) | RR 0.99 (0.06 to 15.76) | 1317 (1 RCT) | ⊕⊝⊝⊝
VERY LOWb,f Due to indirectness and imprecision |
We do not know if there is a difference between PY‐AS and AS‐AQ in bilirubin. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AS‐AQ: artesunate‐amodiaquine; AST: aspartate transaminase; CI: confidence interval; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aSerious adverse events data were not available disaggregated by site to permit their inclusion in this comparison. bDowngraded by one level for serious indirectness: the data are from one study, conducted in six sites in three countries in West Africa. Further studies in Asia would be needed for this result to be fully applicable. cDowngraded by one level for serious imprecision: the CI is large and includes both no effect and clinically important effects. dDowngraded by one level for serious imprecision: the effect estimate is close to no effect, but the CI is wide. eDowngraded by one level for serious imprecision: the low number of events recorded in the study is insufficient to confidently estimate the effect size. However, aggregate analysis of ALT increase across different comparator drugs provides indirect evidence that the point estimate may be accurate. fDowngraded by two levels for very serious imprecision: the CI is very large and includes both no effect and clinically important effects.
Summary of findings 3. Pyronaridine‐artesunate (PY‐AS) compared to mefloquine plus artesunate (MQ + AS) for adults and children with uncomplicated Plasmodium falciparum malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to mefloquine plus artesunate (MQ + AS) for adults and children with uncomplicated Plasmodium falciparum malaria | ||||||
| Patient or population: adults and children with uncomplicated P falciparum malaria Setting: malaria transmission settings Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: mefloquine plus artesunate (MQ + AS) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with MQ + AS | Risk with PY‐AS | |||||
| Total failure: day 28 (PCR‐adjusted) | 22 per 1000 | 8 per 1000 (3 to 23) | RR 0.37 (0.13 to 1.05) | 1117 (1 RCT) | ⊕⊕⊝⊝
LOWa,b,c Due to indirectness and imprecision |
Compared to MQ + AS, PY‐AS may result in fewer PCR‐adjusted failures at day 28. |
| Total failure: day 42 (PCR‐adjusted) | 29 per 1000 | 53 per 1000 (27 to 105) | RR 1.80 (0.90 to 3.57) | 1037 (1 RCT) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
Compared to MQ + AS, PY‐AS may result in more PCR‐adjusted failures at day 42. |
| Total failure: day 28 (unadjusted) | 41 per 1000 | 15 per 1000 (7 to 32) | RR 0.36 (0.17 to 0.78) | 1120 (1 RCT) | ⊕⊕⊕⊝
MODERATEa Due to indirectness |
Compared to MQ + AS, PY‐AS probably results in fewer unadjusted failures at day 28. |
| Total failure: day 42 (unadjusted) | 83 per 1000 | 70 per 1000 (45 to 109) | RR 0.84 (0.54 to 1.31) | 1059 (1 RCT) | ⊕⊕⊝⊝
LOWa,b,d Due to indirectness and imprecision |
There is probably little or no difference between PY‐AS and MQ + AS in unadjusted failures at day 42. |
| Serious adverse events (42 days) | 7 per 1000 | 7 per 1000 (2 to 28) | RR 1.00 (0.25 to 3.97) | 1271 (1 RCT) | ⊕⊕⊝⊝
LOWa,b Due to indirectness and imprecision |
There may be little or no difference between PY‐AS and MQ + AS in serious adverse events. |
| First treatment, abnormal ALT increase (42 days) | 2 per 1000 | 18 per 1000 (2 to 133) | RR 7.48 (0.99 to 56.45) | 1271 (1 RCT) | ⊕⊕⊝⊝
LOWa,e Due to indirectness and imprecision |
Compared to MQ + AS, PY‐AS may result in higher events of abnormal ALT increase. (Aggregate analysis indicates this estimate may be accurate.) |
| First treatment, abnormal AST increase (42 days) | 0 per 1000 | 0 per 1000 (0 to 0) | RR 9.49 (0.55 to 162.64) | 1271 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,f Due to indirectness and imprecision |
We do not know if there is a difference between PY‐AS and MQ + AS in AST. |
| First treatment, abnormal bilirubin increase (42 days) | 2 per 1000 | 8 per 1000 (1 to 67) | RR 3.49 (0.43 to 28.29) | 1271 (1 RCT) | ⊕⊝⊝⊝
VERY LOWa,f Due to indirectness and imprecision |
We do not know if there is a difference between PY‐AS and MQ + AS in bilirubin. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; MQ + AS: mefloquine plus artesunate; PCR: polymerase chain reaction; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for serious indirectness: of the 1271 children and adults aged greater than 5 years enrolled in this trial, 81.3% (1033) were enrolled and treated in trial sites in Asia (Cambodia, India, Thailand, and Vietnam), and only 18.7% (237) in Africa (Burkina Faso, Ivory Coast, and Tanzania). Further studies in African children are necessary for this result to be fully applicable. bDowngraded by one level for serious imprecision: the CI is large and includes both no effect and clinically important effects. cCertainty of the evidence differs from the 2014 review version due to the identification of additional data: the previous review reported no substantial difference between PY‐AS and MQ + AS for this outcome and therefore did not downgrade for imprecision. In this update we have reported a reduced rate in the PY‐AS arm. Because we concluded that there may be a difference, we necessarily downgraded for the imprecision. dCertainty of the evidence differs from the 2014 review version due to alterations in the data extraction protocol: the CI has become less precise, and our decision is more consistent with the certainty of evidence for other outcomes. eDowngraded by one level for serious imprecision: the low number of events recorded in the study is insufficient to confidently estimate the effect size. However, aggregate analysis of ALT increase across different comparator drugs provides indirect evidence that the point estimate may be accurate. fDowngraded by two levels for very serious imprecision: the CI is very large and includes both no effect and clinically important effects.
Summary of findings 4. Pyronaridine‐artesunate (PY‐AS) compared to other antimalarials for adults and children with uncomplicated malaria.
| Pyronaridine‐artesunate (PY‐AS) compared to other antimalarials for adults and children with uncomplicated malaria | ||||||
| Patient or population: adults and children with uncomplicated malaria Setting: high and low transmission settings for P falciparum and P vivax malaria Intervention: pyronaridine‐artesunate (PY‐AS) Comparison: other antimalarials | ||||||
| Outcomesa,b,c | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other antimalarials | Risk with PY‐AS | |||||
| Serious adverse events | 5 per 1000 | 7 per 1000 (3 to 15) | RR 1.24 (0.54 to 2.84) | 3941 (7 RCTs) | ⊕⊕⊕⊝
MODERATEd Due to imprecision |
There is probably little or no difference between PY‐AS and other antimalarials in rate of serious adverse events. |
| First treatment, abnormal ALT increase | 2 per 1000 | 7 per 1000 (4 to 15) | RR 3.59 (1.76 to 7.33) | 6669 (8 RCTs) | ⊕⊕⊕⊕ HIGHe,f | Abnormal ALT increase is more frequent with PY‐AS compared to other antimalarials. |
| First treatment, abnormal AST increase | 3 per 1000 | 7 per 1000 (3 to 13) | RR 2.22 (1.12 to 4.41) | 6669 (8 RCTs) | ⊕⊕⊕⊝
MODERATEf,g Due to imprecision |
There is probably an increased risk of abnormal AST increase with PY‐AS compared to other antimalarials. |
| First treatment, abnormal bilirubin increase | 4 per 1000 | 4 per 1000 (2 to 9) | RR 1.03 (0.49 to 2.18) | 6384 (7 RCTs) | ⊕⊕⊕⊝
MODERATEd Due to imprecision |
There is probably little or no difference between PY‐AS and other antimalarials in bilirubin. |
| Subsequent treatment(s), abnormal ALT increase | 4 per 1000 | 8 per 1000 (3 to 23) | RR 2.18 (0.76 to 6.27) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,h Due to imprecision and indirectness |
When receiving a subsequent treatment, there may be an increased risk of abnormally raised ALT with PY‐AS compared to other antimalarials. |
| Subsequent treatment(s), abnormal AST increase | 6 per 1000 | 11 per 1000 (4 to 27) | RR 1.82 (0.74 to 4.44) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,h Due to imprecision and indirectness |
When receiving a subsequent treatment, there may be an increased risk of abnormally raised AST with PY‐AS compared to other antimalarials. |
| Subsequent treatment(s), abnormal bilirubin increase | 8 per 1000 | 9 per 1000 (3 to 24) | RR 1.13 (0.42 to 3.01) | 1649 (1 RCT) | ⊕⊕⊝⊝
LOWd,h Due to imprecision and indirectness |
There may be little or no difference between PY‐AS and other antimalarials in bilirubin. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ALT: alanine aminotransferase; AST: aspartate transaminase; CI: confidence interval; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOnly adverse event outcomes were considered for this comparison. bA comparison of pyronaridine‐artesunate versus other antimalarials for frequency of electrocardiogram (ECG) abnormalities is reported in Table 5. cThe length of follow‐up varies between studies. Follow‐up times are reported for individual studies in the Characteristics of included studies tables. dDowngraded by one level for serious imprecision: the CI includes both no effect and clinically important effects. eNot downgraded: although the CI is wide, there were few events. fObservational data from non‐randomized studies corroborated the increased risk of raised liver enzymes when treated with pyronaridine‐artesunate. These changes were judged as 'mild' on the liver severity index, and no increase in the risk of adverse events was observed in participants with pre‐existing elevations in liver enzyme compared to those with normal liver enzyme levels at baseline. gDowngraded by one level for serious imprecision: the CI includes both almost no effect and clinically important effect. hDowngraded by one level for serious indirectness: only 232 children aged less than five years were included in this study.
Comparison 1. Pyronaridine‐artesunate versus artemether‐lumefantrine
Four studies with 3341 participants contributed data to this comparison (Kayentao 2012; Roth 2018a; Sagara 2018; Tshefu 2010).
Total treatment failure (PCR‐adjusted)
In the pooled analysis, there were fewer PCR‐adjusted treatment failures at day 28 following treatment with pyronaridine‐artesunate compared to artemether‐lumefantrine, but the confidence interval (CI) crossed the line of no effect (risk ratio (RR) 0.59, 95% CI 0.26 to 1.31; 4 trials, 3068 participants; Analysis 1.1). There was little or no difference between groups at day 42 (RR 0.86, 95% CI 0.49 to 1.51; 4 trials, 2575 participants; Analysis 1.2).
1.1. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 1: Total failure: day 28 (PCR‐adjusted)
1.2. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 2: Total failure: day 42 (PCR‐adjusted)
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% in all trials at day 28. At day 42, the PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was slightly greater than 5% in two studies (Kayentao 2012, 18 events for 275 evaluable participants, 6.5%; Roth 2018a, 4 events for 77 evaluable participants, 5.2%).
Total treatment failure (PCR‐unadjusted)
In the pooled analysis, there were fewer PCR‐unadjusted treatment failures following treatment with pyronaridine‐artesunate compared to artemether‐lumefantrine at day 28 (RR 0.27, 95% CI 0.13 to 0.58; 4 trials, 3149 participants; Analysis 1.3) and at day 42 (RR 0.61, 95% CI 0.46 to 0.82, 4 trials, 3080 participants; Analysis 1.4).
1.3. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 3: Total failure: day 28 (unadjusted)
1.4. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 4: Total failure: day 42 (unadjusted)
Early treatment failure
Two events of early treatment failure occurred in one trial (Kayentao 2012), both in the pyronaridine‐artesunate arm (RR 2.53, 95% CI 0.12 to 52.39; 4 trials, 3149 participants; Analysis 1.5).
1.5. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 5: Early treatment failure
Serious adverse events
We were unable to include data on serious adverse events from one multicentre trial in the meta‐analysis as the data were not disaggregated by trial site (Sagara 2018), and participant randomization did not take place independently from site. Instead, we have summarized the number and nature of the serious adverse events in the trial in Table 7. Across the trials included in the quantitative synthesis, there were six serious adverse events, four of which occurred in participants in the pyronaridine‐artesunate arm and two in participants in the artemether‐lumefantrine arm. There was no significant difference between treatment groups (RR 1.16, 95% CI 0.30 to 4.50; 3 trials, 2004 participants; Analysis 1.6).
3. Serious adverse events.
| Study | Pyronaridine‐artesunate | Comparator(s) |
| Kayentao 2012 | Severe malaria (1)a |
Artemether‐lumefantrine ‐ |
| Nelwan 2015 | Head trauma (1)a Typhoid fever (1)b Nephrolithiasis (1)b |
Artesunate only Metacarpal fracture (1)a Acute gastroenteritis (1)a Suspected ureteric stone (1)b Dihydroartemisinin‐piperaquine Dengue fever (1)a |
| Poravuth 2011 | Pyrexia (1)a Typhoid fever (1)a |
Chloroquine ‐ |
| Ringwald 1996 | ‐ | ‐ |
| Ringwald 1998 | ‐ | ‐ |
| Roth 2018a | ‐ | ‐ |
| Rueangweerayut 2012 | Autoimmune haemolytic anaemia (1)a Cholera (1)a Pneumonia (1)a Acute pyelonephritis (1)a Wound infection (1)a Abortion (1)a Depression (1)a |
Mefloquine plus artesunate Cerebral malaria (1)a Seizure (1)c Grand mal seizure (1)c |
| Tshefu 2010 | Parotitis (1)a Typhoid fever (1)a Urinary tract infection (1)a |
Artemether‐lumefantrine Cerebral malaria (1)a Immunosuppression (1)a |
| Sagara 2018d | Elevated ALT (2)c Elevated AST (2)c Transaminases increased (4)c Drug‐induced liver injury (1)c Hypercreatininaemia (1)c |
Artemether‐lumefantrine Drug‐induced liver injury (1)c Toxic epidermal necrolysis (1)c Artesunate‐amodiaquine Drug‐induced liver injury (1)c Transaminases increased (2)c |
Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase.
aJudged by study authors as unrelated to drug. bJudged by study authors as unlikely to be related to drug. cJudged by study authors as treatment‐related. dThe nature of the serious adverse events judged to be unrelated to drug is not reported. Some of the listed events in the comparator groups may have occurred in comparisons with dihydroartemisinin‐piperaquine, but we were unable to extract data in relation to this.
1.6. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 6: Serious adverse events
Adverse events leading to withdrawal from treatment
We were unable to include data from one trial for the same reason as described above (Sagara 2018). Across the trials included in quantitative synthesis, there were 37 events leading to withdrawal from treatment, 27 of which occurred in participants in the pyronaridine‐artesunate arm and 10 in participants in the artemether‐lumefantrine arm. There was no significant difference between treatment groups (RR 1.41, 95% CI 0.68 to 2.90; 3 trials, 2004 participants; Analysis 1.7).
1.7. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 7: Adverse events leading to withdrawal
Elevated liver function tests
In this review update, we included one new record contributing to Sagara 2018. This led to a slight increase in RR for the proportion of participants with abnormally raised ALT and AST following first treatment.
The proportion of participants with abnormally raised ALT was higher in those treated with pyronaridine‐artesunate compared to artemether‐lumefantrine (RR 3.34, 95% CI 1.33 to 8.39; 4 trials, 3415 participants; Analysis 1.8, Figure 3). There were zero events in either arm of one study (Roth 2018a), so this did not contribute to the risk ratio calculation.
1.8. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 8: First treatment, ALT increase > 5 × ULN
3.

Forest plot of comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, outcome 1.8: ALT increase > 5 × ULN, first treatment.
The proportion of participants with abnormally raised AST was higher in those treated with pyronaridine‐artesunate compared to artemether‐lumefantrine (RR 3.12, 95% CI 1.23 to 7.94; 4 trials, 3415 participants; Analysis 1.9, Figure 4).
1.9. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 9: First treatment, AST increase > 5 × ULN
4.

Forest plot of comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, outcome: 1.9 AST increase > 5 × ULN, first treatment.
There was no significant difference in abnormally raised bilirubin between pyronaridine‐artesunate and artemether‐lumefantrine (RR 0.82, 95% CI 0.33 to 2.04; 3 trials, 3130 participants; Analysis 1.10).
1.10. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 10: First treatment, bilirubin increase > 2.5 × ULN
One trial investigated the rate of elevated liver function tests in participants receiving second or subsequent treatments with pyronaridine‐artesunate compared to artemether‐lumefantrine (Sagara 2018). The rates of such events were low in each treatment arm. In a pooled analysis across the trial sites, we detected no significant differences in the number of abnormally raised ALT, AST, or bilirubin events between pyronaridine‐artesunate and artemether‐lumefantrine (1 trial, 865 participants; Analysis 1.11; Analysis 1.12; Analysis 1.13).
1.11. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 11: Subsequent treatment(s), ALT increase > 5 × ULN
1.12. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 12: Subsequent treatment(s), AST increase > 5 × ULN
1.13. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 13: Subsequent treatment(s), bilirubin increase > 2.5 × ULN
Other adverse events
No data were available for this outcome.
Subgroup analysis
When including only the two trials which studied paediatric populations exclusively (Kayentao 2012; Roth 2018a), we did not find differences between pyronaridine‐artesunate and artemether‐lumefantrine in efficacy (Analysis 1.14; Analysis 1.15; Analysis 1.16; Analysis 1.17; 558 to 693 participants) or safety outcomes (Analysis 1.18; Analysis 1.19; 732 participants). We were unable to extract disaggregated data for children from the other two trials (Sagara 2018; Tshefu 2010).
1.14. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 14: Paediatric trials ‐ total failure: day 28 (PCR‐adjusted)
1.15. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 15: Paediatric trials ‐ total failure: day 42 (PCR‐adjusted)
1.16. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 16: Paediatric trials ‐ total failure: day 28 (unadjusted)
1.17. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 17: Paediatric trials ‐ total failure: day 42 (unadjusted)
1.18. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 18: Paediatric trials ‐ first treatment, ALT increase > 5 × ULN
1.19. Analysis.

Comparison 1: Pyronaridine‐artesunate versus artemether‐lumefantrine, Outcome 19: Paediatric trials ‐ first treatment, AST increase > 5 × ULN
Insufficient studies prevented us from performing further subgroup analyses or investigation of heterogeneity.
