Abstract
Background and Aims:
The impact of different types of food intolerance on gastrointestinal symptoms and quality of life (QOL) is poorly understood. We aimed to investigate associations of food intolerance and type of intolerance with irritable bowel syndrome (IBS), health-related QOL, and psychological symptoms.
Methods:
We conducted an observational study of US-based adults through an online survey. Demographics, culprit foods, symptoms, medical evaluation, Rome IV criteria for IBS, health-related QOL (SF-12), and anxiety and depression scores (HADS) were collected in participants with self-reported food intolerance (lactose, non-lactose food, lactose plus food intolerance), and controls with no intolerance. Univariable associations of group with study endpoints were analyzed with Kruskal-Wallis and Pearson’s chi-square or Fisher’s exact test. Multivariable comparisons were analyzed by logistic and linear regression.
Results:
A total of 197 with (59 lactose, 61 non-lactose food, 77 lactose plus food intolerance) and 273 without intolerance participated. Lactose, wheat, and eggs were the most common food triggers. Gas (54.2%), abdominal pain (40.2%), and diarrhea (37.3%) were frequently reported symptoms of FI. Reactions caused 57.8% to eliminate the food. Rates of IBS, abnormal anxiety scores, and abnormal depression scores were highest in lactose plus food intolerance; SF-12 scores were lowest in lactose plus food intolerance. Multivariable analyses revealed all intolerance subgroups were more likely to have IBS than controls.
Conclusions:
Food intolerance is associated with IBS, anxiety, depression, and decreased health-related QOL and frequently leads to food elimination. Adults with lactose and lactose plus food intolerance have higher rates of IBS, increased psychological symptoms, and poorer QOL.
Keywords: survey, lactose intolerance, depression, anxiety, quality of life
Graphical Abstract
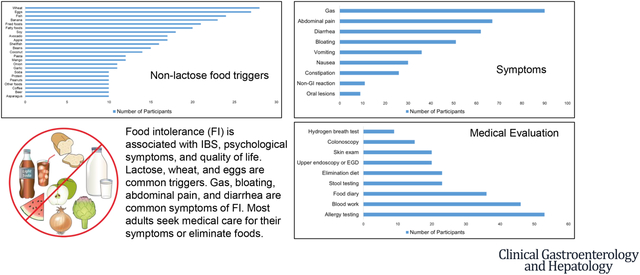
Introduction:
Food intolerance (FI), a non-immunological adverse response to food, is a frequently reported symptom.1 Prevalence rates range from 4–20% of the general population by most study estimates.2–4 Recent data from United States (US) internet users suggests prevalence rates as high as 24.8%.5 The issue of FI is not novel, but exploration of the relationship of this self-perceived condition, including type of FI, with overall health and quality of life (QOL) is ongoing.
The precise pathogenesis of FI or non-allergic food hypersensitivity remains obscure. Enzyme and transport defects, food antigen effect on intestinal barrier, local allergy, chemical reactions, and luminal distention from fermentable carbohydrates all represent potential mechanisms.1, 6–8 Lactose intolerance (LI) is a symptomatic FI secondary to enzymatic deficiency of lactase resulting in maldigestion. Symptoms attributed to FI are variable; flatulence, abdominal pain, bloating, and diarrhea comprise common gastrointestinal complaints.1 Adverse reactions typically resolve when the culprit food is eliminated and return with reintroduction.1 Many consider double-blinded, placebo-controlled oral challenge to be the gold standard for identification of FI; however, this approach poses numerous challenges and clinically useful diagnostics are lacking.1
Earlier literature suggests that particular trigger foods generate gastrointestinal symptoms in irritable bowel syndrome (IBS) in as many as 84% of patients.9, 10 Patients with IBS often report that symptoms worsen or are triggered after a meal and as a result, may exclude foods or limit their diet.11, 12 Food-related IBS symptoms appear to be associated with reduced QOL and more severe disease.9 Dietary interventions improve symptoms for some patients with IBS.13–16 Higher numbers of food triggers are associated with increased severity of IBS symptoms; however, it is unclear if the type of FI reported further influences associations with IBS, QOL, or psychological health.9 Due to the impact of food-related gastrointestinal symptoms on patients’ perceived wellness, we aimed to investigate associations of FI and type of FI (lactose alone, non-lactose food, lactose plus other foods) with IBS, health-related QOL, and psychological symptoms. We hypothesized that FI is associated with IBS, poorer QOL, and psychological distress and that associations may differ by FI type.
Methods:
We conducted an observational online survey study using Amazon’s Mechanical Turk (MTurk), a crowdsourcing website for the completion of requester-directed tasks, from August 2019 to October 2020. Individuals were invited to participate in a survey-based study on “food-related health” in exchange for a small sum. MTurk approximates the demographics of internet users in the United States and is comparable to convenience-based or traditional sampling methods.17–21 Inclusion criteria were adults (18–80 years), a US-based internet protocol address, and an MTurk approval rating >95% (proportion of completed tasks approved as properly done). Participants with high approval ratings answer quality control questions with greater accuracy, which may reduce response bias.22 We excluded those with celiac disease, IgE-mediated food allergies or anaphylaxis, eosinophilic esophagitis, inflammatory bowel disease, or pancreatic insufficiency.
Data were collected on demographics, medical comorbidities (Charlson comorbidity index [CCI]) self-reported FI, culprit foods, symptoms, and medical evaluation (Supplemental Questionnaire). We assessed presence of IBS (Rome IV diagnostic questionnaire), health-related QOL (Short-Form Health Survey 12 [SF-12]), and anxiety and depression (Hospital Anxiety and Depression Scale [HADS]).23–25 Prior to inquiring about FI, we conducted a qualitative analysis using an open-ended question asking how participants defined FI. We then provided participants with our definition of FI and asked them to use it for the survey: any bowel or gut (gastrointestinal tract) related symptom you experience after eating a particular food. This study was approved by the Indiana University Institutional Review Board; consent was implied if individuals decided to participate.
