Abstract
Context:
Targeted identification and treatment of people with latent tuberculosis infection (LTBI) are key components of the US tuberculosis elimination strategy. Because of recent policy changes, some LTBI treatment may shift from public health departments to the private sector.
Objectives:
To (1) develop methodology to estimate initiation and completion of treatment with isoniazid for LTBI using claims data, and (2) estimate treatment completion rates for isoniazid regimens from commercial insurance claims.
Methods:
Medical and pharmacy claims data representing insurance-paid services rendered and prescriptions filled between January 2011 and March 2015 were analyzed.
Participants:
Four million commercially insured individuals 0 to 64 years of age.
Main Outcome Measures:
Six-month and 9-month treatment completion rates for isoniazid LTBI regimens.
Results:
There was an annual isoniazid LTBI treatment initiation rate of 12.5/100 000 insured persons. Of 1074 unique courses of treatment with isoniazid for which treatment completion could be assessed, almost half (46.3%; confidence interval, 43.3-49.3) completed 6 or more months of therapy. Of those, approximately half (48.9%; confidence interval, 44.5-53.3) completed 9 months or more.
Conclusions:
Claims data can be used to identify and evaluate LTBI treatment with isoniazid occurring in the commercial sector. Completion rates were in the range of those found in public health settings. These findings suggest that the commercial sector may be a valuable adjunct to more traditional venues for tuberculosis prevention. In addition, these newly developed claims-based methods offer a means to gain important insights and open new avenues to monitor, evaluate, and coordinate tuberculosis prevention.
Keywords: administrative data, claims data, isoniazid, latent tuberculosis infection, methods, treatment completion
Domestic tuberculosis (TB) elimination is a long-standing component of US public health policy.1–3 Unfortunately, the goal of TB elimination, defined as a rate of newly diagnosed TB less than 1 case per million population,1 remains unmet and progress toward meeting the goal has slowed.4 One important reason for this is the need to better address latent TB infection (LTBI).3 People with LTBI are infected with Mycobacterium Tuberculosis but they do not have active TB disease, they cannot transmit TB to others, and they are asymptomatic. Latent TB infection represents a vast and largely unaddressed reservoir of future TB cases.3 Up to 13 million people in the United States with LTBI remain at elevated risk for developing TB through their lifetime; without treatment, 5% to 10% of these individuals will develop active TB.5,6
Only 14% of domestic TB cases are from recent transmissions of Mycobacterium tuberculosis; most developed TB after a period of LTBI.7 Latent TB infection treatment prevents progression to active TB in a sizable proportion of patients,8 so the majority of cases of active TB in the United States would have been prevented had the LTBI been proactively identified and had LTBI treatment been completed. Accordingly, targeted efforts to identify and treat people with LTBI are increasingly important to domestic TB prevention, control, and elimination strategies.9
The overwhelming majority of TB control and prevention, including LTBI treatment, has traditionally been provided by safety net and local public health agencies.3,10–12 This may be changing with the Affordable Care Act’s deliberate move toward insurance-financed care, and some treatment for LTBI previously occurring in public health departments is likely shifting to private sector health care providers.11–13 This shift will be expedited with the US Preventive Services Task Force’s (USPSTF) recent assignment of a “grade B” rating to the practice of screening for LTBI in populations that are at increased risk of TB.14 With this rating, the Affordable Care Act requires that TB/LTBI testing in these populations be covered by health plans at no out-of-pocket cost to patients.15 Even in the absence of these explicit requirements, the USPSTF recommendation would promote increased testing. The recommendation raises awareness and provides information regarding best practices and evidence-based medicine that facilitate the provision of quality care. These factors will likely drive increased LTBI treatment initiation in the private sector. As activity in the commercial setting grows, it is important for public health authorities and health plans to consider how commercial claims data can be used to inform planning and evaluation of TB prevention.
The authors identified and evaluated LTBI treatment using commercial claims data to provide methods and inform planning around TB prevention occurring in the commercial health care sector. Latent TB infection treatment with daily-dose isoniazid regimens was examined because past research indicates that they have been the overwhelmingly dominant regimens prescribed for LTBI.16 The specific objectives were to (1) develop methodology to identify LTBI-related health care and specifically long-term daily-dose isoniazid treatment using medical and pharmacy claims data, and (2) estimate the treatment initiation and completion rates from a large commercial claims data set.
Methods
The institutional review board of University of North Texas Health Science Center reviewed and approved this project as exempt category research.