Narrative synthesis of other reported outcomes
Three studies also reported fever and parasite clearance times, which were broadly comparable between pyronaridine‐artesunate and artemether‐lumefantrine (Table 8). Differences in reporting precluded quantitative synthesis.
4. Pyronaridine‐artesunate (PY‐AS) versus artemether‐lumefantrine (AL): other reported outcomes.
| Trial | Fever clearance time | Parasite clearance time | ||
| PY‐AS | AL | PY‐AS | AL | |
| Kayentao 2012 | Median 8.1 h (95% CI 8.0 to 8.1) | Median 8.1 h (95% CI 8.0 to 15.8) | Median 24.1 h (95% CI 24.0 to 24.1) | Median 24.2 h (95% CI 24.1 to 32.0) |
| Roth 2018a | Median 1 day (1 to 1) | Median 1 day (1 to 1) | Median 1 day (1 to 2) | Median 2 days (1 to 2) |
| Tshefu 2010 | Mean 13.6 h (SD 8.9) | Mean 14.8 h (SD 10.1) | Mean 23.3 h (SD 8.8) | Mean 26.5 h (SD 10.1) |
Abbreviations: AL: artemether‐lumefantrine; CI: confidence interval; PY‐AS: pyronaridine‐artesunate; SD: standard deviation.
Comparison 2. Pyronaridine‐artesunate versus artesunate‐amodiaquine
One study with 1336 participants contributed data to this comparison (Sagara 2018). We extracted data disaggregated by site as described in Results of the search, and presented data for each site separately in our meta‐analyses.
Total treatment failure (PCR‐adjusted)
In the pooled analysis across the multiple sites, there were fewer PCR‐adjusted treatment failures at day 28 for pyronaridine‐artesunate compared to artesunate‐amodiaquine, but the CI crossed the line of no effect (RR 0.55, 95% CI 0.11 to 2.77; 1 trial, 1245 participants; Analysis 2.1). There was little or no difference between groups in PCR‐adjusted treatment failure at day 42 (RR 0.98, 95% CI 0.20 to 4.83; 1 trial, 1091 participants; Analysis 2.2).
2.1. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 1: Total failure: day 28 (PCR‐adjusted)
2.2. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 2: Total failure: day 42 (PCR‐adjusted)
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% in all sites at both day 28 and day 42.
Total treatment failure (PCR‐unadjusted)
In pooled analysis, there were fewer PCR‐unadjusted treatment failures with pyronaridine‐artesunate compared to artesunate‐amodiaquine at day 28 (RR 0.49, 95% CI 0.30 to 0.81; 1 trial, 1257 participants; Analysis 2.3). There was little or no difference between groups at day 42 (RR 0.98, 95% CI 0.78 to 1.23; 1 trial, 1235 participants; Analysis 2.4).
2.3. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 3: Total failure: day 28 (unadjusted)
2.4. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 4: Total failure: day 42 (unadjusted)
Early treatment failure
There was no early treatment failure reported in either the pyronaridine‐artesunate arm or the artesunate‐amodiaquine arm across all study sites (1336 participants, 1 trial).
Serious adverse events, adverse events leading to withdrawal from treatment
We were unable to include trial data on serious adverse events and adverse events leading to withdrawal in a meta‐analysis for the same reason described above. We summarized the number and nature of the serious adverse events in Sagara 2018 in Table 7.
Elevated liver function tests
Following first treatment, there was no significant difference between pyronaridine‐artesunate and artesunate‐amodiaquine in abnormally raised ALT (RR 1.41, 95% CI 0.28 to 7.09; 1 trial, 1317 participants; Analysis 2.5); abnormally raised AST (RR 0.40, 95% CI 0.08 to 2.07; 1 trial, 1317 participants, Analysis 2.6); or abnormally raised bilirubin (RR 0.99, 95% CI 0.06 to 15.76; 1 trial, 1317 participants; Analysis 2.7).
2.5. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 5: First treatment, ALT increase > 5 × ULN
2.6. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 6: First treatment, AST increase > 5 × ULN
2.7. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 7: First treatment, bilirubin increase > 2.5 × ULN
Similarly, on second or subsequent treatments, we detected no significant difference in the number of abnormally raised ALT, AST, or bilirubin events between pyronaridine‐artesunate and artesunate‐amodiaquine treatment arms (784 participants, 1 trial; Analysis 2.8; Analysis 2.9; Analysis 2.10).
2.8. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 8: Subsequent treatment(s), ALT increase > 5 × ULN
2.9. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 9: Subsequent treatment(s), AST increase > 5 × ULN
2.10. Analysis.

Comparison 2: Pyronaridine‐artesunate versus artesunate‐amodiaquine, Outcome 10: Subsequent treatment(s), bilirubin increase > 2.5 × ULN
Other adverse events
No data were available for this outcome.
Narrative synthesis of other reported outcomes
In this review update, one new record contributing to the Sagara 2018 study also reported parasite clearance times, which were broadly comparable between pyronaridine‐artesunate and artesunate‐amodiaquine (Table 9).
5. Pyronaridine‐artesunate (PY‐AS) versus artesunate‐amodiaquine (AS‐AQ): other reported outcomes.
| Trial | Parasite clearance time | |
| PY‐AS | AS‐AQ | |
| Sagara 2018 | 24.1 h (95% CI 24.0 to 24.2 h) | 23.9 h (95% CI 23.8 to 24.0 h) |
Abbreviations: CI: confidence interval; PY‐AS: pyronaridine‐artesunate; AS‐AQ: artesunate‐amodiaquine.
Comparison 3. Pyronaridine‐artesunate versus mefloquine plus artesunate
One study with 1271 participants contributed data to this comparison (Rueangweerayut 2012).
Total treatment failure (PCR‐adjusted)
There were fewer PCR‐adjusted treatment failures at day 28 for pyronaridine‐artesunate compared to mefloquine plus artesunate, but the CI crossed the line of no effect (RR 0.37, 95% CI 0.13 to 1.05; 1 trial, 1117 participants; Analysis 3.1). There were more PCR‐adjusted treatment failures at day 42 with pyronaridine‐artesunate compared to mefloquine plus artesunate, but the CI crossed the line of no effect (RR 1.80, 95% CI 0.90 to 3.57; 1 trial, 1037 participants; Analysis 3.2).
3.1. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 1: Total failure: day 28 (PCR‐adjusted)
3.2. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 2: Total failure: day 42 (PCR‐adjusted)
The PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was less than 5% at day 28. At day 42, the PCR‐adjusted treatment failure rate for pyronaridine‐artesunate was slightly greater than 5% (37 events for 698 evaluable participants, 6.5%).
Total treatment failure (PCR‐unadjusted)
There were fewer PCR‐unadjusted treatment failures with pyronaridine‐artesunate compared to mefloquine plus artesunate at day 28 (RR 0.36, 95% CI 0.17 to 0.78; 1 trial, 1120 participants; Analysis 3.3). There was little or no difference between pyronaridine‐artesunate and mefloquine plus artesunate at day 42 (RR 0.84, 95% CI 0.54 to 1.31; 1 trial, 1059 participants; Analysis 3.4).
3.3. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 3: Total failure: day 28 (unadjusted)
3.4. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 4: Total failure: day 42 (unadjusted)
Early treatment failure
There was one early treatment failure in the mefloquine plus artesunate arm of the study, and none in the pyronaridine‐artesunate arm.
Serious adverse events, adverse events leading to withdrawal from treatment
There was little or no difference in serious adverse events between pyronaridine‐artesunate and mefloquine plus artesunate (RR 1.00, 95% CI 0.25 to 3.97; 1 trial, 1271 participants; Analysis 3.5). There was no significant different between groups in adverse events leading to withdrawal from treatment (1271 participants, 1 trial; Analysis 3.6).
3.5. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 5: Serious adverse events
3.6. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 6: Adverse events leading to withdrawal
Elevated liver function tests
There were higher rates of abnormally raised ALT in the pyronaridine‐artesunate arm compared to the mefloquine plus artesunate arm, but the CIs crossed the line of no effect (RR 7.48, 95% CI 0.99 to 56.45; 1 trial, 1271 participants; Analysis 3.7). We did not find a significant difference in the rate of abnormally raised AST (RR 9.49, 95% CI 0.55 to 162.64; 1 trial, 1271 participants; Analysis 3.8) or bilirubin (RR 3.49, 95% CI 0.43 to 28.29; 1 trial, 1271 participants; Analysis 3.9).
3.7. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 7: First treatment, ALT increase > 5 × ULN
3.8. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 8: First treatment, AST increase > 5 × ULN
3.9. Analysis.

Comparison 3: Pyronaridine‐artesunate versus mefloquine plus artesunate, Outcome 9: First treatment, bilirubin increase > 2.5 × ULN
Other adverse events
No data were available for this outcome.
Narrative synthesis of other reported outcomes
The Rueangweerayut 2012 study also reported fever, parasite and gametocyte clearance times, which were broadly comparable between pyronaridine‐artesunate and mefloquine plus artesunate (Table 10).
6. Pyronaridine‐artesunate (PY‐AS) versus mefloquine plus artesunate (MQ + AS): other reported outcomes.
| Trial | Fever clearance time | Parasite clearance time | Gametocyte clearance time | |||
| PY‐AS | MQ + AS | PY‐AS | MQ + AS | PY‐AS | MQ + AS | |
| Rueangweerayut 2012 | Mean 19.3 h (SD 12.9) | Mean 19.2 h (SD 12.5) | Mean 35.9 h (SD 19.8) | Mean 38.5 h (SD 20.1) | Mean 25.5 h (SD 23.3) | Mean 30.9 h (SD 19.9) |
Abbreviations: MQ + AS: mefloquine plus artesunate; PY‐AS: pyronaridine‐artesunate; SD: standard deviation.
Comparison 4. Pyronaridine‐artesunate versus any other antimalarial
Eight RCTs with 6614 participants contributed data to a safety meta‐analysis comparing pyronaridine‐artesunate to any other antimalarial. The comparators were artesunate alone, artemether‐lumefantrine, dihydroartemisinin‐piperaquine, chloroquine, mefloquine plus artesunate, and artesunate‐amodiaquine. For the Sagara 2018 trial, we extracted data disaggregated by site, as described in Results of the search, and have presented data for each site separately in meta‐analysis.
An additional two RCTs compared pyronaridine monotherapy to chloroquine (Ringwald 1996; Ringwald 1998), and contributed to the quantitative and qualitative synthesis of other adverse events (excluding serious adverse events or liver enzymes).
All trials contributing to the safety meta‐analysis excluded individuals with baseline hepatic impairment.
Serious adverse events
We detected little or no difference in the rate of serious adverse events with pyronaridine‐artesunate compared to other antimalarials (RR 1.24, 95% CI 0.54 to 2.84; 7 trials, 3941 participants; Analysis 4.1). We were unable to include data from the Sagara 2018 trial in this meta‐analysis, as explained above.
4.1. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 1: Serious adverse events
To provide a narrative synthesis, we summarized the nature and number of serious adverse events in Table 7. In the pyronaridine‐artesunate arm, there were 26 serious adverse events across the 10 included trials (Kayentao 2012; Nelwan 2015; Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018a; Rueangweerayut 2012; Sagara 2018; Shin 2011; Tshefu 2010). Of these 26 serious adverse events, we judged 10 events to be related to treatment with pyronaridine‐artesunate or pyronaridine alone, with each of these occurring in the Sagara 2018 trial. In comparison, we judged two of four events in the artemether‐lumefantrine arm, Kayentao 2012; Sagara 2018; Tshefu 2010; three of three events in the artesunate‐amodiaquine arm, Sagara 2018; and two of three events in the mefloquine plus artesunate arm, Rueangweerayut 2012, to be related to the treatment. We did not judge the sole serious adverse event seen with dihydroartemisinin‐piperaquine and the three serious adverse events seen in the artesunate‐only arm to be related to treatment (Nelwan 2015). No serious adverse events were seen in the chloroquine arm (Poravuth 2011; Ringwald 1996; Ringwald 1998; Roth 2018a).
Adverse events leading to withdrawal from treatment
We detected little or no difference between pyronaridine‐artesunate and other antimalarials in the rate of adverse events leading to withdrawal from treatment (RR 1.06, 95% CI 0.58 to 1.94; 6 trials, 3911 participants; Analysis 4.2).
4.2. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 2: Adverse events leading to withdrawal
Elevated liver function tests
Quantitative synthesis
Different studies defined rises in ALT, AST, and bilirubin as important at different levels, ranging from 3 x upper limit of normal (ULN) to 10 x ULN. The differences between definitions of important rises are shown in Table 6.
Following first treatment, pyronaridine‐artesunate was associated with a greater incidence of abnormally raised ALT compared to other antimalarials (RR 3.59, 95% CI 1.76 to 7.33; 8 trials, 6669 participants; Analysis 4.3, Figure 5). Pyronaridine‐artesunate was also associated with a greater incidence of abnormally raised AST compared to other antimalarials (RR 2.22, 95% CI 1.12 to 4.41; 8 trials, 6669 participants; Analysis 4.4). We detected little or no difference between groups in abnormally raised bilirubin events (RR 1.03, 95% CI 0.49 to 2.18; 7 trials, 6384 participants; Analysis 4.5).
4.3. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 3: First treatment, ALT increase > 5 × ULN
5.

Forest plot of comparison 4: Pyronaridine‐artesunate versus other antimalarials, outcome: 4.3 ALT increase > 5 × ULN, first treatment.
4.4. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 4: First treatment, AST increase > 5 × ULN
4.5. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 5: First treatment, bilirubin increase > 2.5 × ULN
For second or subsequent treatments, we detected no significant differences in the number of abnormally raised ALT, AST, or bilirubin events between pyronaridine‐artesunate and other antimalarials (1649 participants, 1 trial; Analysis 4.6; Analysis 4.7; Analysis 4.8). There were small numbers in each arm.
4.6. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 6: Subsequent treatment(s), ALT increase > 5 × ULN
4.7. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 7: Subsequent treatment(s), AST increase > 5 × ULN
4.8. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 8: Subsequent treatment(s), bilirubin increase > 2.5 × ULN
A sensitivity analysis confined to only those trials that used the same grading for severely raised ALT also found a greater incidence of abnormally raised ALT in those treated with pyronaridine‐artesunate compared to other antimalarials (RR 4.44, 95% CI 1.99 to 9.87; 4 trials, 5746 participants; Analysis 4.9).
4.9. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 9: Sensitivity analysis: first treatment, ALT increase > 5 × ULN
Qualitative (narrative) synthesis
Ringwald 1996 reported that five out of 40 participants given pyronaridine had elevated bilirubin levels, compared to zero out of 41 given chloroquine. It should be noted, however, that this study used pyronaridine monotherapy, and a higher dose than that which is currently recommended. The report did not provide any further details of the extent of the increase.
As an indication of the magnitude of ALT increases, the highest reported ALT values in individual studies were 612 international units (IU)/L in Rueangweerayut 2012 and 1229 IU/L in Sagara 2018. These were in individual participants, and are not indicative of the population as a whole. The study with the longest follow‐up recruited 180 participants (Nelwan 2015). In the 60 participants receiving pyronaridine‐artesunate, observed increases in median ALT and AST values had returned to baseline by day 14, and no clinical consequences of these liver enzyme increases were reported over one year of follow‐up. None of the 60 participants experienced abnormally raised ALT or AST. It should be noted, however, that this was a study of pyronaridine‐artesunate plus primaquine, which could impact the findings. Sagara 2018 reported one case in which raised ALT occurred with raised bilirubin in a two‐year‐old girl. The safety monitoring board concluded that this event was an acute hepatocellular liver injury, and was reported as a serious adverse event (Table 7).
Other adverse events
All other reported adverse events are summarized in Analysis 4.10. To enable graphical display of these adverse events by MedDRA System Organ Class and High Level Term (MedDRA 2016), we have presented the results in a forest plot based on meta‐analysed subtotals in Figure 6, according to PRISMA guidance (Zorzela 2016). There was a lower risk of electrocardiograph (ECG) abnormality, including QT prolongation, with pyronaridine‐artesunate compared to each of the other antimalarials used as comparator drugs. A summary of the proportion of participants experiencing ECG abnormalities in each treatment group is provided in Table 5. The greatest differences were seen in the Sagara 2018 study when pyronaridine‐artesunate was compared to artemether‐lumefantrine and artesunate‐amodiaquine; the rates of observed QT prolongation were much higher in this study compared to other included studies.
4.10. Analysis.

Comparison 4: Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only), Outcome 10: Other adverse events
6.
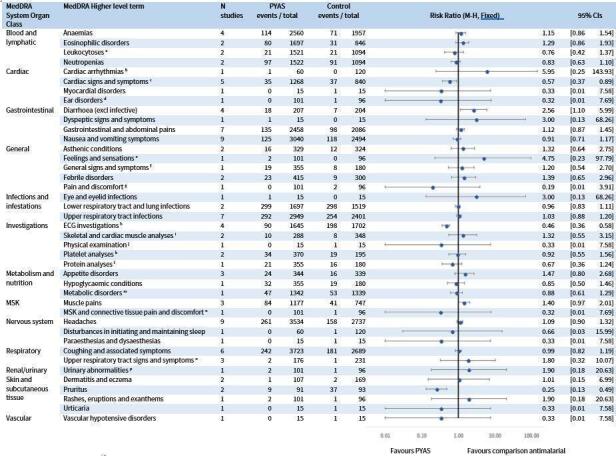
A comparison of adverse events following treatment with pyronaridine‐artesunate versus other antimalarials, based on the reporting guidelines in PRISMA harms (Zorzela 2016). Adverse events are categorized according to MedDRA system organ class and high level terms (MedDRA 2016). Where specific low level terms were reported, we have used footnotes to indicate the condition described. Where trials reported more than one low level term belonging to the same high level term, we have reported the low level term with the highest frequency. aIncludes basophilia and monocytosis. bAsymptomatic unifocal ventricular ectopics. cIncludes dizziness and palpitations. dEar pain. e"Chills". fInfluenza‐like illness. gChest pain. hProlonged QTc, t wave inversion. iElevated creatine phosphokinase. jWeight decreased. kThrombocytopenia. lHypoalbuminaemia. mRaised creatinine. nNeck pain. oThroat pain, cold, postnasal drip. pDark urine.