Data were summarized using frequencies (percentages), medians ± interquartile range (IQR), or mean ± standard deviation (SD). Assuming the prevalence of FI to be ≥20%, a target sample size of n=375 was calculated a priori to detect an odds ratio (OR) of 2.3 for the association between a binary outcome and FI (where the prevalence of the binary outcome among participants without FI is 20–65%) and to detect differences of 0.4 SD in mean values for continuous outcomes with at least 80% power at the 5% significance level. Study endpoints were compared between participants with and without FI using the Wilcoxon rank sum test, two-sample t-test, and Pearson’s chi-square test. Univariable comparisons across subgroups (controls, lactose intolerance [LI] only, non-lactose FI only, LI plus other FI) were performed using the Kruskal-Wallis test or ANOVA F-test and Pearson’s chi-square or Fisher’s exact test. When overall comparisons were significant, we performed pairwise comparisons using the Bonferroni approach to adjust for multiple comparisons. Multivariable comparisons adjusted for age, sex, sociodemographics, CCI, and body mass index were performed by logistic and linear regression. Because GI symptoms, mood, and QOL may be related to one another and therefore mediate associations of FI with IBS, psychological symptoms, or QOL, we conducted a secondary multivariable analysis incorporating study endpoints of interest as covariates. To further examine the impact of sex, exploratory multivariable analyses were conducted among men and women separately. Analyses were conducted with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results:
Study Population:
We collected 599 completed surveys (Figure 1). After excluding ineligible participants (n=67) those with invalid responses (n=62), there were 470 valid survey responses from n=273 participants with no FI and n=197 participants with any FI (n=59 with LI only, n=61 with a non-lactose FI, and n=77 with LI plus FI).
Figure 1.
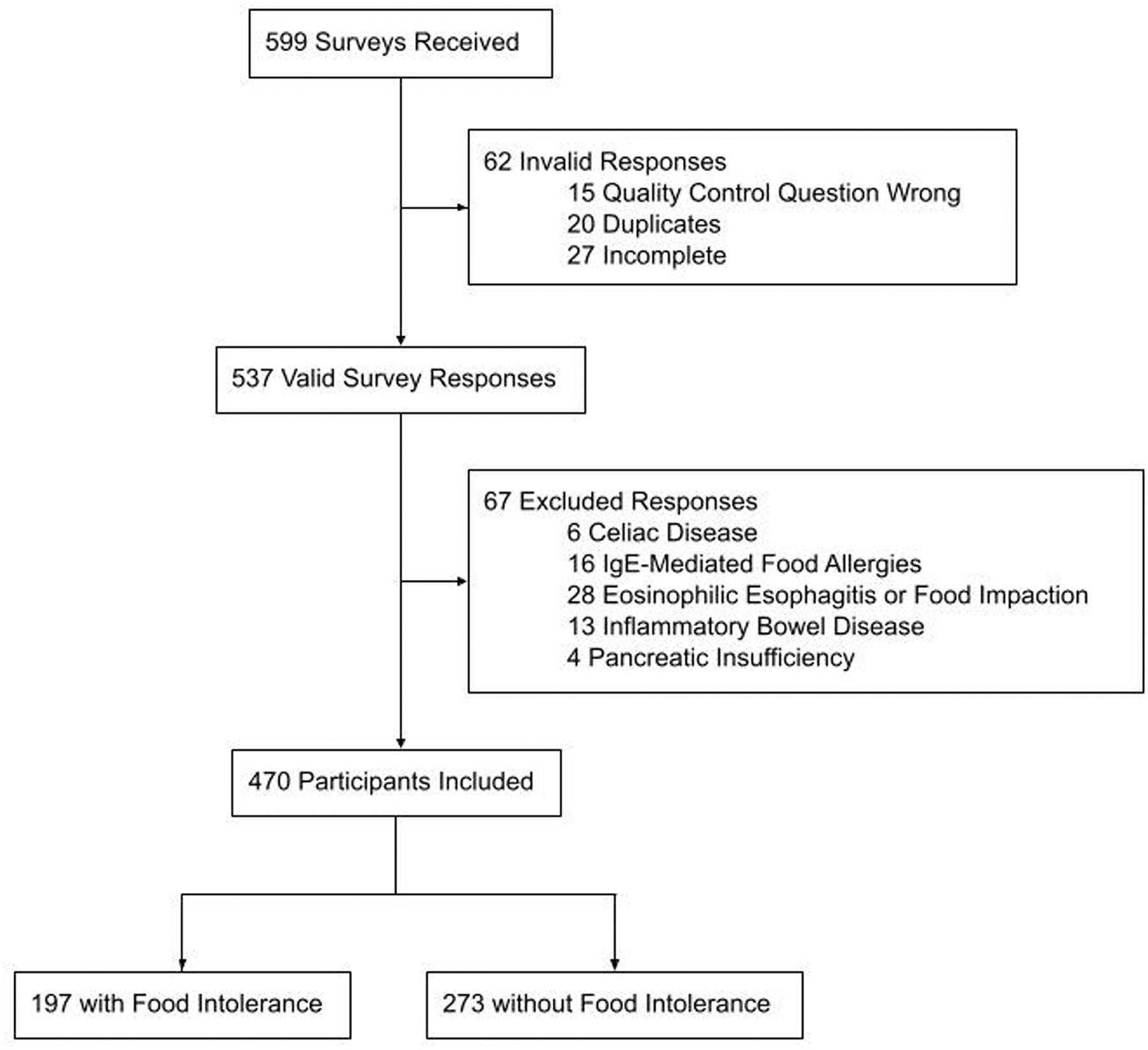
Flow Diagram
No food intolerance vs. any food intolerance:
Clinical Characteristics:
Age, gender, education, household income, and region of residence were not different between participants with any FI and controls (Table 1). Participants with any FI were less frequently Caucasian (p<0.001), more frequently Hispanic (p=0.03), more frequently born outside of the US (p=0.004), and more frequently had CCI scores ≥ 1.
Table 1.