Data source
The authors analyzed a deidentified, randomly selected sample of paid medical and pharmacy claims and health insurance enrollment data for 4 million continuously commercially insured individuals 0 to 64 years of age from the Optum Impact National Research Database.17 This database includes claims data for approximately 30.6 million commercially insured people, which is roughly 19% of the commercially insured population in the United States. These claims represent insurance-covered office visits, inpatient and outpatient facility and professional services, independent laboratory services, transportation services, prescriptions, and other health care services reimbursed by commercial insurers. In addition, the Optum data contain basic information about the individuals (ie, sex, age group, census division) and their health insurance enrollment time spans.
All people in the sample had continuous health insurance enrollment with both medical and pharmacy coverage beginning January 1, 2011, and ending no earlier than December 31, 2013. The data reflect insurance-covered services rendered and prescriptions filled from January 2011 through the end of each person’s health insurance coverage period or March 31, 2015, whichever came first. Of the 4 million people in the sample, 2 390 112 (59.7%) were continuously enrolled through March 2015. In total, the sample represented 186 670 279 person/months of commercial insurance-covered health care utilization. The distribution of individuals in the sample roughly approximated the 2010 US population distribution based on census divisions.18
The authors developed an algorithm to identify people initiating and those completing 6 to 9 months of isoniazid. This algorithm identifies billing codes suggestive of LTBI treatment within the claims data and then applies increasingly sensitive logic to retain only those episodes of care that can be confidently attributed to LTBI treatment for final analysis. For those meeting the inclusion criteria for final analyses, treatment completion and initiation rates were calculated. The authors defined “LTBI treatment” as 6-month or 9-month daily-dose isoniazid LTBI treatment regimens.
Process to identify LTBI treatment
The algorithm to identify individuals eligible to be included in the LTBI treatment completion analyses is depicted in Figure 1. The algorithm requires a 6-month preperiod and a 1-year postperiod to assess treatment completion, so 18 months of each individual’s data are used (Figure 2). Justifications for the exclusions made at each point in the process are detailed in Supplemental Digital Content 1, available at http://links.lww.com/JPHMP/A356,16,19–23 and Supplemental Digital Content 2, available at http://links.lww.com/JPHMP/A357, details the sensitivity testing related to cell F in Figure 1. Specific billing codes for each step are available in Supplemental Digital Content 3, available at http://links.lww.com/JPHMP/A358.
FIGURE 1.
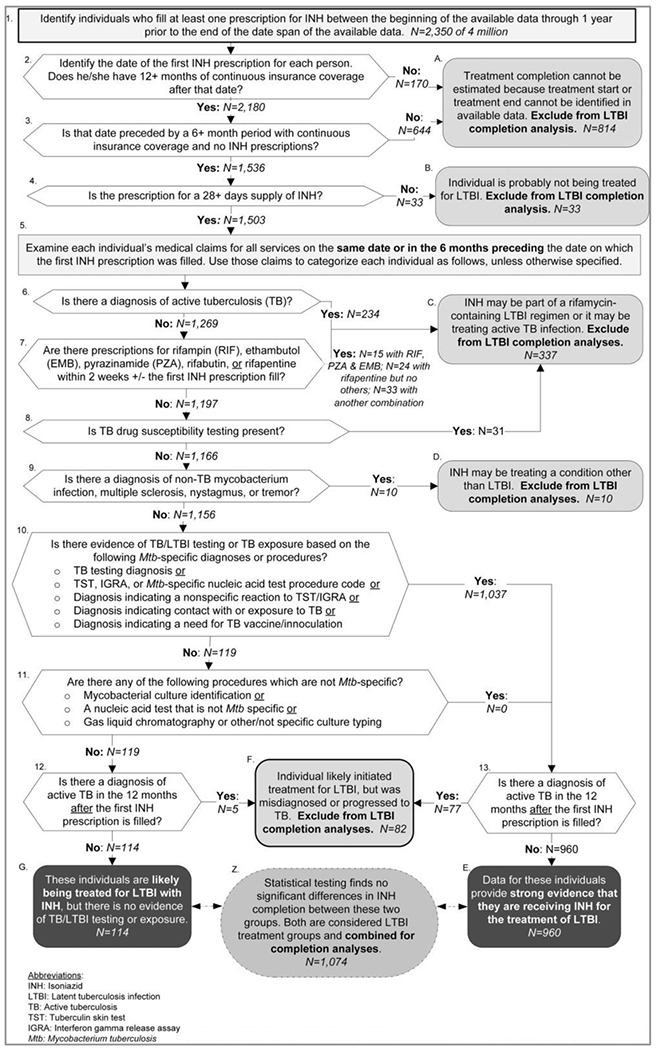
Process Identifying Individuals Initiating LTBI Treatment With Isoniazid for Whom Treatment Completion Can Be Assesseda
Abbreviations: IGRA, interferon gamma release assay; INH, isoniazid; LTBI, latent tuberculosis infection; TST, tuberculin skin test.
aThe process was applied to Optum Impact National Research Database claims data for 4 million commercially insured people. The data represented services from January 2011 through March 2015.
