Abstract
We investigated the direct and indirect effects of mono-, di-, and trivalent salts (NaCl, MgCl2, and AlCl3) on the adsorption of several viruses (MS2, PRD-1, φX174, and poliovirus 1) to microporous filters at different pH values. The filters studied included Millipore HA (nitrocellulose), Filterite (fiberglass), Whatman (cellulose), and 1MDS (charged-modified fiber) filters. Each of these filters except the Whatman cellulose filters has been used in virus removal and recovery procedures. The direct effects of added salts were considered to be the effects associated with the presence of the soluble salts. The indirect effects of the added salts were considered to be (i) changes in the pH values of solutions and (ii) the formation of insoluble precipitates that could adsorb viruses and be removed by filtration. When direct effects alone were considered, the salts used in this study promoted virus adsorption, interfered with virus adsorption, or had little or no effect on virus adsorption, depending on the filter, the virus, and the salt. Although we were able to confirm previous reports that the addition of aluminum chloride to water enhances virus adsorption to microporous filters, we found that the enhanced adsorption was associated with indirect effects rather than direct effects. The increase in viral adsorption observed when aluminum chloride was added to water was related to the decrease in the pH of the water. Similar results could be obtained by adding HCl. The increased adsorption of viruses in water at pH 7 following addition of aluminum chloride was probably due to flocculation of aluminum, since removal of flocs by filtration greatly reduced the enhancement observed. The only direct effect of aluminum chloride on virus adsorption that we observed was interference with adsorption to microporous filters. Under conditions under which hydrophobic interactions were minimal, aluminum chloride interfered with virus adsorption to Millipore, Filterite, and 1MDS filters. In most cases, less than 10% of the viruses adsorbed to filters in the presence of a multivalent salt and a compound that interfered with hydrophobic interactions (0.1% Tween 80 or 4 M urea).
The effects of salts on virus adsorption to microporous filters have been studied for many years and have been discussed in several reviews (4, 13, 16, 17, 28). In previous studies, the researchers concluded that addition of aluminum ions (or magnesium ions) enhanced viral adsorption to microporous filters, and from these results it was concluded that the presence of salts is necessary for optimum viral adsorption to the filters (4, 13, 16, 17, 27, 28). However, we found two problems with the earlier studies. First, relatively few viruses and few microporous filters were studied. Much of the information was information concerning the adsorption of poliovirus to nitrocellulose filters (Millipore filters). However, due to recent developments in filter technology, new filters that have substantially different properties than the filters previously studied are currently being used. Therefore, it is necessary to evaluate the effects of salts on these filters.
Second, previous studies on the effects of salts on virus adsorption did not distinguish between direct and indirect effects of the salts that influenced viral adsorption. The direct effects that have been proposed include formation of salt bridges between the viruses and the filters (16) and alteration of the charge of a filter (14). The indirect effects include (i) a decrease in the pH due to addition of aluminum salts to purified water (28); (ii) the formation of flocs that adsorb viruses and are then physically trapped by the filters (9, 28); and (iii) the reaction between aluminum ions and humic materials that interfere with virus adsorption (10, 25).
In order to better understand the forces involved in viral adsorption to solids, we investigated the adsorption of four viruses to four commercially available filters having different compositions and different physical characteristics. Two of the filters used in this study (Filterite and 1MDS filters) are currently used for recovering viruses from water (1, 2). The Filterite filters have a net negative charge at pH values near neutrality, in contrast to the 1MDS filters, which are positively charged or have a slight negative charge at similar pH values (14, 24). Nitrocellulose filters (Millipore HA filters) also have a net negative charge at pH 7 (14) and have been used in studies on virus adsorption and to recover viruses from water (6, 7, 12, 28). Cellulose filters (Whatman filters) were included as an example of filters that poorly adsorb viruses. These filters are made from material that is electronegative at pH 7 (26) and have little ability to adsorb viruses unless they are modified (11).
Mono- and multivalent salts were used at different pH values, and compounds that have been shown to disrupt hydrophobic interactions were also used. In addition, the effects of different concentrations of aluminum and the effects of different concentrations of magnesium on pH and viral adsorption, respectively, were studied. The previously described direct and indirect effects were minimized by controlling flocculation and pH by filtering and buffering. All virus stocks and salt solutions were prefiltered through 0.2-μm-pore-size filters prior to each experiment to reduce the effects of aggregation and flocculation. The pH was monitored and controlled, and only purified water was used. Under these conditions, magnesium chloride was found to promote virus adsorption, to interfere with virus adsorption, and to have little or no effect on virus adsorption, depending on the virus and the microporous filter tested. The most consistent effect of sodium chloride and aluminum chloride on virus adsorption was to interfere with virus adsorption to 1MDS filters.
MATERIALS AND METHODS
Filtration of samples.
All viral stocks and solutions were prefiltered through a 0.2-μm-pore-size filter (Millipore GS; Millipore Corp., Bedford, Mass.) that had been prewashed with 20 ml of deionized water before use.
