Abstract
Background/Aims:
To assess and compare long-term reproducibility of optic nerve head (ONH) and macula optical coherence tomography angiography (OCTA) vascular parameters and optical coherence tomography (OCT) thickness parameters in stable primary open angle glaucoma (POAG), glaucoma suspect, and healthy eyes.
Methods:
Eighty-eight eyes (15 healthy, 38 glaucoma suspect, and 35 non-progressing POAG) of 68 subjects who had at least 3 visits within 1 to 1.5 years with OCTA and OCT imaging (Angiovue; Optovue Inc., Fremont, CA) on the same day were included. A series of vascular and thickness parameters were measured including macular parafoveal vessel density (pfVD), ONH circumpapillary capillary density (cpCD), macular parafoveal ganglion cell complex (pfGCC), and ONH circumpapillary retinal nerve fibre layer (cpRNFL). A random effects analysis of variance model was used to estimate intraclass correlation (ICC) coefficients and long-term variability estimates.
Results:
ICC was lower for OCTA (pfVD 0.823 (95% confidence interval: 0.736, 0.888) and cpCD 0.871 (0.818, 0.912)) compared to OCT (pfGCC 0.995 (0.993, 0.997) and cpRNFL 0.975 (0.964, 0.984)). Within-subject test-retest standard deviation was 1.17 and 1.22 % for pfVD and cpCD, and 0.57 and 1.22 μm for pfGCC and cpRNFL. Older age and lower SSI were associated with decreasing long-term variability of vessel densities.
Conclusions:
OCTA-measured macula and ONH vascular parameters have good long-term reproducibility, supporting the use of this instrument for longitudinal analysis. OCTA long-term reproducibility is less than OCT-measured thickness reproducibility. This needs to be taken into consideration when serial OCTA images are evaluated for change.
Synopsis
OCTA-measured vascular parameters have good long-term reproducibility, supporting the use of this instrument for monitoring of glaucoma.
Keywords: long-term reproducibility, optical coherence tomography, optical coherence tomography angiography, glaucoma
Introduction
Glaucoma is a progressive optic neuropathy caused by loss of retinal ganglion cells and their axons with characteristic visual field (VF) defects.[1 2] Optical coherence tomography (OCT) is a widely accepted method for monitoring retinal nerve fibre layer (RNFL) thinning and optic nerve head (ONH) changes over time in glaucoma patients and glaucoma suspects.[3] Optical coherence tomography angiography (OCTA) has emerged recently as a non-invasive modality for imaging retinal and choroidal vasculature, and studies have suggested that OCTA may be useful in glaucoma monitoring and management.[4 5] However, studies on the long-term reproducibility of this modality are lacking.
Detecting glaucoma progression relies on the ability to distinguish true change from test-retest variability. Since glaucoma is typically a chronic progressive disease, true change is not expected to occur in a relatively short timeframe. Though characterizing the short-term reproducibility of OCTA is important,[6–9] it does not assess the long-term reproducibility which is one of the important criteria for a reliable glaucoma diagnostic test with regard to monitoring progression of the disease. Determining long-term variability by repeatedly testing glaucoma eyes – usually within a short period of several months has been used to calculate the confidence limits or tolerance intervals of variability for detection of visual field progression,[10 11] and structural change over time.[12–14]
Previously, Urata et al. reported that the long-term variability of OCT is higher than short-term variability in 43 glaucoma subjects using Spectralis spectral domain OCT imaging system. The average standard deviation of the residuals – used as a measure of variability – was significantly higher with annual long-term testing compared to weekly short-term testing for cpRNFL thickness.[13] Some studies have evaluated the short-term reproducibility of OCTA-measured vessel densities of the ONH[6–8 15 16] and the macular[6 7] regions. Manalastas et al. reported that short-term CV of intravisit and intervisit vessel density measurements ranges from 2.3 to 4.1 % for ONH and 3.2 to 7.9 % for macula in 14 glaucoma subjects using Avanti Angiovue imaging system.[6] Liu et al reported the short-term reproducibility of cpCD in 12 normal and 12 glaucoma eyes using Avanti Angiovue imaging system and reported a CV of 1.9 % and 4.0 %, respectively.[17] In their reports, the short-term reproducibility of OCTA ONH and macular vessel density measurements is good, but as our results suggest, often worse than OCT. To the best of our knowledge, the long-term reproducibility of OCTA in glaucoma patients has not been reported.
In this study, the long-term variability of the OCT ONH and macula thickness parameters and OCTA vessel density in primary open-angle glaucoma (POAG) eyes were assessed and compared using Avanti Angiovue imaging system.
Methods
Participants
This was a longitudinal study of healthy, glaucoma suspect, and POAG patients enrolled in Diagnostic Innovations in Glaucoma Study (DIGS)[18 19] who underwent OCTA (Angiovue; Optovue Inc., Fremont, CA) from January 2015 to August 2019. All participants from the DIGS study who met the inclusion criteria described below were enrolled. Informed consent was obtained from all participants. The University of California San Diego Human Subjects Committee approved all protocols (NCT00221897), and the methods described adhered to the tenets of the Declaration of Helsinki.