FIGURE 2.
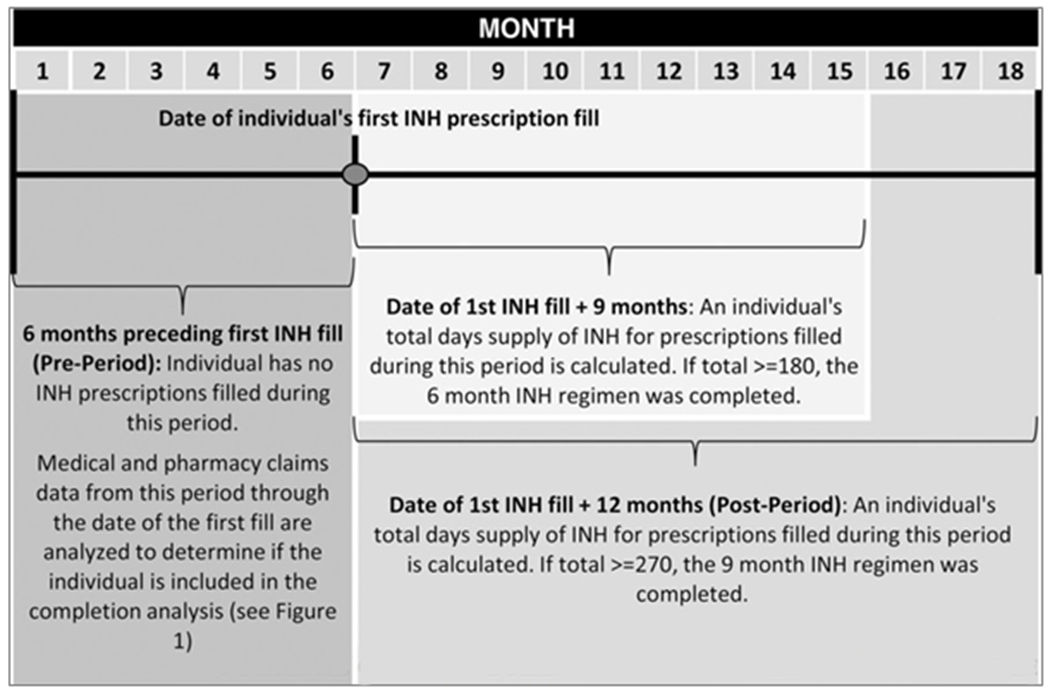
Illustration of How 18 Months of Each Individuals’ Data Were Used to Identify Latent Tuberculosis Infection Treatment With Isoniazid
Abbreviation: INH, isoniazid.
The process begins by identifying people with prescriptions for isoniazid. By the end of the process, 2 groups of individuals remain. One group’s data contain strong evidence that they were receiving isoniazid-only LTBI treatment (Figure 1, terminal cell E). A smaller group of individuals who fill prescriptions for isoniazid were not disqualified (Figure 1, cells 1-9 and 12), but their claims data histories contained no specific evidence that they were being treated for LTBI (Figure 1, cells 10 and 11). No uses for isoniazid other than those previously noted21–23 were identified, so it is likely that the individuals in this group were also being treated for LTBI.
Statistical assessment of group equivalency
The authors used 2 types of statistical tests to compare the number of isoniazid prescriptions filled to determine whether (1) people without evidence of LTBI testing or diagnoses (Figure 1, terminal cell G), and (2) those with LTBI testing or diagnoses (Figure 1, terminal cell E) both consist of people taking isoniazid for LTBI treatment. First, bivariate and multivariable ordinal logit regression models were used to determine whether there were differences in 3 levels of completion-–completion of neither the 6-month regimen nor the 9-month regimen, completion of the 6-month regimen but not the 9-month regimen, and completion of the 9-month regimen. Second, bivariate and multivariable zero-truncated negative binomial models were used to determine whether they differed in terms of the number of months of isoniazid prescriptions filled. All models were calculated using robust standard errors, and multivariable models included sex, age group, region, and year as covariates. A lack of significant differences based on all of these tests would be used as evidence that the 2 groups were equally likely to represent individuals being treated for LTBI. In that case, both groups would be included in the treatment initiation and completion analyses. Conversely, if significant differences were identified, LTBI treatment initiation and completion would be calculated only for the group of individuals with LTBI testing procedures or diagnoses. In addition, the authors generated frequency distributions to examine individuals included in the completion analysis and those completing isoniazid treatment.
