Abstract
Limited researches focused on the application of laparoscopic gastrectomy (LG) in locally advanced gastric cancer (LAGC) patients following neoadjuvant chemotherapy (NACT). In this study, we aimed at illustrating the surgical and survival outcome of LG in LAGC patients following NACT. We performed a retrospective study of patients with LAGC who received either LG following NACT or upfront LG at Fujian Provincial Hospital between March 2013 and October 2018. Perioperative parameters, short-term and long-term outcomes were compared. The Kaplan–Meier estimator was used to describe the survival curves, and the differences were examined by the log-rank test. In total, 76 consecutive patients were enrolled into the NACT-LG (41 patients) and LG (35 patients) group. The postoperative hospital stay was significantly longer for LG than for NACT-LG (11.0 vs. 12.0 day, P = 0.031). Significant difference was found in Grade ≥ III severe postoperative complications in two groups (0 vs. 17.1%, P = 0.001). No patient died of postoperative complications in the NACT-LG group, and one patient (1/35, 2.9%) died of postoperative complications in the LG group. A forest plot revealed that most subgroups of LG group were at great risks of postoperative complications. Compared with the LG group, the NACT-LG group had a significantly better DFS (14.4% vs. 5.7%, P = 0.0299) and better OS (34.1% vs. 8.6%, P = 0.0061) at 3 years. NACT increased the safety of LG for patients with LAGC and offer better disease-free and overall survival. For patients with LAGC, LG following NACT should be the priority treatment.
Subject terms: Diseases, Oncology
Introduction
Gastric cancer is a life-threatening disease, and surgical resection remains the only curative treatment1,2. However, the long-term outcome is still far from satisfactory for patients who receive surgery alone, especially in patients with locally advanced gastric cancer (LAGC). Approximately 70% patients with LAGC died within 5 years after surgery3,4. Therefore, perioperative therapy is imperatively required to improve the survival.
Neoadjuvant chemotherapy (NACT) is generally accepted to benefit prognosis by downstaging tumor, increasing complete resection rate and eradicating micro-metastases5,6. The MAGIC study has illustrated a survival benefit of perioperative chemotherapy and consequently opened the era of neoadjuvant therapy in patients with LAGC7. Although NACT has demonstrated several strengths as mentioned above, surgeons concerned about its negative effects on the surgical safety. Destruction of anatomical dissection plane induced by tissue edema and fibrotic changes may complicate laparoscopic surgeries. In addition, chemotherapy related adverse effects deteriorate the nutritional and immune status of patients, which may impair the prognosis of patients8–10.
With the development of laparoscopic technique and accumulation of evidence from clinical trials in recent years, laparoscopic surgery has been recommended to patients with LAGC11. Nevertheless, limited studies focused on the application of laparoscopic gastrectomy (LG) in patients receiving NACT. There is an urgent need for researches comparing patients receiving laparoscopic surgery after NACT or upfront laparoscopic surgery. However, conducting prospective trials targeting on the issue is impractical for the reason that NACT has been widely accepted in patients with locally advanced gastric cancer (LAGC) due to its positive effects on patients’ survival12–14.
Therefore, this retrospective study was conducted to evaluate the safety and efficacy of LG for patients with LAGC following NACT, focusing on whether NACT increased the safety of LG for patients with LAGC.
Patients and methods
Patients
We reviewed our prospectively maintained gastric cancer database at Fujian Provincial Hospital. Clinical data of LAGC patients underwent either LG following NACT or upfront LG between March 2013 and October 2018 were analyzed. Patients were randomly assigned to the 2 groups. The inclusion criteria were listed as follows: (1) stomach adenocarcinoma, histologically confirmed by endoscopic biopsy; (2) clinical stage III (cT3/4a, N+, M0) according to the 8th edition of the AJCC/UICC staging system15, diagnosed using computed tomography (CT), endoscopic ultrasonography (EUS), or laparoscopic exploration; (3) totally laparoscopic gastrectomy. The exclusion criteria were listed as follows: (1) cancer of the esophagogastric junction; (2) residual gastric cancer; (3) malignant tumor history; and (4) emergency surgery due to complications (obstruction, bleeding, or perforation); (5) laparoscopy-assisted gastrectomy; (6) incomplete clinical and pathological data.