Viruses.
The phages used in this study, their isoelectric points (1), and their hosts are as follows: MS2 (= ATCC 15597-B1), pl 3.9, Escherichia coli C-3000 (= ATCC 15597); φX174 (= ATCC 13706-B1), pl 6.6, E. coli ATCC 13607; and PRD-1, pl 4.2, Salmonella typhimurium ATCC 19585. Numbers of phage PFU were determined by using the appropriate hosts and a soft-agar overlay (23). Poliovirus 1 (pI, approximately 4 and 7 [13]) was grown on BGM cells, and the number of poliovirus PFU was determined by an agar overlay method (22).
Microporous filters.
The flowing microporous filters were used: Millipore HA filters (nitrocellulose; Millipore Corp.); Filterite 0.20-μm-pore-size filters (bound fiberglass; Filterite Corp., Timonium, Md.); Whatman no. 5 filters (cellulose; Fischer Scientific, Pittsburgh, Pa.); and 1MDS filters (charge-modified fibers; AMF Cuno, Meridan, Conn.). The Filterite and 1MDS filters were purchased as cartridge filters. These filters were broken down, and smaller filters were cut from the filter material. The Millipore, Filterite, and Whatman filters are negatively charged at pH values near neutrality; the 1MDS filters are positively or slightly negatively charged at the same pH values (14, 19, 24, 26).
Solutions.
Solutions of sodium chloride, magnesium chloride, aluminum chloride, urea, and Tween 80 were prepared with a buffer solution (0.02 M imidazole–0.02 M glycine, unless otherwise indicated). The solutions were adjusted to the required pH by adding 1 M NaOH or 1 M HCl. Tap water was dechlorinated by adding 10 mg of sodium thiosulfate per liter. Deionized water (>15 MΩ-cm) was obtained from a Barnstead NANOpure II unit.
Aluminum chloride and magnesium chloride titration experiments.
For aluminum chloride and magnesium chloride titration experiments (Fig. 1 through 3), aliquots of a 1.00 M aluminum chloride or 1.00 M magnesium chloride solution were added to a 0.002 M glycine–0.002 M imidazole solution or to purified water to obtain the desired concentrations. Since the concentrations of salts used in these experiments were lower than the concentrations used in other experiments, a lower concentration of buffer was used to reduce interference with the metallic ions by buffer ions.
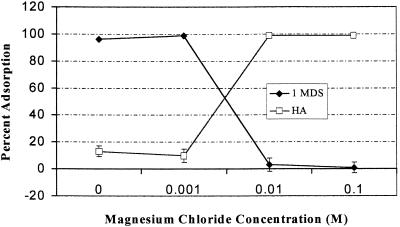
FIG. 1.
Influence of magnesium chloride concentration on adsorption of poliovirus 1 to 1MDS and Millipore HA filters at pH 7.
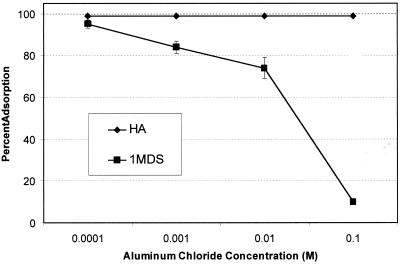
FIG. 3.
Influence of aluminum chloride concentration on adsorption of poliovirus 1 to 1MDS and Millipore HA filters at pH 3.5.
In addition, aluminum chloride was added to deionized water, and the pH was recorded. The pH values of samples of deionized water were then adjusted with HCl to values that matched the pH values of the solutions described above that contained aluminum chloride. Virus adsorption experiments were then conducted by using samples containing aluminum chloride and samples containing HCl at the same pH value (Fig. 4).
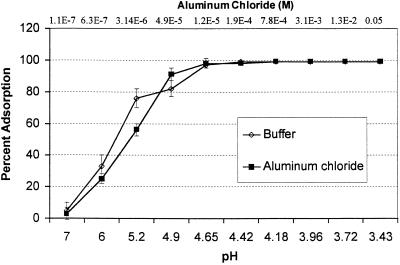
FIG. 4.
Influence of aluminum chloride concentration and the corresponding pH of a buffer solution on adsorption of poliovirus 1 to Millipore HA filters. The pH values used corresponded to the pH values resulting from dissolution of aluminum chloride at the concentrations used.
The pH values of all solutions were measured at the beginning and at the end of each experiment.
Experimental procedure.