Completion and initiation rate calculations
The authors calculated 6-month and 9-month completion rates. Claims data do not provide the information needed to determine whether a patient was prescribed a 6- or 9-month regimen of isoniazid, so both of these regimens were assessed for each individual. Completion of the 9-month and 6-month daily isoniazid treatment regimen was defined as at least 270 and 180 doses received within 12 and 9 months, respectively.16 Completion rates were calculated as the proportion of completed treatments among those individuals who initiated treatment in the final analyses.
To calculate the LTBI treatment initiation rate within commercially insured persons, the authors identified all treatment initiations, even where completion could not be evaluated, by rerunning the algorithm depicted in Figure 1 but this time retaining individuals without 12 months of continuous insurance coverage in the postperiod (Figure 1, cells 2 and A). The result was used as the numerator, and the treatment initiation rate was denominated in person-years.
Results
Assessment of group equivalency
Comparing the 2 groups that were potentially eligible to be included in the completion analysis, 960 had LTBI-testing procedures or diagnoses while 114 did not have these procedures or diagnoses. The results of the ordinal logit models that examined differences in 3 levels of completion indicated that there were no significant differences between the 2 groups in the levels of treatment completion achieved (unadjusted odds ratio, 1.22; confidence interval [CI], 0.84-1.78; adjusted odds ratio, 1.176; CI, 0.801-1.722). Nonsignificant Brant tests indicated that the parallel regression assumptions of the ordinal logit models were not violated.
Similarly, the results of the zero-truncated negative binomial models indicated that there was no significant difference between the 2 groups in terms of the number of isoniazid prescriptions that they filled (unadjusted incidence rate ratio: 1.15, CI: 0.99-1.34; Adjusted incidence rate ratio: 1.13, CI: 0.96-1.32). Given the lack of statistically significant differences, the authors concluded that both groups consist of people taking isoniazid for LTBI treatment. The groups were combined to estimate the proportion of these individuals who completed each of the 2 treatment regimens.
Treatment initiation and completion rates
The authors identified 1197 LTBI treatments initiated during 9555 858 commercially insured person-years over 33 months of observation, an annual treatment initiation rate of 12.5/100 000. Completion could be evaluated for 1074 of these; 497 (46.3%; CI, 43.3-49.3) completed at least the 6-month regimen. Of those, 243 (48.9%; CI, 44.5%-53.3%) completed the 9-month regimen (Table). Thus, the 9-month treatment completion rate was 22.6% (243/1074; CI, 20.2%-25.2%). The characteristics of those evaluated for treatment completion are detailed in Supplemental Digital Content 4, available at http://links.lww.com/JPHMP/A359.
TABLE.
Isoniazid-Only Latent Tuberculosis Infection Treatment Initiation and Completion Estimates, Based on Individuals in the Optum National Research Database Medical and Pharmacy Claims Data Sample With an Initial Isoniazid Prescription Filled Between July 2011 and March 2014
| Measure Description | Measure Value |
|---|---|
| Person/years during which treatment initiation was assessed | 9 555 856.6 |
|
| |
| Number initiating treatment | 1197 |
|
| |
| Annual treatment initiation rate per 100 000 insured persons | 12.53 |
|
| |
| Number initiating treatment for which completion could be assessed | 1074 |
|
| |
| Number completing ≥6 mo of isoniazid treatment | 497 |
|
| |
| Percent completing ≥6 mo of isoniazid treatment | 46.3% (95% CI, 43.3-49.3) |
|
| |
| Of those completing ≥6 mo of isoniazid treatment | |
| Number completing ≥6 mo but ≤9 mo | 254 |
| Percent completing ≥6 mo but ≤9 mo | 51.1% (95% CI, 46.7-55.5) |
| Number completing ≥9 mo | 243 |
| Percent completing at ≥9 mo | 48.9% (95% CI, 44.5-53.3) |
Abbreviation: CI, confidence interval.
Discussion
The authors successfully used claims data to identify and evaluate LTBI treatment among a large national sample of commercially insured individuals. The 6-month LTBI treatment completion rates among patients initiating commercial insurance-covered LTBI treatment are on the lower end of the 39% to 79% range of isoniazid-only LTBI treatment completion rates observed in retrospective studies conducted in public health settings.16,24–36 Almost half of those initiating treatments in the sample completed a 6-month or longer regimen. Almost half of those, roughly one-quarter of all those initiating treatment, completed 9 or more months. Just as importantly, the authors quantified LTBI treatment rates in the commercial sector based on a sizable and roughly geographically representative national sample of data. Previous research on LTBI treatment in the private sector has focused only on limited geographic areas or select samples of providers.10
These results indicate that LTBI treatment was occurring in the commercial health care setting prior to the USPSTF recommendations. Commercial health care is likely a valuable adjunct to more traditional venues for TB prevention efforts. An increasing proportion of the US population has commercial insurance, and currently more than 70% of adults and more than 56% of children in the US population are commercially insured.37 The commercially insured US population includes many of the persons at highest risk of LTBI and TB, including foreign-born persons.38 More than 59% of foreign-born individuals in the United States have private health insurance, and this rate is increasing.39 As more people become commercially insured and as health care financing and policy evolve, management of LTBI will likely become increasingly common within the commercial health care sector. It is important to understand treatment patterns in the commercial sector for LTBI as much to identify opportunities as to assess care quality. This study provides important evidence toward both. The authors did not evaluate how well LTBI treatments in the sample were targeted to persons at risk for progression to active TB, and work should be done to gauge the potential effectiveness of those efforts. But, given that medication adherence is one measure of care quality,40–42 the finding that the rates of completion of at least 6 months of isoniazid treatment are similar in commercial and public settings suggests that LTBI treatment through commercial health care is of comparable quality to that in public health setting by at least that common indicator.