1. Pyronaridine‐artesunate (PY‐AS) versus other antimalarials: electrocardiogram (ECG) abnormalities.
| Comparator drug | Trial | Pyronaridine‐artesunate | Comparator | ||
| ECG abnormalities | Number of participants | ECG abnormalities | Number of participants | ||
| Artemether‐lumefantrine | Sagara 2018 | 55 | 673 | 99 | 671 |
| Amodiaquine‐artesunate | Sagara 2018 | 34 | 669 | 91 | 668 |
| Chloroquine | Poravuth 2011a | 1 | 228 | 6 | 228 |
| Shin 2011a | 0 | 15 | 1 | 15 | |
| Dihydroartemisinin‐piperaquine or artesunate alone | Nelwan 2015b | 0 | 60 | 1 | 120 |
| Total | 90 | 1645 | 198 | 1702 | |
Abbreviations: ECG: electrocardiogram.
aPlasmodium vivax participants only. bPyronaridine alone used as study drug.
Rates were similar between pyronaridine‐artesunate and comparators for most types of adverse events. Differences observed included the following.
Lower risk of cardiac symptoms. This category included dizziness and palpitations. The difference observed related to high instances of dizziness in the control group (mefloquine plus artesunate) in one study (Rueangweerayut 2012).
Lower risk of pruritis. This occurred in comparisons to chloroquine, for which pruritis is a commonly recognized adverse event.
Higher risk of diarrhoea. Most cases of diarrhoea were contributed by one study (Ringwald 1996), a monotherapy study using higher doses of pyronaridine than that which is currently recommended.
NRS safety review
All included NRS reported the frequency of adverse events, and five studies categorized adverse events by severity. Serious adverse events were reported by participants infrequently (total serious adverse events; 0.4%), and were most commonly blood and lymphatic system disorders (8/8267). A small number of participants reported adverse effects related (or possibly related) to the study drug (702/7805, 9.0%). The two studies reporting changes in liver enzymes demonstrated small increases with pyronaridine‐artesunate in a small number of participants (Bui 2020; Leang 2016). These were infrequent, judged as 'mild' on the liver severity index, and were reported by Bui 2020 to have returned to normal levels by the end of the study period.
In four studies that did not exclude individuals with increased liver enzyme levels at baseline, including one study that subgrouped participants by their baseline liver enzyme levels (normal compared to abnormal), there was no obvious evidence of an increase in adverse events. In particular, participants with abnormal pre‐existing liver enzyme levels showed no evidence of increased frequency of adverse effects compared to those with normal baseline liver enzymes, providing reassuring evidence for the safety of pyronaridine‐artesunate in this group. A full report of the results from NRS is provided in Appendix 1.
Discussion
Summary of main results
See Table 1; Table 2; Table 3; Table 4.
Efficacy analysis (RCTs)
Overall, pyronaridine‐artesunate appears to have similar efficacy to other ACTs (artemether‐lumefantrine, artesunate‐amodiaquine, mefloquine plus artesunate). In most of the included trials, pyronaridine‐artesunate had a lower than 5% PCR‐adjusted treatment failure rate at day 28 and day 42.
Treatment with pyronaridine‐artesunate may lead to fewer PCR‐adjusted failures at day 28 compared to artemether‐lumefantrine, artesunate‐amodiaquine, and mefloquine plus artesunate (low‐certainty evidence). In all these instances, the CIs crossed the line of no effect.
Treatment with pyronaridine‐artesunate may lead to fewer PCR‐unadjusted failures at day 28 compared to artemether‐lumefantrine (low certainty evidence), and probably leads to fewer failures compared to artesunate‐amodiaquine and mefloquine plus artesunate (moderate‐certainty evidence). The PCR‐unadjusted outcome reflects the post‐treatment effect of the drug in preventing new infections.
There may be little or no difference in the rate of PCR‐adjusted failure at day 42 with pyronaridine‐artesunate compared to artemether‐lumefantrine or artesunate‐amodiaquine (low‐certainty evidence), but pyronaridine‐artesunate may lead to higher rates of failure than mefloquine plus artesunate (low‐certainty evidence).
Pyronaridine‐artesunate may lead to a lower rate of PCR‐unadjusted treatment failure at day 42 compared to artemether‐lumefantrine (low‐certainty evidence), suggesting that the drug combination may reduce the likelihood of re‐infection during the treatment period. For this outcomes, there may be little or no difference with pyronaridine compared to mefloquine plus artesunate (low‐certainty evidence), and probably little or no difference with pyronaridine compared to artesunate‐amodiaquine (moderate‐certainty evidence).
Safety analysis (RCTs)
Abnormally raised ALT is more frequent with pyronaridine‐artesunate compared to other antimalarials (high‐certainty evidence). Abnormally raised AST probably also occurs more frequently with pyronaridine‐artesunate (moderate‐certainty evidence). There is probably little or no difference in abnormally raised bilirubin between pyronaridine‐artesunate and other antimalarials (moderate‐certainty evidence). There was one reported case in which raised ALT occurred with raised bilirubin. Qualitative evidence from one trial, with a cohort of 180 participants and a follow‐up of one year, indicated that raised liver enzymes were not prolonged and did not lead to clinical sequelae, though it should be noted that pyronaridine‐artesunate was administered concurrently with primaquine.
ECG abnormalities were less commonly seen with pyronaridine‐artesunate compared to other antimalarials. Regarding the remaining safety outcomes, there appears to be little or no difference in safety between pyronaridine‐artesunate and other ACTs (artemether‐lumefantrine, artesunate‐amodiaquine, mefloquine plus artesunate, dihydroartemisinin‐piperaquine) or non‐ACT antimalarials (artesunate alone, chloroquine).
NRS safety review
The review of safety from observational studies demonstrates low and reassuring estimates of serious adverse events and drug‐specific adverse effects. Data on liver enzymes show mild and infrequent increases in liver enzyme levels, which quickly return to baseline. Data from the study including people with evidence of liver injury demonstrates consistency in safety outcomes for pyronaridine‐artesunate between this group and those with no evidence of liver injury at baseline. When added to the data from RCTs in this Cochrane Review, the observational study data effectively double the numbers of people in whom safety is monitored.
Overall completeness and applicability of evidence
Completeness and applicability of efficacy findings
Five trials contributed 5711 participants to quantitative synthesis for efficacy analyses in this review. There were 4465 participants from 13 trial sites in Africa (Burkina Faso, Democratic Republic of the Congo, Gabon, Cote d'Ivoire, Kenya, Mali, Tanzania, The Gambia, Ghana, Mozambique, Senegal, Guinea, Mali) and 1246 participants from five sites in Asia (the Philippines, Cambodia, Indonesia, Thailand, Vietnam). The large number of included sites broadens the applicability of the efficacy findings. The actual number of participants recruited at the country level was small, precluding evaluation of efficacy at this level.
A key limitation on the applicability of review findings on efficacy was participant age, as the included trials mostly recruited older children and adults. The total number of participants definitively aged less than five years remains at just 527 in the pyronaridine‐artesunate arm and 472 in the comparator arm.
Trials additionally reported a number of outcomes relating to fever clearance, parasite clearance, and gametocyte carriage, which did not form a priori outcomes for this version of the review, in a change to the previously published protocol. We encountered different modes of reporting, different units of measurement, and incomplete reporting of these outcomes. This limits the contribution of these outcomes to the evidence.
Completeness and applicability of safety findings
Eight trials contributed to the quantitative synthesis of key safety outcomes in this review (serious adverse events and liver function tests), and a further two trials contributed data to an analysis of all safety outcomes. All trials contributing to the safety meta‐analysis excluded individuals with baseline hepatic impairment, and most trials listed viral hepatitis as a specific exclusion criterion. Similarly, Sagara 2018 excluded individuals with raised liver enzymes from second and subsequent treatments. Screening for baseline hepatic impairment, or for hepatic impairment during treatment, may not be feasible in many malaria‐endemic settings where resources are limited. This may limit the applicability of the findings. Four trials explicitly listed HIV as an exclusion criterion (Kayentao 2012; Poravuth 2011; Sagara 2018; Tshefu 2010). Such exclusions represent standard practice for phase III trials. Given the high seroprevalence of such conditions in malaria‐endemic areas, this may also limit the applicability of the safety findings.
However, observational data from studies included in the NRS safety review suggest that these findings may be applicable to those with baseline hepatic impairment or HIV‐positive individuals. The two studies that did not exclude individuals with increased liver enzyme levels at baseline found no increase in adverse events in participants with pre‐existing elevations in liver enzyme levels (Han 2020; Lutete 2021a). Similarly, Lutete 2021a reported little difference in the frequency of adverse events between healthy populations and potentially vulnerable subgroups, including those with HIV.
Certainty of the evidence
We assessed the certainty of the evidence in the review using the GRADE approach, and presented it in Table 1, Table 2, Table 3, and Table 4.
Regarding the efficacy of pyronaridine‐artesunate, the studies included in this review provided moderate‐ to low‐certainty evidence due to inconsistency, with frequent quantitative and qualitative heterogeneity between studies, and indirectness, given that children under five years were underrepresented (especially in Asia).
Regarding the safety of pyronaridine‐artesunate, we judged the certainty of evidence for the outcome abnormally raised ALT to be high, deciding not to downgrade for imprecision, as although the CI is wide, there were few events. We found moderate‐certainty evidence that pyronaridine‐artesunate increases the proportion of participants experiencing abnormally raised AST, and moderate‐certainty evidence that there is little or no difference between treatments in the proportion of participants experiencing abnormally raised bilirubin. We downgraded both of these outcomes for imprecision. Observational data from the NRS safety review did not contradict the findings from the RCTs, and so supported the above evaluations of the certainty of evidence.
Potential biases in the review process
This represents the third update of this review. For this update we repeated the screening process, increasing the likelihood that we identified all relevant studies.
The largest trial included in this review, Sagara 2018, did not randomize participants to all comparisons at all sites, and we were unable to obtain disaggregated data for all outcomes at all sites. However, we were able to do so for the outcomes most pertinent to this review (efficacy and liver function data), and so do not consider that this introduces significant bias to the review process.
As shown in Table 6, different trial authors used different grading for the severity of adverse events. Most trial authors considered ALT > 5 x ULN as important, and reported at this threshold. The three studies that reported at a lower threshold recorded zero events, so we retained these in the analysis for completeness (Nelwan 2015; Roth 2018a; Shin 2011). Kayentao 2012 reported at a higher threshold, and so may underdetect events. When we performed a sensitivity analysis excluding these four trials, the risk ratio was similar (Analysis 4.9). It should be noted that the grading for raised ALT does not correspond with international definitions for drug‐induced liver injury (Appendix 5) (Aithal 2011). Similarly, the thresholds for raised bilirubin also differed, both from each other and from the 2 x ULN used in international definitions of moderate or severe drug‐induced liver injury (Appendix 5) (Aithal 2011). As we are unable to provide case‐by‐case analysis for raised ALT > 5 x ULN, we cannot exclude the possibility that in some instances these occurred in conjunction with symptoms of bilirubin > 2 x ULN (given that the reported threshold was 2.5 in most studies). Qualitative synthesis does not suggest this was the case.
We had planned to conduct a sensitivity analysis altering the denominator for the efficacy outcomes according to Appendix 4. However, we were unable to reliably extract data pertinent to missing or indeterminate PCR values. As PCR is unlikely to differentially misclassify recrudescences as re‐infections between comparison groups, we do not feel that this is likely to have introduced bias to the main outcome of PCR‐adjusted treatment failure.
For the safety analysis, we used MedDRA to create analogous definitions to allow comparison (MedDRA 2016). This may lead to misclassification and loss of detail. However, we feel that the overview is more useful and meaningful to the clinical reader.
Agreements and disagreements with other studies or reviews
Our search identified an individual patient data analysis published by authors from Medicines for Malaria Venture and Shin Poong Pharmaceuticals, who developed the pyronaridine‐artesunate combination (Duparc 2013). The efficacy analysis in Duparc 2013 included four of the RCTs included in our review (Kayentao 2012; Poravuth 2011; Rueangweerayut 2012; Tshefu 2010). The safety analysis included an additional two studies: a non‐randomized dose‐finding study excluded from this review (Ramharter 2008), and a randomized dose‐finding study that was published as a conference abstract which assessed pyronaridine‐artesunate without a comparator (Looareesuwan 2007). This integrated analysis is not a formal systematic review, and as such did not have an a priori protocol or a formal search strategy. It did not make assessments of risk of bias. It is unclear whether the authors have included all potentially relevant studies, or whether it represents a convenience sample of available data at the time of the analysis. The safety findings of this integrated analysis do not report risk ratios or anticipated absolute effects of raised ALT, instead reporting incidence at day three and day seven. The day seven incidence of 0.9% (24/2709) is similar to our anticipated absolute effect of 0.8%. Our review agrees with the conclusion from this integrated analysis, that pyronaridine‐artesunate has good efficacy for uncomplicated P falciparum malaria and was well tolerated with a similar adverse event profile to comparators, but was associated with transient increases in transaminases in a relatively small proportion of patients.
Authors' conclusions
Implications for practice.
Pyronaridine‐artesunate was efficacious against uncomplicated Plasmodium falciparum malaria; achieved a polymerase chain reaction (PCR)‐adjusted treatment failure rate of < 5% at days 28 and 42; and may be at least as good as or better than existing artemisinin‐based combination therapies (ACTs).
Pyronaridine‐artesunate causes a four‐fold increase in the risk of abnormally raised alanine aminotransferase (ALT) and probably causes a two‐fold increase in the risk of abnormally raised aspartate transaminase (AST). This meets clinical chemistry criteria for drug‐induced liver injury (Appendix 5) (Aithal 2011). In the absence of abnormally raised bilirubin, and in the absence of symptoms, this corresponds to mild drug‐induced liver injury only. There was one reported case in which raised ALT occurred with raised bilirubin, meeting the criteria for moderate drug‐induced liver injury.
The previous version of this review, Pryce 2019, highlighted the raised ALT and AST as a safety signal warranting further evaluation in cohort studies. Since publication of the previous review, an open‐label cohort event monitoring study of 7154 patients has reported no protocol‐defined hepatic events (Lutete 2021b). We conclude that although raised ALT and AST is a safety signal indicating a theoretical risk of severe drug‐induced liver injury, no such cases have been reported amongst over 6000 otherwise‐healthy trial participants to date. As such, the raised ALT and AST may reflect a capacity for pyronaridine‐artesunate to cause only mild, asymptomatic, and reversible injury.
World Health Organization guidelines recommend the use of pyronaridine‐artesunate for the treatment of uncomplicated malaria in adults and children weighing 5 kg and over in all malaria‐endemic areas. The guideline states that it is a strong recommendation, based on high‐certainty evidence, but then states that specifically for artesunate + pyronaridine is "currently un GRADEd, anticipated to be updated in 2022" (WHO 2022).
As authors we believe this judgement is reasonable, as the benefit in this clinical scenario outweighs a theoretical risk. The findings of this review cannot fully inform a risk‐benefit assessment for an unselected population. Readers should remain aware of this uncertainty when considering the use of pyronaridine‐artesunate in patients with known or suspected pre‐existing liver dysfunction, and when co‐administering with other medications that may cause liver dysfunction.
Implications for research.
Armed Forces Research Institute of Medical Sciences Thailand is conducting an ongoing randomized controlled trial, NCT03726593, that aims to recruit 252 participants and will analyse hepatic safety data amongst other outcomes. This trial should provide further evidence to inform the recommendation of pyronaridine‐artesunate, particularly on the basis of safety.
There is limited published data in children aged less than five years of age. Future studies should aim to redress this.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2022 | New search has been performed | Two new authors joined the author team, and updated the search to 27 October 2021. The authors also included a review of non‐randomized studies on safety. |
| 27 May 2022 | New citation required and conclusions have changed | The author team used the same search strategy to identify new studies published or registered from 8 May 2018 to 27 October 2021. This resulted in the inclusion of two new records, which related to the previously included study Sagara 2018. They replaced unpublished data for one trial site (Bobo‐dioulasso) with that recently published by Compaore 2021. |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 3, 2014
| Date | Event | Description |
|---|---|---|
| 8 January 2019 | New citation required and conclusions have changed | We included four new studies: three published after the previous version of this review, Bukirwa 2014, and one that was not identified in the previous review. During re‐extraction of data from the previous studies we used a transparent, itemized, and replicable procedure that differed from that used in Bukirwa 2014. The inclusion of new studies has allowed a new comparison of pyronaridine‐artesunate and artesunate‐amodiaquine, and has led to changes in the certainty of the evidence in relation to the primary outcomes. This review update gives higher‐certainty evidence in relation to the effect of pyronaridine‐artesunate on raised alanine aminotransferase (ALT). |
| 8 January 2019 | New search has been performed | There is a new author team: Joseph Pryce, Paul Hine (new contact person). We use the term 'pyronaridine‐artesunate' in preference to 'artesunate‐pyronaridine' to reflect how most authors refer to the intervention. We have updated the Background to reflect changes in global epidemiology, current World Health Organization (WHO) guidelines, and other developments. We added the term 'pyramax' to the search strategy. We have replaced the quantitative analysis of secondary outcomes parasite clearance, fever clearance, and gametocyte carriage with a narrative synthesis, and comment on the reasons for doing so. We simplified the adverse events outcomes to reflect that elevated liver function tests are a primary area of interest. We itemized the procedure for data extraction to ensure that the process is transparent and replicable in future review updates. We have added further commentary regarding the data extraction process. We simplified the risk of bias assessment for adverse events. We abbreviated the content of tables to enable clear and succinct presentation. We incorporated risk of bias for adverse events assessments into the main risk of bias tables. We added an appendix to summarize our risk of bias assessment process. |
| 11 November 2008 | Amended | We converted to the new review format with minor editing. |
Acknowledgements
Editorial and peer‐reviewer contributions
The following people conducted the editorial process for this review update.