Healthy subjects were defined as having intraocular pressure (IOP) of 21mmHg or lower, without history of elevated IOP; normal-appearing optic discs, intact neuroretinal rims and retinal nerve fibre layer; and normal visual field test results, defined as pattern standard deviation (PSD) within the 95% confidence limits and glaucoma hemifield test (GHT) result within normal limits. Glaucoma suspects were defined as having suspicious-appearing optic discs without the presence of repeatable glaucomatous visual field damage. POAG was defined as the presence of repeatable and reliable (fixation losses and false negatives ≤ 33% and false positives ≤ 15%) abnormal standard automated perimetry tests using the 24–2 Swedish Interactive Thresholding Algorithm with either a PSD outside the 95% normal limits or a GHT result outside the 99% normal limit. Glaucoma disease severity was classified as early (24–2 VF MD>−6 dB), moderate (−6 dB≥24–2 VF MD>−12 dB), and advanced (24–2 VF MD≤−12 dB).
Healthy subjects and clinically stable glaucoma suspect and POAG patients who met the following criteria were included: (1) older than 18 years of age, (2) open angles on gonioscopy, (3) best-corrected visual acuity of 20/40 or better, and (4) at least 2 years of follow-up with a minimum of four follow-up OCTA scanning sessions. Exclusion criteria were: (1) history of trauma or intraocular surgery (except for uncomplicated cataract surgery or glaucoma surgery), (2) coexisting retinal disease including diabetic retinopathy, (3) uveitis, or (4) non-glaucomatous optic neuropathy. Participants with the diagnosis of Parkinson’s disease, Alzheimer’s disease, dementia, or a history of stroke were also excluded. Eyes with an axial length of more than 26 mm or spherical equivalent of less than −6 dioptre were also excluded.
Glaucoma Stability Assessment
Clinically-stable glaucoma suspects and POAG patients who met eligibility criteria were included for longitudinal reproducibility. Clinical stability was determined on the basis of serial stereo optic disc photographs and VF progression analysis assessments by the independent masked observers (T.N. and S.M.). The presence of changes or functional changes in the glaucomatous disc and/or RNFL was confirmed by consensus between the two observers. All colour stereophotographs were taken using a Nidek Stereo Camera Model 3-DX (Nidek Inc, Palo Alto, CA) after maximal pupil dilation. Glaucomatous structural progression included progressive optic disc changes – such as focal or diffuse narrowing or notching of the neuroretinal rim, increased cup-to-disc ratio, adjacent vasculature position shift, or optic disc haemorrhage – and progressive RNFL changes, including an appearance of a new defect or worsening of an existing defect.[20] In cases of disagreement on progression status, consensus between the observers was obtained.
Functional progression was based on serial evaluation of visual fields using event-based and trend-based methods. Event-based analysis determines whether significant visual field progression has occurred, and this method is employed by the commercially available Guided Progression Analysis (GPA) software from the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA). Using the GPA, “possible progression” or “likely progression” was identified if three or more test points showed a change exceeding variability limits expected based on two consecutive baseline measurements. Trend-based analysis provides estimates of rates of visual field progression based on linear regression analysis of the visual field index. In this method, visual field progression was defined as a rate showing significantly negative slope (p< 0.05). In this study, POAG eyes were included if they had at least 5 years of follow-up and 6 visual fields before the last OCTA, without GPA-based possible or likely progression or a statistically significant visual field index slope.
Imaging
Subjects were enrolled who had at least 3 visits within 1 to 1.5 years and both OCTA and OCT (Optovue Inc, Fremont, California, USA) imaging on the same day. The diagnosis of healthy, glaucoma suspects, or POAG was defined at the time of baseline visit for the OCTA and OCT scan extracted with the aforementioned criteria. Macula and ONH microvasculature were evaluated using the AngioVue OCT system (software version 2018.1.0.43). This system has been described previously.[21] Macula 3 × 3-mm2 scans (304 B-scans × 304 A-scans per B-scan) centered on the fovea and ONH 4.5 × 4.5-mm2 scans (304 B-scans × 304 A-scans per B-scan) centered on the ONH were obtained with this system. Vessel density was automatically calculated as the proportion of measured area occupied by flowing blood defined as pixels having decorrelation values acquired by the split-spectrum amplitude-decorrelation angiography algorithm above the threshold level. Image quality review was done on all scans according to the University of California, San Diego, Imaging Data Evaluation and Analysis Reading Center standard protocol. Trained graders reviewed scans and excluded poor-quality images. The detail of the review previously has been published.[22] In brief, poor quality images were defined as images with: (1) a signal strength index of less than 48; (2) defocus (decrease in reflective intensity and clear visualization of the details of small vessels on the angiogram; (3) eye movement artifacts visible as irregular vessel pattern or disc boundary on the en face angiogram, (4) shadow (decreased intensity of retinal layers in isolated areas, often due to vitreous floaters or corneal opacities; (5) segmentation errors that could not be corrected. The location of the disc margin in the ONH and macula scans was reviewed for accuracy and was adjusted manually if required. The location of the disc margin in the ONH and macula scans was reviewed for accuracy and was adjusted manually if required.
A series of vascular and thickness parameters were measured including macula whole image vessel density (wiVD), macula parafoveal vessel density (pfVD), ONH whole image capillary density (wiCD), ONH circumpapillary capillary density (cpCD), macular whole image ganglion cell complex (wiGCC), macular parafoveal ganglion cell complex (pfGCC), and ONH circumpapillary retinal nerve fibre layer (cpRNFL).