Administrative claims data are ubiquitous and accessible. At the same time, they are collected to facilitate payment, not to inform clinical decision making, and realizing their potential can be challenging.43 Unlike data collected from disease reporting or ad hoc program evaluation in the public health setting, LTBI-related health care is not directly identified in claims data. Instead, it must be inferred from claims data rather than simply counted, and the authors are unaware of prior work that either provides methods or that has done so. By designing and testing the logic and methodologies necessary to tap into this resource, the authors enable public health researchers and health plans to explore their own data and build on this work.
The authors analyzed commercial insurance claims data, but the codes used to represent diagnoses, procedures, and medications found on medical and pharmacy claims are generally consistent across private and public third party payers.44 The methods developed in the current study may be as useful to analyze public payer claims data from Medicare and Medicaid as from commercial payers. Claims data from public and private payers are widely available; researchers can gain access to or receive files of these data by purchasing the data or paying an access fee. Private companies have developed claims databases containing public and private payers’ data, public payer claims data are available from the Centers for Medicare and Medicaid Services or directly from states, 6 states currently have all-payer claims databases available to researchers, and the majority of the remaining states are developing or are interested in developing such databases.17,45–49 In addition, as public health departments and other safety net agencies begin to bill third party payers for services,13 these new methods can guide them in program evaluation.
The uninsurance rate in the United States is decreasing and organizations such as the USPSTF are recognizing the benefits of targeted LTBI screening. As a result, insurance-covered LTBI-related care is becoming available to and will likely be used by more people. Consequently, claims-based methods to examine LTBI in the private sector are increasingly relevant.39 As the coordinating discipline for protections against TB in the United States, it is important that public health authorities use claims-based methods to monitor TB-related health care services taking place beyond their clinics and seek to include such data in their decision making. In so doing, the public health community will continue its important leadership role to encourage LTBI treatment in both the public and private sectors and more effectively drive progress toward US elimination goals.
There are numerous opportunities for future research exploring LTBI treatment in the private sector. The current study develops a methodology to identify isoniazid treatment within claims data, but it does not identify treatment with other regimens. Claims-based methods to identify and evaluate treatment completion in regimens with shorter treatment durations will become increasingly important, given that the CDC’s Division of Tuberculosis Elimination strategic plan involves scaling up the use of shorter-course LTBI treatments.9 In addition, the associations between treatment completion and clinical or sociodemographic characteristics of interest (eg, immunosuppression, age) were not examined. Estimating the proportion of LTBI treatment taking place in the private sector and how these proportions change over time are other topics in need of research.
This study does have limitations. Only patients who fill at least 1 prescription become visible to medication adherence studies that rely on claims data, and the authors cannot assess nonacceptance of treatment or determine how many people accepted treatment but failed to fill their first prescription.50 The bias introduced by this missing information limits the ability to make more broad comparisons to public sector quality on the basis of treatment acceptance but would not challenge the basic findings of the initiated treatment rate and outcomes. One also cannot know whether a patient filled a prescription but did not ingest the medication, but this is true for any setting not using directly observed preventive therapy.50 Similarly, claims data do not contain information needed to determine whether a 6- or 9-month regimen was initiated. In the public sector, the majority of patients are prescribed a 9-month regimen16; it cannot be determined from claims data whether the same is true in the private sector. Also, for individuals who initiate LTBI treatment but are subsequently diagnosed with active TB, one cannot determine whether there was an initial misdiagnosis, a progression to active TB during treatment, or a progression to active TB after treatment completion or discontinuation. However, the algorithm is robust to variations in assumptions for these individuals (see Supplemental Digital Content 2, available at http://links.lww.com/JPHMP/A357). Furthermore, some variables could not be examined. As is typical with claims data,43 detailed demographic information (eg, race/ethnicity, income) was unavailable. Information about individuals’ locations was limited due to the deidentification of the data set.