Most experiments were conducted at pH 7.0 or 3.5. The lower pH (pH 3.5) was selected since it has been used in several other studies and is the recommended pH for recovering viruses in water when Filterite filters are used (2, 3, 18, 27). Also, the aluminum salts used (at the concentrations used) are soluble at pH 3.5. Aluminum salts were not used at pH 7 (Table 1 and 2) since they are relatively insoluble and form flocs that can adsorb viruses (8, 9). Viruses were added to solutions to obtain an initial titer of approximately 105 PFU/ml. The viruses in the solutions were assayed following dilution in 1% tryptic soy broth (Difco) for phages or in minimal essential medium supplemented with 2% fetal calf serum for poliovirus. In each case the dilution was sufficient to raise the pH of the sample to approximately 7. To prevent flocculation when experiments were performed with aluminum chloride, an initial 1/10 dilution with deionized water was prepared, and then a second dilution with the dilution media described above was prepared. The filters used were 25-mm circles or filters cut into 25-mm circles and placed into stainless steel filter holders. One layer of Millipore HA filter material, two layers of Filterite filter material, two layers of Whatman no. 5 filter material, or three layers of 1MDS filter material were placed in each holder for the experiment. Each filter preparation was first rinsed with 60 ml of deionized water. Then 25 ml of the salt solution containing viruses was passed through each filter by using a mechanical syringe infusion pump (Harvard Apparatus Co., Millis, Mass.) at a flow rate 1.5 ml/s. The filter effluents were assayed for viruses, and the percentage removed was determined. A portion of each sample that had not been passed through any filter was assayed at the beginning and at the end of each experiment to determine if the solutions inactivated the viruses. The numbers of viruses in the unfiltered and filtered samples were used to determine the percentages removed by the filters. Each experiment was performed in triplicate. In addition, each experiment was performed at least twice. Therefore, each value reported below represents a mean based on at least six determinations. The error associated with the values reported was less than √n, where n is the number of the PFU counted. A statistical analysis of the data obtained (standard deviations, slopes, correlation, and general t test probabilities) was performed by using PSI-Plot software (Poly Software International, Salt Lake City, Utah).
TABLE 1.
Effects of salts on virus adsorption to microporous filters at pH 7a
| Filter | Virus | % Virus adsorption
|
||
|---|---|---|---|---|
| Buffer | Buffer containing 0.1 M NaCl | Buffer containing 0.1 M MgCl2 | ||
| Millipore HA | MS2 | 8 Ab | 18 B | 99 C |
| PRD-1 | 6 A | 8 A | 59 B | |
| φX174 | 4 A | 40 B | 57 C | |
| Poliovirus 1 | 3 A | 98 B | 99 B | |
| Mean | 5 | 41 | 79 | |
| Filterite | MS2 | 6 A | 21 B | 23 B |
| PRD-1 | 11 A | 9 A | 10 A | |
| φX174 | 3 A | 3 A | 5 A | |
| Poliovirus 1 | 10 A | 9 A | 99 B | |
| Mean | 7 | 11 | 34 | |
| Whatman | MS2 | 18 A | 12 A | 19 A |
| PRD-1 | 7 A | 3 A | 6 A | |
| φX174 | 5 A | 9 A | 38 B | |
| Poliovirus 1 | 8 A | 6 A | 5 A | |
| Mean | 10 | 8 | 17 | |
| 1MDS | MS2 | 96 A | 10 B | 7 B |
| PRD-1 | 97 A | 13 B | 13 B | |
| φX174 | 29 A | 12 B | 18 C | |
| Poliovirus 1 | 79 A | 7 B | 9 B | |
| Mean | 75 | 11 | 12 | |
We added approximately 105 PFU of virus to 25-ml portions of buffer, buffer containing 0.1 M NaCl, and buffer containing 0.1 M MgCl2, and each type of solution was passed through the different types of filters at a rate of 1.5 ml/s. The numbers of viruses in the influent and effluent were measured in order to determine the percentage of adsorption in each case.
Values on the same line followed by the same letter are not significantly different at P = 0.05.
TABLE 2.
Effects of 0.1 M MgCl3 and 4 M urea on virus adsorption to microporous filters at pH 7a
| Filter | Virus | % Adsorption in the presence of:
|
||
|---|---|---|---|---|
| Buffer | Buffer containing 4 M urea | Buffer containing 4 M urea plus 0.1 M MgCl2 | ||
| Millipore HA | MS2 | 8 Ab | 6 A | 1 B |
| PRD-1 | 6 A | 3 A | 3 A | |
| φX174 | 4 A | 4 A | 2 A | |
| Poliovirus 1 | 3 A | 7 A | 2 A | |
| Mean | 5 | 5 | 2 | |
| Filtrite | MS2 | 6 A | 8 A | 3 B |
| PRD-1 | 11 A | 6 A | 2 B | |
| φX174 | 3 A | 3 A | 1 A | |
| Poliovirus 1 | 10 A | 11 A | 10 A | |
| Mean | 8 | 7 | 4 | |
| Whatman | MS2 | 18 A | 4 B | 5 B |
| PRD-1 | 7 A | 4 A | 4 A | |
| φX174 | 5 A | 5 A | 7 A | |
| Poliovirus 1 | 8 A | 4 A | 7 A | |
| Mean | 10 | 4 | 6 | |
| 1MDS | MS2 | 96 A | 96 A | 6 B |
| PRD-1 | 97 A | 90 A | 11 B | |
| φX174 | 29 A | 21 A | 10 B | |
| Poliovirus 1 | 79 A | 68 A | 8 B | |
| Mean | 75 | 69 | 9 | |
We added approximately 105 PFU of virus to 25-ml portions of buffer, buffer containing 4 M urea, and buffer containing 4 M urea plus 0.1 M MgCl2, and each type of solution was passed through the different types of filters at a rate of 1.5 ml/s. The numbers of viruses in the influent and effluent were measured in order to determine the percentage of adsorption in each case.