Contact Editor: Dr Hellen Gelband
Sign‐off Editor (final editorial decision): Professor Paul Garner
Managing Editor (collated comments, provided editorial guidance to authors, edited the article): Dr Deirdre Walshe
Copy Editor (copy editing and production): Lisa Winer, Cochrane Copy Edit Support
JP, MT, TF, and PH were supported by the Research, Evidence and Development Initiative (READ‐It) project. READ‐It and the Cochrane Infectious Diseases Group editorial base are funded by UK aid from the UK government for the benefit of low‐ and middle‐income countries (project number 300342‐104). The views expressed do not necessarily reflect the official policies of the UK government.
This 2021 review update was supported in part by a grant from the Global Malaria Programme (GMP), World Health Organization (WHO); WHO GMP Programme Agreement for Performance of Work (APW) grant (202710314).
We thank the authors of the previously published versions of this Cochrane Review (Bukirwa 2014; Unnikrishnan 2007).
We thank Rebecca Thomas for helping with the screening for this review update.
Appendices
Appendix 1. Pyronaridine‐artesunate: non‐randomized studies safety review
Background
The objective of this review was to assess safety outcomes of pyronaridine‐artesunate for treating uncomplicated Plasmodium falciparum malaria in an extended population, including safety data from studies other than cluster‐randomized controlled trials (cRCTs). We conducted this systematic review to present the adverse effects of pyronaridine‐artesunate reported in the public domain from non‐randomized, observational studies.
Methods
Types of studies
Prospective cohort studies including single‐arm and comparative studies. Retrospective cohort studies of individual case series and case reports.
Participants
Adults, including pregnant women, and children. Healthy participants, or with co‐existing disease (HIV, tuberculosis, or liver disease).
Interventions
Pyronaridine‐artesunate (PY‐AS), pyronaridine in combination with other antimalarials, or pyronaridine monotherapy as treatment.
Main outcomes
Clinically important adverse effects (effects related to the study drug); elevated bilirubin concentration (> 2 x upper limit of normal (ULN))*, elevated liver enzymes (alanine aminotransferase (ALT) and aspartate transaminase (AST) > 3 x ULN), other evidence of liver failure.
Other adverse events (potentially unrelated to the study drug).
*Following the Aithal 2011 clinical chemistry criteria for drug‐induced liver injury (Appendix 5), elevated bilirubin alone does not indicate liver injury.
Search methods
Vittoria Lutje (CIDG Information Specialist) searched the following databases on 2 September 2021: Cochrane Central Register of Controlled Trials (CENTRAL; Issue 9 of 12, 2021), published in the Cochrane Library; MEDLINE (Ovid); Embase (Ovid); and Scopus (Elsevier). She also searched the WHO International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov) for trials in progress. Further details of the search strategy are provided in Annex 1 of this appendix. Our database searches identified 374 records, of which 266 records remained after removal of duplicates. Two review authors (TF and MT) independently screened all titles and abstracts.
Contacting study authors
We contacted study authors in the case of insufficient detail.
Risk of bias assessment
We assessed risk of bias of the included studies using methods adapted from the 2017 Cochrane Review ‘Mefloquine for preventing malaria during travel to endemic areas’.
Data extraction
One review author (TF) extracted all data. We aimed to extract the following data for safety outcomes.
-
Frequency of participants experiencing adverse events, including:
serious adverse events;
life‐threatening adverse events;
adverse events leading to discontinuation of the study drug.
-
Frequency of participants experiencing increases in liver function tests for:
ALT;
AST;
bilirubin.
Results of the search
Excluded studies
We excluded 27 studies after full‐text screening. Nineteen studies were excluded due to an incorrect study design, including 15 RCTs, 3 discussion articles, and 1 review; 4 studies reported no relevant outcomes; 2 were duplicates; and 2 studies were published only in a language other than English.
Included studies
Seven studies met our inclusion criteria and were included in this report. See 'Characteristics of included studies' for details (Table 1 in this appendix).
Included studies
Study design
The characteristics of included studies are summarized in Table 1. We included five single‐arm observational studies with a total of 1007 participants (Bui 2020; Han 2020; Leang 2016; Leang 2019a; Leang 2019b). We also included one cohort event monitoring study with 7746 participants (Lutete 2021a), and one dose‐escalation study with 59 participants (Ramharter 2008). No case reports were identified.
The included studies were conducted between 2006 and 2019. Six studies had a length of follow‐up of 42 days. Lutete 2021a had a length of follow‐up of 28 days, which was sufficient to capture most treatment failures. Three studies were conducted in Cambodia; one in each of Vietnam, Myanmar, and Gabon; and one multinational study was conducted across Cameroon, Democratic Republic of Congo, Gabon, Côte d'Ivoire, and Republic of Congo.
Participants
All studies included participants from malaria‐endemic areas with microscopically or rapid diagnostic test‐confirmed acute uncomplicated malaria and fever or history of fever. All seven studies included participants with Plasmodium falciparum infection. Three studies additionally included participants with P vivax infection (n = 321) (Han 2020; Leang 2019a; Lutete 2021a). One study further included participants with P falciparum/P ovale co‐infections (n = 75), P falciparum/P malariae co‐infections (n = 55), and P ovale/P malariae co‐infections (n = 1) (Lutete 2021a).
Six studies included adults and children as participants, whilst one study included children aged 2 to 14 years only (Ramharter 2008).
One study included and specifically reported outcomes for several potentially vulnerable subgroups that would be expected to be found in a population treatment group but that were excluded from most of the other included studies (Lutete 2021a). These included HIV‐positive participants, those with low bodyweight (< 20 kg), malnourished individuals, young children (1 to < 5 years), and those experiencing their first malaria episode.
Liver function tests
Three studies specifically excluded individuals with underlying hepatic dysfunction (exclusion criteria: Bui 2020 ALT or AST values > 2.5 x ULN; Leang 2016 ALT or AST values > 2.5 x ULN; and Ramharter 2008 liver function test results that were > 3 times above normal levels). Leang 2019a, Leang 2019b, and Han 2020 excluded participants with known severe disease, such as kidney or liver disease, but did not specifically test for baseline levels of ALT/AST.
Lutete 2021a excluded individuals with clinical signs or symptoms of hepatic injury or known severe liver disease but measured baseline liver enzyme levels in all other eligible participants. In order to compare the frequency of adverse events and severe adverse events in those with abnormal baseline liver enzyme levels tests against those with normal baseline levels (normal baseline ALT/AST < 2 x ULN; abnormal baseline ALT/AST > 2 x ULN), they did not exclude those with abnormal liver enzymes.
Intervention
All studies used pyronaridine‐artesunate as Pyramax, supplied by Shin Poong Pharmaceuticals. Six studies used PY‐AS for prophylaxis, dosed according to bodyweight. Participants in all studies were treated once daily for three days. Participants included in Ramharter 2008 were administered PY‐AS using a dose‐escalation method, starting at 48 mg pyronaridine plus 16 mg artesunate and increasing in dose up to 96 mg pyronaridine plus 32 mg artesunate, over a three‐day period.
Outcomes
Adverse events
All seven studies (9546 participants) reported the frequency of adverse events for the duration of the study. Five studies (8267 participants) also reported the frequency of severe adverse events (Leang 2016; Leang 2019a; Leang 2019b; Lutete 2021a; Ramharter 2008). Three studies detailed the numbers of specific adverse events (Leang 2016; Leang 2019a; Leang 2019b), and three studies reported the number of adverse events at different time points during the study period (Bui 2020; Leang 2016; Leang 2019a). The methods used to report safety outcomes of treatment for malaria with PY‐AS differed across the included studies, resulting in wide variation in the frequency of adverse events reported.
Raised liver enzymes
Two studies reported liver function test results including ALT, AST, and bilirubin levels (Bui 2020; Leang 2016). Bui 2020 reported the absolute values and the frequency of increases from baseline for ALT, AST, and total bilirubin.
Leang 2016 conducted biochemical analyses at baseline and on days 3, 7, 14, and 28 to measure ALT, AST, and bilirubin levels. All cases of ALT and/or AST levels > 3 x ULN plus peak total bilirubin levels > 2 x ULN, or ALT levels 5 x ULN were deemed serious adverse events.
Although the authors of Lutete 2021a measured baseline liver enzyme levels to compare the frequency of adverse events and severe adverse events in those with abnormal baseline liver enzyme levels tests against those with normal baseline levels, they did not report changes in liver enzyme levels following treatment.
Risk of bias assessment
Results of the risk of bias assessment are summarized in Table 2.
Results: treatment safety
Adverse events
The proportion of participants experiencing adverse events in each included study is summarized in Table 3.
Adverse events of any severity
Across the seven studies, 1877 out of 9546 participants (19.7%) reported experiencing adverse events of any severity at any time point throughout the study. This includes participants reporting symptoms at baseline, and commonly included headache, fever, and vomiting, which may be consistent with malaria.
Serious adverse events
In the five studies categorizing adverse events by severity, a limited number of participants (31/8267, 0.4%) were reported to have experienced severe or serious symptoms. These included blood and lymphatic system disorders (n = 8), gastrointestinal disorders (n = 1), general disorders and administration site conditions (n = 2), infections and infestations (n = 17), nervous system disorders (n = 1), pregnancy, puerperium, and perinatal conditions (n = 1), respiratory, thoracic, and mediastinal disorders (n = 1), skin and subcutaneous tissue disorders (n = 1), and vascular disorders (n = 2). Three of the five studies reported no severe or serious adverse events in their study population (Leang 2016; Leang 2019a; Leang 2019b).
Adverse events related to study drug (adverse effects)
In the two studies that reported adverse events related to pyronaridine‐artesunate (Lutete 2021a; Ramharter 2008), a small number of participants reported symptoms related (or possibly related) to the study drug (702/7805, 9.0%) (Table 3).
Adverse events in potentially vulnerable populations
Lutete 2021a reported the frequency of adverse events in several potentially vulnerable subgroups, including HIV‐positive participants (frequency of adverse events 26.7%, compared to 24.8% in healthy population); those with low bodyweight (< 20 kg) (20.4%, compared to 20.8% in > 20 kg population); malnourished individuals (19.8%, compared to 20.8% in healthy population); young children (1 to 5 years) (22.4%, compared to 27.0% in > 18 years population); and those experiencing their first malaria episode (19.0%, compared to 16.6% in population with previous malaria infection). The results suggested little difference in the percentage of participants experiencing adverse events in the vulnerable versus invulnerable populations.
Liver function tests
Two studies reported changes in liver enzymes throughout the study period (summarized in Table 4), although both excluded individuals with ALT or AST values > 2.5 x ULN at baseline. We have provided a judgement on whether these liver function test results represent mild, moderate, severe, or fatal liver injury severity based on the drug‐induced liver injury severity index (Appendix 5). We judged that all the participants experiencing increases in AST, ALT or bilirubin during the study were experiencing liver injury of mild severity. There were no cases of moderate, severe, or fatal liver injury.
Leang 2016 reported changes from baseline for ALT, AST, and total bilirubin (n = 123). Overall results of mean ALT values across the study population rose from 27.5 international units (IU)/L (standard deviation (SD) 20.0) at baseline, peaking at 50.5 IU/L (SD 64.3) on day 7, and returning to normal by day 28, 23.2 IU/L (SD 12.7). Very little effect was reported on AST or bilirubin levels. Study authors specifically reported three participants who experienced an increase in ALT > 2 x ULN on day 7, one participant experiencing bilirubin increases to > 3 x ULN on day 3, and six participants experiencing an increase in AST to > 3 x ULN between 0 and 7 days. None of these was classified as potential Hy’s law cases, and all were consistent with recovery from malaria.
Bui 2020 also reported changes in ALT, AST, and bilirubin levels. The authors reported that all participants had ALT and AST levels < 2.5 x ULN, and 97.3% of participants had bilirubin levels < 2.5 x ULN at baseline. They reported that on day 7, 12 participants (7.8%) had increases in ALT > 2.5 x ULN; 8 participants (5.1%) had increases in AST > 2.5 x ULN; and 2 participants (1.5%) had bilirubin levels > 2.5 x ULN. By day 28, this had changed to 2 (1.4%), 7 (4.8%), and 5 (3.8%) participants experiencing transient liver enzyme levels > 2.5 x ULN for ALT, AST, and bilirubin, respectively. There were no potential Hy’s law cases at day 7. One participant had ALT 5.1 x ULN and bilirubin 3.2 x ULN at day 28. The authors reported that this participant had falciparum malaria recrudescence at day 28, and the increased liver enzymes were not thought to be treatment related.
Lutete 2021a reported the frequency of adverse events in participants with normal compared to participants abnormal liver function enzyme levels at baseline. We summarized data from both participant groups from this study for drug‐related adverse effects, adverse events leading to discontinuation of treatment, severe adverse events, life‐threatening adverse events, blood and lymphatic system disorders, cardiac disorders, and gastrointestinal disorders (Table 5). The results suggest that reporting of adverse events was similar in participants with abnormal baseline liver enzyme levels compared to those with normal baseline liver function tests. For example, drug‐related adverse events were reported in 9.5% of participants in the normal baseline group compared to 7.6% of participants in the abnormal baseline group, and adverse events leading to discontinuation of the study drug were reported in 0.6% of participants in the normal group compared to 1.3% of participants in the abnormal group. The descriptive nature of the results limits our ability to analyse differences between the two groups, and the absence of liver function tests in participants during the study period beyond baseline means we cannot further investigate the relationship between the study drug and liver disease indicators.
Discussion
The methods used to report safety outcomes of treatment for malaria with pyronaridine‐artesunate differed across the included studies, resulting in the large variation in frequency of adverse events reported (0 to 100%). These events are most frequently of little concern, such as headache and fever (sometimes reported at baseline), which do not prevail for long periods of time. The frequency of serious adverse events and adverse effects related to the study drug may provide more reliable estimates of safety outcomes, as they include more specific reporting criteria, with results demonstrating the infrequent occurrence of serious adverse events (avg. 0.4%) and adverse effects (avg. 9.0%).
This analysis includes three studies that did not specifically perform liver function tests at baseline to exclude individuals from the study, and one study that intentionally included participants with both normal and abnormal baseline liver function test results, allowing the investigation of safety outcomes in normal populations. In two studies that reported increases in liver enzyme levels throughout the study period (although excluded individuals with ALT/AST > 2.5 x ULN at baseline), the results demonstrate that liver enzyme levels did not exceed mild liver injury and occurred infrequently in the study populations. Bui 2020 reported that all increases in liver enzyme levels had either returned to baseline or had improved by the end of the study period, suggesting mild and temporary effects that may be related to the study drug.