In addition, the initiation rate is based on all commercially insured individuals rather than those at risk of TB/LTBI, tested for TB/LTBI, or offered LTBI treatment because data exploration indicated that testing and test results are inconsistently coded in claims and offers of treatment are not coded. The initiation rate should be considered a conservative estimate, given that the authors excluded individuals with a diagnosis of active TB. While diagnoses on claims generally represent clinical conditions and are of great use in research and surveillance,43 they are imperfect because providers may code diagnoses presumptively or as justifications for further testing.51 Thus, a subset of individuals excluded because of TB diagnosis codes may have had LTBI rather than active TB.
Finally, it is likely that at least some people receiving LTBI-related medical care do so using a mix of public and commercial services. Presumably, some individuals’ LTBI treatment in the commercial sector occurs only after interaction with the public sector (eg, being identified by the health department as a contact to a person with infectious TB). Pharmacy claims in the sample did not contain information about the prescribing provider, so the authors were unable to discern between isoniazid prescribed by private and public sector practitioners. In this analysis, 10.6% of the people initiating LTBI treatment with isoniazid had no LTBI-related diagnoses or procedures in the medical claims. These individuals may have been prescribed isoniazid by public health departments but filled their prescription through private pharmacies using insurance benefits; conversely, private sector providers may have prescribed these individuals isoniazid for LTBI without coding-associated diagnoses or procedures on medical claims. Still, while these limitations might cloud the understanding of how prevention efforts are distributed among public and private agencies, they do not challenge this study’s basic findings regarding insurance paid treatment incidence and completion rates.
Supplementary Material
Implications for Policy & Practice.
Latent tuberculosis infection treatment is occurring in the private health care sector, with completion rates within the range of rates observed in public health settings.
The study findings suggest a shared purpose between public and commercial health and the opportunity to develop commercial health care as a valuable adjunct to more traditional TB prevention venues.
The claims-based methods developed by the authors offer a means to gain important insights and open new avenues to monitor, evaluate, and coordinate TB prevention.
These methods facilitate the goal of domestic TB elimination.
Acknowledgments
The authors gratefully acknowledge the support of the US Centers for Disease Control and Prevention’s Division of Tuberculosis Elimination and its Tuberculosis Epidemiologic Studies Consortium (Atlanta, Georgia), which provided valuable intellectual and other contributions. In addition, the research reported in this publication was developed in collaboration with Magellan Health, Inc (Scottsdale, Arizona). The authors thank Magellan for their invaluable contributions to this work.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention or Magellan Health, Inc. Mention of company names or products does not imply endorsement by the US Centers for Disease Control and Prevention.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (http://www.JPHMP.com).
Contributor Information
Erica L. Stockbridge, Department of Health Behavior and Health Systems, School of Public Health, University of North Texas Health Science Center, Fort Worth, Texas; Department of Advanced Health Analytics and Solutions, Magellan Health, Inc, Scottsdale, Arizona.
Thaddeus L. Miller, Department of Health Behavior and Health Systems, School of Public Health, University of North Texas Health Science Center, Fort Worth, Texas.
Erin K. Carlson, College of Nursing and Health Innovation, University of Texas at Arlington, Arlington, Texas.
Christine Ho, Division of Tuberculosis Elimination, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- 1.Centers for Disease Control and Prevention. A strategic plan for the elimination of tuberculosis in the United States. MMWR Suppl. 1989;38(16):269–272. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Tuberculosis elimination revisited: obstacles, opportunities, and a renewed commitment. Advisory Council for the Elimination of Tuberculosis (ACET). MMWR Recomm Rep. 1999;48(RR-9):1–13. [PubMed] [Google Scholar]
- 3.Institute of Medicine Committee on the Elimination of Tuberculosis in the United States. Ending Neglect: The Elimination of Tuberculosis in the U.S Washington, DC: National Academy of Sciences; 2000. [Google Scholar]
- 4.Salinas JL, Mindra G, Haddad MB, Pratt R, Price SF, Langer AJ. Leveling of tuberculosis incidence—United States, 2013–2015. MMWR Morb Mortal Wkly Rep. 2016;65(11):273–278. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso JD, Diffenderfer JM, Ghassemieh BJ, Horne DJ, Kao TC. The prevalence of latent tuberculosis infection in the United States. Am J Respir Crit Care Med. 2016;194(4):501–509. [DOI] [PubMed] [Google Scholar]
- 6.Miramontes R, Hill AN, Yelk Woodruff RS, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011-2012. PLoS One. 2015;10(11):e0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen CM, Kammerer JS, Marks K, Navin TR, France AM. Recent transmission of tuberculosis—United States, 2011-2014. PLoS One. 2016;11(4):e0153728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999;3(10):847–850. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Division of tuberculosis elimination strategic plan 2016-2020. http://www.cdc.gov/tb/about/strategicplan.htm. Published 2015. Accessed April 6, 2017.