Values on the same line followed by the same letter are not significantly different at P = 0.05.
Measurement of aluminum concentrations.
Solutions were analyzed to determine total aluminum contents before and after filtration by workers at the Analytical Research Laboratory of the University of Florida.
Contact angle measurements.
Contact angles for chloroform on filters were measured as previously described (21).
RESULTS
The effects of solutions of salts at pH 7 on the adsorption of the viruses studied to the filters depended on the filter type and the salt added (Table 1). In general, addition of salts increased the adsorption of viruses to Millipore filters and interfered with the adsorption to 1MDS filters. Except for φX174, the salts did not increase viral adsorption to Whatman filters. Magnesium chloride greatly increased the adsorption of poliovirus to Filterite filters; the salts had little or no effect on adsorption of the phages tested to these filters.
The effects of buffer and buffer containing a salt (sodium chloride, magnesium chloride, or aluminum chloride) at a concentration of 0.1 M on adsorption of viruses at pH 3.5 are shown in Table 3. None of the salts affected viral adsorption to Millipore or Whatman filters. More than 95% of the viruses tested adsorbed to Millipore filters, and less than 10% adsorbed to Whatman filters under all of the conditions tested; however, the salts interfered with viral adsorption to 1MDS and Filterite filters. The degree of interference depended on the virus and the salt used. Aluminum chloride interfered with adsorption of viruses to 1MDS filters more than magnesium chloride or sodium chloride interfered with such adsorption. Only aluminum chloride interfered significantly with virus adsorption to Filterite filters.
TABLE 3.
Effects of salts on virus adsorption to microporous filters at pH 3.5a
| Filter | Virus | % Virus adsorption
|
|||
|---|---|---|---|---|---|
| Buffer | Buffer containing 0.1 M NaCl | Buffer containing 0.1 M MgCl2 | Buffer containing 0.1 M AlCl3 | ||
| Millipore HA | MS2 | >99 Ab | >99 A | >99 A | >99 A |
| φX174 | 98 A | >99 A | >99 A | >99 A | |
| Poliovirus 1 | >99 A | >99 A | >99 A | >99 A | |
| Mean | >99 | >99 | >99 | >99 | |
| Filtrite | MS2 | >99 A | 98 AB | >99 A | 96 B |
| φX174 | 95 A | 97 A | 98 A | 23 B | |
| Poliovirus 1 | 99 A | >99 A | 97 A | 10 B | |
| Mean | 98 | 98 | 98 | 43 | |
| Whatman | MS2 | 3 A | 4 A | 3 A | 4 A |
| φX174 | 6 A | 2 A | 2 A | 4 A | |
| Poliovirus 1 | 9 A | 7 A | 5 A | 7 A | |
| Mean | 6 | 4 | 3 | 5 | |
| 1MDS | MS2 | 95 A | 60 B | 44 C | 5 D |
| φX174 | 85 A | 75 B | 73 B | 10 C | |
| Poliovirus 1 | 99 A | 95 A | 93 A | 10 B | |
| Mean | 93 | 76 | 70 | 14 | |
We added approximately 105 PFU of virus to 25-ml portions of buffer, buffer containing 0.1 M NaCl, buffer containing 0.1 M MgCl2, and buffer containing 0.1 M AlCl3, and each type of solution was passed through the different types of filters at a rate of 1.5 ml/s. The numbers of viruses in the influent and effluent were measured in order to determine the percentage of adsorption in each case.
Values on the same line followed by the same letter are not significantly different at P = 0.05.
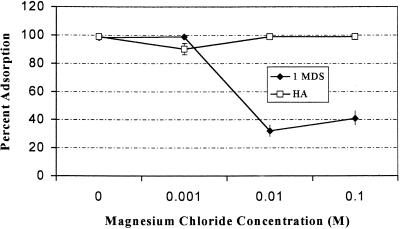
The effect of the concentration of magnesium chloride on the adsorption of poliovirus to filters is shown in Fig. 1 and 2. At pH 7, increasing the concentration of magnesium chloride increased poliovirus 1 adsorption to Millipore HA filters but decreased poliovirus 1 adsorption to 1MDS filters (Fig. 1). At pH 3.5, increasing the concentration of magnesium chloride (Fig. 2) did not affect adsorption of poliovirus 1 to Millipore HA filters but decreased poliovirus 1 adsorption to 1MDS filters.
FIG. 2.
Influence of magnesium chloride concentration on adsorption of poliovirus 1 to 1MDS and Millipore HA filters at pH 3.5.