Statistical analysis
Patient and eye characteristics data were presented as mean (95% confidence interval (CI)) for continuous variables and count (%) for categorical variables. To determine long-term variability of OCTA and OCT measurements, a linear random effects analysis of variance model was used to estimate the intraclass correlation coefficient (ICC), root mean squared error (RMSE), within-subject test-retest standard deviation (Sw), and coefficient of variation (CV).[7 13 23] The ICC summarizes the reproducibility of a measurement for a group of subjects based on the variance between subjects divided by total random effect and error variation.[24] A cutoff value of 0.70 for ICC is considered acceptable,[25] with larger values demonstrating better reproducibility. The Sw was calculated as the square root of the within-subject mean square for error (the unbiased estimator of the component of variance due to random error) in a mixed-effects model. The CV (%) was calculated as 100 × (Sw / overall mean). Comparison of rates of change in OCTA and OCT parameters for each diagnosis group was performed using a linear mixed model to take into account within-subject variability.[26–28] To see the effect of age, SSI, 24–2 VF MD, and IOP on the repeatability of OCTA and OCT measurements, regression estimates were calculated using linear mixed effects models. Time-dependent values rather than baseline values were used to see if there was any variability due to changes in the variables. Statistical analyses were performed using Stata version 15.1 (StataCorp, College Station, TX), and R version 3.6.3. P values of less than 0.05 were considered statistically significant for all analyses.
Results
A total of 88 eyes (15 healthy, 38 glaucoma suspect, and 35 non-progressive POAG) of 68 subjects were enrolled in this study. Demographics and baseline clinical characteristics are summarized in Table 1. Macula cohort included 9 healthy, 17 glaucoma suspects, and 17 POAG eyes of 42 subjects, and ONH cohort included 13 healthy, 30 glaucoma suspects, and 30 POAG eyes of 60 subjects. The mean follow-up (95% CI) was 1.2 (1.2, 1.2) years, and the mean number of visits (95% CI) was 3.1 (3.0, 3.2) for the macula cohort and 3.0 (3.0, 3.1) for the ONH cohort. The mean age (95% CI [range]) was 68.8 (65.9, 71.6 [37.3–88.9]) years, and the mean baseline 24–2 VF MD (95% CI) was −0.82 (−1.17, −0.46) dB. For the macula cohort, the average wiVD, pfVD, wiGCC, and pfGCC (95% CI) were 45.4 (44.2, 46.6) %, 48.3 (47.1, 49.4) %, 97.3 (94.2, 100.5) μm, and 102.7 (99.4, 106) μm, respectively. For the ONH cohort, the average wiCD, cpCD, and cpRNFL (95% CI) were 44.7 (43.7, 45.6) %, 46.5 (45.4, 47.5) %, and 90.8 (87.2, 94.3) μm, respectively.
Table 1.
Demographics and Baseline Characteristics of Subjects
| Macula Cohort (n = 42, 49 Eyes) | ONH cohort (n = 60, 73 Eyes) | Combined Cohort (n = 68, 88 Eyes) | |
|---|---|---|---|
|
| |||
| Age (years) | 66.6 (62.6, 70.6) | 68.8 (65.7, 71.9) | 68.8 (65.9, 71.6) |
| Gender (M/F) | 15/27 | 23/37 | 25/43 |
| Race | |||
| African Descents (%) | 11 (26.2%) | 16 (26.7%) | 19 (27.9%) |
| European Descents (%) | 24 (57.1%) | 37 (61.7%) | 39 (57.4%) |
| Others (%) | 7 (16.7%) | 7 (11.7%) | 10 (14.7%) |
| Axial Length (mm) | 24 (23.8, 24.3) | 24.0 (23.8, 24.3) | 24.0 (23.8, 24.2) |
| CCT (μm) | 539.4 (529.9, 548.8) | 539.6 (530.1, 549.1) | 537.1 (528.6, 545.6) |
| Spherical Equivalent (D) | −0.95 (−1.41, −0.49) | −0.73 (−1.13, −0.32) | −0.79 (−1.15, −0.43) |
| Baseline IOP (mmHg) | 15.0 (13.8, 16.2) | 14.7 (13.5, 15.8) | 15.1 (14.0, 16.1) |
| Diagnosis by subject | |||
| Healthy, n (%) | 9 (18.4%) | 13 (17.8%) | 15 (17.0%) |
| Glaucoma suspect, n (%) | 23 (46.9%) | 30 (41.1%) | 38 (43.2%) |
| Glaucoma, n (%) | 17 (34.7%) | 30 (41.1%) | 35 (39.8%) |
| Baseline 24-2 MD (dB) | −0.47 (−0.85, −0.08) | −0.85 (−1.25, −0.45) | −0.82 (−1.17, −0.46) |
| Average SSI | 70.1 (68.1, 72.1) | 64.2 (62.4, 66.1) | |
| Average wiVD (%) | 44.8 (43.7, 46.0) | ||
| Average pfVD (%) | 47.8 (47.0, 48.9) | ||
| Average wiGCC (μm) | 97.0 (93.7, 100.1) | ||
| Average pfGCC (μm) | 102.3 (98.9, 105.6) | ||
| Average wiCD (%) | 44.7 (43.7, 45.6) | ||
| Average cpCD (%) | 46.5 (45.4, 47.5) | ||
| Average cpRNFL (μm) | 90.8 (87.2, 94.3) | ||
| Follow-up for OCTA (years) | 1.2 (1.2, 1.2) | 1.2 (1.2, 1.2) | |
| Visits | 3.1 (3.0, 3.2) | 3.0 (3.0, 3.1) | |
| Visual field follow-up period for glaucoma patients | 8.7 (6.0, 11.5) | 10.1 (8.5, 11.7) | |
| Visual field follow-up period for glaucoma suspect subject | 9.7 (7.2, 12.3) | 9.6 (7.1, 12.0) | |
CCT = central corneal thickness; CD = capillary density; cp = circumpapillary; D = diopter; F = female; GCC = ganglion cell complex; IOP = intraocular pressure; ONH = optic nerve head; pf = parafoveal; RNFL = retinal nerve fiber layer; SSI = signal strength index; VD = vessel density; wi = whole image; M = male; MD = mean deviation. Values are shown in mean (95% confident interval), unless otherwise indicated.