Sample characteristics overall participants 0
| Total (n = 470) | Food or lactose Intolerance | ||
|---|---|---|---|
| No (n = 273) | Yes (n = 197) | ||
| Age, mean (SD) | 37.1 (11.4) | 37.9 (11.8) | 36.0 (10.8) |
| Female | 195 (41.5%) | 113 (41.4%) | 82 (41.6%) |
| Caucasian*, n=3 prefer not to say | 365 (78.2%) | 235 (86.4%) | 130 (66.7%) |
| Race** | |||
| African | 2 (0.4%) | 1 (0.4%) | 1 (0.5%) |
| African American | 37 (7.9%) | 10 (3.7%) | 27 (13.7%) |
| Asian | 27 (5.7%) | 10 (3.7%) | 17 (8.6%) |
| Caucasian | 365 (77.7%) | 235 (86.1%) | 130 (66.0%) |
| Native American | 22 (4.7%) | 6 (2.2%) | 16 (8.1%) |
| Native Hawaiian | 1 (0.2%) | 1 (0.4%) | 0 (0%) |
| Pacific Islander | 1 (0.2%) | 1 (0.4%) | 0 (0%) |
| Multiple | 12 (2.6%) | 8 (2.9%) | 4 (2.0%) |
| Prefer not to say | 3 (0.6%) | 1 (0.4%) | 2 (1.0%) |
| Hispanic* | 48 (10.2%) | 21 (7.7%) | 27 (13.7%) |
| Education | |||
| High school | 51 (10.9%) | 33 (12.1%) | 18 (9.1%) |
| College | 370 (78.7%) | 218 (79.9%) | 152 (77.2%) |
| Above college | 49 (10.4%) | 22 (8.1%) | 27 (13.7%) |
| Household income | |||
| <$50,000 | 234 (49.8%) | 141 (51.6%) | 93 (47.2%) |
| $50,000–$99,999 | 181 (38.5%) | 96 (35.2%) | 85 (43.1%) |
| $100,000+ | 55 (11.7%) | 36 (13.2%) | 19 (9.6%) |
| Body mass index (kg/m2)*, mean (sd), n=24 missing | 27.0 (6.7) | 27.6 (7.0) | 26.1 (6.1) |
| Region | |||
| Midwest | 96 (20.4%) | 58 (21.2%) | 38 (19.3%) |
| Northeast | 93 (19.8%) | 57 (20.9%) | 36 (18.3%) |
| South | 152 (32.3%) | 92 (33.7%) | 60 (30.5%) |
| West | 129 (27.4%) | 66 (24.2%) | 63 (32.0%) |
| Born in USA* | 425 (90.4%) | 256 (93.8%) | 169 (85.8%) |
| Charlson Comorbidity Index Score* | |||
| 0 | 336 (71.5%) | 213 (78%) | 123 (62.4%) |
| 1 | 81 (17.2%) | 37 (13.6%) | 44 (22.3%) |
| 2+ | 53 (11.3%) | 30 (8.4%) | 30 (15.2%) |
Statistically significant difference <0.05. P-values were calculated based on the Wilcoxon rank sum test or two-sample t-test for continuous variables.
P-value for individual race categories not reported due to sparse data. For categorical variables, we used the Pearson’s chi-square test. Mean and standard deviation (SD) are reported for normally distributed continuous variables. Region is derived based on the state of current residence.
Foods:
A total of 136 participants reported LI and 138 reported non-lactose FI. Wheat (20.3%), eggs (19.6%), fish (17.4%), banana (16.7%), fried foods (15.2%), and fatty foods (14.5%) were commonly selected non-lactose culprit foods (Figure 2A). Intolerance to fruits, vegetables, grains, and lactose were most common in decreasing order.
Figure 2.
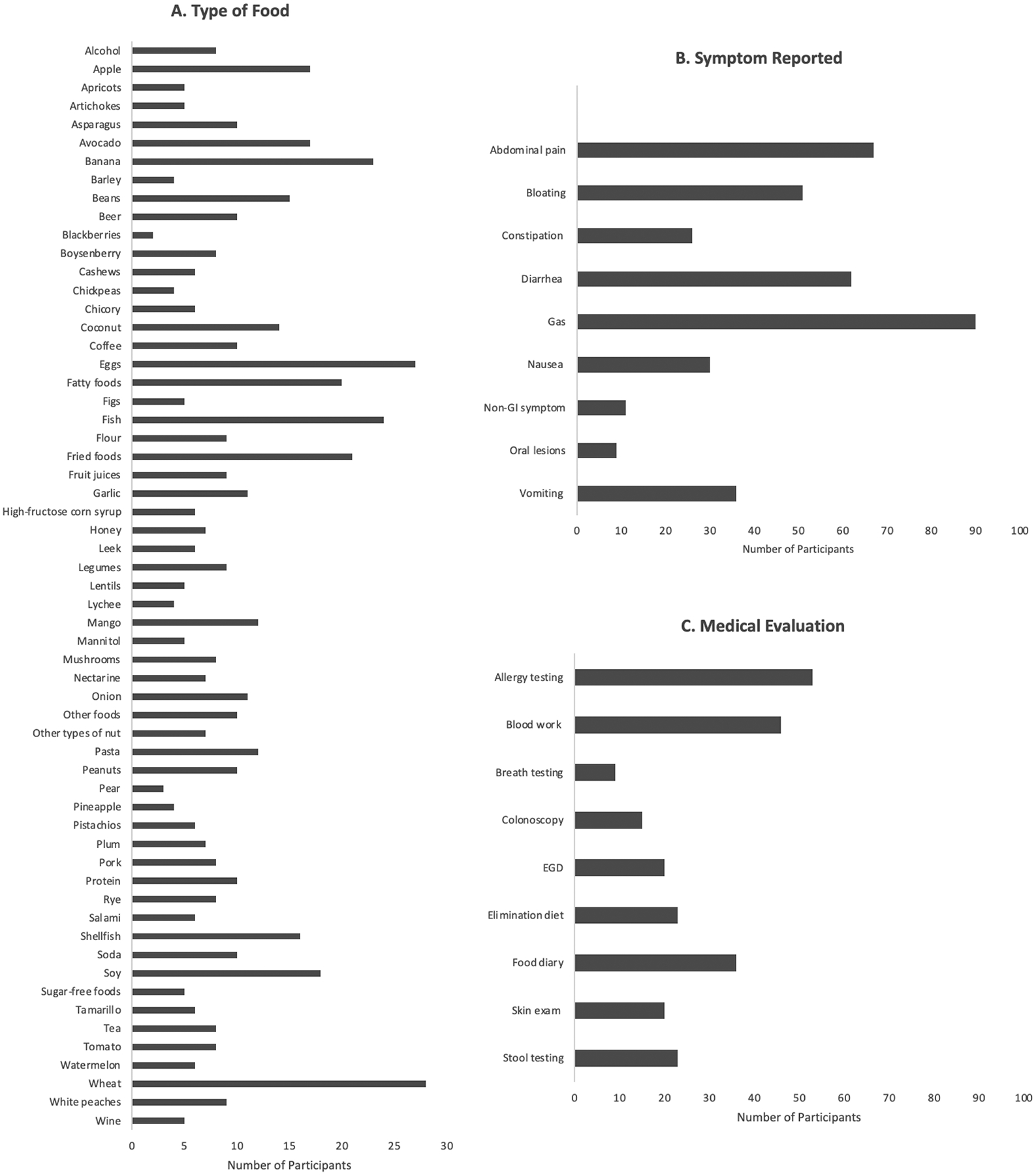
A. Culprit foods, B. Symptoms, C. Medical Evaluation for Participants with Food Intolerance
Symptoms:
Among participants with FI (Figure 2B), the most common gastrointestinal symptoms were gas (54.2%), abdominal pain (40.4%), and diarrhea (37.3%). Symptoms typically occurred within minutes (56.0%) or hours (39.8%) after food ingestion and lasted hours (57.2%) in most, but for minutes in many (32.5%). Mean age of onset of intolerance was 20 (± 10.9) years. Symptoms occurred “most of the time the food is eaten (>50%)” in 45.8% and “every time the food is eaten (100%)” in 24.1%. The amount of food needed to cause symptoms was noted to be a “small amount (few bites)” in 43.4% or a “moderate amount (one portion)” in 42.8%. Mean symptom severity was 61.3 (±19.3) on a visual analog scale (0 [least severe] to 100 [most severe]). Most with FI (62.9%) underwent some form of medical evaluation (Figure 2C). Consequences of FI were as follows: elimination of the food (57.8%), lost sleep (42.8%), missed work (36.1%), doctor’s appointment (17.5%), and hospitalization (15.1%). Among 96 who eliminated foods, most (93%) reported symptom frequencies of ≥50%.
IBS, Psychological Symptoms and QOL:
Higher rates of IBS (p<0.001), higher HADS anxiety and depression scores (p<0.001), and lower SF-12 physical and mental component scores (p<0.001) were observed in those with any FI (Table 2) in univariable and multivariable analysis. Any FI was associated with a greater likelihood of IBS [OR 6.08 (95% CI, 2.83–12.78)], higher HADS anxiety [1.93 (95% CI, 0.98–2.88)] and depression estimates [1.004 (95% CI, 0.164–1.844)], and lower physical [−3.94 (95% CI, −5.51-[−2.37])] and mental [−3.06 (95% CI, −5.26-[−0.85])] component scores (worse physical and mental status) compared to controls.
Table 2.
Study outcomes
| Total (n = 470) | Food or Lactose Intolerance | P-value | ||
|---|---|---|---|---|
| No (n = 273) | Yes (n = 197) | |||
| Irritable bowel syndrome (IBS) | 56 (11.9%) | 11 (4.0%) | 45 (22.8%) | <0.001 |
| HADS anxiety score, median (IQR) | 7.0 (2.0 – 11.0) | 4.0 (1.0 – 9.0) | 9.0 (5.0 – 12.0) | <0.001 |
| HADS anxiety | <0.001 | |||
| Normal (0–7) | 256 (54.5%) | 179 (65.6%) | 77 (39.1%) | |
| Borderline abnormal (8–10) | 85 (18.1%) | 41 (15.0%) | 44 (22.3%) | |
| Abnormal (11–21) | 129 (27.4%) | 53 (19.4%) | 76 (38.6%) | |
| HADS depression score, median (IQR) | 5.0 (1.0 – 9.0) | 3.0 (1.0 – 8.0) | 7.0 (3.0 – 10.0) | <0.001 |
| HADS depression | <0.001 | |||
| Normal (0–7) | 306 (65.1%) | 197 (72.2%) | 109 (55.3%) | |
| Borderline abnormal (8–10) | 98 (20.9%) | 45 (16.5%) | 53 (26.9%) | |
| Abnormal (11–21) | 66 (14.0%) | 31 (11.4%) | 35 (17.8%) | |
| SF-12, mean (sd) | ||||
| Physical component score | 48.6 (8.6) | 50.8 (7.9) | 45.6 (8.5) | <0.001 |
| Mental component score | 46.5 (11.4) | 48.3 (11.8) | 43.9 (10.4) | <0.001 |
HADS, Hospital Anxiety and Depression Scale; SF-12, Short Form 12. Median and interquartile range (IQR) are reported for continuous variables with a skewed distribution and mean and standard deviation (SD) are reported for normally distributed continuous variables. P-values are calculated based on the Wilcoxon rank sum test or two-sample t-test for continuous variables. For categorical variables, we used the Pearson’s chi-square test.
Comparisons by food intolerance type:
Clinical Characteristics:
There were significant associations of race, education, birthplace, and comorbidities across FI subgroups (Table 3). While presence of FI was associated with Hispanic ethnicity, there was no difference across subgroups. IBS, anxiety and depression, and health-related QOL scores were significantly associated with FI subgroups (Table 4).
Table 3.