The effects of different concentrations of aluminum chloride on the adsorption of poliovirus to Millipore and 1MDS filters are shown in Fig. 3. Increasing the concentration of aluminum chloride had little effect on poliovirus adsorption to Millipore filters but interfered with adsorption of this virus to 1MDS filters.
The Millipore and 1MDS filters differed in hydrophobicity. The contact angle for chloroform on Millipore filters was 144.2 ± 3.2°, and the contact angle for chloroform on 1MDS filters was 151.8 ± 1.6°, which showed that the Millipore filters were more hydrophobic.
At pH 7 and in absence of salt ions, we observed little adsorption of poliovirus 1 in deionized water to Millipore HA filters (Fig. 4). As the concentration of aluminum chloride was increased, the adsorption of poliovirus increased. The pH values of the solutions also decreased as aluminum chloride was added. When the pH values of samples of deionized water were decreased by adding HCl, a similar trend in virus adsorption was observed. The decrease in the pH of the solution that was caused by the addition of aluminum chloride was sufficient to explain the observed increase in virus adsorption associated with the addition of aluminum chloride.
Adding urea in the absence of salt ions did not have a significant effect on viral adsorption to the filters at pH 7 (Table 2). However, viral adsorption was greatly reduced in the presence of both 4 M urea and a salt at a concentration of 0.1 M.
We observed a similar effect at pH 3.5; urea alone had little effect on virus adsorption (Table 4). Adding 0.1 M magnesium chloride or 0.1 M aluminum chloride to solutions of urea reduced the adsorption of both MS2 and poliovirus 1 to the filters tested. Tween 80 at a concentration of 0.1% had the same effect on viral adsorption as 4 M urea had (data not shown). The effects of prefiltering aluminum chloride solutions made with tap water before viruses were added are shown in Table 5. When a 0.0001 M aluminum chloride solution was prepared with tap water at pH 7, viruses added to the solution were removed by a Millipore HA filter. If the aluminum chloride solution was first passed through a 0.2-μm-pore-size Millipore GS filter before the viruses were added, then no significant viral adsorption to Millipore HA filters was observed. The prefiltering step decreased the concentration of aluminum chloride from 0.0001 M to less than the detectable level (<0.00003 M). In contrast, no change in the aluminum chloride concentration was observed following filtration of 0.0001 M aluminum chloride solutions through the same type of filter at pH 3.5 (where aluminum chloride is more soluble).
TABLE 4.
Effects of 0.1 M AlCl3 or 0.1 M MgCl3 and 4 M urea on virus adsorption to microporous filters at pH 3.5a
| Filter | Virus | % Adsorption in the presence of:
|
|||
|---|---|---|---|---|---|
| Buffer | Buffer containing 4 M urea | Buffer containing 4 M urea plus 0.1 M MgCl2 | Buffer containing 4 M urea plus 0.1 M AlCl3 | ||
| Millipore HA | MS2 | >99 Ab | 98 A | 15 B | 13 B |
| φX174 | 98 A | 95 A | 9 B | 7 B | |
| Poliovirus 1 | >99 A | >99 A | 12 B | 15 B | |
| Mean | 99 | 97 | 12 | 12 | |
| Filtrite | MS2 | >99 A | 95 B | 14 B | 11 B |
| φX174 | 95 A | 92 B | 7 B | 8 B | |
| Poliovirus 1 | 99 A | 99 A | 13 B | 15 B | |
| Mean | 98 | 95 | 11 | 11 | |
| Whatman | MS2 | 3 A | 9 A | 11 A | 7 A |
| φX174 | 6 A | 4 A | 3 A | 6 A | |
| Poliovirus 1 | 9 A | 7 A | 8 A | 4 A | |
| Mean | 6 | 7 | 7 | 6 | |
| 1MDS | MS2 | 95 A | 95 A | 10 B | 8 B |
| φX174 | 85 A | 69 A | 48 B | 30 C | |
| Poliovirus 1 | 99 A | 99 A | 50 B | 10 C | |
| Mean | 93 | 88 | 36 | 16 | |
We added approximately 105 PFU of virus to 25-ml portions of buffer, buffer containing 4 M urea, buffer containing 4 M urea plus 0.1 M MgCl2, and buffer containing 4 M urea plus 0.1 M AlCl3, and each type of solution was passed through the different types of filters at a rate of 1.5 ml/s. The numbers of viruses in the influent and effluent were measured in order to determine the percentage of adsorption in each case.
Values on the same line followed by the same letter are not significantly different at P = 0.05.
TABLE 5.
Effect of prefiltering the aluminum chloride solutions used in viral adsorption experiments on viral adsorption to Millipore HA filters at pH 7a
| Virus | % Viral adsorption
|
|
|---|---|---|
| Not prefiltered | Prefiltered | |
| MS2 | >99 Ab | 13 B |
| PRD-1 | 98 A | 8 B |
| φX174 | 95 A | 14 B |
| Poliovirus 1 | >99 A | 16 B |
| Mean | 98 | 13 |
The concentration of aluminum chloride in 100 ml of dechlorinated water containing 105 PFU of virus per ml was adjusted to 0.0001 M. Fifty milliliters was passed through a Millipore HA filter at a rate of 1.5 ml/s. The remaining 50 ml was first passed through a glass fiber prefilter (Millipore GS). This solution was then passed through a Millipore HA filter. In each experiment the numbers of viruses in the influent and effluent were measured, and the corresponding percentage of adsorption was determined.