- 10.Sterling TR, Bethel J, Goldberg S, et al. The scope and impact of treatment of latent tuberculosis infection in the United States and Canada. Am J Respir Crit Care Med. 2006;173(8):927–931. [DOI] [PubMed] [Google Scholar]
- 11.Ehman M, Flood J, Barry PM. Tuberculosis treatment managed by providers outside the public health department: lessons for the Affordable Care Act. PLoS One. 2014;9(10):e110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balaban V, Marks SM, Etkind SC, et al. Tuberculosis elimination efforts in the United States in the era of insurance expansion and the Affordable Care Act. Public Health Rep. 2015;130(4):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bovbjerg RR, Ormond BA, Waidmann TA. What directions for public health after the Affordable Care Act? Urban Institute Policy Brief. http://www.urban.org/research/publication/what-directions-public-health-under-affordable-care-act. Published 2011. Accessed April 6, 2017. [Google Scholar]
- 14.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for Latent Tuberculosis Infection in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316(9):962–969. [DOI] [PubMed] [Google Scholar]
- 15.H.R. 3590. The Patient Protection and Affordable Care Act, Sec. 2713, coverage of preventive services. https://www.gpo.gov/fdsys/pkg/BILLS-111hr3590enr/pdf/BILLS-111hr3590enr.pdf. Published 2010. Accessed April 6, 2017.
- 16.Horsburgh CR Jr, Goldberg S, Bethel J, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest. 2010;137(2):401–409. [DOI] [PubMed] [Google Scholar]
- 17.Optum. Retrospective databases. https://www.optum.com/life-sciences/develop-evidence/data-assets/retrospective-databases.html. Published 2015. Accessed April 6, 2017.
- 18.US Department of Commerce. United States summary: 2010 population and housing unit counts. https://www.census.gov/prod/cen2010/cph-2-1.pdf. Published 2012. Accessed April 6, 2017.
- 19.Liberman JN, Girdish C. Recent trends in the dispensing of 90-day-supply prescriptions at retail pharmacies: implications for improved convenience and access. Am Health Drug Benefits. 2011;4(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 20.Moro RN, Borisov AS, Saukkonen J, et al. Factors associated with noncompletion of latent tuberculosis infection treatment: experience from the PREVENT TB Trial in the United States and Canada. Clin Infect Dis. 2016;62(11):1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Tuberculosis treatment. http://www.cdc.gov/tb/topic/treatment/. Published 2016. Accessed April 6, 2017.
- 22.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. [DOI] [PubMed] [Google Scholar]
- 23.Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007;(1):CD005029. [DOI] [PubMed] [Google Scholar]
- 24.Sandgren AA, Noordegraaf-Schouten MV, van Kessel F, Stuurman A, Oordt-Speets A, van der Werf MJ. Initiation and completion rates for latent tuberculosis infection treatment: a systematic review. BMC Infect Dis. 2016;16(1):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young KH, Ehman M, Reves R, et al. Tuberculosis contact investigations—United States, 2003-2012. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1369–1374. [DOI] [PubMed] [Google Scholar]
- 26.Aatola H, Hutri-Kahonen N, Juonala M, et al. Prospective relationship of change in ideal cardiovascular health status and arterial stiffness: the Cardiovascular Risk in Young Finns Study. J Am Heart Assoc. 2014;3(2):e000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page KR, Sifakis F, Montes de Oca R, et al. Improved adherence and less toxicity with rifampin vs isoniazid for treatment of latent tuberculosis: a retrospective study. Arch Intern Med. 2006;166(17):1863–1870. [DOI] [PubMed] [Google Scholar]
- 28.Clerk N, Sisson K, Antunes G. Latent tuberculosis: concordance and duration of treatment regimens. Br J Nurs. 2011;20(13):824–827. [DOI] [PubMed] [Google Scholar]
- 29.Smith BM, Schwartzman K, Bartlett G, Menzies D. Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ. 2011;183(3):E173–E179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lardizabal A, Passannante M, Kojakali F, Hayden C, Reichman LB. Enhancement of treatment completion for latent tuberculosis infection with 4 months of rifampin. Chest. 2006;130(6):1712–1717. [DOI] [PubMed] [Google Scholar]