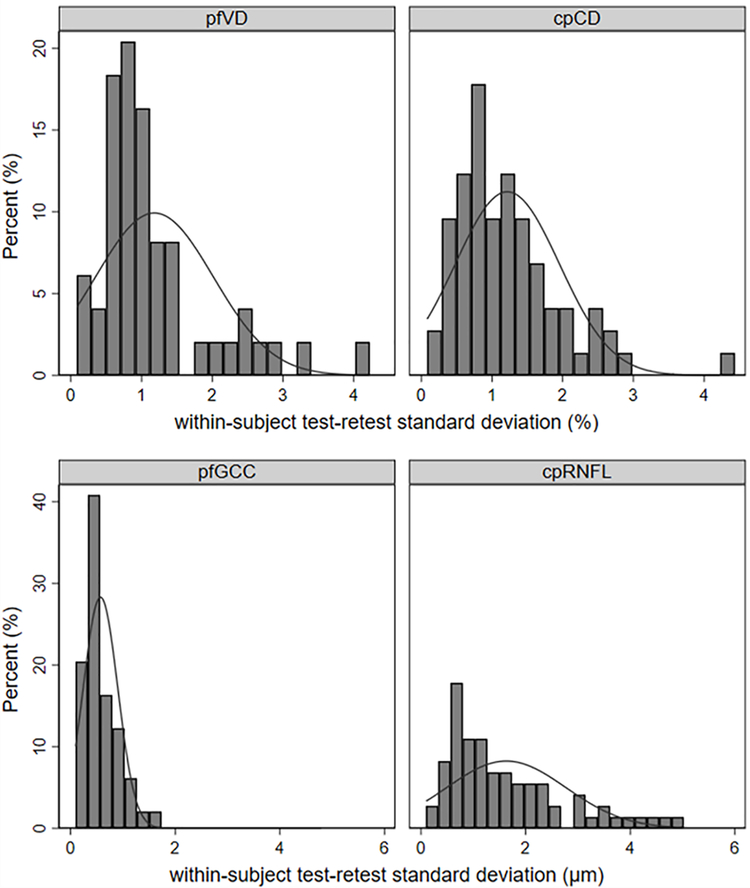
No significant pfVD or cpCD loss, or cpRNFL thinning (95% CI) was observed (−0.45 (−0.98, 0.08) %/year; p=0.094, −0.37 (−0.85, 0.12) %/year; p=0.137, and 0.51 (−1.18, 0.16) μm/year; p=0.137, respectively), while wiVD or wiCD loss, and wiGCC or pfGCC thinning was detectable over time (−0.55 (−1.06. −0.03) %/year; p=0.037, −0.60 (−1.04, −0.15) %/year; p=0.009, −0.77 (−1.00, −0.54) μm/year; p<0.001, and −0.69 (−0.90, −0.47) μm/year; p<0.001, respectively, Supplemental table 1). However, no statistically significant differences were found in rates of vessel density and thickness change among healthy, glaucoma suspects, and POAG groups (p>0.05) (Supplemental table 1). ICC among healthy, glaucoma suspects, and POAG groups was shown in Supplemental table 2. Table 2 shows the repeatability estimates of the macula and ONH parameters. ICC (95% CI) was lower for OCTA (wiVD 0.839 (0.758, 0.899), pfVD 0.823 (0.736, 0.888), wiCD 0.861 (0.804, 0.905), and cpCD 0.871 (0.818, 0.912)) compared to OCT (wiGCC 0.994 (0.991, 0.996), pfGCC 0.995 (0.993, 0.997), and cpRNFL 0.975 (0.964, 0.984)). RMSE was between 1.34 and 1.43 % for OCTA, and 0.70, 0.65, and 2.00 μm for wiGCC, pfGCC, and cpRNFL. Sw was between 1.17 and 1.22 % for OCTA, and 0.61, 0.57, and 1.63 μm for wiGCC, pfGCC, and cpRNFL. Long-term test-retest variability was between 2.29 and 2.39 % for vascular parameters. CV was higher for OCTA parameters (between 2.48 and 2.62 %) compared to OCT parameters (wiGCC 0.63, pfGCC 0.56, and cpRNFL 1.79 %). Figure shows the standard deviation of residuals of macula and ONH parameters. There was a greater spread in the distribution of the standard deviation of residuals for cpRNFL compared to pfGCC, while the result of cpCD and pfVD was similar.