All patients signed written informed consent, and this study was approved by the ethics committee of the Fujian Provincial Hospital. The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, the national legislation, and the institutional requirements.
Treatment
Patients in the NACT-LG group received three to six cycles of NACT (XELOX regimen) at three-weekly intervals. XELOX regimen consisted of oral fluoropyrimidine capecitabine (825 mg/m2, twice daily on days 1–14) and intravenous oxaliplatin (130 mg/m2 on day 1). Surgery was performed four to five weeks after the last cycle. Patients in the LG group received upfront surgery after preoperative evaluation. Laparoscopic total or distal gastrectomy with D2 lymphadenectomy was performed for patients according to the location of tumor. Reconstruction of the gastrointestinal tract was all completed using Roux-en-Y anastomosis. All the operations were performed by one experienced surgeon team. Within five weeks after surgery, patients were treated with three to six cycles of postoperative chemotherapy (XELOX regimen) at three-weekly intervals.
Clinical and pathologic assessment
Clinicopathological variables, such as age, sex, body mass index (BMI), Eastern Clinical Oncology Group performance status (ECOG PS)16, tumor size, tumor location, Borrmann type, Lauren type, cT stage, as well as perioperative data, such as surgical type, incision length, operation time, blood loss, first aerofluxus time, drainage duration, postoperative hospital stay, and surgical radicalness were analyzed. Clavien-Dindo classification system was applied to evaluate postoperative complication, which occurs within 30 days17,18. Postoperative mortality was defined as death of any cause within 30 days after surgery. Pathological findings including number of harvested and metastatic lymph nodes, pathological stage, and tumor regression grade (TRG) were also compared between groups. The TRG was evaluated according to the NCCN guideline12.
Follow-up
All the patients received postoperative follow-up every 3 months within 2 years after surgery and every 6 months for the next 3 years. Recurrence was defined as local recurrence identified by contrast CT scan or endoscopy biopsy, or distant recurrence identified by CT scan, ECT bone scan or PET/CT. Immediate follow-up occurred in patients with worsening or new symptoms. The disease-free survival (DFS) was calculated from surgery to recurrence, death, or last follow-up; the overall survival (OS) was calculated from beginning of treatment to death or last follow-up.
Statistical analysis
Continuous variables with normal distribution were expressed as the mean ± standard deviation (SD) while non-normal variables were expressed as the median (range). T-test or Mann–Whitney rank-sum test was used to evaluate differences between the two groups according to the distribution of data. The Kaplan–Meier method was performed using a log-rank test to estimate differences in DFS and OS. SPSS version 20 was used for the statistical analysis, and P values less than 0.05 (two-sided) were considered statistically significant.
Results
Patients
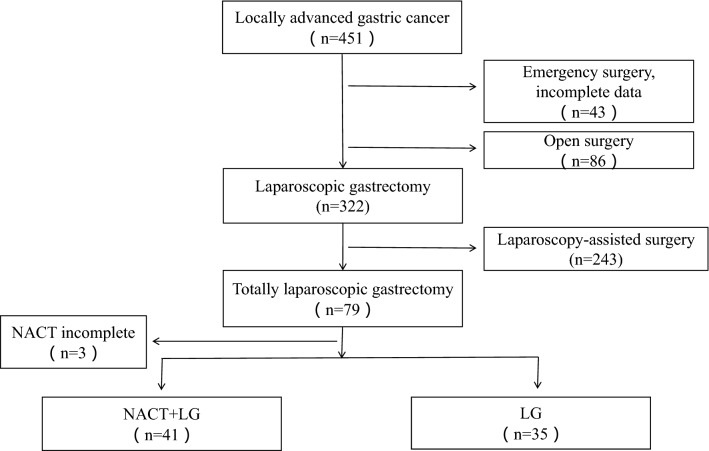
During the study period, we identified 451 patients with locally advanced gastric cancer. 375 patients were excluded due to emergency surgery and incomplete data (n = 43), open surgery (n = 86), laparoscopy-assisted surgery (n = 243), NACT incomplete which results from intolerable responses to chemotherapies (n = 3). We obtained data on76 patients in this study, 41 (53.9%) patients in the NACT-LG group and 35 (46.1%) patients in the LG group, respectively (Fig. 1). The characteristics of patients were detailed in Table 1. The two groups were balanced in baseline characteristics in term of age, sex, BMI and ECOG PS (all P > 0.05). There were also no differences between groups in tumor size, tumor location, Borrmann type, Lauren type, differentiation, cT stage and postoperative chemotherapy cycles (all P > 0.05).