Table 1. Characteristics of included studies
| Study design |
Author (year) |
Country | Patient selection | Sample size (per protocol pop.) | Length of follow‐up (days) | Trial sponsor | Baseline liver function tests? | Excluded participants with raised liver enzymes |
| Single arm | Bui 2020 | Vietnam | Adults + children | 155 | 42 | BMG USAID | Yes | Yes |
| Han 2020 | Myanmar | Adults + children (≥ 6 years) | 390 | 42 | BMG USAID | No | No | |
| Leang 2016 | Cambodia | Adults + children | 117 | 42 | BMG | No | Yes | |
| Leang 2019a | Cambodia | Adults + children (≥ 7 years) | 224 | 42 | BMG USAID | No | Yes | |
| Leang 2019b | Cambodia | Adults + children (≥ 7 years) | 121 | 42 | BMG | No | Yes | |
| Cohort event monitoring | Lutete 2021a | Cameroon, Democratic Republic of the Congo, Gabon, Côte D'Ivoire, Republic of the Congo | Adults + children | 7746 | 28 | MMV | Yes | No |
| Dose escalation | Ramharter 2008 | Gabon | Children (2 to 14 years) | 59 | 42 | MMV Shin Poong Pharma | Yes | Yes |
| All studies used Pyramax as the study drug, which was supplied by Shin Poong Pharmaceuticals. All studies used a dose of 180 mg pyronaridine plus 60 mg artesunate, except Ramharter 2008, which was a dose‐escalation study using four doses: 48 mg of pyronaridine tetraphosphate and 16 mg of artesunate; 72 mg of pyronaridine tetraphosphate and 24 mg of artesunate; 96 mg of pyronaridine tetraphosphate and 32 mg of artesunate; 60 mg of pyronaridine tetraphosphate and 20 mg of artesunate. Abbreviations: BMG: Bill & Melinda Gates Foundation; MMV: Medicines for Malaria Venture. | ||||||||
Table 2. Summary of risk of bias assessments for included studies
| Study ID | Was monitoring active or passive? | Was data collection prospective or retrospective? |
Was the number of symptoms assessments adequate? (Baseline and at least 1 value at follow‐up) |
Was the number of liver function tests adequate? (Baseline and at least 1 value at follow‐up) | Were harms predefined using standardized or precise definitions? | Was ascertainment technique adequately described? |
| Bui 2020 | Active | Prospective | Adequate | Adequate | Inadequate | Adequate |
| Han 2020 | Active | Prospective | Adequate | Inadequate | Inadequate | Adequate |
| Leang 2016 | Active | Prospective | Adequate | Adequate | Adequate | Adequate |
| Leang 2019a | Active | Prospective | Unclear | Inadequate | Inadequate | Adequate |
| Leang 2019b | Active | Prospective | Adequate | Inadequate | Inadequate | Adequate |
| Lutete 2021a | Active | Prospective | Adequate | Inadequate | Adequate | Adequate |
| Ramharter 2008 | Active | Prospective | Adequate | Adequate | Inadequate | Inadequate |
Table 3. Summary of adverse events across all included studies
| Author (year) | No. of participants | Participant experiencing adverse events* % (n) | ||
| Any severity | Serious adverse event | Related to drug | ||
| Bui 2020 | 155 | 0.0 (0) | ‐ | ‐ |
| Han 2020 | 390 | 0.0 (0) | ‐ | ‐ |
| Leang 2016 | 117 | 91.1 (112) | 0.0 (0) | ‐ |
| Leang 2019a | 224 | 100.0 (120) | 0.0 (0) | ‐ |
| Leang 2019b | 121 | 87.6 (106) | 0.0 (0) | ‐ |
| Lutete 2021a | 7746 | 20.8 (1490) | 0.4 (29) | 9.6 (685) |
| Ramharter 2008 | 59 | 83.0 (49) | 3.4 (2) | 28.8 (17) |
| *Includes adverse events reported at baseline in some studies. | ||||
Table 4. Liver function test results and liver injury severity judgement
| Author (year) | Day | Elevation (x ULN) | Participants with raised liver enzymes % (n) | Liver injury severity | |||
| ALT | AST | Bilirubin |
Participants with > 1 raised liver enzyme (n) |
Severity index judgement | |||
| Bui 2020 | 0 | > 2.6 | 0 (0) | 0 (0) | 2.7 (4) | ‐ | ‐ |
| Bui 2020 | 7 | > 2.6 | 7.8 (12) | 4.8 (8) | 1.5 (2) | 0 | Mild |
| Bui 2020 | 28 | > 2.6 | 1.4 (2) | 4.8 (7) | 3.8 (5) | 1 | Mild1 |
| Leang 2016 | 0 to 7 | > 3 | ‐ | 4.9 (6) | ‐ | 0 | Mild |
| Leang 2016 | 7 | > 3 | 2.4 (3) | ‐ | ‐ | 0 | Mild |
| Leang 2016 | 3 | > 2 | ‐ | ‐ | 0.8 (1) | 0 | None2 |
| Abbreviations: ALT: alanine aminotransferase; AST: aspartate transaminase; ULN: upper limit of normal. | |||||||
| 1The 1 participant with > 1 raised liver enzyme result had ALT 5.1 x ULN and bilirubin 3.2 x ULN at day 28 (Bui 2020). The authors reported that this participant had falciparum malaria recrudescence at day 28, and that the increased liver enzymes were not thought to be treatment‐related. 2Elevated bilirubin alone does not indicate liver injury. | |||||||
Table 5. Summary of findings of adverse events in Lutete 2021
| Adverse event | Participants experiencing adverse event % (n) | |||
| Baseline liver function results | ||||
| Normal | Abnormala | Unknown | All | |
| Any adverse event | 16.3 (167) | 18.8 (3) | 33.3 (4) | 16.6 (174) |
| Drug‐related adverse effect | 9.5 (663) | 7.6 (12) | 28.6 (10) | 9.6 (685) |
| Adverse events leading to discontinuation of study drug | 0.6 (42) | 1.3 (2) | 28.6 (10) | 0.8 (54) |
| Severe adverse event | 0.5 (35) | 0 (0) | 0 (0) | 0.5 (35) |
| Life‐threatening adverse event | 0.1 (7) | 0.6 (1) | 2.9 (1) | 0.1 (9) |
| Blood and lymphatic system disorders | 0.4 (31) | 0.6 (1) | 5.7 (2) | 0.5 (34) |
| Cardiac disorders | 0.1 (6) | 0 (0) | 0 (0) | 0.1 (6) |
| Gastrointestinal disorders | 7.1 (494) | 7.0 (11) | 28.6 (10) | 7.2 (515) |
| Abbreviations: ALT: alanine aminotransferase; AST: aspartate aminotransferase; ULN: upper limit of normal. | ||||
| aAbnormal baseline liver function test results defined as ALT/AST > 2 x ULN. | ||||
Annex 1: Search strategy
Database: Ovid MEDLINE(R) and In‐Process, In‐Data‐Review & Other Non‐Indexed Citations <1946 to July 30, 2021>
Search strategy
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (pyronaridine or pyramax).mp. or "pyronaridine tetraphosphate artesunate drug combination".mp
2 (safe or safety).tw.
3 side effect*.tw.
4 ((adverse or undesirable or harms* or serious or toxic) adj3 (effect* or reaction* or event* or outcome*)).tw.
5 exp clinical trials, phase iv/
6 exp poisoning/
7 exp Substance‐Related Disorders/
8 exp drug toxicity/
9 exp abnormalities, drug induced/
10 exp drug monitoring/
11 exp drug hypersensitivity/
12 (toxicity or complication* or noxious or tolerability).tw.
13 (undesirable adj1 effect*).mp. or (treatment adj1 emergent).tw.
14 (tolerability or tolerance or tolerate or adrs or adr or harm or harms or harmful or complication* or risk or risks or (unintended adj1 event*) or (unintended adj1 effect*)).tw
15 Bilirubin/ or bilirubin.tw or Hyperbilirubinemia.tw. or Hyperbilirubinemia/
16 "Chemical and Drug Induced Liver Injury"/ or Liver Diseases/
17 hepatotoxicity.mp. or (liver injury or liver injuries or liver disease*).tw
18 liver enzymes.mp. or hepatic enzymes.mp
19 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20 1 and 19
Appendix 2. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | Embaseb | LILACSb |
| 1 | Malaria | Malaria ti, ab, kw | Malaria ti, ab, Mesh | Malaria.mp Emtree | malaria |
| 2 | pyronaridine | Pyronaridine ti, ab, kw | "pyronaridine"ti, ab, Supplementary Concept | pyronaridine/ or artesunate plus pyronaridine/ or pyronaridine.mp. | pyronaridine |
| 3 | pyramax | Pyramax ti, ab, kw | "Naphthyridines" Mesh | Pyramax.mp | pyramax |
| 4 | 2 or 3 | 2 or 3 | Pyramax ti, ab | 2 or 3 | 2 or 3 |
| 5 | 1 and 4 | 1 and 4 | 2 or 3 or 4 | 1 and 4 | 1 and 4 |
| 6 | — | — | 1 and 5 | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by Cochrane (Lefebvre 2011).
Appendix 3. Review authors' risk of bias judgement
| Potential bias | Review authors' judgementa |
| Random sequence generation (selection bias) | High: not randomized or quasi‐randomized Unclear: states 'randomized', but does not report method Low: describes method of randomization |
| Allocation concealment (selection bias) | High: not concealed, open‐label trial for individually randomized, method of concealment not adequate Unclear: details of method not reported or insufficient detail Low: central allocation; sequentially numbered, opaque, sealed envelopes |
| Blinding (performance bias and detection bias) | High: personnel, participants, or outcome assessors not blinded Unclear: no details reported, insufficient details reported Low: personnel, participants, and outcome assessors blinded |
| Incomplete outcome data (attrition bias) | High: losses to follow‐up not evenly distributed across intervention and control group Unclear: no details reported, insufficient details reported Low: losses to follow‐up evenly distributed across groups, reasons for loss to follow‐up and exclusions clearly stated |
| Selective reporting (reporting bias) | High: did not fully report measured or relevant outcomes Unclear: insufficient information to permit judgement Low: all stated outcomes reported |
| Other bias | High: other source of bias identified by review authors Low: no obvious other source of bias of concern to review authors |
| Adverse event monitoring (detection bias) | High: passive methods relying on spontaneous patient report only, undefined adverse events Unclear: insufficient information to permit judgement Low: key adverse events defined, prespecified active detection method |
| Incomplete adverse event reporting (reporting bias) | High: adverse event severity undefined, combination of treatment groups, post hoc cut‐offs for reporting adopted Unclear: insufficient information to permit judgement Low: clear reporting on important adverse events with numerical data by intervention group |
aIndicative reasons for judgements only; this list is not intended to be exhaustive.
Appendix 4. Planned sensitivity analysis
| Analysisa | Participants | PCRb‐unadjusted | PCR‐adjusted | ||
| Numerator | Denominator | Numerator | Denominator | ||
| Primary analysis | Exclusions after enrolment | Excludedb | Excluded | Excluded | Excluded |
| Missing or indeterminate PCR | Included as failures | Included | Excluded | Excluded | |
| New infections | Included as failures | Included | Excluded | Excluded | |
| Sensitivity analysis 1c | As ‘Primary analysis' except: missing or indeterminate PCR | — | — | Included as failures | Included |
| Sensitivity analysis 2d | As ‘Sensitivity analysis 1' except: new infections | — | — | Included as successes | Included |
| Sensitivity analysis 3e | As ‘Sensitivity analysis 2' except: exclusions after enrolment | Included as failures | Included | Included as failures | Included |
| Sensitivity analysis 4f | As ‘Sensitivity analysis 2' except: exclusions after enrolment | Included as successes | Included | Included as successes | Included |
Abbreviations: PCR: polymerase chain reaction.
aRemove participants that did not satisfy the inclusion criteria after randomization from all calculations. b‘Excluded' means removed from the calculation. cTo reclassify all indeterminate or missing PCR results as treatment failures in the PCR‐adjusted analysis. dTo reclassify all PCR‐confirmed new infections as treatment successes in the PCR‐adjusted analysis. (This analysis may overestimate efficacy, as PCR is not wholly reliable, and some recrudescences may be falsely classified as new infections. Also some participants may have gone on to develop a recrudescence after the new infection.) eTo reclassify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment failures. For PCR‐unadjusted total failure, this represents a true worse‐case scenario. fTo reclassify all exclusions after enrolment (losses to follow‐up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment successes.
Appendix 5. Case definition in drug‐induced liver injury
Aithal 2011 is a consensus statement from an Expert Working Group of clinicians and scientists on the case definition of drug‐induced liver injury. The criteria are as follows.
Clinical chemistry criteria for drug‐induced liver injury
Any one of the following.
More than or equal to five‐fold elevation above the ULN for ALT.
More than or equal to two‐fold elevation above the ULN for ALP (particularly with accompanying elevations in concentrations of 5′‐nucleotidase or γ‐glutamyl transpeptidase in the absence of known bone pathology driving the rise in ALP level).
More than or equal to three‐fold elevation in ALT concentration and simultaneous elevation of bilirubin concentration exceeding 2 × ULN.
Drug‐induced liver injury severity index
| Category | Severity | Description |
| 1 | Mild | Elevated ALT/ALP concentration reaching clinical chemistry criteria for drug‐induced liver injury, but bilirubin concentration < 2 × ULN |
| 2 | Moderate | Elevated ALT/ALP concentration reaching criteria for drug‐induced liver injury, and bilirubin concentration ≥ 2 × ULN, or symptomatic hepatitis |
| 3 | Severe | Elevated ALT/ALP concentration reaching criteria for drug‐induced liver injury, bilirubin concentration ≥ 2 × ULN, and 1 of the following.
|
| 4 | Fatal or transplantation | Death or transplantation due to drug‐induced liver injury |
Abbreviations: ALP: alkaline phosphatase; ALT: alanine aminotransferase; ULN: upper limit of normal.
Data and analyses
Comparison 1. Pyronaridine‐artesunate versus artemether‐lumefantrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total failure: day 28 (PCR‐adjusted) | 7 | 3068 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.31] |
| 1.2 Total failure: day 42 (PCR‐adjusted) | 7 | 2575 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.51] |
| 1.3 Total failure: day 28 (unadjusted) | 7 | 3149 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.13, 0.58] |
| 1.4 Total failure: day 42 (unadjusted) | 7 | 3080 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.46, 0.82] |
| 1.5 Early treatment failure | 7 | 3149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.12, 52.39] |
| 1.6 Serious adverse events | 3 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.30, 4.50] |
| 1.7 Adverse events leading to withdrawal | 3 | 2004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.68, 2.90] |
| 1.8 First treatment, ALT increase > 5 × ULN | 7 | 3415 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.33, 8.39] |
| 1.9 First treatment, AST increase > 5 × ULN | 7 | 3415 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [1.23, 7.94] |
| 1.10 First treatment, bilirubin increase > 2.5 × ULN | 6 | 3130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.33, 2.04] |
| 1.11 Subsequent treatment(s), ALT increase > 5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [0.49, 13.10] |
| 1.12 Subsequent treatment(s), AST increase > 5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [0.50, 7.06] |
| 1.13 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 4 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.16, 3.63] |
| 1.14 Paediatric trials ‐ total failure: day 28 (PCR‐adjusted) | 2 | 684 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.13, 7.84] |
| 1.15 Paediatric trials ‐ total failure: day 42 (PCR‐adjusted) | 2 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.41, 2.59] |
| 1.16 Paediatric trials ‐ total failure: day 28 (unadjusted) | 2 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.18] |
| 1.17 Paediatric trials ‐ total failure: day 42 (unadjusted) | 2 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.33] |
| 1.18 Paediatric trials ‐ first treatment, ALT increase > 5 × ULN | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.03, 8.06] |
| 1.19 Paediatric trials ‐ first treatment, AST increase > 5 × ULN | 2 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.16, 14.52] |
Comparison 2. Pyronaridine‐artesunate versus artesunate‐amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total failure: day 28 (PCR‐adjusted) | 6 | 1245 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.77] |
| 2.2 Total failure: day 42 (PCR‐adjusted) | 6 | 1091 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.20, 4.83] |
| 2.3 Total failure: day 28 (unadjusted) | 6 | 1257 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.30, 0.81] |
| 2.4 Total failure: day 42 (unadjusted) | 6 | 1235 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.23] |
| 2.5 First treatment, ALT increase > 5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.28, 7.09] |
| 2.6 First treatment, AST increase > 5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.07] |
| 2.7 First treatment, bilirubin increase > 2.5 × ULN | 6 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.76] |
| 2.8 Subsequent treatment(s), ALT increase > 5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.48, 7.76] |
| 2.9 Subsequent treatment(s), AST increase > 5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.49, 6.62] |
| 2.10 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 6 | 784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.33, 9.83] |
Comparison 3. Pyronaridine‐artesunate versus mefloquine plus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Total failure: day 28 (PCR‐adjusted) | 1 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.13, 1.05] |
| 3.2 Total failure: day 42 (PCR‐adjusted) | 1 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.90, 3.57] |
| 3.3 Total failure: day 28 (unadjusted) | 1 | 1120 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.17, 0.78] |
| 3.4 Total failure: day 42 (unadjusted) | 1 | 1059 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 3.5 Serious adverse events | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.25, 3.97] |
| 3.6 Adverse events leading to withdrawal | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.17, 2.31] |
| 3.7 First treatment, ALT increase > 5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.48 [0.99, 56.45] |
| 3.8 First treatment, AST increase > 5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.49 [0.55, 162.64] |
| 3.9 First treatment, bilirubin increase > 2.5 × ULN | 1 | 1271 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [0.43, 28.29] |
Comparison 4. Pyronaridine‐artesunate versus other antimalarials for all malaria subtypes (safety outcomes only).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Serious adverse events | 7 | 3941 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.54, 2.84] |
| 4.2 Adverse events leading to withdrawal | 6 | 3911 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.58, 1.94] |
| 4.3 First treatment, ALT increase > 5 × ULN | 14 | 6669 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [1.76, 7.33] |
| 4.4 First treatment, AST increase > 5 × ULN | 14 | 6669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.12, 4.41] |
| 4.5 First treatment, bilirubin increase > 2.5 × ULN | 13 | 6384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.18] |
| 4.6 Subsequent treatment(s), ALT increase > 5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.76, 6.27] |
| 4.7 Subsequent treatment(s), AST increase > 5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.74, 4.44] |
| 4.8 Subsequent treatment(s), bilirubin increase > 2.5 × ULN | 7 | 1649 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.42, 3.01] |
| 4.9 Sensitivity analysis: first treatment, ALT increase > 5 × ULN | 10 | 5746 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [1.99, 9.87] |
| 4.10 Other adverse events | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.10.1 Blood and lymphatic: anaemias | 4 | 4517 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.54] |
| 4.10.2 Blood and lymphatic: eosinophilic disorders | 2 | 2543 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.86, 1.93] |
| 4.10.3 Blood and lymphatic: leukocytosis | 2 | 2615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.37] |
| 4.10.4 Blood and lymphatic: neutropenias | 2 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 4.10.5 Cardiac: cardiac arrhythmias | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.95 [0.25, 143.93] |
| 4.10.6 Cardiac: cardiac signs and symptoms | 5 | 2108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.89] |
| 4.10.7 Cardiac: myocardial disorders | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.10.8 Ear: ear disorders | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 4.10.9 Gastrointestinal: diarrhoea (excl infective) | 4 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [1.10, 5.99] |
| 4.10.10 Gastrointestinal: dyspeptic signs and symptoms | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 68.26] |
| 4.10.11 Gastrointestinal: gastrointestinal and abdominal pains | 7 | 4544 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.87, 1.45] |
| 4.10.12 Gastrointestinal: nausea and vomiting symptoms | 9 | 5534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.17] |
| 4.10.13 General: asthenic conditions | 2 | 653 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.64, 2.75] |
| 4.10.14 General: general signs and symptoms | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.54, 2.70] |
| 4.10.15 General: feelings and sensations | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [0.23, 97.79] |
| 4.10.16 General: febrile disorders | 2 | 715 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.65, 2.96] |
| 4.10.17 General: pain and discomfort | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.91] |
| 4.10.18 Infections and infestations: eye and eyelid | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 68.26] |
| 4.10.19 Infections and infestations: lower respiratory tract infection and lung | 2 | 3216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 4.10.20 Infections and infestations: upper respiratory tract infection | 7 | 5350 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.88, 1.20] |
| 4.10.21 Investigations: ECG | 4 | 3347 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.36, 0.58] |
| 4.10.22 Investigations: skeletal/cardiac muscle analyses | 2 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.55, 3.15] |
| 4.10.23 Investigations: physical exam | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.10.24 Investigations: platelet | 2 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.55, 1.56] |
| 4.10.25 Investigations: protein | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.24] |
| 4.10.26 Metabolism and nutrition: appetite disorders | 3 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.80, 2.68] |
| 4.10.27 Metabolism and nutrition: hypoglycaemia conditions | 1 | 535 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.46] |
| 4.10.28 Metabolism and nutrition: metabolic disorders | 1 | 2681 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.29] |
| 4.10.29 Musculoskeletal and connective tissue: muscle pains | 3 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.97, 2.01] |
| 4.10.30 Musculoskeletal and connective tissue: pain and discomfort | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 4.10.31 Nervous system: headaches | 9 | 6271 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.32] |
| 4.10.32 Nervous system: sleep disturbance | 1 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.03, 15.99] |
| 4.10.33 Nervous system: paraesthesias and dysaesthesias | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.10.34 Respiratory: coughing and assoc symptoms | 6 | 6412 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 4.10.35 Respiratory: upper respiratory tract signs and symptoms | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.32, 10.07] |