Table 2.
ICC, RMSE, within-subject test-retest standard deviation, and CV for Macula and ONH parameters
| ICC (95% CI) | RMSE (%/μm) | Sw (%/μm) | CV (%) | |
|---|---|---|---|---|
|
| ||||
| Macula | ||||
| wiVD | 0.839 (0.758, 0.899) | 1.39 | 1.17 | 2.62 |
| pfVD | 0.823 (0.736, 0.888) | 1.43 | 1.18 | 2.48 |
| wiGCC | 0.994 (0.991, 0.996) | 0.70 | 0.61 | 0.63 |
| pfGCC | 0.995 (0.993, 0.997) | 0.65 | 0.57 | 0.56 |
| ONH | ||||
| wiCD | 0.861 (0.804, 0.905) | 1.34 | 1.17 | 2.61 |
| cpCD | 0.871 (0.818, 0.912) | 1.41 | 1.22 | 2.61 |
| cpRNFL | 0.975 (0.964, 0.984) | 2.00 | 1.63 | 1.79 |
CI = confidence interval; CD = capillary density; cp = circumpapillary; CV = coefficient of variation; GCC = ganglion cell complex; ICC = intra-class correlation; ONH = optic nerve head; pf = parafoveal; RMSE = root mean squared error; RNFL = retinal nerve fiber layer; Sw = within-subject test-retest standard deviation; VD = vessel density; wi = whole image.
Figure 1.
Distribution of the within-subject test–retest SD for optic nerve head (left) and macula (right), and vessel density (upper) and thickness (lower). cpCD, circumpapillary capillary density; cpRNFL, circumpapillary retinal nerve fibre layer; pfVD, parafoveal vessel density; pfGCC, parafoveal ganglion cell complex.
Tables 3 and 4 show the effect of age, SSI, 24–2 VF MD, and IOP on the repeatability of OCTA and OCT measurements for macular and ONH parameters from univariable mixed models. The significant negative coefficients associated with age indicated that the variability of vessel density and thickness parameters increased significantly with older age (all p<0.05, except for pfGCC (p=0.05)). Additionally, the significant positive associations between SSI and vessel density were found in wiVD (r-squared (95% CI): 0.35 (0.23, 0.46), p<0.001), pfVD (0.29 (0.18, 0.41), p<0.001), and wiCD (0.05 (0.01, 0.12), p=0.002), except for cpCD (p=0.247).
Table 3.
Effect of age, SSI, 24-2 VF MD, and IOP on the repeatability of OCTA and OCT measurements for macular parameters
| Coefficient, 95% CI | R-squared, 95% CI | p value | |
|---|---|---|---|
|
| |||
| wiVD | |||
| Age | −0.18 (−0.26, −0.11) | 0.48 (0.37, 0.58) | <0.001 |
| SSI | 0.21 (0.15, 0.26) | 0.35 (0.23, 0.46) | <0.001 |
| 24-2 VF MD | 0.56 (0.00, 1.12) | 0.08 (0.02, 0.18) | 0.072 |
| IOP | 0.00 (−0.26, 0.25) | 0.00 (0.00, 0.04) | 0.975 |
| pfVD | |||
| Age | −0.17 (−0.25, −0.09) | 0.43 (0.32, 0.54) | <0.001 |
| SSI | 0.19 (0.13, 0.25) | 0.29 (0.18, 0.41) | <0.001 |
| 24-2 VF MD | 0.36 (−0.20, 0.92) | 0.04 (0.00, 0.12) | 0.228 |
| IOP | −0.05 (−0.30, 0.20) | 0.01 (0.00, 0.06) | 0.681 |
| wiGCC | |||
| Age | −0.28 (−0.54,−0.02) | 0.70 (0.63, 0.76) | 0.041 |
| SSI | 0.04 (0.00, 0.08) | 0.02 (0.00, 0.08) | 0.054 |
| 24-2 VF MD | 2.85 (1.92, 3.78) | 0.75 (0.69, 0.80) | <0.001 |
| IOP | 0.13 (−0.62, 0.87) | 0.05 (0.00, 0.14) | 0.743 |
| pfGCC | |||
| Age | −0.28 (−0.56, −0.01) | 0.72 (0.65, 0.78) | 0.050 |
| SSI | 0.04 (0.01, 0.08) | 0.02 (0.00, 0.09) | 0.022 |
| 24-2 VF MD | 2.65 (1.65, 3.65) | 0.73 (0.67, 0.79) | <0.001 |
| IOP | 0.12 (−0.65, 0.89) | 0.05 (0.00, 0.14) | 0.765 |
CD = capillary density; CI = confidence interval; cp = circumpapillary; GCC = ganglion cell complex; IOP = intraocular pressure; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; pf = parafoveal; SSI = signal strength index; VD = vessel density; VF = visual field; wi = whole image. Values are shown in β coefficient (95% confident interval). Negative coefficient demonstrates that reproducibility decreased with increasing the values of the putative factor. Statistically significant p value is shown in bold.
Table 4.