Figure 1.
A flowchart presenting the selection procedure.
Table 1.
Patient baseline characteristics in the NACT-LG and LG groups.
| NACT-LG (n = 41) | LG (n = 35) | P | |
|---|---|---|---|
| Age (years) | 0.112 | ||
| < 60 | 19 (46.3) | 10 (28.6) | |
| ≥ 60 | 22 (53.7) | 25 (71.4) | |
| Sex | 0.231 | ||
| Male | 32 (78.0) | 23 (65.7) | |
| Female | 9 (22.0) | 12 (34.3) | |
| BMI (kg/m2) | 23.2 ± 1.9 | 22.6 ± 2.8 | 0.238 |
| ECOG PS | 0.760 | ||
| 0 | 26 (63.4) | 21 (60.0) | |
| 1 | 15 (36.6) | 14 (40.0) | |
| Tumor size (cm) | 0.193 | ||
| < 5 | 26 (63.4) | 17 (48.6) | |
| ≥ 5 | 15 (36.6) | 18 (51.4) | |
| Tumor location | 0.187 | ||
| Upper third | 15 (36.6) | 6 (17.1) | |
| Middle third | 14 (34.1) | 14 (40.0) | |
| Lower third | 12 (29.3) | 14 (40.0) | |
| Total | 0 (0) | 1 (2.9) | |
| Borrmann type | 0.117 | ||
| I | 1 (2.4) | 2 (5.7) | |
| II | 7 (17.2) | 1 (2.9) | |
| III | 32 (78.0) | 29 (82.9) | |
| IV | 1 (2.4) | 3 (8.6) | |
| Lauren type | 0.913 | ||
| Diffused | 9 (22.0) | 9 (25.7) | |
| Intestinal | 18 (43.9) | 14 (40.0) | |
| Mixed | 14 (34.1) | 12 (34.3) | |
| cT stage | 0.367 | ||
| T3 | 18 (43.9) | 19 (54.3) | |
| T4 | 23 (56.1) | 16 (45.7) | |
| Chemotherapy cycles | |||
| Preoperative | 4 (3–6) | NA | NA |
| Postoperative | 3 (3–6) | 4 (3–6) | 0.160 |
Fisher’s exact test was used as an alternative to Chi-square test when the number in one of the cells is smaller than 5.
NACT neoadjuvant chemotherapy, LG laparascopic gastrectomy, BMI body mass index, ECOG PS Eastern Clinical Oncology Group performance status, NA not applicable.
Surgical procedures
All patients received an LG with D2 lymphadenectomy. The surgical data were compared in Table 2. No significant difference was found in the surgical trauma in terms of incision length operation time, blood loss, and postoperative recovery in terms of first aerofluxus time, and first time on liquid diets between the two groups (all P > 0.05). Patients in LG group had longer postoperative hospital stay (11.0 vs. 12.0d, P = 0.031). In addition, the NACT-LG exhibited a significantly greater R0 resection rate (95.1% vs. 77.1%, P < 0.05). R1 resection was performed in two (4.9%) patients in the NACT-LG group with positive proximal margins. Eight (22.9%) patients with R1 resection were found in the LG group, including four (11.4%) patients with intramural extension to esophagus and four (11.4%) patients with intramural extension to duodenum.. No similar situations were found in the NACT-LG group.
Table 2.