Values on the same line followed by different letters are significantly different at P = 0.05.
In order to separate the effects of salt type from the effects of ionic strength, we performed experiments in the presence of added salt at a constant ionic strength. As shown in Table 6, magnesium chloride solutions were better at promoting adsorption of MS2 and PRD-1 than sodium chloride solutions at the same ionic strength were. Sodium chloride and magnesium chloride both promoted adsorption of φX174 to nitrocellulose filters.
TABLE 6.
Effect of salt type on virus adsorption to Millipore HA filters at pH 7
Increasing the ionic strength of solutions of magnesium chloride and sodium chloride to the ionic strength of aluminum chloride decreased the adsorption of viruses to 1MDS filters (Table 7). At a constant ionic strength of 0.6, all of the salts significantly reduced the virus adsorption that was observed in solutions containing buffer alone. However, the greatest interference with adsorption was observed with solutions of aluminum chloride.
TABLE 7.
Effect of salt type on virus adsorption to 1MDS filters at pH 3.5a
DISCUSSION
Based on early studies on the influence of salts on virus adsorption, it was suggested that salts promote virus adsorption to microporous filters by promoting electrostatic interactions between the viruses and the filters. The possible mechanisms that were suggested to explain the observations made were salt bridging and charge neutralization of the filters. It was also suggested that the ability of salts to promote virus adsorption was related to the valence of the cation involved. Trivalent cations (Al3+) were better than divalent cations (Mg2+), which were better than monovalent cations (Na+), at promoting virus adsorption when the cations were used at the same concentration (4, 13, 16, 17, 27, 28).
In examining the early studies on the effects of salts on virus adsorption, we found three main problems. These were (i) the role of salts in promoting hydrophobic interactions was not always considered; (ii) the indirect effects of adding salts on the pH values of solutions and flocculation of the salts were not always determined; and (iii) few viruses and few filter types were studied. Below we discuss these problems in relation to our study.
Hydrophobic interactions.
Previous studies on virus adsorption to microporous filters led to the suggestion that metal chelators could interfere with virus adsorption promoted by salts (14, 16, 17). Later studies showed that chelators, such as the citrate ion, did not elute viruses adsorbed to Millipore HA filters (7). In fact, chelators have been found to promote, rather than interfere with, virus adsorption to microporous filters (6). The study of Farrah (6) and other studies showed that increased virus adsorption to certain microporous filters in the presence of cations and anions was influenced by hydrophobic interactions (6, 12, 21). Therefore, the adsorption of MS2 and poliovirus 1 in the presence of salts to filters was best explained by the presence of hydrophobic interactions between the filters and the viruses. The effect of added magnesium chloride on virus adsorption was to strengthen hydrophobic interactions rather than electrostatic interactions (6).
In this study, urea had little effect on virus adsorption. This compound did not interfere with or promote adsorption of viruses to the filters tested at pH 7.0 or 3.5. In contrast, it greatly reduced virus adsorption when salts were present. The neutral detergent Tween 80 had an effect similar to that of urea, even though it was used at much lower concentrations. As discussed above, the salts probably promoted hydrophobic interactions and interfered with electrostatic interactions between the viruses and the filters (5, 12, 21).
Indirect effects.
Adding salts can change the pH of a solution or cause the formation of flocs, or salts can interact with organic material present in the solution. This is especially true for aluminum salts and makes determining the direct role of aluminum ions on virus adsorption difficult. As shown in Fig. 4, the effect of increasing the aluminum chloride concentration on virus adsorption reported by Wallis et al. (28) can be explained by the effect of aluminum chloride on the pH of the solution. Lowering the pH of deionized water by adding either aluminum chloride or an acid gave similar results for virus adsorption. It was not possible to reproduce exactly the effects of different concentrations of aluminum chloride on virus adsorption reported by Wallis et al. (28) since these authors did not report the pH values of the solutions which they used. However, the similarities are substantial, and the trend is identical.
Adding aluminum chloride to tap water or other solutions can produce flocs (8, 9). Removing these flocs from water may also remove viruses, and this process can be used to concentrate viruses from tap water. Previously, other authors (9, 27) have described removal of viruses in tap water following addition of aluminum chloride. This was more than likely the result of entrapment of flocs during filtration rather than enhancement of adsorption by soluble ions. As shown in Table 5, good viral removal is obtained if a pH 7 aluminum chloride solution (0.0001 M) containing viruses is passed through a Millipore filter. The ability to enhance removal is lost when the solution is prefiltered before the viruses are added and passed through the filter used to test for adsorption. It is likely that prefiltration removes flocs rather than soluble ions since passage of aluminum chloride solutions at pH 3.5 (in which aluminum chloride is more soluble) does not change the concentration of aluminum.