Effect of age, SSI, 24-2 VF MD, and IOP on the variability of OCTA and OCT measurements for ONH parameters
| Coefficient, 95% CI | R-squared, 95% CI | p value | |
|---|---|---|---|
|
| |||
| wiCD | |||
| Age | −0.14 (−0.22, −0.06) | 0.29 (0.20, 0.39) | 0.001 |
| SSI | 0.58 (0.23, 0.94) | 0.05 (0.01, 0.12) | 0.002 |
| 24-2 VF MD | 0.65 (0.11, 1.19) | 0.17 (0.09, 0.26) | 0.022 |
| IOP | −0.08 (−0.27, 0.10) | 0.03 (0.00, 0.09) | 0.386 |
| cpCD | |||
| Age | −0.15 (−0.24, −0.06) | 0.29 (0.20, 0.39) | 0.002 |
| SSI | 0.23 (−0.16, 0.61) | 0.01 (0.00, 0.05) | 0.247 |
| 24-2 VF MD | 0.62 (0.02, 1.21) | 0.14 (0.06, 0.23) | 0.045 |
| IOP | −0.12 (−0.32, 0.08) | 0.05 (0.01, 0.12) | 0.237 |
| cpRNFL | |||
| Age | −0.43 (−0.75, −0.11) | 0.49 (0.40, 0.57) | 0.011 |
| SSI | 0.26 (−0.31, 0.83) | 0.00 (0.00, 0.03) | 0.374 |
| 24-2 VF MD | 1.08 (−0.89, 3.04) | 0.12 (0.05, 0.20) | 0.287 |
| IOP | 0.01 (−0.64, 0.65) | 0.00 (0.00, 0.02) | 0.984 |
CD = capillary density; CI = confidence interval; cp = circumpapillary; IOP = intraocular pressure; MD = mean deviation; OCT = optical coherence tomography; OCTA = optical coherence tomography angiography; ONH = optic nerve head; RNFL = retinal nerve fiber layer; SSI = signal strength index; VD = vessel density; VF = visual field; wi = whole image. Values are shown in β coefficient (95% confident interval). Negative coefficient demonstrates that reproducibility decreased with increasing the values of the putative factor. Statistically significant p value is shown in bold.
Discussion
The present study finds that both OCTA-measured macula and ONH vascular parameters have good long-term reproducibility using Avanti Angiovue imaging system, although it is generally worse than the OCT reproducibility. Our results indicate that measurements over an average of 1.2 years are reproducible, supporting the use of the OCTA as an additional tool in the long-term follow up of glaucoma patients.
In order to interpret a change in serial OCTA or OCT as true glaucomatous progression, it is essential to differentiate a true change from long-term variability. One of the purposes of this longitudinal study was to determine a cutoff value for variation, which may help in the early detection of progression. Our results suggest that glaucomatous progression may be identified when the change in vascular parameters is greater than the long-term test-retest variability of 1.9% in one year. While the ICCs of OCTA-measured vascular parameters were above the threshold for acceptability, they were lower than those of OCT-measured thickness parameters. One possible explanation for this finding is that OCTA may be more sensitive to image quality than OCT, as preprocessing and postprocessing signals of OCTA are different,[29] and postprocessing signal in OCTA may relatively more impacted by signal strength compared with OCT.[30] Venugopal et al evaluated short-term reproducibility in 42 eyes of 27 normal subjects and 45 eyes of 26 glaucoma patients, and reported significant positive associations between SSI and better reproducibility of vessel densities for vessel density parameters.[7] In our study of long-term reproducibility, we found similar associations between higher SSI and better reproducibility of vessel densities for most vessel density parameters, while for OCT only pfGCC is correlated with SSI. Even in the current study when only good quality scans were included, higher SSI was associated with better reproducibility. In addition, our study focused on averaged global values from OCTA over sectoral values. It has been reported parameters from regions vulnerable to glaucomatous progression such as the inferotemporal area have a higher diagnostic accuracy for glaucoma compared to the global average, though localized sectors may demonstrate greater variability.[31 32] Further study is needed to investigate the long-term reproducibility of sectoral parameters.
In this study, our goal was to evaluate clinically-stable POAG patients. As such, we chose to designate a follow-up period of 1.5 years in order to limit the possibility of age-related changes and glaucomatous progression that may have been a more significant factor in a longer study.[12] Although we reviewed the VF and photos taken over 5 years prior to study inclusion to exclude eyes with disease progression, we may not have been able to fully rule out progression given the chronic nature of the disease. However, if we take into account that the present study may include some eyes with potential progression, then the results may overstate the variability of the OCTA and OCT measurements and the long-term reproducibility of OCTA and OCTA in truly stable subjects may be better.
Of note, in comparing OCTA and OCT reproducibility, care should be taken when evaluating the RMSE and Sw from this study. The RMSE and Sw are not comparable between OCTA and OCT as they depend on the magnitude of the mean value. Therefore, the ICC and CV are more appropriate for comparing the reproducibility of the two modalities. For this reason, we included ICC as comparing OCTA and OCT reproducibility.