Comparison of surgical procedures between the NACT-LG and LG groups.
| NACT-LG (n = 41) | LG (n = 35) | P | |
|---|---|---|---|
| Surgical type | 0.327 | ||
| LTG | 30 (73.2) | 21 (60.0) | |
| LDG | 11 (26.8) | 14 (40.0) | |
| Incision length (cm) | 6.0 (3.0–8.0) | 5.0 (4.0–7.0) | 0.109 |
| Operation time (min) | 260.0 (205.0–346) | 250.0(140.0–360.0) | 0.281 |
| Estimated blood loss (mL) | 100.0 (20.0–450.0) | 100.0 (30.0–2500.0) | 0.395 |
| The first aerofluxus time (days) | 3.0 (1.0–6.0) | 3.0 (1.0–6.0) | 0.303 |
| Time to pull drainage (days) | 8.0 (5.0–13.0) | 7.0 (5.0–14.0) | 0.061 |
| First time on liquid diets (days) | 3.0 (2.0–12.0) | 3.0 (1.0–12.0) | 0.118 |
| Hospital stay after surgery (days) | 11.0 (6.0–18.0) | 12.0 (6.0–77.0) | 0.031 |
| Surgical radicalness | 0.049 | ||
| R0 | 39 (95.1) | 27 (77.1) | |
| R1 | 2 (4.9) | 8 (22.9) | |
Fisher’s exact test was used as an alternative to Chi-square test when the number in one of the cells is smaller than 5.
NACT neoadjuvant chemotherapy, LG laparascopic gastrectomy, LTG laparoscopic total gastrectomy, LDG laparoscopic distal gastrectomy.
Surgery morbidity and mortality
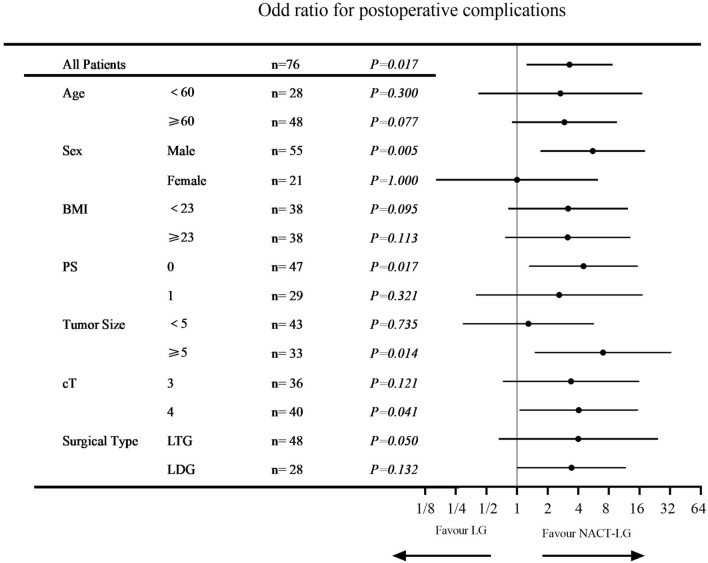
The postoperative complications were detailed in Table 3. Grade II postoperative complications occurred similarly in two groups, 9 patients (22.0%) in the NACT-LG group and 10 patients (28.5%) in the LG group (P = 0.680). The most common postoperative complications were intra-abdominal infection (9/76, 11.8%), followed by pulmonary infection (5/76, 6.6%) and transfusion (4/76, 5.3%), respectively. While significant difference was found in Grade ≥ III severe postoperative complications in two groups (0 vs. 17.1%, P = 0.001). Six (17.1%) patients suffered major complications (grade III–V) requiring invasive interventions in the LG group; three (8.6%) patients with anastomotic leakage recovered after active abodominal drainage; two (5.7%) patients with pulmonary infection recovered after ICU care, and one (2.9%) patient died of respiratory failure. No patient died of postoperative complications in the NACT-LG group. Subgroup analyses were performed to investigate the impact of safety about NACT-LG. It was found that most subgroups of LG group were at great risks of postoperative complications, especially in male, PS = 0, Tumor size ≥ 5 and cT4 groups (Fig. 2).
Table 3.
Postoperative complications in the NACT-LG and LG groups.
| NACT-LG (n = 41) | LG (n = 35) | P | |
|---|---|---|---|
| Grade I | 0 (0) | 0 (0) | NA |
| Grade II | 9 (22.0) | 10 (28.5) | 0.680 |
| Transfusion | 2 (4.9) | 2 (5.7) | |
| Pulmonary infection | 2 (4.9) | 3 (8.6) | |
| Intra-abdominal infection | 4 (9.8) | 5 (14.2) | |
| Intra-abdominal hemorrhage | 1 (2.4) | 0 (0) | |
| Grade III/IV/V | 0 (0) | 6 (17.1) | 0.001 |
| Anastomotic leakage | 0 (0) | 3 (8.6) | |
| Pulmonary infection | 0 (0) | 2 (5.7) | |
| Respiratory failure | 0 (0) | 1 (2.9) |
Fisher’s exact test was used as an alternative to Chi-square test when the number in one of the cells is smaller than 5.