| 4.10.36 Renal and urinary: urinary abnormalities | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.18, 20.63] |
| 4.10.37 Skin and subcutaneous tissue: dermatitis and eczema | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.15, 6.99] |
| 4.10.38 Skin and subcutaneous tissue: pruritis | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.13, 0.49] |
| 4.10.39 Skin and subcutaneous tissue: rashes, eruptions, exanthems | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.18, 20.63] |
| 4.10.40 Skin and subcutaneous tissue: urticaria | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.10.41 Vascular: vascular hypotensive disorders | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kayentao 2012.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, November 2007 to November 2008 |
|
| Participants | Children with Plasmodium falciparum malaria Number: 535, randomized 2:1 Inclusion criteria: age ≤ 12 years; bodyweight 5 kg to 25 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; other clinically significant disorder; severe vomiting; severe diarrhoea; viral hepatitis/HIV; malnutrition; QTc ≥ 450 ms; other febrile conditions; hepatic impairment (AST/ALT > 2.5 x ULN); renal impairment; electrolyte imbalance; anaemia (haemoglobin < 8 g/dL); allergy to study drugs; antimalarial therapy in previous 2 weeks, investigational drug in previous 4 weeks; taking any drug metabolized by cytochrome enzyme CYP2D6; previous participation in pyronaridine‐artesunate studies; pregnancy/lactation; unable to comply with follow‐up visits Diagnosis: microscopy (asexual parasite density 1000 to 200,000/µL blood) Children under 5: 152 (pyronaridine‐artesunate); 64 (artemether‐lumefantrine) |
|
| Interventions |
|
|
| Outcomes |
†2 consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Africa (n = 514, 96%) and Asia (n = 21, 4%). Africa: Burkina Faso, Democratic Republic of the Congo, Gabon, Côte d’Ivoire, Kenya, Mali. Asia: the Philippines Setting: local hospitals and clinics Malaria endemicity: high Resistance profile: not described Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs of similar appearance masked on allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants not blinded ("drugs given open‐label"). Clinical and parasitological assessments blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest. |
| Other bias | Low risk | 3 authors declared conflicting interests (employees of funders); blind to treatment allocation. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not fully describe method for detection of adverse events, includes biochemical and ECG monitoring. Authors do not give definitions of all adverse events. Uses higher ULN values for grading of hepatic enzyme severity compared to other studies |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | All adverse events reported. Supplementary tables detail laboratory variables at each assessment. |
Nelwan 2015.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, 4 months. March 2013 to July 2014 |
|
| Participants | Adult soldiers with Plasmodium vivax malaria Number: 180 Inclusion criteria: age 18 to 65 years; travelled to NE Papua within 12 months; bodyweight 40 kg to 90 kg Exclusion criteria: G6PD deficiency; Plasmodium falciparum monoinfection, hospitalization, anaemia (haemoglobin < 7 g/dL), planned absence from military base; clinically significant disorders; QTc ≥ 450 ms; family history prolonged QTc/sudden death; concomitant drugs known to prolong QT; hepatic impairment (AST/ALT > 2.5 x ULN); renal impairment; viral hepatitis; allergy to study drugs; previous participation; recent antimalarials Diagnosis: microscopy of P vivax, confirmed by a second microscopist |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Indonesia, in travellers returning from Papua Setting: army base Malaria endemicity: no known risk of malaria in study site Resistance profile: the infections were by the chloroquine‐resistant and primaquine‐tolerant Chesson‐like P vivax strains Source of funding: sponsored by the ALERT Asia Foundation (Indonesia), funded by Medicines for Malaria Venture (Switzerland) Follow‐up: 1 year |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Statistician block‐allocated treatment assignments by varying blocking number at random" |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if participants or assessing clinicians were blinded Parasitological assessments blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Prospective registration, reports all a priori outcomes |
| Other bias | Low risk | MMV played an advisory role, but had no role in study conduct or analysis. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not fully describe schedule for detection of adverse events, includes biochemical and ECG monitoring. Authors do not clearly define adverse events, including significant raise in hepatic enzymes. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Reports numbers of adverse events by grade, but does not define grading used |
Poravuth 2011.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, March 2007 to March 2008 |
|
| Participants | Adults and children with Plasmodium vivax malaria Number: 456 Inclusion criteria: age 3 to 60 years; fever or history of fever within 24 hours; bodyweight 20 kg to 90 kg Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; severe vomiting; other clinical condition recurring hospitalization; other clinically significant disorder; viral hepatitis/HIV; malnutrition; QTc ≥ 450 ms; other febrile conditions; hepatic impairment (AST/ALT > 2.5 x ULN); renal impairment; anaemia (haemoglobin < 8 g/dL); allergy to study drugs; antimalarial therapy in previous 2 weeks (or antibacterial with antimalarial effect); investigational drug in previous 4 weeks; previous participation in pyronaridine‐artesunate studies; pregnancy/lactation; unable to comply with follow‐up visits Diagnosis: microscopy of P vivax (parasite density ≥ 250/mL blood, including > 50% asexual parasites) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review †2 consecutive normal readings taken between 7 and 25 hours apart |
|
| Notes | Location: Asia (Cambodia, India, Indonesia, and Thailand) Setting: local hospitals Malaria endemicity: high Resistance profile: not described Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization scheme |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Doubly‐dummy ‐ study drug and matching placebo, packaged similarly All study investigators, laboratory technicians, and participants blind to treatment assignment. Investigator calculated the appropriate dose, and study drug was administered by a different member of staff. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest. |
| Other bias | Low risk | 3 authors declared conflicting interests (employees of funders); blind to treatment allocation. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not fully describe method for detection of adverse events, but do describe interval for ECG assessment. Not all definitions of adverse events provided. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | All‐cause adverse events enumerated. Report table only includes adverse events occurring in ≥ 2% (or ≥ 1% if judged to be drug related). |
Ringwald 1996.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, 1 month: April 1994 to May 1995 |
|
| Participants | Adults (> 15 years) with Plasmodium falciparum malaria Number: 96 Inclusion criteria: fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; recent self‐medication; pregnancy Diagnosis: thin film microscopy of P falciparum (asexual parasite density > 5000/µL blood) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Cameroon Setting: dispensary (outpatients) Malaria endemicity: high Resistance profile: 57% of isolates chloroquine‐resistant Source of funding: French Ministere de la Co‐operation (Grant 93A43); pyronaridine was supplied by the Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shanghai, China Follow‐up: 14 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (blocks of 10); from communication with authors recorded in previous version of this review |
| Allocation concealment (selection bias) | Low risk | Central randomization; from communication with authors recorded in previous version of this review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded, but tablets were different, and participants treated with chloroquine suffered pruritis; from communication with authors recorded in previous version of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions; unlikely to have differentially influenced liver toxicity outcomes used in this review. |
| Selective reporting (reporting bias) | Low risk | Trial not prospectively registered, trial protocol not available; however, all outcomes stated in methods reported. |
| Other bias | Low risk | None identified. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not fully describe method for detection of adverse events. Not all definitions of adverse events provided. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors give brief narrative description of adverse events only. Report does not detail extent of elevation in liver transaminases or the proportions retested at day 14. |
Ringwald 1998.
| Study characteristics | ||
| Methods | RCT Duration not stated. Year 1996 |
|
| Participants | Children (< 15 years) with Plasmodium falciparum malaria Number: 88 Inclusion criteria: fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; recent self‐medication; pregnancy; anaemia (haemoglobin < 5 g/dL), moderate to severe malnutrition Diagnosis: thin film microscopy of P falciparum (asexual parasite density > 5000/µL blood) |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Cameroon Setting: dispensary (outpatients) Malaria endemicity: high Resistance profile: 49% of isolates chloroquine‐resistant Source of funding: French Ministere de la Co‐operation (Grant 93A43); pyronaridine was supplied by the Institute of Parasitic Diseases, Chinese Academy of Preventive Medicine, Shanghai, China Follow‐up: 14 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization (blocks of 10); from communication with authors recorded in previous version of this review |
| Allocation concealment (selection bias) | Low risk | Central randomization; from communication with authors recorded in previous version of this review |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded, but tablets were different, and participants treated with chloroquine suffered pruritis; from communication with authors recorded in previous version of this review |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report and subsequent correspondence from authors lists reasons for attrition and exclusions; unlikely to have differentially influenced liver toxicity outcomes used in this review. |
| Selective reporting (reporting bias) | Low risk | Trial not prospectively registered, trial protocol not available; however, all outcomes stated in methods reported. |
| Other bias | Low risk | None identified. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not fully describe method for detection of adverse events. Not all definitions of adverse events provided. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors give brief narrative description of adverse events only. Report does not detail extent of elevation in liver transaminases or the proportions retested at day 14. |
Roth 2018a.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, 5 months. October 2015 to June 2016, January 2017 to August 2017 |
|
| Participants | Children with Plasmodium falciparum malaria Number: 197 Inclusion criteria: age 6 months to 12 years; bodyweight ≥ 5 kg Exclusion criteria: severe/complicated malaria; non‐falciparum/mixed Plasmodium infection; other clinically significant disorder; malnutrition; hepatic impairment (AST/ALT not specified); renal impairment; anaemia (haemoglobin < 6 g/dL); allergy to study drugs; current participation in other antimalarial study; previous participation in study, not available for follow‐up Diagnosis: microscopically confirmed P falciparum monoinfection (asexual parasite density 1000 µL to 200,000/µL) Children under 5: 31 (pyronaridine‐artesunate); 31 (artemether‐lumefantrine) |
|
| Interventions |
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †2 consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Kenya Setting: Local clinic Malaria endemicity: high Resistance profile: not described Source of funding: EU FP7‐Health‐2013. 0‐1 Project “Translation of the direct‐on‐blood PCR‐NALFIA system into an innovative near point‐of‐care diagnostic for malaria” (DIAGMAL) (Grant Number 601714) Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule provided by sponsor. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants probably not blinded (drugs were administered by pharmacy personnel aware of group
assignments). Clinical and parasitological assessments performed by study staff blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Authors report fever clearance time, which was not in trial registration document. Unlikely to have introduced significant bias |
| Other bias | Low risk | Target recruitment not reached. Shin Poong Pharmaceutical Company (Seoul, South Korea) provided pyronaridine–artesunate tablets and granules, but had no further role in study design, data collection, data analysis, and writing of the report. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors describe full schedule for ALT/AST monitoring. Due to logistic constraints, ALT and AST were only measured for the first 150 participants. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors do not give reporting threshold for adverse events. |
Rueangweerayut 2012.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, 10 months. January 2007 to October 2008 |
|
| Participants | Adults and children with Plasmodium falciparum malaria Number: 1271 Inclusion criteria: age 3 to 60 years; bodyweight 20 kg to 90 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; anaemia (haemoglobin < 8 g/dL); severe vomiting; diarrhoea; pregnancy/lactation; other clinically significant disorder; hepatic impairment (undefined); renal impairment; antimalarial therapy in previous 2 weeks; investigational drug in previous 4 weeks; previous participation in study; allergy to study drugs Diagnosis: thick and thin film microscopy of P falciparum (asexual parasite density 1000 mm3 to 100,000 mm3 blood) |
|
| Interventions | Randomized in a 2:1 ratio to:
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †2 consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Asia (81%) and Africa (19%). Asia: Cambodia, India, Thailand, Vietnam. Africa: Bukina Faso, Ivory Coast, Tanzania Setting: local hospitals and health centres Malaria endemicity: high Resistance profile: in Cambodia, significantly extended parasite clearance times (for both treatment arms) were suggestive of in vivo resistance to artemisinin Source of funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs Randomization communicated by investigator to a third party who administered the correct amount of tablets. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unclear if participants were blinded Outcome assessors blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes all prestated outcomes of interest. |
| Other bias | Low risk | Some authors employed by trial sponsors, but all authors assumed responsibility for reporting accuracy. |
| Adverse event monitoring (detection bias) Adverse events | Unclear risk | Authors do not describe methods for monitoring adverse events, but includes biochemical monitoring. Authors do describe time point of assessments in protocol. |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Report percentage of adverse events. Supplementary data table provided. Reports "any cause" adverse events if they occurred in > 2% of participants. Reports "treatment related" adverse events if they occurred in > 1% of participants. Does not report method of judging relation of adverse events to treatment |
Sagara 2018.
| Study characteristics | ||
| Methods | RCT* Duration: 4 years, 4 months: 24 October 2011 to 1 February 2016 *Different arms at different study centres, therefore we requested disaggregated data |
|
| Participants | Adults and children with Plasmodium falciparum malaria Number: 4710 (2640 within pyronaridine‐artesunate subsection) Inclusion criteria: age > 2 years*; bodyweight ≥ 15 kg (decreased to ≥ 5 kg after review); fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; severe vomiting; severe diarrhoea; other clinically significant disorder including QTc ≥ 450 ms, active tuberculosis, jaundice and others; anaemia (haemoglobin < 70 g/dL); other febrile conditions; allergy to study drugs; antimalarial therapy in previous 2 weeks; investigational drug in previous 4 weeks; pregnancy/lactation; alcohol abuse; viral hepatitis/HIV; hepatic impairment (ALT > 2 x ULN); renal impairment (1.5 x ULN) Diagnosis: positive microscopy for Plasmodium spp. (> 0 to < 200,000 parasites/μL blood) *For pyronaridine‐artesunate group, inclusion criteria changed during the study. (i) Beginning of the study, inclusion age of 15 years or older and bodyweight of 24 kg or over, (ii) after 20 retreatments, inclusion age of 2 years or older and bodyweight of 15 kg or over, and (iii) after 40 retreatments, inclusion age of at least 6 months with bodyweight of 5 kg or over Children under 5: 344 (PY‐AS); 129 (AL); 249 (AS‐AQ) The total number of participants receiving at least 1 study treatment in each comparison are below.
The breakdown of total participants receiving each treatment at each site is below.
The total numbers of participants and numbers disaggregated by each site were obtained from the trial authors in response to a request for further information in May 2017. |
|
| Interventions |
*Not compared against pyronaridine‐artesunate in this trial |
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: West Africa (Burkina Faso, Guinea, Mali), conducted by the West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) Setting: tertiary health facilities Malaria endemicity: high Resistance profile: not described Source of funding: European and Developing Countries Clinical Trial Partnership, Medicines for Malaria Venture, United Kingdom Medical Research Councils, Swedish International Development Co‐operation Agency, German Ministry for Education and Research, University Claude Bernard (France), University of Science Techniques and Technologies of Bamako, Centre National de Recherche et de Formation sur le Paludisme (Burkina Faso), Institut de Recherche en Sciences de la Santé (Bobo‐Dioulasso, Burkina Faso), and Centre National de Formation et de Recherche en Santé Rurale (Republic of Guinea) Follow‐up: 42 days active; 2 year passive |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list for each site within each country was used; block size of 2 |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque, sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open‐label: participants and investigators not blinded to treatment allocation Microscopists assessing parasite outcomes masked to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons provided for all withdrawals across study arms. Withdrawal numbers small and balanced across the intervention arms with reasons for withdrawal similar between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as listed in the trial register; however, day 63 outcomes are not reported. |
| Other bias | Low risk | Some authors employed by trial sponsors, but all authors assumed responsibility for reporting accuracy. |
| Adverse event monitoring (detection bias) Adverse events | Low risk | Authors report that physical examinations made and adverse events recorded at all assessments. Describes ECG and biochemistry monitoring schedule. Used Medical Dictionary for Regulatory Activities (MedDRA) |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | Authors enumerate adverse events clearly, and report events of interest. Supplementary tables are provided. We were unable to extract adverse events by study site. |
Sagara 2018 (Bobo‐Doiulasso, Burkina Faso).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 232 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Bobo, Burkina Faso | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Bougoula, Mali).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 619 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Bougoula, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Djoliba, Mali).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 172 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Djoliba, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Kolle, Mali).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 195 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Kolle, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Mafrinyah, Guinea).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 468 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Mafrinyah, Guinea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Ouagadougou, Burkina Faso).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 429 | |
| Interventions |
|
|
| Outcomes | ‐ | |
| Notes | Location: Ouagadougou, Burkina Faso | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Sagara 2018 (Sotuba, Mali).
| Study characteristics | ||
| Methods | RCT* ‐ represents disaggregated data from Sagara 2018 (see above) | |
| Participants | Number receiving at least 1 study treatment: 325 | |
| Interventions | Comparison 1
Comparison 2
|
|
| Outcomes | ‐ | |
| Notes | Location: Sotuba, Mali | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Represents disaggregated data from Sagara 2018 (see above) |
| Allocation concealment (selection bias) | Low risk | ‐ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Selective reporting (reporting bias) | Low risk | ‐ |
| Other bias | Low risk | ‐ |
| Adverse event monitoring (detection bias) Adverse events | Low risk | ‐ |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | ‐ |
Shin 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Adults and children with Plasmodium vivax malaria Number: 30 |
|
| Interventions |
|
|
| Outcomes |
*Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Korea Setting: unknown Malaria endemicity: unstable Resistance profile: not described Source of funding: unknown Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned in ascending order a randomization number according to order recruited. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for 14 of 15 participants in pyronaridine‐artesunate arm, and 15 of 15 participants in chloroquine arm. |
| Selective reporting (reporting bias) | Low risk | Authors provided all requested data. |
| Other bias | Unclear risk | Authors from pharmaceutical company manufacturing pyronaridine‐artesunate |
| Adverse event monitoring (detection bias) Adverse events | Low risk | Full schedule of safety monitoring |
| Incomplete adverse event reporting (reporting bias) Adverse events | Low risk | Authors provided all requested data. |
Tshefu 2010.