With good long-term reproducibility, OCTA is suitable for the monitoring and management of glaucoma. However, our study has a few limitations. First, a significant number of OCTA scans were excluded due to poor quality. Prior studies have reported a high number of poor-quality images using OCTA.[15 22 33] In the clinical setting, consistent high-quality images may not always be attainable – thus, greater variability may be seen than in this study. Second, our study did not evaluate high-density mode (400 B-scans × 400 A-scans per B-scan) OCTA, which may improve OCTA scan reproducibility and may be an interesting future direction of study. Third, we used 3 × 3-mm2 scans for macula and the software version 2018. Previously, You et al. showed that 6 × 6-mm2 scans showed significantly higher diagnostic accuracy than the 3 × 3-mm2 scans for glaucoma.[34] Further study is needed on reproducibility for different scan sizes with newer software versions. Fourth, this study includes many cases of glaucoma in relatively early stages. Caution should be exercised when extrapolating the results to moderate-to-severe stages of glaucoma. Finally, our results were obtained from OCTA and OCT scans performed with Optovue. The results may not be generalizable to other platforms and algorithms.
Although serial measurements of OCTA macula and ONH vascular parameters are not as high generally as the reproducibility of OCT parameters, our results demonstrate their good long-term reproducibility and support their use for longitudinal analysis. Additional studies are needed to determine whether they are complementary to OCT, VF, and fundus photographs in the monitoring and management of glaucoma.
Supplementary Material
Acknowledgments / Financial Disclosures:
c. Grant information
Funding/Support:
National Institutes of Health/National Eye Institute Grants R01EY029058, R01EY011008, U10EY14267, R01EY026574, R01EY019869 and R01EY027510; Core Grant P30EY022589; an Unrestricted Grant (no grant number) from Research to Prevent Blindness (New York, NY); UC Tobacco Related Disease Research Program (T31IP1511); German Research Foundation (DFG, research fellowship grant RE 4155/1-1); and grants for participants’ glaucoma medications from Alcon, Allergan, Pfizer, Merck, and Santen. The sponsor or funding organizations had no role in the design or conduct of this research.
Footnotes
STATEMENTS
a. Commercial Disclosures:
Takashi Nishida: none; Sasan Moghimi: none; Huiyuan Hou: none; James A. Proudfoot: none; Aimee C. Chang: none; Ryan Caezar C. David: none; Alireza Kamalipour: none; Nevin El-Nimri: none; Jasmin Rezapour: none; Christopher Bowd: none; Linda M. Zangwill: Financial support (research instruments) - Heidelberg Engineering, Carl Zeiss Meditec, Optovue, Topcon; Robert N. Weinreb: Financial support (research instruments) - Heidelberg Engineering, Carl Zeiss Meditec, Konan Medical, Optovue, Centervue, Bausch & Lomb; Consultant – Aerie Pharmaceuticals, Allergan, Equinox, Eyenovia; Patent – Toromedes, Carl Zeiss Meditec.
e. Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Weinreb RN, Leung CK, Crowston JG, et al. Primary open-angle glaucoma. Nat Rev Dis Primers 2016;2:16067 doi: 10.1038/nrdp.2016.67. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA 2014;311(18):1901–11 doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Use of progressive glaucomatous optic disk change as the reference standard for evaluation of diagnostic tests in glaucoma. Am J Ophthalmol 2005;139(6):1010–8 doi: 10.1016/j.ajo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes. Invest Ophthalmol Vis Sci 2016;57(9):OCT451–9 doi: 10.1167/iovs.15-18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoji T, Zangwill LM, Akagi T, et al. Progressive Macula Vessel Density Loss in Primary Open-Angle Glaucoma: A Longitudinal Study. Am J Ophthalmol 2017;182:107–17 doi: 10.1016/j.ajo.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manalastas PIC, Zangwill LM, Saunders LJ, et al. Reproducibility of Optical Coherence Tomography Angiography Macular and Optic Nerve Head Vascular Density in Glaucoma and Healthy Eyes. J Glaucoma 2017;26(10):851–59 doi: 10.1097/IJG.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venugopal JP, Rao HL, Weinreb RN, et al. Repeatability of vessel density measurements of optical coherence tomography angiography in normal and glaucoma eyes. Br J Ophthalmol 2018;102(3):352–57 doi: 10.1136/bjophthalmol-2017-310637. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Jiang C, Ko T, et al. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 2015;253(9):1557–64 doi: 10.1007/s00417-015-3095-y. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121(7):1322–32 doi: 10.1016/j.ophtha.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artes PH, O’Leary N, Nicolela MT, Chauhan BC, Crabb DP. Visual field progression in glaucoma: what is the specificity of the Guided Progression Analysis? Ophthalmology 2014;121(10):2023–7 doi: 10.1016/j.ophtha.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 11.De Moraes CG, Paula JS, Blumberg DM, et al. Detection of Progression With 10–2 Standard Automated Perimetry: Development and Validation of an Event-Based Algorithm. Am J Ophthalmol 2020;216:37–43 doi: 10.1016/j.ajo.2020.03.046. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros FA, Doshi R, Zangwill LM, Vasile C, Weinreb RN. Long-term variability of GDx VCC retinal nerve fiber layer thickness measurements. J Glaucoma 2007;16(3):277–81 doi: 10.1097/IJG.0b013e3180391a3c. [DOI] [PubMed] [Google Scholar]