Another indirect effect is the formation of complexes with humic matter in water by the cations added (10, 25). This may explain the requirement for adding aluminum chloride to water in virus concentration procedures (2, 3). It may also explain why the concentrations of aluminum chloride required for recovering viruses from organic matter-rich estuarine water (18) are higher than the concentrations required for recovering viruses from cleaner tap water.
Viruses and filters studied.
In many of the previous studies, adsorption of poliovirus and MS2 to Millipore HA filters was examined. These studies led to the conclusion that salts promote virus adsorption to microporous filters.
In this study, we used three filters composed of materials that are electronegative at pH values near neutrality (Millipore, Filterite, and Whatman filters) and one filter that is more electropositive under the same conditions (13, 21, 23).
The difference between a negatively charged filter that is relatively hydrophobic (a Millipore HA filter) and a positively charged filter that less hydrophobic (a 1MDS filter) is clearly shown in Fig. 1 through 3. At pH 7, little adsorption of negatively charged viruses (above their isoelectric point) to a negatively charged filter (a Millipore filter) occurs. Increasing the salt concentration increases the hydrophobic interactions between the viruses and the Millipore filter, which increases adsorption. The salt also decreases electrostatic interactions between the viruses and the 1MDS filters, which decreases adsorption. The results obtained with 1MDS filters at pH 3.5 were similar. However, viruses adsorbed to Millipore filters at pH 3.5 under all of the conditions tested. Without salts, the viruses were positively charged (below their isoelectric points) and adsorbed to the negatively charged filters. Increasing the concentration of salts probably decreased the electrostatic interactions but also increased the hydrophobic interactions, so a constant, high percentage of the viruses adsorbed. The fact that the salts interfered with electrostatic interactions and increased hydrophobic interactions was evident when urea or Tween 80 was added and adsorption decreased substantially (Table 2 and 4).
From the results of this and other studies (6, 12, 20, 21) we concluded that certain salt cations promote hydrophobic interactions between a virus and a filter or substrate. The cations that are most effective are smaller, monovalent or multivalent ions (6, 12). These salt cations also interfere with the electrostatic interactions that might occur between a virus and a filter. This is probably a result of screening of charges by ions in solution (5) and has been observed in other studies (15, 20, 21). Electrostatic interactions influence viral adsorption in many cases. Such situations include situations in which a filter surface is electropositive and the pH value of the solution is near or greater than the virus isoelectric point (19, 24). Electrostatic interactions also are important when the filter surface is electronegative and the pH value of the solution is below the virus isoelectric point (2, 3, 8, 18). The presence of both urea and a multivalent salt is sufficient to overcome most of the forces responsible for viral adsorption to solid surfaces.
The type of salt appears to be more important than the ionic strength of the salt in promoting adsorption to Millipore filters for some viruses (MS2 and PRD-1) but not for others (φX174 and poliovirus 1). Both the ionic strength and the type of salt appear to influence virus adsorption to 1MDS filters. Solutions of sodium chloride, magnesium chloride, and aluminum chloride having the same ionic strength all interfered with virus adsorption, but solutions of aluminum chloride resulted in the most interference.
In summary, the results of this study show that salts can promote virus adsorption, interfere with virus adsorption, or have little effect on virus adsorption. Virus adsorption to solids is the result of many interactions; therefore, theories on the effects of salts on virus adsorption should account for the multiple effects observed.
ACKNOWLEDGMENTS
We acknowledge the financial contribution of the Engineering Research Center (ERC) for Particle Science and Technology at the University of Florida, National Science Foundation grant EEC-94-02989, and the Industrial Partners of the ERC.
Footnotes
Paper number R-07534 from the Florida Agricultural Experiment Station, Gainesville.