Sample characteristics by food intolerance (FI) subgroup (no FI, lactose intolerance only (LI), non-lactose FI, LI plus FI)
| Food Intolerance | ||||
|---|---|---|---|---|
| No FI (n = 273) | LI (n = 59) | Non-lactose FI (n = 61) | LI plus FI (n = 77) | |
| Age, mean (SD) | 37.9 (11.8) | 36.1 (10.9) | 37.0 (11.4) | 35.1 (10.2) |
| Female | 113 (41.4%) | 26 (44.1%) | 25 (41.0%) | 31 (40.3%) |
| Caucasian*, n=3 missing | 235 (86.4%) | 36 (62.1%) | 42 (70.0%) | 52 (67.5%) |
| Hispanic | 21 (7.7%) | 7 (11.9%) | 8 (13.1%) | 12 (15.6%) |
| Education* | ||||
| High school | 33 (12.1%) | 11 (18.6%) | 5 (8.2%) | 2 (2.6%) |
| College | 218 (79.9%) | 40 (67.8%) | 46 (75.4%) | 66 (85.7%) |
| Above college | 22 (8.1%) | 8 (13.6%) | 10 (16.4%) | 9 (11.7%) |
| Household income | ||||
| <$50,000 | 141 (51.6%) | 32 (54.2%) | 28 (45.9%) | 33 (42.9%) |
| $50,000–$99,999 | 96 (35.2%) | 20 (33.9%) | 24 (39.3%) | 41 (53.2%) |
| $100,000+ | 36 (13.2%) | 7 (11.9%) | 9 (14.8%) | 3 (3.9%) |
| Body mass index (kg/m2), mean (sd), n=24 missing | 27.6 (7.0) | 26.6 (5.5) | 26.2 (5.2) | 25.5 (7.3) |
| Region | ||||
| Midwest | 58 (21.2%) | 13 (22.0%) | 12 (19.7%) | 13 (16.9%) |
| Northeast | 57 (20.9%) | 13 (22.0%) | 9 (14.8%) | 14 (18.2%) |
| South | 92 (33.7%) | 18 (30.5%) | 23 (37.7%) | 19 (24.7%) |
| West | 66 (24.2%) | 15 (25.4%) | 17 (27.9%) | 31 (40.3%) |
| Born in USA* | 256 (93.8%) | 52 (88.1%) | 49 (80.3%) | 68 (88.3%) |
| Charlson Comorbidity Index Score* | ||||
| 0 | 213 (78.0%) | 44 (74.6%) | 41 (67.2%) | 38 (49.4%) |
| 1 | 37 (13.6%) | 10 (16.9%) | 11 (18.0%) | 23 (29.9%) |
| 2+ | 23 (8.4%) | 5 (8.5%) | 9 (14.8%) | 16 (20.8%) |
Statistically significant difference <0.05. Overall p-values reported for comparisons across all groups; IQR, interquartile range. P-values were calculated based on the Kruskal-Wallis test or ANOVA F-test and Pearson’s chi-square or Fischer’s exact test. Mean and standard deviation (SD) are reported for normally distributed continuous variables. Region is derived based on the state of current residence.
Table 4.
Study outcomes by food intolerance (FI) subgroup (no FI, lactose intolerance only (LI), non-lactose FI, LI plus FI)
| Food Intolerance | *P-Value | ||||
|---|---|---|---|---|---|
| No FI (n = 273) | LI (n = 59) | Non-lactose FI (n = 61) | LI plus FI (n = 77) | ||
| Irritable bowel syndrome (IBS) | 11 (4.0%) | 9 (15.3%)† | 7 (11.5%)α | 29 (37.7%)†β | <0.001 |
| HADS anxiety score, median (IQR) | 4.0 (1.0–9.0) | 8.0 (4.0–12.0)γ | 6.0 (2.0–11.0) | 10.0 (8.0–13.0) β | <0.001 |
| HADS anxiety | <0.001 | ||||
| Normal (0–7) | 179 (65.6%) | 27 (45.8%) | 35 (57.4%) | 15 (19.5%) | |
| Borderline abnormal (8–10) | 41 (15.0%) | 9 (15.3%) | 10 (16.4%) | 25 (32.5%) | |
| Abnormal (11–21) | 53 (19.4%) | 23 (39.0%) | 16 (26.2%) | 37 (48.1%) | |
| HADS depression score, median (IQR) | 3.0 (1.0–8.0) | 7.0 (2.0–10.0) | 4.0 (1.0–8.0) | 8.0 (6.0–10.0)β | 0.023 |
| HADS depression | <0.001 | ||||
| Normal (0–7) | 197 (72.2%) | 33 (55.9%) | 42 (68.9%) | 34 (44.2%) | |
| Borderline abnormal (8–10) | 45 (16.5%) | 15 (25.4%) | 11 (18.0%) | 27 (35.1%) | |
| Abnormal (11–21) | 31 (11.4%) | 11 (18.6%) | 8 (13.1%) | 16 (20.8%) | |
| SF-12, mean (sd) | |||||
| Physical component score | 50.8 (7.9) | 48.2 (8.3) | 48.1 (7.8) | 41.6 (7.8)β | <0.001 |
| Mental component score | 48.3 (11.8) | 43.1 (12.1)γ | 47.5 (9.9) | 41.7 (8.6)β | <0.001 |
Overall p-values reported for comparisons across all groups; IQR, interquartile range; HADS, Hospital Anxiety and Depression Scale; SF-12, Short Form 12. Pairwise comparisons between groups performed using multivariable logistic or linear regression:
p<0.01 for pairwise group comparison vs. controls;
p=0.02 for food intolerance (FI) only vs. controls;
p<0.01 for FI plus lactose intolerance (LI) vs. FI only;
p<0.05 for LI vs FI only.
Symptoms:
Reported symptoms did not differ by FI subgroup. However, food elimination was most common among those with non-lactose FI (Supplemental Figure).
IBS:
In multivariable analysis, the likelihood of IBS was higher with all types of FI and highest in those with LI plus FI [OR 12.01 (95% CI, 5.01–28.76)] compared to controls. Comparisons between FI subgroups demonstrated that LI plus FI [OR 3.62 (95% CI, 1.34–9.76)] was associated with a higher likelihood of IBS than non-lactose FI; no significant differences were observed between LI and non-lactose FI groups.
Psychological Symptoms:
HADS anxiety and depression scores were highest in those with LI plus FI (Table 4). Multivariable analysis demonstrated higher anxiety and depression scores (estimates, 95% CI) in LI [anxiety 2.28 (95% CI, 0.94–3.63), depression 1.56 (95% CI, 0.37–2.75)]. Higher scores were seen in LI plus FI [anxiety 3.25 (95% CI, 1.89–4.61), depression 1.87 (95% CI, 0.66–3.08)] than controls. Adjusted comparisons between FI subgroup demonstrated higher anxiety and depression scores in LI [anxiety 2.01 (95% CI, 0.3–3.72), depression 1.98 (95% CI, 0.46–3.50)]. Higher scores were also seen in LI plus FI [anxiety 2.98 (95% CI, 1.30–4.66), depression 2.29 (95% CI, 0.80–3.78)] than in non-lactose FI.