| Study characteristics | ||
| Methods | RCT Duration: 1 year, 3 months: January 2007 to April 2008 |
|
| Participants | Adults and children with Plasmodium falciparum malaria Number: 1272 Inclusion criteria: age 3 to 60 years; bodyweight 20 kg to 90 kg; fever or history of fever within 24 hours Exclusion criteria: severe/complicated malaria; mixed Plasmodium infection; malnutrition; anaemia (haemoglobin < 8 g/dL); severe vomiting; severe diarrhoea; other clinically significant disorder; hepatic impairment (limit not stated); renal impairment; other febrile conditions; viral hepatitis/HIV; electrolyte imbalance; allergy to study drugs; antimalarial therapy in previous 2 weeks, investigational drug in previous 4 weeks; taking any drug metabolized by cytochrome enzyme CYP2D6; previous participation in pyronaridine‐artesunate studies; pregnancy/lactation Diagnosis: microscopy (asexual parasite density 1000 to 100,000/µL blood) |
|
| Interventions | Randomized in a 2:1 ratio to:
|
|
| Outcomes |
*Adequate clinical and parasitological response rate †2 consecutive normal readings taken between 7 and 25 hours apart ‡Not assessed in quantitative synthesis in this review |
|
| Notes | Location: Africa (n = 1080, 85%) and Asia (n = 192, 15%). Africa: Democratic Republic of the Congo, The Gambia, Ghana, Kenya, Mali, Mozambique, Senegal. Asia: Indonesia, the Philippines Setting: local hospitals and clinics Malaria endemicity: high Resistance profile: not described Funding: Medicines for Malaria Venture, Shin Poong Pharmaceutical Company Ltd, Seoul, Republic of Korea Follow‐up: 42 days |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization schedule. Block randomization of 9 by study centre |
| Allocation concealment (selection bias) | Low risk | Individually numbered treatment packs Randomization communicated by investigator to a third party who administered the correct amount of tablets, and who was not involved in clinical assessment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded: artemether‐lumefantrine placebo dosed twice daily to maintain blinding. Food not required for artemether‐lumefantrine dosing to retain blinding. Outcome assessors blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Report lists reasons for attrition and exclusions. |
| Selective reporting (reporting bias) | Low risk | Prospectively registered. Report includes prestated outcomes of interest. Day 42 efficacy outcomes and gametocyte counts not listed in trial registration document; listed in the report as exploratory. |
| Other bias | Unclear risk | Sponsors designed the trial, were responsible for data collection and analysis, and developed the report; all authors had access to trial data. Participants on artemether‐lumefantrine were not expected to take medication after food; unclear if this reduced bioavailability of lumefantrine. |
| Adverse event monitoring (detection bias) Adverse events | Low risk | Reports that adverse events were recorded during treatment and at all follow‐up visits |
| Incomplete adverse event reporting (reporting bias) Adverse events | Unclear risk | Authors report all‐cause adverse events as percentages. Report table only includes adverse events occurring in ≥ 5% (or ≥ 1% if judged to be drug related). Authors explain method for determining relation of adverse events to study drug. |
Abbreviations: ACPR: adequate clinical and parasitological response; AL: artemether‐lumefantrine; ALT: alanine aminotransferase; AS‐AQ: artesunate‐amodiaquine; AST: aspartate transaminase; ECG: electrocardiogram; MMV: Medicines for Malaria Venture; PCR: polymerase chain reaction; QT: QT interval on electrocardiogram; QTc: corrected QT interval on electrocardiogram; PY‐AS: pyronaridine‐artesunate; RCT: randomized controlled trial; ULN: upper limit of normal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bui 2020 | Not an RCT |
| Han 2020 | Not an RCT |
| Huang 1988 | Quasi‐RCT. Randomized according to order of admission |
| Huang 1989 | Quasi‐RCT. Odd and even numbers used for allocation |
| Huang 1993 | RCT: conducted in people with complicated falciparum malaria |
| Laman 2014 | Trial registration (ACTRN12610000913077) planned to use pyronaridine‐artesunate, but not available due to concerns over hepatotoxicity at time of trial. |
| Leang 2016 | Not an RCT: single‐arm observational study |
| Leang 2019a | Not an RCT |
| Leang 2019b | Not an RCT |
| Looareesuwan 1996 | Not an RCT: clinical trial of 2 doses of pyronaridine monotherapy, with group given second dose recruited after results of first dose were analysed |
| Looareesuwan 2007 | RCT: phase II dose‐ranging trial |
| Lutete 2021a | Not an RCT |
| Piola 2008 | Not an RCT: phase II dose‐ranging study |
| Ramharter 2008 | Not an RCT: open‐label dose‐escalation study |
| Roth 2018b | Did not report our outcomes of interest |
| Sagara 2014 | Not an RCT: pooled analysis |
Abbreviations: RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT03726593.
| Study name | Drug Combinations of Atovaquone‐Proguanil (AP) With ACT (APACT) |
| Methods | RCT |
| Participants | Adults with Plasmodium falciparum malaria Number: 252; randomised 1:1:1 Inclusion criteria: understands Khmer spoken language; male or female (18 to 70 years old); able to take oral medications; haemoglobin on day of enrolment ≥ 9.0 g/dL; agree to follow‐up, including inpatient hospitalization and 6‐weekly follow‐up; written permission for those on active military duty Exclusion criteria: allergy to study drugs; pregnant/lactating, females of childbearing age who do not agree to use contraception during study period and follow‐up; severe vomiting; severe malaria; abnormal liver function test results; isolated AST or ALT or total bilirubin > 2 x ULN; known significant cardiovascular, liver, or renal abnormality or any other clinically significant illness; treatment for malaria within the last 4 weeks; unable to provide informed consent; judged by the investigator to be otherwise unsuitable for study participation (to include, but not limited to, taking other medications that are known to cause serious drug‐drug interactions with the study drugs, or having suspected medical condition or taking other drugs that may affect test results interpretation or put the volunteer at much higher risk) Diagnosis: microscopic confirmation of asexual stages of P falciparum or mixed infection with P falciparum, with baseline asexual parasite densities between 100/µL and 200,000/µL |
| Interventions | Artesunate‐pyronaridine: once daily for 3 days, following standard weight‐based dosing per drug label. All volunteers with P falciparum monoinfection will receive single dose of primaquine (PQ) (15 mg) for transmission blocking. Atovaquone‐proguanil (AP) + artesunate‐pyronaridine (ASPY): once daily for 3 days, following standard weight‐based dosing per drug label for each drug. All volunteers with P falciparum monoinfection will receive single dose of PQ (15 mg) for transmission blocking. Atovaquone‐proguanil (AP) + artesunate‐mefloquine (ASMQ): ASMQ once daily for 3 days (D0, D1, D2), following standard weight‐based dosing per drug label. Subsequently, volunteers will continue their treatment with AP once daily starting on day 3, for 3 additional days (D3, 4, 5). All volunteers with P falciparum monoinfection will receive single dose of PQ (15 mg) for transmission blocking. |
| Outcomes | Primary outcome: ACPR day 42 (PCR‐adjusted) Secondary outcomes:
|
| Starting date | 4 October 2018 |
| Contact information | Mariusz Wojnarski (MARIUSZ.WOJNARSKI.MIL@AFRIMS.ORG) Norman Waters (Norman.Waters.mil@afrims.org) |
| Countries of recruitment | Cambodia |
| Notes |
NCT03814616.
| Study name | Pyramax in asymptomatic carriers of P. falciparum monoinfections |
| Methods | RCT |
| Participants | Asymptomatic adults with Plasmodium falciparum malaria Number: 300; randomised 1:1:1 Inclusion criteria: asymptomatic infection with P falciparum monoinfection; absence of any clinical symptoms of malaria at the time of enrolment and within 72 hours before enrolment; age > 5 years old and > 20 kg bodyweight; ability to swallow oral medication; evidence of informed consent document national regulations; compliance with scheduled visits, treatment plan, laboratory tests, and other study procedures Exclusion criteria: haemoglobin < 7 g/dL (measured at screening); previous antimalarial treatment up to 6 weeks prior to study period; herbal or traditional therapy 14 days prior to treatment; allergy to study drugs; pregnancy/lactation; severe malnutrition; participation in other studies within 30 days before the current study begins or during study participation, or both; inability to comprehend and/or unwillingness to follow the study protocol; previously randomized in this study; severe acute or chronic medical or psychiatric condition or laboratory abnormality that may increase the risk associated with study participation; individual the investigator considers at particular risk of participating in the study Diagnosis: thick and thin blood smears with parasite density between 20/µL and 50,000/µL |
| Interventions |
|
| Outcomes | Primary outcomes: PCR‐adjusted APR at day 28 Seconary outcomes:
|
| Starting date | 3 October 2018 |
| Contact information | Study Director: Jang Sik Shin; Shin Poong |
| Countries of recruitment | The Gambia and Zambia |
| Notes |
ALT: alanine aminotransferase; APR: adequate parasitological response; AST: aspartate transaminase; AUC: area under the curve; Cmax: maximum serum concentration; ETF: early treatment failure; LCTF: late clinical treatment failure; LPTF: late parasitological treatment failure; LTF: late treatment failure; PCR: polymerase chain reaction; qPCR: quantitative polymerase chain reaction; RCT: randomized controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; WHO: World Health Organization.
Differences between protocol and review
Differences between protocol and 2014 review
We stated in the protocol that we intended to assess the methods used to generate the allocation sequence and conceal allocation concealment as adequate, inadequate, or unclear according to Juni 2001, and note who was blinded to the interventions in each trial. However, since the introduction of Review Manager 5, we have made these assessments using the methods described in Higgins 2011.
In keeping with Cochrane policy to use summary of findings tables, which was introduced after publication of the protocol, we generated these tables using GRADEpro GDT (GRADEpro GDT), and interpreted the evidence for each outcome and comparison using the GRADE approach (Schünemann 2019).
We revised the list of outcomes to reflect current World Health Organization standards for assessing outcomes in antimalarial trials.
Although gametocyte carriage was not included as an outcome in the protocol, we included it as a secondary outcome because of its importance in malaria transmission.
In the protocol we stated that we intended to assess the effectiveness of pyronaridine both as a monotherapy and in combination with an artemisinin. However, this was revised to focus only on pyronaridine‐artemisinin combinations. In addition, due to concerns regarding the effect of pyronaridine on the liver, assessment of the effects of the comparisons on liver function now include randomized comparisons in both Plasmodium falciparum and Plasmodium vivax malaria. Accordingly, we updated the Background and Methods sections considerably to reflect the changing scenario in malaria policies and epidemiology.
PT and HB joined the review team. Rajeev Aravindakshan withdrew from the team due to conflicting demands on his time.
Differences between protocol and 2018 review update
There is a new author team: Joseph Pryce and Paul Hine.
We used the term pyronaridine‐artesunate in preference to artesunate‐pyronaridine to reflect how most authors refer to the intervention, and changed the title of the review accordingly. We did not proceed with quantitative analysis of secondary outcomes parasite clearance, fever clearance, and gametocyte carriage. The original protocol did not clearly define these outcomes, including whether they refer to durations, rates, or proportions of patients at given time points. We encountered considerable heterogeneity in these measures between studies, and therefore presented a narrative synthesis. We simplified the adverse events outcomes to reflect areas which were of most interest.
We added the term 'pyramax' to the search strategy. We simplified the risk of bias assessment for adverse events.
Differences between 2018 review update and 2022 review update
Melissa Tayor and Tilly Fox joined the author team.
We used the same search strategy to identify new studies published or registered from 8 May 2018 to 27 October 2021. This resulted in the inclusion of two new records which related to the previously included study Sagara 2018. We replaced unpublished data for one trial site (Bobo‐dioulasso) with that recently published by Compaore 2021. This resulted in no changes to the treatment efficacy outcomes, and a minor increase in risk ratios for the proportion of participants with raised liver enzymes. It also led to no changes in the certainty of the evidence, except that for pyronaridine‐artesunate compared to artemether‐lumefantrine for first treatment, the certainty of evidence for abnormal aspartate transaminase (AST) levels following treatment was changed from very low to low. In the aggregate analysis of pyronaridine‐artesunate compared to any drug, this small change had no effect on the overall GRADE of moderate for this outcome. We also included a qualitative summary of parasite clearance times using data reported by Soulama 2019.
We also included a review of non‐randomized studies on safety. Estimates of serious adverse effects were low and therefore reassuring. When added to the data from randomized controlled trials in this review, they effectively double the numbers of people in whom safety is monitored, and are also important because two of the studies did not exclude people with evidence of liver injury.
Contributions of authors
For this update, Melissa Taylor (MT) screened all newly identified studies and extracted data for those eligible for inclusion. Joseph Pryce (JP) and Paul Hine (PH) screened and extracted data from all studies previously included in this review and performed all risk of bias assessments. PH and JP wrote the Background, Methods, Discussion, and Authors' conclusions, which have been updated by MT. PH, JP, and MT completed the analysis, summary of findings tables, and results. Tilly Fox (TF) completed the safety review on non‐randomized studies which is included in this update. All review authors read and approved the final manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK
External sources
-
Foreign, Commonwealth, and Development Office (FCDO), UK
Project number 300342‐104
-
World Health Organization (WHO), Switzerland
WHO Global Malaria Programme Agreement for Performance of Work (APW) grant (202710314) for this review update version
Declarations of interest
JP is a CIDG Editor, and was not involved in the editorial process of this review update. He has no known conflicts of interest.
MT has no known conflicts of interest.
TF has no known conflicts of interest.
PH is a CIDG Editor, and was not involved in the editorial process of this review update. He was previously employed full time by Cochrane Infectious Diseases Group (CIDG), and currently works full time within the UK National Health Service (NHS). To the best of his knowledge, no financial or non‐financial conflicts of interests have influenced the current submitted work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Kayentao 2012 {published data only}
- Kayentao K, Doumbo OK, Pénali LK, Offianan AT, Bhatt KM, Kimani J, et al. Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malaria Journal 2012;11:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayentao K. Pyronaridine-artesunate versus artemether/lumefantrine: efficacy in malaria patients with uncomplicated acute falciparum malaria: results of a pivotal Phase III trial. American Journal of Tropical Medicine and Hygiene 2008;79(Suppl 6):114. [Google Scholar]
- NCT00541385. Pyronaridine artesunate 3:1 granule formulation vs. Coartem© crushed tablets in P. falciparum malaria pediatric patients. www.clinicaltrials.gov/show/NCT00541385 (first received 10 October 2007).
Nelwan 2015 {published data only}
- Nelwan EJ, Ekawati LL, Tjahjono B, Setiabudy R, Sutanto I, Chand K, et al. Randomized trial of primaquine hypnozoitocidal efficacy when administered with artemisinin-combined blood schizontocides for radical cure of Plasmodium vivax in Indonesia. BMC Medicine 2015;13:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poravuth 2011 {published data only}
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:51-100. [Google Scholar]
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:101-50. [Google Scholar]
- Poravuth Y, Socheat D, Rueangweerayut R, Uthaisin C, Pyae Phyo A, Valecha N, et al. Pyronaridine-artesunate versus chloroquine in patients with acute Plasmodium vivax malaria: a randomized, double-blind, non-inferiority trial. PLOS ONE 2011;6(1):e14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringwald 1996 {published data only}
- Ringwald P, Bickii J, Basco L. Randomised trial of pyronaridine versus chloroquine for acute uncomplicated falciparum malaria in Africa. Lancet 1996;347(8993):24-8. [DOI] [PubMed] [Google Scholar]
- Ringwald P, Meche FS, Basco LK. Short report: effects of pyronaridine on gametocytes in patients with acute uncomplicated falciparum malaria. American Journal of Tropical Medicine and Hygiene 1999;61(3):446-8. [DOI] [PubMed] [Google Scholar]
Ringwald 1998 {published data only}
- Ringwald P, Bickii J, Basco LK. Efficacy of oral pyronaridine for the treatment of acute uncomplicated falciparum malaria in African children. Clinical Infectious Diseases 1998;26(4):946-53. [DOI] [PubMed] [Google Scholar]
Roth 2018a {published data only}
- Roth JM, Sawa P, Makio N, Omweri G, Osoti V, Okach S, et al. Pyronaridine–artesunate and artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a randomized controlled non‑inferiority trial. Malaria Journal 2018;17(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rueangweerayut 2012 {published data only}
- NCT00403260. Pyronaridine artesunate (3:1) versus mefloquine artesunate in P. falciparum malaria patients. www.clinicaltrials.gov/show/NCT00403260 (first received 21 November 2006).
- Rueangweerayut R, Phyo AP, Uchaisin C, Poravuth Y, Quang Binh T, Tinto H, et al. A randomized clinical trial comparing the efficacy and safety of fixed-dose pyronaridine-artesunate versus mefloquine plus artesunate in uncomplicated Plasmodium falciparum malaria. Submitted under review; personal communication by email from Isabelle Borghini Fuhrer, Medicines for Malaria Venture.
- Rueangweerayut R, Phyo AP, Uchaisin C, Socheat D, Quang Binh T, Tinto H, et al. Efficacy and safety of pyronaridine/artesunate fixed-dose combination compared with mefloquine plus artesunate in patients with acute uncomplicated Plasmodium falciparum malaria: results of a pivotal phase III trial. Tropical Medicine and International Health 2009;14 Suppl 2:30-97. [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine 2012;366(14):1298-309. [DOI] [PubMed] [Google Scholar]
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, et al. Supplement to: Pyronaridine–artesunate versus mefloquine plus artesunate for malaria. New England Journal of Medicine 2012;366(14):1298-309. [DOI] [PubMed] [Google Scholar]
Sagara 2018 {published and unpublished data}
- Beshir KB, Diallo N, Fofana B, Traore A, Kodio A, Togo A, et al. Measuring the efficacy of four ACT regimens in Mali using qPCR-based estimates of Plasmodium falciparum clearance time. In: 63rd Annual Meeting of the American Society of Tropical Medicine and Hygiene; 2013 Nov 2-6; New Orleans. Arlington (VA): American Society of Tropical Medicine and Hygiene, 2014.