- 13.Urata CN, Mariottoni EB, Jammal AA, et al. Comparison of Short- And Long-Term Variability in Standard Perimetry and Spectral Domain Optical Coherence Tomography in Glaucoma. Am J Ophthalmol 2020;210:19–25 doi: 10.1016/j.ajo.2019.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bowd C, Zangwill LM, Weinreb RN, Medeiros FA, Belghith A. Estimating Optical Coherence Tomography Structural Measurement Floors to Improve Detection of Progression in Advanced Glaucoma. Am J Ophthalmol 2017;175:37–44 doi: 10.1016/j.ajo.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollo G Intrasession and Between-Visit Variability of Sector Peripapillary Angioflow Vessel Density Values Measured with the Angiovue Optical Coherence Tomograph in Different Retinal Layers in Ocular Hypertension and Glaucoma. PLoS One 2016;11(8):e0161631 doi: 10.1371/journal.pone.0161631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JC, Grisafe DJ, Burkemper B, et al. Intrasession repeatability and intersession reproducibility of peripapillary OCTA vessel parameters in non-glaucomatous and glaucomatous eyes. Br J Ophthalmol 2020:bjophthalmol-20 doi: 10.1136/bjophthalmol-2020-317181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L, Jia Y, Takusagawa HL, et al. Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma. JAMA Ophthalmol 2015;133(9):1045–52 doi: 10.1001/jamaophthalmol.2015.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sample PA, Girkin CA, Zangwill LM, et al. The African Descent and Glaucoma Evaluation Study (ADAGES): design and baseline data. Arch Ophthalmol 2009;127(9):1136–45 doi: 10.1001/archophthalmol.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girkin CA, Sample PA, Liebmann JM, et al. African Descent and Glaucoma Evaluation Study (ADAGES): II. Ancestry differences in optic disc, retinal nerve fiber layer, and macular structure in healthy subjects. Arch Ophthalmol 2010;128(5):541–50 doi: 10.1001/archophthalmol.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowd C, Weinreb RN, Zangwill LM. Evaluating the optic disc and retinal nerve fiber layer in glaucoma. I: Clinical examination and photographic methods. Semin Ophthalmol 2000;15(4):194–205 doi: 10.3109/08820530009037871. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Jian G, Bao W, et al. Analysis of Foveal Microvascular Abnormalities in Diabetic Retinopathy Using Optical Coherence Tomography Angiography with Projection Artifact Removal. J Ophthalmol 2018;2018:3926745 doi: 10.1155/2018/3926745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamalipour A, Moghimi S, Hou H, et al. Optical Coherence Tomography Angiography Artifacts in Glaucoma. Ophthalmology 2021. doi: 10.1016/j.ophtha.2021.03.036. [DOI] [Google Scholar]
- 23.Kim KE, Yoo BW, Jeoung JW, Park KH. Long-Term Reproducibility of Macular Ganglion Cell Analysis in Clinically Stable Glaucoma Patients. Invest Ophthalmol Vis Sci 2015;56(8):4857–64 doi: 10.1167/iovs.14-16350. [DOI] [PubMed] [Google Scholar]
- 24.Mwanza JC, Chang RT, Budenz DL, et al. Reproducibility of peripapillary retinal nerve fiber layer thickness and optic nerve head parameters measured with cirrus HD-OCT in glaucomatous eyes. Invest Ophthalmol Vis Sci 2010;51(11):5724–30 doi: 10.1167/iovs.10-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vet HCWd, Terwee C, Mokkink W, Knol DL. Measurement in medicine : a practical guide, 2015.
- 26.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–74. [PubMed] [Google Scholar]
- 27.Laird NM, Donnelly C, Ware JH. Longitudinal studies with continuous responses. Stat Methods Med Res 1992;1(3):225–47 doi: 10.1177/096228029200100302. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Dastiridou A, Francis BA, et al. Baseline Fourier-Domain Optical Coherence Tomography Structural Risk Factors for Visual Field Progression in the Advanced Imaging for Glaucoma Study. Am J Ophthalmol 2016;172:94–103 doi: 10.1016/j.ajo.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CL, Ishikawa H, Wollstein G, et al. Histogram Matching Extends Acceptable Signal Strength Range on Optical Coherence Tomography Images. Invest Ophthalmol Vis Sci 2015;56(6):3810–9 doi: 10.1167/iovs.15-16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirasawa K, Smith CA, West ME, et al. Discrepancy in Loss of Macular Perfusion Density and Ganglion Cell Layer Thickness in Early Glaucoma. Am J Ophthalmol 2021;221:39–47 doi: 10.1016/j.ajo.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Rao HL, Pradhan ZS, Weinreb RN, et al. Regional Comparisons of Optical Coherence Tomography Angiography Vessel Density in Primary Open-Angle Glaucoma. Am J Ophthalmol 2016;171:75–83 doi: 10.1016/j.ajo.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with stratus OCT in glaucomatous eyes. Ophthalmology 2008;115(4):661–66 e4 doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Suh MH, Zangwill LM, Manalastas PI, et al. Optical Coherence Tomography Angiography Vessel Density in Glaucomatous Eyes with Focal Lamina Cribrosa Defects. Ophthalmology 2016;123(11):2309–17 doi: 10.1016/j.ophtha.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You QS, Tan O, Pi S, et al. Effect of algorithms and covariates in glaucoma diagnosis with optical coherence tomography angiography. Br J Ophthalmol 2021. doi: 10.1136/bjophthalmol-2020-318677. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.