- 31.Fresard I, Bridevaux PO, Rochat T, Janssens JP. Adverse effects and adherence to treatment of rifampicin 4 months vs isoniazid 6 months for latent tuberculosis: a retrospective analysis. Swiss Med Wkly. 2011;141:w13240. [DOI] [PubMed] [Google Scholar]
- 32.Rennie TW, Bothamley GH, Engova D, Bates IP. Patient choice promotes adherence in preventive treatment for latent tuberculosis. Eur Respir J. 2007;30(4):728–735. [DOI] [PubMed] [Google Scholar]
- 33.Young H, Wessolossky M, Ellis J, Kaminski M, Daly JS. A retrospective evaluation of completion rates, total cost, and adverse effects for treatment of latent tuberculosis infection in a public health clinic in central Massachusetts. Clin Infect Dis. 2009;49(3):424–427. [DOI] [PubMed] [Google Scholar]
- 34.Gilroy SA, Rogers MA, Blair DC. Treatment of latent tuberculosis infection in patients aged > or = 35 years. Clin Infect Dis. 2000;31(3):826–829. [DOI] [PubMed] [Google Scholar]
- 35.Parsyan AE, Saukkonen J, Barry MA, Sharnprapai S, Horsburgh CR Jr. Predictors of failure to complete treatment for latent tuberculosis infection. J Infect. 2007;54(3):262–266. [DOI] [PubMed] [Google Scholar]
- 36.LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. Am J Respir Crit Care Med. 2003; 168(4):443–447. [DOI] [PubMed] [Google Scholar]
- 37.Cohen RA, Martinez ME, Zammitti EP. Health Insurance Coverage: Early Release of Estimates From the National Health Interview Survey, 2015. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2016. http://www.cdc.gov/nchs/data/nhis/earlyrelease/insur201605.pdf. Accessed April 6, 2017. [Google Scholar]
- 38.Cain KP, Benoit SR, Winston CA, MacKenzie WR. Tuberculosis among foreign-born persons in the United States. JAMA. 2008;300(4):405–412. [DOI] [PubMed] [Google Scholar]
- 39.Barnett JCV, Marina S. Health insurance coverage in the United States: 2015. http://www.census.gov/content/dam/Census/library/publications/2016/demo/p60-257.pdf. Published 2016. Accessed April 6, 2017.
- 40.Seabury SA, Lakdawalla DN, Dougherty JS, Sullivan J, Goldman DP. Medication adherence and measures of health plan quality. Am J Manag Care. 2015;21(6):e379–e389. [PubMed] [Google Scholar]
- 41.National Quality Forum. Improving patient medication: compact action brief. http://www.qualityforum.org/Publications/2011/03/Improving_Patient_Medication_Adherence_CAB.aspx. Published 2011. Accessed April 6, 2017. [Google Scholar]
- 42.National Committee for Quality Assurance. PCMH care linked to better medication adherence. http://www.ncqa.org/newsroom/details/pcmh-care-linked-to-better-medication-adherence?ArtMID=11280&ArticleID=62&tabid=2659. Published 2016. Accessed April 6, 2017.
- 43.Virnig BAB. Administrative data for public health surveillance and planning. Annu Rev Public Health. 2001;22(1):213–230. [DOI] [PubMed] [Google Scholar]
- 44.Cleverley WO, Cleverley JO, Song PH. Essentials of Health Care Finance. 7th ed. Burlington, MA: Jones & Bartlett; 2010. [Google Scholar]
- 45.Manos D CMS makes Medicare, Medicaid data easier for researchers to access. Healthcare IT News. http://www.healthcareitnews.com/news/cms-makes-medicare-medicaid-data-accessible-virtually-data-health-population-research. Published 2013. Accessed April 6, 2017. [Google Scholar]
- 46.Burda D Releasing the power of the health care claim. Twin Cities Business. http://tcbmag.com/Opinion/Columns/Explanation-of-Benefits/Releasing-The-Power-Of-The-Health-Care-Claim. Published 2016. Accessed April 6, 2017. [Google Scholar]
- 47.Porter J, Love D, Peters A, Sachs J, Costello A. The Basics of All-Payer Claims Databases: A Primer for States. Princeton, NJ: All Payer Claims Database Council & the Robert Wood Johnson Foundation; 2014. http://www.rwjf.org/content/dam/farm/reports/issue_briefs/2014/rwjf409988. Accessed April 6, 2017. [Google Scholar]
- 48.Research Data Assistance Center. About ResDAC. http://www.resdac.org/about-resdac. Published 2016. Accessed April 6, 2017.
- 49.Truven Health Analytics. Putting research data into your hands with the MarketScan databases. http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases. Published 2017. Accessed April 6, 2017.
- 50.McGinnis B, Kauffman Y, Olson KL, Witt DM, Raebel MA. Interventions aimed at improving performance on medication adherence metrics. Int J Clin Pharm. 2014;36(1):20–25. [DOI] [PubMed] [Google Scholar]
- 51.O’Malley KJ, Cook KF, Price MD, Wildes KR, Hurdle JF, Ashton CM. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5, pt 2):1620–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