REFERENCES
- 1.Ackermann H W, Michael S D. Viruses of procaryotes. Boca Raton, Fla: CRC Press; 1987. pp. 173–201. [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater. New York, N.Y: American Public Health Association; 1998. pp. 9:117–9:119. [Google Scholar]
- 3.Berg G, Safferman R S, Dahling D R, Berman D. U. S. EPA manual of methods for virology. Publication EPA-600/4-84-013. Cincinnati, Ohio: Environmental and Support Laboratory; 1984. [Google Scholar]
- 4.Bitton G. Adsorption of viruses to surfaces: technological and ecological implications. In: Bitton G, Marshall K C, editors. Adsorption of microorganisms to surfaces. New York, N.Y: John Wiley & Sons; 1980. pp. 332–374. [Google Scholar]
- 5.Cantor C R, Schimmel P R. Biophysical chemistry. San Francisco, Calif: W. H. Freeman and Company; 1980. pp. 676–678. [Google Scholar]
- 6.Farrah S R. Chemical factors influencing adsorption of bacteriophage MS2 to membrane filters. Appl Environ Microbiol. 1982;43:659–663. doi: 10.1128/aem.43.3.659-663.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farrah S R, Bitton G. Elution of poliovirus adsorbed to membrane filters. Appl Environ Microbiol. 1978;36:982–984. doi: 10.1128/aem.36.6.982-984.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farrah S R, Gerba C P, Wallis C, Melnick J L. Concentration of viruses from large volumes of tap water using pleated membrane filters. Appl Environ Microbiol. 1976;31:221–226. doi: 10.1128/aem.31.2.221-226.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrah S R, Goyal S M, Gerba C P, Wallis C, Melnick J L. Concentration of poliovirus from tap water onto membrane filters with filters with aluminum chloride at ambient pH levels. Appl Environ Microbiol. 1978;36:624–626. doi: 10.1128/aem.35.3.624-626.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrah S R, Goyal S M, Gerba C P, Wallis C, Shaffer P T B. Characteristics of humic acid and organic compounds concentrated from tap water using the Aquella virus concentrator. Water Res. 1976;10:897–901. [Google Scholar]
- 11.Farrah S R, Preston D R. Concentration of viruses from water by using cellulose filters modified by in situ precipitation of ferric and aluminum hydroxide. Appl Environ Microbiol. 1985;50:1502–1504. doi: 10.1128/aem.50.6.1502-1504.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farrah S R, Shah D O, Ingram L O. Effects of chaotropic and antichaotropic agents on elution of poliovirus adsorbed on membrane filters. Proc Natl Acad Sci USA. 1981;78:1229–1232. doi: 10.1073/pnas.78.2.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerba C P. Applied and theoretical aspects of virus adsorption to surfaces. Adv Appl Microbiol. 1984;30:133–168. doi: 10.1016/s0065-2164(08)70054-6. [DOI] [PubMed] [Google Scholar]
- 14.Kessick M A, Wagner R A. Electrophoretic mobilities of virus adsorbing filter materials. Water Res. 1978;12:263–268. [Google Scholar]
- 15.Lytle C D, Routson L B, Jain N B, Myers M R, Green B L. Virus passage through track-etch membranes modified by salinity and a nonionic surfacant. Appl Environ Microbiol. 1999;65:2773–2775. doi: 10.1128/aem.65.6.2773-2775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mix T W. The physical chemistry of membrane-virus interaction. Dev Ind Microbiol. 1974;15:136–142. [Google Scholar]
- 17.Mix T W. Mechanism of adsorption and elution of viruses to and from surfaces. In: Berg G, editor. Methods for recovering viruses from the environment. Boca Raton, Fla: CRC Press; 1987. pp. 127–137. [Google Scholar]
- 18.Payment P, Gerba C P, Wallis C, Melnick J L. Methods for concentrating viruses from large volumes of estuarine water on pleated membrane filters. Water Res. 1976;10:893–896. [Google Scholar]
- 19.Preston D R, Vasudevan T V, Bitton G, Farrah S R, Morel J-L. Novel approach for modifying microporous filters for virus concentration from water. Appl Environ Microbiol. 1988;54:1325–1329. doi: 10.1128/aem.54.6.1325-1329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shields P A, Farrah S R. Influence of salts on electrostatic interactions between poliovirus and membrane filters. Appl Environ Microbiol. 1983;45:526–531. doi: 10.1128/aem.45.2.526-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shields P A, Ling T F, Tjatha V, Shah D O, Farrah S R. Comparison of positively charged membrane filters and their use in concentrating bacteriophages in water. Water Res. 1986;20:145–151. [Google Scholar]
- 22.Smith E M, Gerba C P. Laboratory methods for the growth and detection of animal viruses. In: Gerba C P, Goyal S M, editors. Methods in environmental virology. New York, N.Y: Marcel Dekker, Inc.; 1982. pp. 15–47. [Google Scholar]
- 23.Snustad S A, Dean D S. Genetic experiments with bacterial viruses. W. H. San Francisco, Calif: Freeman and Co.; 1971. [Google Scholar]
- 24.Sobsey M D, Glass J S. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980;40:201–210. doi: 10.1128/aem.40.2.201-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sobsey M D, Glass J S. Influence of water quality on enteric virus concentration by microporous filter methods. Appl Environ Microbiol. 1984;47:956–960. doi: 10.1128/aem.47.5.956-960.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stana-Kleinschek K, Ribitsch V. Electrokinetic properties of processed cellulose fiber. Colloids Surf A Physiochem Eng Aspects. 1998;140:127–138. [Google Scholar]
- 27.Wallis C, Henderson M, Melnick J L. Enterovirus concentration on cellulose membranes. Appl Microbiol. 1972;23:476–480. doi: 10.1128/am.23.3.476-480.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wallis C, Melnick J L, Gerba C P. Concentration of viruses from water by membrane chromatography. Annu Rev Microbiol. 1979;33:413–437. doi: 10.1146/annurev.mi.33.100179.002213. [DOI] [PubMed] [Google Scholar]