NACT neoadjuvant chemotherapy, LG laparascopic gastrectomy, NA not applicable.
Figure 2.
Forest plot evaluating the impact of the treatment selections on postoperative complications.
Pathological outcomes
A comparison of pathologies between the two groups was summarized in Table 4. The number of resected lymph nodes did not differ significantly between the two groups, whereas significantly less metastatic lymph nodes were found in the NACT-LG group than in the LG group (1 vs. 8, P = 0.001). Among all patients, there was a greater proportion of less advanced pT stage (T0–2; 29.3% vs. 0%, P < 0.01), less advanced pN stage (N0; 36.6% vs. 0%, P < 0.01), and less advanced pTNM stage (pTNM 0–II; 51.2% vs. 0%, P < 0.01) in the NACT-LG group than in the LG group. In the NACT-LG group, the pathological response was 65.9%. Over half of the patients (51.2%) obtained tumor downstaging in the NACT-LG group. Four patients (9.8%) were considered pathological complete responders after preoperative chemotherapy.
Table 4.
Comparison of pathologies between the NACT-LG and LG groups.
| NACT-LG (n = 41) | LG (n = 35) | P | |
|---|---|---|---|
| Number of resected lymph nodes | 37 (12–91) | 32 (12–69) | 0.081 |
| Number of metastatic lymph nodes | 1 (0–36) | 8 (1–27) | 0.001 |
| pT stage | 0.008 | ||
| 0–2 | 12 (29.3) | 0 (0) | |
| 3–4 | 29 (70.7) | 35 (100.0) | |
| pN stage | < 0.01 | ||
| 0 | 15 (36.6) | 0 (0) | |
| 1–3 | 26 (63.4) | 35 (100.0) | |
| pTNM stage | < 0.01 | ||
| 0–II | 21 (51.2) | 0 (0) | |
| III | 20 (48.8) | 35 (100.0) | |
| Tumor regression grades | NA | ||
| 0 | 4 (9.8) | NA | |
| 1 | 13 (31.7) | NA | |
| 2 | 10 (24.4) | NA | |
| 3 | 14 (34.1) | NA | |
| Pathological complete response | 4 (9.8) | NA | |
Fisher’s exact test was used as an alternative to Chi-square test when the number in one of the cells is smaller than 5.
NACT neoadjuvant chemotherapy, LG laparascopic gastrectomy, NA not applicable.
Survival
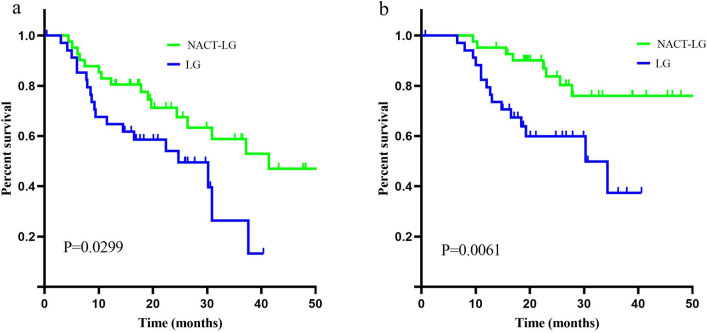
The overall median follow-up was 23.0 months (6.6–71.5) in this study, 25.6 months (9.5–71.5) in the NACT-LG group, and 18.6 months (6.6–40.6) in the LG group, respectively. The survival curves were shown in Fig. 3. The 3-year disease-free survival rates in the NACT-LG group and LG group were 14.4% and 5.7% (P = 0.0299), respectively. The 3-year overall survival rates in the NACT-LG group and LG group were 34.1% and 8.6% (P = 0.0061), respectively (Fig. 3).
Figure 3.
Kaplan–Meier estimates of (a) disease-free survival and (b) overall survival.