QOL:
SF-12 physical and mental component scores were lowest (poorest) in the LI plus FI subgroup (Table 4). Differences between FI subgroups remained statistically significant in multivariable analyses; lower scores were observed in all FI subgroups compared to controls with the exception of physical and mental component scores (estimates, 95% CI) for the non-lactose FI group which did not differ significantly from controls [LI physical −2.82 (95% CI, −5.04-[−0.60]), LI mental −4.58 (95% CI, −7.71-[−1.44]), FI physical −2.11 (95% CI, −4.35–0.14), FI mental 0.59 (95% CI, −2.57–3.76), LI plus FI physical −7.04 (95% CI, −9.29-[−4.79]), LI plus FI mental −5.15 (95% CI-[−8.32[1.98])]. Compared to those with a non-lactose FI, those with LI plus FI had lower physical [−4.93 (95% CI, −7.71-[−2.15])] and mental health scores [−5.74 (95% CI, −9.66[−1.83])] and those with LI had lower mental component scores [−5.17 (95% CI, −9.16-[−1.18])].
Secondary and exploratory analysis:
Multivariable analyses incorporating study endpoints as independent predictors demonstrated decreased, but significant associations (both p<0.001) of any FI with IBS (OR=4.58, 95% CI, 2.11–9.928 [p<0.001]) and poorer physical component scores (estimate −2.63, 95% CI, −4.18-[−1.08]). Associations of FI with psychological symptoms and mental component scores were no longer significant. Exploratory analyses stratified by sex showed significant associations between FI and IBS, anxiety, depression, and QOL in both men and women (Supplemental Tables)
Qualitative Data:
Participants defined FI as: “symptoms or discomfort that are attributable to a particular food,” “a negative physical reaction from your body when it consumes certain foods or ingredients,” “inability to digest food properly,” “allergy to food,” “not an allergy per say, but something that upsets your stomach or gives you other unpleasant (though non-life threatening) symptoms,” “aversion,” “any gastrointestinal irritation caused by the ingestion of food substances,” and “food poisoning.” Most, but not all participants made a distinction between FI and food allergy.
Discussion:
In this survey-based study, we observed significant associations of any FI with IBS, psychological symptoms, and health-related QOL. FI frequently caused participants to eliminate foods (57.8%), miss work (36.1%), and seek medical care (doctor’s appointment [17.5%] and hospitalization [15.1%]). Subgroup analysis revealed that the highest rates of IBS, highest anxiety and depression scores, and lowest physical and mental component scores were in participants with LI plus FI. Compared to controls, both LI and LI plus FI were significantly associated with IBS, greater anxiety and depression, and poorer QOL. Comparisons between FI subgroups demonstrated higher rates of IBS, increased psychological symptoms, and poorer QOL in adults with LI plus FI than in adults with a non-lactose FI. While increased psychological symptoms and poorer mental component scores were also observed in those with LI compared to those with a non-lactose FI, no significant differences in likelihood of IBS were observed.
Non-immunologically mediated reactions to food such as enzymatic defects leading to lactose maldigestion have been hypothesized to play a role in IBS. However, the clinical significance of true lactose maldigestion in IBS remains unclear, and prior studies suggest that perception of LI may be of greater importance.26, 27 Our findings suggest that while self-reported LI may be associated with IBS, the impact of perceived LI in IBS does not differ from the impact of a non-lactose FI. A higher likelihood of IBS in those with LI plus FI may further suggest that a higher number of self-reported intolerances correlates with increased IBS symptoms as reported by others.9 Similar to a study by Bohn et al, lactose-containing items, fried or fatty foods, and wheat were commonly identified by participants as problem foods.9 Puente-Fernandez et al in Mexico noted dairy products, vegetables, and fruits as the groups most associated with FI, similar to our findings.28 Our study further revealed eggs and fish to be frequent triggers, which may indicate a role for histamine-release that warrants further study. Overall, results reinforce LI as the most commonly and consistently reported FI; however, pathophysiologic mechanisms including histamine-release (eggs, fish, fruits), altered gastrointestinal motor responses (high calorie and fatty foods), and fermentation of poorly absorbed carbohydrates may play a part in mediating perceived FI among adults.
Previous studies noted relationships between FI and psychological symptoms, and QOL. Bohn et al. concluded that FI was associated with significant symptom burden and reduced QOL, but not anxiety.9 Other data are conflicting. Our findings differ from Bohn and Monsbakken et al, but concur with others who demonstrated associations of FI with anxiety and depression11, 12, 29–31 and corroborate the link between FI and poorer health-related QOL.32, 33 Our subgroup analysis suggests that LI plus FI has the most profound impact on QOL and psychological symptoms, while LI is associated with poorer mental health. Relationships of LI with decreased QOL34, 35 and with anxiety and depression36–39 have been reported. Taken together, findings indicate that perceived LI may disproportionately impact on QOL and mental health. The mechanisms linking specific intolerances to mental health are unclear, although some have hypothesized a role for L-tryptophan metabolism affecting serotonin biosynthesis;40, 41 effects of anxiety on perception of FI symptoms;37 or changes in diet quality that may modulate pathways of inflammation, oxidative stress, cortisol production, and neurogenesis.42 Diet quality in FI is of interest as >50% of those with FI reported food elimination in our study and others.12 We attempted to explore relationships of FI with GI symptoms, mental health, and QOL through secondary analyses to find that psychological symptoms may partly, but not fully, explain the association between FI and IBS (GI symptoms) and that psychological and GI symptoms may partly, but not fully, explain the association between FI and physical QOL. Conversely, relationships between FI and mental well-being appeared to be heavily, if not entirely, influenced by GI symptoms (IBS) and physical QOL. Further exploration of food avoidance, nutritional adequacy, health care utilization, causal relationships, and overall wellness are avenues for continued research.