- Kabore TN, Barry N, Compaore YD, Nikiema F, Kabre Z, Fofana A. Randomized trial to assess the effect on qtc interval of repeated treatment of uncomplicated malaria with acts in Bobo-Dioulasso, Burkina Faso: relation between parasitemia and prolonged qtc. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):94. [Google Scholar]
- Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, et al. Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 2018;391(10128):1378-90. [DOI: 10.1016/S0140-6736(18)30291-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara I, Beavogui AH, Zongo I, Soulama I, Borghini-Fuhrer I, Fofana B, et al. Safety and efficacy of re-treatments with pyronaridine-artesunate in African patients with malaria: a substudy of the WANECAM randomised trial. Lancet Infectious Diseases 2016;16(2):189-98. [DOI: 10.1016/S1473-3099(15)00318-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulama I, Coulibaly AS, Kabore JM, Ouattara MS, Bougouma EC, Sanon S. Assessment of the dynamics of Plasmodium falciparum parasitemia regarding three artemisinin combination regimens for acute uncomplicated malaria treatment, Banfora, Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;97(5 Suppl 1):317. [Google Scholar]
- Soulama I, Coulibaly AS, Kabore MJ, Ouattara M, Bougouma EC, Sanon S, et al. Safety and efficacy of repeated administration of pyronaridine-artesunate or dihydroartemisinin-piperaquine vs artesunate-amodiaquine in children and adult patients with acute uncomplicated Plasmodium sp malaria over of two years period at Banfora/Niangoloko site in Burkina Faso. American Journal of Tropical Medicine and Hygiene 2017;95(5 Suppl 1):479. [Google Scholar]
- Soulama I, Sirima SB. SBOC 8459 Assessment of parasite clearance after repeated treatment with artesunate amodiaquine, dihydroartemisinin-piperaquine, pyronaridine-artesunate in malaria patients in Burkina Faso. BMJ Global Health 2019;4:A7-8. [DOI: 10.1136/bmjgh-2019-EDC.17] [DOI] [Google Scholar]
- Tekete M, Djimde A, Borrmann S. A phase IIIb/IV comparative, randomised, multi-centre, open label, parallel 3-arm clinical study to assess the safety and efficacy of repeated administration of four artemisinin-based combination therapy (ACT) over a two-year period in children and adult patients with acute uncomplicated Plasmodium sp. malaria. Jahrestreffen der AG Malaria der Sektion Antiparasitare Chemotherapie der Paul-Ehrlich-Gesellschaft fur Chemotherapie 2012;9. [Google Scholar]
Sagara 2018 (Bobo‐Doiulasso, Burkina Faso) {published and unpublished data}
- Compaoré YD, Zongo I, Somé AF, Barry N, Nikiema F, Kabore TN, et al. Hepatic safety of repeated treatment with pyronaridine‐artesunate versus artemether–lumefantrine in patients with uncomplicated malaria: a secondary analysis of the WANECAM 1 data from Bobo-Dioulasso, Burkina Faso. Malaria Journal 2021;20:64. [DOI: 10.1186/s12936-021-03593-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANECAM trial authors. Site data: Bobo, Burkina Faso (as supplied 6 July 2017). Data on file.
Sagara 2018 (Bougoula, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Bougala, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Djoliba, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Djoliba, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Kolle, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Kolle, Mali (as supplied 6 July 2017). Data on file.
Sagara 2018 (Mafrinyah, Guinea) {published and unpublished data}
- WANECAM trial authors. Site data: Mafrinyah, Guinea (as supplied 6 July 2017). Data on file.
Sagara 2018 (Ouagadougou, Burkina Faso) {published and unpublished data}
- WANECAM trial authors. Site data: Ouaga, Burkina Faso (as supplied 6 July 2017). Data on file.
Sagara 2018 (Sotuba, Mali) {published and unpublished data}
- WANECAM trial authors. Site data: Sotuba, Mali (as supplied 6 July 2017). Data on file.
Shin 2011 {published and unpublished data}
- Shin CS, Kwak YG, Lee KD, Borghini Fuhrer I, Miller RM, Duparc S. Treatment of Korean vivax malaria patients with the fixed-dose combination of pyronaridine:artesunate. Tropical Medicine and International Health 2011;16 Suppl 1:97-384. [ABSTRACT NO.: 1.1-169] [Google Scholar]
Tshefu 2010 {published data only}
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Efficacy of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:51-100. [Google Scholar]
- Duparc S, Borghini-Fuhrer I, Craft JC, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety of pyronaridine/artesunate in clinical trials in patients with uncomplicated acute Plasmodium falciparum or Plasmodium vivax malaria: results of an integrated analysis. American Journal of Tropical Medicine and Hygiene 2009;81 Suppl:101-50. [Google Scholar]
- Tshefu AK, Gaye O, Kayentao K, Thompson R, Bhatt KM, Sesay SS, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised non-inferiority trial. Lancet 2010;375(9724):1457-67. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bui 2020 {published data only}
- Bui PQ, Huynh QH, Tran DT, Le DT, Nguyen TQ, Truong HV, et al. Pyronaridine-artesunate efficacy and safety in uncomplicated Plasmodium falciparum malaria in areas of Artemisin-resistant falciparum in Viet Nam (2017-2018). Clinical Infectious Diseases 2020;70(10):2187. [DOI] [PubMed] [Google Scholar]
Han 2020 {published data only}
- Han KT, Lin K, Han ZY, Myint MK, Aye KH, Thi A, et al. Efficacy and safety of Pyronaridine-artesunate for the treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax malaria in Myanmar. American Journal of Tropical Medicine and Hygiene 2020;103(3):1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 1988 {published data only}
- Huang ZS, Shao BR, Meng F, Zeng LH, Ye XY, Huang J, et al. Effects of combined dose of pyronaridine/sulfadoxine/pyrimethamine on falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1988;6(4):285-8. [PubMed] [Google Scholar]
Huang 1989 {published data only}
- Huang ZS, Feng Z, Meng F, Zeng LH, Lin X, Zhen Y, et al. Therapeutic effect of pyronaridine in plain tablets and enteric coated tablets in falciparum malaria patients. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 1989;7(1):19-21. [PubMed] [Google Scholar]
Huang 1993 {published data only}
- Huang Z, Shao B, Meng F, Shi X. Comparison of different regimen of pyronaridine and sulfadoxine combined with pyrimethamine in the treatment of malignant malaria. Chinese Journal of Infectious Diseases 1993;11(Suppl 3):175-7. [ISSN: CN-00256187] [Google Scholar]
Laman 2014 {published data only}
- Laman M, Moore BR, Benjamin JM, Yadi G, Bona C, Warrel J. Artemisinin-naphthoquine versus artemether-lumefantrine for uncomplicated malaria in Papua New Guinean children: an open-label randomized trial. PLOS Medicine 2014;11(12):e1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leang 2016 {published data only}
- Leang R, Canavati SE, Khim N, Vestergaard LS, Borghini Fuhrer I, Kim S, et al. Efficacy and safety of pyronaridine-artesunate for the treatment of uncomplicated Plasmodium falciparum malaria in western Cambodia. Antimicrobial Agents and Chemotherapy 2016;60(7):3884-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leang 2019a {published data only}
- Leang R, Khim N, Chea H, Huy R, Mairet-Khedim M, Bouth DM, et al. Efficacy and safety of Pyronaridine-artesunate plus single-dose Primaquine for the treatment of malaria in Western Cambodia. Antimicrobial Agents and Chemotherapy 2019;63:1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leang 2019b {published data only}
- Leang R, Mairet-Khedim M, Chea H, Huy R, Khim N, Bouth DM, et al. Efficacy and safety of Pyronaridine-artesunate plus single-dose Primaquine for treatment of uncomplicated Plasmodium falciparum malaria in Eastern Cambodia. Antimicrobial Agents and Chemotherapy 2019;63:2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Looareesuwan 1996 {published data only}
- Looareesuwan S, Kyle DE, Viravan C, Vanijanonta S, Wilairatana P, Wernsdorfer WH. Clinical study of pyronaridine for the treatment of acute uncomplicated falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene 1996;54(2):205-9. [DOI] [PubMed] [Google Scholar]
Looareesuwan 2007 {published data only}
- Looareesuwan S, Gaye O, Tjitra E, Bojang K, Socheat D, Piola P. Results of a randomized, multicentre, phase II, dose-ranging, clinical study to assess the safety and efficacy of fixed dose, orally administered pyronaridine and artesunate in adult patients with acute uncomplicated Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 2007;77(5):1004. [Google Scholar]
Lutete 2021a {published data only}
- Lutete GT, Mombo-Ngoma G, Assi SB, Bigoga JD, Koukouikila-Koussounda F, Ntamabyaliro NY, et al. Pyronaridine-artesunate real-world safety, tolerability, and effectiveness in malaria patients in 5 African countries: a single-arm, open-label, cohort event monitoring study. PLOS Medicine 2021;16(6):e1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piola 2008 {published data only}
- Piola P, Fleckenstein L. Pharmacokinetics, clinical and safety outcomes of pyronaridine/artesunate treatment of acute Plasmodium falciparum malaria in Uganda. American Journal of Tropical Medicine and Hygiene 2008;79(6):855. [Google Scholar]
Ramharter 2008 {published data only}
- Ramharter M, Kurth F, Schreier AC, Nemeth J, Glasenapp Iv, Bélard S, et al. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. Journal of Infectious Diseases 2008;198(6):911-9. [DOI] [PubMed] [Google Scholar]
Roth 2018b {published data only}
- Roth JA, Sawa P, Omweri J, Makio N, Osoti V, Jong MD, et al. Molecular detection of residual parasitemia after pyronaridine–artesunate or artemether–lumefantrine treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children. American Journal of Tropical Medicine and Hygiene 2018;99(4):970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagara 2014 {published data only}
- Sagara I, Piarroux R, Djimde A, Giorgi R, Kayentao K, Doumbo OK, et al. Delayed anemia assessment in patients treated with oral artemisinin derivatives for uncomplicated malaria: a pooled analysis of clinical trials data from Mali. Malaria Journal 2014;13(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT03726593 {unpublished data only}
- NCT03726593. Drug combinations of Atovaquone-Proguanil (AP) with ACT (APACT). clinicaltrials.gov/ct2/show/NCT03726593 (first received 31 October 2018).
NCT03814616 {unpublished data only}
- NCT03814616. Pyramax in asymptomatic carriers of P. falciparum monoinfections. clinicaltrials.gov/ct2/show/NCT03814616 (first received 24 January 2019).
Additional references
Aithal 2011
- Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clinical Pharmacology & Therapeutics 2011;89(6):806-15. [DOI] [PubMed] [Google Scholar]
Basco 1992
- Basco LK, Le Bras J. In vitro activity of pyronaridine against African strains of Plasmodium falciparum. Annals of Tropical Medicine and Parasitology 1992;86(5):447-54. [DOI] [PubMed] [Google Scholar]
Chang 1992
- Chang C, Lin-Hua T, Jantanavivat C. Studies on a new antimalarial compound: pyronaridine. Transactions of the Royal Society of Tropical Medicine and Hygiene 1992;86(1):7-10. [DOI] [PubMed] [Google Scholar]
Chavalitshewinkoon‐Petmitr 2000
- Chavalitshewinkoon-Petmitr P, Pongvilairat G, Auparakkitanon S, Wilairat P. Gametocytocidal activity of pyronaridine and DNA topoisomerase II inhibitors against multidrug-resistant Plasmodium falciparum in vitro. Parasitology International 2000;48(4):275-80. [DOI] [PubMed] [Google Scholar]
Childs 1988
- Childs GE, Häusler B, Milhous W, Chen C, Wimonwattrawatee T, Pooyindee N, et al. In vitro activity of pyronaridine against field isolates and reference clones of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene 1988;38(1):24-9. [DOI] [PubMed] [Google Scholar]
Croft 2012
- Croft SL, Duparc S, Arbe-Barnes SJ, Craft JC, Shin CS, Fleckenstein L, et al. Review of pyronaridine anti-malarial properties and product characteristics. Malaria Journal 2012;11:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dondorp 2009
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine 2009;351(5):455-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duparc 2013
- Duparc S, Borghini-Fuhrer I, Craft CJ, Arbe-Barnes S, Miller RM, Shin CS, et al. Safety and efficacy of pyronaridine-artesunate in uncomplicated acute malaria: an integrated analysis of individual patient data from six randomized clinical trials. Malaria Journal 2013;12:70. [DOI: 10.1186/1475-2875-12-70] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fu 1991
- Fu S, Xiao SH. Pyronaridine: a new antimalarial drug. Parasitology Today 1991;7(11):310-3. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 11 November 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Juni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurth 2009
- Kurth F, Pongratz P, Bélard S, Mordmüller B, Kremsner PG, Ramharter M. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti-malarial drug susceptibility assays. Malaria Journal 2009;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Loke 2007
- Loke YK, Price D, Herxheimer A, Cochrane Adverse Effects Methods Group. Systematic reviews of adverse effects: framework for a structured approach. BMC Medical Research Methodology 2007;7(32):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubell 2014
- Lubell Y, Dondorp A, Guerin PJ, Drake T, Meek S, Ashley E, et al. Artemisinin resistance - modelling the potential human and economic costs. Malaria Journal 2014;13(452):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutete 2021b
- Lutete TG, Mombo-Ngoma G, Assi SB, Bigoga JD, Koukouikila-Koussounda F, Ntamabyaliro NY, et al. Pyronaridine–artesunate real-world safety, tolerability, and effectiveness in malaria patients in 5 African countries: a single-arm, open-label, cohort event monitoring study. PLOS Medicine 2021;18(6):e1003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
MedDRA 2016 [Computer program]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Medical Dictionary for Regulatory Activities. Version accessed 19 October 2018. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, 2016. Available at www.meddra.org.
MMV 2002
- TDR/Medicines for Malaria Venture/Shin Poong Pharmaceuticals Inc. WHO TDR/Medicines for Malaria Venture/Shin Poong Pharmaceuticals Inc. sign agreement for development of pyronaridine-artesunate for treatment of malaria. www.mmv.org/newsroom/press-releases/tdrmedicines-malaria-ventureshin-poong-pharmaceuticals-inc-sign-agreement (accessed 17 April 2018).
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noedl 2008
- Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. New England Journal of Medicine 2008;359(24):2619-20. [DOI] [PubMed] [Google Scholar]
Peters 1997
- Peters W, Robinson BL. The chemotherapy of rodent malaria. LV. Interactions between pyronaridine and artemisinin. Annals of Tropical Medicine and Parasitology 1997;91(2):141-5. [DOI] [PubMed] [Google Scholar]
Pradines 1998
- Pradines B, Tall A, Parzy D, Spiegel A, Fusai T, Hienne R, et al. In-vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. Journal of Antimicrobial Chemotherapy 1998;42(3):333-9. [DOI] [PubMed] [Google Scholar]
Price 2010
- Price RN, Marfurt J, Chalfein F, Kenangalem E, Piera KA, Tjitra E, et al. In vitro activity of pyronaridine against multidrug-resistant Plasmodium falciparum and Plasmodium vivax. Antimicrobial Agents and Chemotherapy 2010;54(12):5146-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Ringwald 1999
- Ringwald P, Eboumbou EC, Bickii J, Basco LK. In vitro activities of pyronaridine, alone and in combination with other antimalarial drugs, against Plasmodium falciparum. Antimicrobial Agents and Chemotherapy 1999;43(6):1525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019 :403-31. [Google Scholar]
Sinclair 2009
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007483. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tickell‐Painter 2017
- Tickell‐Painter M, Maayan N, Saunders R, Pace C, Sinclair D. Mefloquine for preventing malaria during travel to endemic areas. Cochrane Database of Systematic Reviews 2017, Issue 10. Art. No: CD006491. [DOI: 10.1002/14651858.CD006491.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vivas 2008
- Vivas L, Rattray L, Stewart L, Bongard E, Robinson BL, Peters W, et al. Anti-malarial efficacy of pyronaridine and artesunate in combination in vitro and in vivo. Acta Tropica 2008;105(3):222-8. [DOI] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. December 2003. www.who.int/malaria/publications/atoz/whohtmrbm200350/en/ (accessed 1 September 2017).
WHO 2006
- World Health Organization. WHO guidelines for the treatment of malaria. 2006. archives.who.int/publications/2006/9241546948_eng.pdf.
WHO 2009
- World Health Organization. Methods for surveillance of antimalarial drug efficacy. November 2009. www.who.int/malaria/publications/atoz/9789241597531/en/ (accessed 1 September 2017).
WHO 2011
- World Health Organization. Global plan for artemisinin resistance containment (GPARC). www.who.int/malaria/publications/atoz/9789241500838/en/index.html (accessed 4 February 2011).
WHO 2015
- World Health Organization. Guidelines for the treatment of malaria - 3rd edition. April 2015. www.who.int/malaria/publications/atoz/9789241549127/en/ (accessed 1 September 2017).
WHO 2017
- World Health Organization. World Malaria Report 2017. www.who.int/malaria/publications/world-malaria-report-2017/en/.
WHO 2019
- World Health Organization. ACSOMP recommendations 2019. www.who.int/medicines/regulation/medicines-safety/publications/ACSoMP_16.pdf (accessed 4 November 2021).
WHO 2020
- World Health Organization. World Malaria Report 2020. www.who.int/publications/i/item/9789240015791 (accessed 4 November 2021).
WHO 2022
- World Health Organization. WHO guidelines for malaria. Geneva: World Health Organization; 31 March 2022. WHO/UCN/GMP/ 2022.01 Rev.1.
Zorzela 2016
- Zorzela L, Loke YK, Ioannidis JP, Golder S, Santaguida P, Altman DG, et al, PRISMA Harms Group. PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ 2016;352(157):1-17. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bukirwa 2014
- Bukirwa H, Unnikrishnan B, Kramer CV, Sinclair D, Nair S, Tharyan P. Artesunate plus pyronaridine for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD006404. [DOI: 10.1002/14651858.CD006404.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pryce 2019
- Pryce J, Hine P. Pyronaridine‐artesunate for treating uncomplicated Plasmodium falciparum malaria. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD006404. [DOI: 10.1002/14651858.CD006404.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Unnikrishnan 2007
- Unnikrishnan B, Nair S, Aravindakshan R. Pyronaridine for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD006404. [DOI: 10.1002/14651858.CD006404] [DOI] [PMC free article] [PubMed] [Google Scholar]