Discussion
LAGC is characterized by low radical resection rate, high relapse rate and high mortality. Laparoscopic surgery combined with perioperative therapy has been widely accepted as the mainstream treatment. However, only few studies focused on the safety and efficacy of laparoscopic surgery in patients after NACT. To our best of knowledge, the present study is the first head-to-head comparison of NACT-LG and LG, and further evaluate the impact of treatment selections on postoperative complications. The present study revealed that NACT-LG increased safety and offered survival benefits in patients with LAGC.
We found that LG following NACT did not increase the severity of surgical trauma, and shorten duration of postoperative hospital stay, which results from less postoperative complications. In addition, operation time was comparable between NACT-LG and LG, and the postoperative complications decreased significantly in NACT-LG group, which was different with a previous study reported by An et al.8. It was reported that the negative effects of chemotherapy on operation might be overcome by laparoscope. Laparoscopic surgery offers amplifying visual, better exposure of anatomical hierarchy, and then contributes to delicate anatomy of blood vessels and lymphatic vessels19. To further evaluate the impact of NACT-LG regarding postoperative complications, we performed a subgroup analysis and found that patients undergoing NACT-LG were at less risk of developing postoperative complications in most subgroups, especially in male, PS = 0, Tumor size ≥ 5 and cT4 groups. Better compliance and potential benefits to preoperative chemotherapy and reduction of the primary tumor in these patients may be the reasons of less postoperative complications. On the other hand, adverse effects caused by chemotherapy can be attenuated by optimized perioperative care. For instance, malnutrition caused by gastrointestinal side effects can be treated by adequate perioperative nutrition.
Micro-metastases outside the surgical region and microscopic positive margin were the main causes of treatment failure in LAGC20–22. NACT may benefit these patients by eradicating metastases and potentially downstaging tumor, thereby increasing the R0 resection rate. Among 41 patients receiving NACT in this study, the pathological response was 65.9%, in concordance with previous studies23,24. Over half of patients achieved tumor downstaging, which seemed attributable to the effects of NACT. Moreover, our study demonstrated the benefits of LG following NACT compared with immediate surgery, with an increase of 18 percent in the R0 resection rate (95.1% vs. 77.1%, P = 0.049) and a reduction in the number of metastatic lymph nodes (1 vs. 8, P = 0.001).
The MAGIC trial and the EORTC study disagreed about the survival benefits of perioperative chemotherapy in resectable gastric cancer patients7,25. Since proportions of D2 dissection were different among two studies (MAGIC: 42.5%; EORTC: 95.7%), whether patients with D2 gastrectomy benefited from NACT remained unclear. The present study, with all patients received a D2 lymph node dissection, showed that 3-year DFS and OS were superior for NACT-LG than LG (DFS: 14.4% vs. 5.7%, OS: 34.1% vs. 8.6%). We speculated that sufficient preoperative chemotherapy courses and better compliance to chemotherapy may enhance the positive effects of NACT on survival. However, multi-center, large-sample clinical trials were required to verify this hypothesis in the future.
Despite positive findings mentioned above, there were several limitations in this study. First, as a retrospective study with non-randomized patient groups, selection bias were inevitable in this study. Second, the generalizability of findings drawn from a single-center study may be limited. Third, the follow-up period was too short to analyze the longer survival situation.
Conclusions
In summary, for patients with LAGC who underwent LG, this study provided supportive evidence favoring the application of NACT. NACT increase the safety of LG for patients with LAGC and offer higher R0 resection rate and better disease-free and overall survival. For patients with LAGC, LG following NACT should be the priority treatment.
Author contributions
L.L.: Contributed to conception and design; contributed to acquisition, analysis and interpretation; and drafted the manuscript. C.W.: Contributed to interpretation; and critically revised the manuscript. F.L.: Contributed to analysis; critically revised the manuscript. X.Z.: Contributed to conception; and critically revised the manuscript. X.C.: Contributed to analysis, S.L.: Contributed to analysis, Y.L.: Contributed to analysis, C.Y.: Contributed to analysis, W.L.: Contributed to conception and design; contributed to acquisition, analysis and interpretation; and critically revised the manuscript All authors gave their final approval and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Lihang Liu, Chuandong Wang, Feng Li and Xiaojuan Zhang.