Compared to the existing literature, this study surveyed a general population, featured more men than women, and explicitly defined FI to attempt to distinguish it from allergy. In our study, sex was not associated with FI, which was different and exploratory analyses stratified by sex demonstrated similar associations between FI and IBS, psychological distress, and QOL in men and women. Many previous studies were predominantly female or surveyed women only. Bohn et al found that women reported more food items to which they were intolerant.9 Other papers report higher prevalence of FI in women.2, 11, 28 Despite these unique aspects of our patient population and survey design, our findings are consistent with other population-based studies that have demonstrated that gastrointestinal manifestations of FI are common (68%).4, 33 Similar to others, we identified gas, abdominal pain, and diarrhea as predominant food-associated symptoms.10, 11 Responses to the open-ended question revealed that many participants perceived any negative physical reaction to food ingestion as FI. Future iterations of FI surveys may consider inquiring about non-GI and non-allergic physical reactions and including specific digestive symptoms (early satiety, fullness) and nature/location of pain (epigastric pain, lower abdominal cramping).
Study strengths include the large sample size, diverse geographic coverage, use of quality control methods, validated survey tools, and the comprehensive survey nature.43 There were >20% non-Caucasian and >10% Hispanic participants. A qualitative aspect was included to collect participant-provided definitions of FI. The study had limitations. As with any survey-based study, it is subject to selection bias, which could manifest as over-reporting. Findings may not be generalizable to the general US population and represent individuals with more severe GI symptoms given the relatively high prevalence (11.9%) of IBS. But most US adults have internet access and MTurk has been shown to be at least as representative as traditional subject pools. The length of the survey may have deterred some and we were unable to verify self-reported diagnoses, prior surgeries, medications, or reasons for hospitalization, which were surprisingly high. Other disorders that may be linked to food such as functional dyspepsia were not assessed. Although we attempted to exclude participants with food allergies, our provided definition of food intolerance did not explicitly differentiate intolerance from allergy which could have impacted results. Lastly, the study was not specifically powered for subgroup analysis and some groups were small (<10); however, our final sample size (n=470) far exceeded our a priori goal (n=375) and increased our confidence in the findings.
Overall, results of this study demonstrate that FI is common issue with substantial consequences. Among US adults, IBS, anxiety and depression, and health-related QOL are significantly associated with self-reported FI; relationships are especially evident in adults with LI plus another FI. QOL and psychological symptoms are worse with LI, but LI alone is not associated with higher rates of IBS than non-lactose FI. More studies would be useful to define the directionality of the relationship between QOL or psychological distress and FI, develop validated tools for evaluating FI, and examine the nature and consistency of food-related symptoms and their impact on avoidant/restrictive eating behaviors or diet quality in those endorsing FI.
Supplementary Material
What You Need to Know.
Background:
Food intolerances are common. It is unknown if relationships with irritable bowel syndrome (IBS), health-related quality of life (QOL), and psychological symptoms vary by the type of intolerance reported.
Findings:
All types of intolerance are associated with IBS, psychological symptoms, poorer QOL, and food elimination.
Higher rates of IBS are seen in those with food plus lactose intolerance.
Implications for Patient Care:
Patients reporting food intolerances appear to be at higher risk of IBS and concurrent psychological symptoms.
Frequent elimination of foods could encourage avoidant/restrictive food behaviors and impact diet adequacy.
Grant Support:
AS is supported by NIH K23DK122015. No other study support.
Abbreviations:
- CI
Confidence Interval
- FI
Food Intolerance
- HADS
Hospital Anxiety and Depression Scale
- IBS
Irritable Bowel Syndrome
- LI
Lactose Intolerance
- MTurk
Mechanical Turk
- QOL
Quality of Life
- SF
Short-Form
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest or disclosures to declare.
Data Transparency Statement:
Data, methods, materials will be available to other researchers by contacting corresponding author.
References
- 1.Lomer MC. Review article: the aetiology, diagnosis, mechanisms and clinical evidence for food intolerance. Aliment Pharmacol Ther. 2015;41(3):262–75. [DOI] [PubMed] [Google Scholar]
- 2.Young E, Stoneham MD, Petruckevitch A, et al. A population study of food intolerance. Lancet. 1994;343(8906):1127–30. [DOI] [PubMed] [Google Scholar]
- 3.Woods RK, Abramson M, Bailey M, et al. International prevalences of reported food allergies and intolerances. Comparisons arising from the European Community Respiratory Health Survey (ECRHS) 1991–1994. Eur J Clin Nutr. 2001;55(4):298–304. [DOI] [PubMed] [Google Scholar]
- 4.Gislason D, Bjoernsson E, Gislason T. [Allergy and intolerance to food in an Icelandic urban population 20–44 years of age.]. Laeknabladid. 2000;86(12):851–7. 5. [PubMed] [Google Scholar]
- 5.Jansson-Knodell CL, White M, Lockett C, et al. High prevalence of food intolerances among US internet users. Public Health Nutr. 2021;24(3):531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chey WD. Food: The Main Course to Wellness and Illness in Patients With Irritable Bowel Syndrome. Am J Gastroenterol. 2016;111(3):366–71. [DOI] [PubMed] [Google Scholar]
- 7.Fritscher-Ravens A, Pflaum T, Mosinger M, et al. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology. 2019;157(1):109–18 e5. [DOI] [PubMed] [Google Scholar]
- 8.Aguilera-Lizarraga J, Florens MV, Viola MF, et al. Local immune response to food antigens drives meal-induced abdominal pain. Nature. 2021;590(7844):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohn L, Storsrud S, Tornblom H, et al. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108(5):634–41. [DOI] [PubMed] [Google Scholar]