References
- 1.Lutz MP, et al. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer—Differential treatment strategies for subtypes of early gastroesophageal cancer. Eur. J. Cancer (Oxford, England : 1990) 2012;48:2941–2953. doi: 10.1016/j.ejca.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, et al. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann. Oncol. 2011;22(Suppl 5):v1–9. doi: 10.1093/annonc/mdr284. [DOI] [PubMed] [Google Scholar]
- 3.Sant M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur. J. Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, et al. Gastric cancer, version 2.2013: Featured updates to the NCCN guidelines. J. Natl. Comprehens. Cancer Netw. JNCCN. 2013;11:531–546. doi: 10.6004/jnccn.2013.0070. [DOI] [PubMed] [Google Scholar]
- 5.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 6.Miao ZF, et al. Effect of neoadjuvant chemotherapy in patients with gastric cancer: A PRISMA-compliant systematic review and meta-analysis. BMC Cancer. 2018;18:118. doi: 10.1186/s12885-018-4027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 8.An JY, et al. Surgical complications in gastric cancer patients preoperatively treated with chemotherapy: Their risk factors and clinical relevance. Ann. Surg. Oncol. 2012;19:2452–2458. doi: 10.1245/s10434-012-2267-9. [DOI] [PubMed] [Google Scholar]
- 9.Awad S, et al. Marked changes in body composition following neoadjuvant chemotherapy for oesophagogastric cancer. Clin. Nutr. (Edinburgh, Scotland) 2012;31:74–77. doi: 10.1016/j.clnu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Tegels JJ, De Maat MF, Hulsewé KW, Hoofwijk AG, Stoot JH. Improving the outcomes in gastric cancer surgery. World J. Gastroenterol. 2014;20:13692–13704. doi: 10.3748/wjg.v20.i38.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, et al. Effect of laparoscopic vs open distal gastrectomy on 3-year disease-free survival in patients with locally advanced gastric cancer: The CLASS-01 randomized clinical trial. JAMA. 2019;321:1983–1992. doi: 10.1001/jama.2019.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu, H. & Zhou, Z. Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5. Zhonghua Wei Chang Wai Ke Za Zhi (Chinese J. Gastrointest. Surg.)21, 160–164 (2018). [PubMed]
- 13.Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 24, 1–21. 10.1007/s10120-020-01042-y (2021). [DOI] [PMC free article] [PubMed]
- 14.Wang FH, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. (London, England) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeckelmann N, et al. Prognostic implications of the 8th edition American Joint Committee on Cancer (AJCC) staging system in oral cavity squamous cell carcinoma. Oral Oncol. 2018;85:82–86. doi: 10.1016/j.oraloncology.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111:518–526. [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, et al. Assessment of laparoscopic distal gastrectomy after neoadjuvant chemotherapy for locally advanced gastric cancer: A randomized clinical trial. JAMA Surg. 2019;154:1093–1101. doi: 10.1001/jamasurg.2019.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang SY, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann. Surg. Oncol. 2009;16:2738–2743. doi: 10.1245/s10434-009-0616-0. [DOI] [PubMed] [Google Scholar]
- 21.Bickenbach KA, Gonen M, Strong V, Brennan MF, Coit DG. Association of positive transection margins with gastric cancer survival and local recurrence. Ann. Surg. Oncol. 2013;20:2663–2668. doi: 10.1245/s10434-013-2950-5. [DOI] [PubMed] [Google Scholar]
- 22.Nagata T, et al. Prognostic impact of microscopic positive margin in gastric cancer patients. J. Surg. Oncol. 2011;104:592–597. doi: 10.1002/jso.22022. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y, et al. A phase I/II study of NAC with docetaxel, cisplatin, and S-1 for stage III gastric cancer. Anticancer Res. 2018;38:6015–6021. doi: 10.21873/anticanres.12951. [DOI] [PubMed] [Google Scholar]
- 24.Shinkai M, et al. Phase II trial of neoadjuvant chemotherapy with intraperitoneal paclitaxel, S-1, and intravenous cisplatin and paclitaxel for stage IIIA or IIIB gastric cancer. J. Surg. Oncol. 2019;119:56–63. doi: 10.1002/jso.25295. [DOI] [PubMed] [Google Scholar]
- 25.Schuhmacher C, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010;28:5210–5218. doi: 10.1200/jco.2009.26.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.