- 10.Locke GR 3rd, Zinsmeister AR, Talley NJ, et al. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 2000;95(1):157–65. [DOI] [PubMed] [Google Scholar]
- 11.Simren M, Mansson A, Langkilde AM, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63(2):108–15. [DOI] [PubMed] [Google Scholar]
- 12.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60(5):667–72. [DOI] [PubMed] [Google Scholar]
- 13.Altobelli E, Del Negro V, Angeletti PM, et al. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients. 2017;9(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146(1):67–75 e5. [DOI] [PubMed] [Google Scholar]
- 15.Eswaran SL, Chey WD, Han-Markey T, et al. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am J Gastroenterol. 2016;111(12):1824–32. [DOI] [PubMed] [Google Scholar]
- 16.Staudacher HM, Lomer MCE, Farquharson FM, et al. A Diet Low in FODMAPs Reduces Symptoms in Patients With Irritable Bowel Syndrome and A Probiotic Restores Bifidobacterium Species: A Randomized Controlled Trial. Gastroenterology. 2017;153(4):936–47. [DOI] [PubMed] [Google Scholar]
- 17.Paolacci GCJ, Ipeirotis PG. Running Experiments on Amazon Mechanical Turk. Judgment and Decision Making. 2010;5:411–9. [Google Scholar]
- 18.Berinsky AJHG, Lenz GS. Evaluating Online Labor Markets for Experimental Research: Amazon.com’s Mechanical Turk. Political Analysis. 2012;20(3):351–68. [Google Scholar]
- 19.Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A New Source of Inexpensive, Yet High-Quality, Data? Perspect Psychol Sci. 2011;6(1):3–5. [DOI] [PubMed] [Google Scholar]
- 20.Mortensen K, Hughes TL. Comparing Amazon’s Mechanical Turk Platform to Conventional Data Collection Methods in the Health and Medical Research Literature. J Gen Intern Med. 2018;33(4):533–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullinix KJ LT, Druckman JN, Freese J. The Generalizability of Survey Experiments. The Journal of Experimental Political Science. 2015;2(2):109–38. [Google Scholar]
- 22.Peer E, Vosgerau J, Acquisti A. Reputation as a sufficient condition for data quality on Amazon Mechanical Turk. Behav Res Methods. 2014;46(4):1023–31. [DOI] [PubMed] [Google Scholar]
- 23.Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150(6):1257–61. [DOI] [PubMed] [Google Scholar]
- 24.Ware J Jr., Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. [DOI] [PubMed] [Google Scholar]
- 25.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Deng Y, Chu H, et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11(3):262–8 e1. [DOI] [PubMed] [Google Scholar]
- 27.Varju P, Gede N, Szakacs Z, et al. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: A meta-analysis. Neurogastroenterol Motil. 2019;31(5):e13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puente-Fernandez C, Maya-Hernandez RL, Flores-Merino MV, et al. Self-reported prevalence and risk factors associated with food hypersensitivity in Mexican young adults. Ann Allergy Asthma Immunol. 2016;116(6):523–7 e3. [DOI] [PubMed] [Google Scholar]
- 29.Elieson LM, Domotor Z, Koteles F. Health anxiety mediates the connection between somatosensory amplification and self-reported food sensitivity. Ideggyogy Sz. 2017;70(9–10):307–14. [DOI] [PubMed] [Google Scholar]
- 30.Lillestol K, Berstad A, Lind R, et al. Anxiety and depression in patients with self-reported food hypersensitivity. Gen Hosp Psychiatry. 2010;32(1):42–8. [DOI] [PubMed] [Google Scholar]
- 31.Knibb RC, Armstrong A, Booth DA, et al. Psychological characteristics of people with perceived food intolerance in a community sample. J Psychosom Res. 1999;47(6):545–54. [DOI] [PubMed] [Google Scholar]
- 32.Jakobsen MD, Braaten T, Obstfelder A, et al. Self-Reported Food Hypersensitivity: Prevalence, Characteristics, and Comorbidities in the Norwegian Women and Cancer Study. PLoS One. 2016;11(12):e0168653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lind R, Arslan G, Eriksen HR, et al. Subjective health complaints and modern health worries in patients with subjective food hypersensitivity. Dig Dis Sci. 2005;50(7):1245–51. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X, Chu H, Cong Y, et al. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neurogastroenterol Motil. 2015;27(8):1138–46. [DOI] [PubMed] [Google Scholar]
- 35.Casellas F, Aparici A, Perez MJ, et al. Perception of lactose intolerance impairs health-related quality of life. Eur J Clin Nutr. 2016;70(9):1068–72. [DOI] [PubMed] [Google Scholar]
- 36.Addolorato G, Marsigli L, Capristo E, et al. Anxiety and depression: a common feature of health care seeking patients with irritable bowel syndrome and food allergy. Hepatogastroenterology. 1998;45(23):1559–64. [PubMed] [Google Scholar]
- 37.Yang J, Fox M, Cong Y, et al. Lactose intolerance in irritable bowel syndrome patients with diarrhoea: the roles of anxiety, activation of the innate mucosal immune system and visceral sensitivity. Aliment Pharmacol Ther. 2014;39(3):302–11. [DOI] [PubMed] [Google Scholar]
- 38.Ledochowski M, Sperner-Unterweger B, Fuchs D. Lactose malabsorption is associated with early signs of mental depression in females: a preliminary report. Dig Dis Sci. 1998;43(11):2513–7. [DOI] [PubMed] [Google Scholar]
- 39.Schiffner R, Kostev K, Gothe H. Do patients with lactose intolerance exhibit more frequent comorbidities than patients without lactose intolerance? An analysis of routine data from German medical practices. Ann Gastroenterol. 2016;29(2):174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledochowski M, Widner B, Murr C, et al. Fructose malabsorption is associated with decreased plasma tryptophan. Scand J Gastroenterol. 2001;36(4):367–71. [DOI] [PubMed] [Google Scholar]
- 41.Varea V, de Carpi JM, Puig C, et al. Malabsorption of carbohydrates and depression in children and adolescents. J Pediatr Gastroenterol Nutr. 2005;40(5):561–5. Epub 2005/04/30. [DOI] [PubMed] [Google Scholar]
- 42.Marx W, Lane M, Hockey M, et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2021;26(1):134–50. [DOI] [PubMed] [Google Scholar]
- 43.Palsson OS, Whitehead WE, van Tilburg MAL, et al. Development and Validation of the Rome IV Diagnostic Questionnaire for Adults. Gastroenterology. 2016;150(6):1481–91. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data, methods, materials will be available to other researchers by contacting corresponding author.


