Abstract
Purpose: We aimed to evaluate the expression level of programmed death ligand-1 (PD-L1) and its effects on prognosis in acute myeloid leukemia. Methods: The flow cytometry was used to detect PD-L1 expression on leukemic cells of 86 de novo acute myeloid leukemia patients with longitudinal follow-up. Results: Median follow-up was 13 (0–73) months. The mean of expression level was 3.22 ± 0.47 at diagnosis and ranged from 0 to 28%. PD-L1 expression tended to be lower in patients with acute promyelocytic leukemia (2.47 ± 1.08, p = 0.09) but there was no significant difference between neither diagnostic nor cytogenetic subgroups. There was no difference in PD-L1 levels between the patients who achieved complete remission (3.4 ± 0.61) and those who did not (2.91 ± 0.72, p = 0.94). The patients with low PD-L1 at diagnosis (median 25 mo [95% CI; 0–56.7]) had a longer overall survival compared with high PD-L1 (median 13 mo [95% CI; 5.52–25.17], p = 0.079). PD-L1 expression was lower at relapse (2.04 ± 0.79) compared to initial diagnosis (4.52 ± 0.93, p = 0.049). The patients who had overall survival longer than 1 year showed lower PD-L1 expression at relapse (0.66 ± 0.93) compared with who had not (5.06 ± 4.28, p = 0.052). A negative correlation between CD33 and PD-L1 (r = − 0.303, p = 0.005) was detected. Conclusion: Despite its low expression levels, PD-L1 appears to be a clinically important prognostic factor. The negative correlation determined between PD-L1 and CD33 supports the combination approach of PD-L1 inhibitors and CD33 targeted immunotherapies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12288-021-01473-2.
Keywords: PD-L1, B7-H1, CD274, Immune checkpoint, Flow cytometry
Introduction
Acute myeloid leukemia (AML) is a myeloid progenitor cell malignancy where immature hematopoietic cells proliferate and accumulate in bone marrow, peripheral blood and other tissues. Curative treatments can be applied only to a small number of patients who are young and fit. Although several novel therapies (which are expected to impact patients outcomes) have been approved, overall survival (OS) is (24% per 5 year) still remains poor [1].
In recent years, immune checkpoint therapy has shown remarkable clinical results for many solid tumors [2, 3]. Immune checkpoint inhibitors have also taken a place in the treatment of hematological cancers such as Hodgkin’s lymphoma, and provided complete changes in the treatment algorithms [4, 5]. Programmed death 1 (PD-1) inhibitors also have been evaluated in several clinical trials either as monotherapy or in combination therapies in AML patients with the overall response rates varying between 0 and 77%. [6] One of the reasons for the difference in response rates may be the varying expression levels of programmed death ligand-1 (PD-L1). It was shown that PD-L1 expression correlated with response to anti PD-1 therapy in many cancer types [7]. However, there is limited data in the literature about PD-L1 expression and its prognostic significance in patients with AML. Recently Dong et al. showed weak expression of PD-L1 protein on AML patients blast cells and its promoting effect on conversion and expansion of regulatory T (Treg) cells. While the effect of high PD-1+ Treg cells on overall survival was demonstrated, the effect of PD-L1 could not be demonstrated. [8]
The aim of current study is to evaluate the expression level and prognostic significance of PD-L1 in patients with AML.
Materials and Methods
The patients who were diagnosed de novo AML between 2014 and 2017 in our institution were studied retrospectively. The medical records and the results of flow cytometric analyses of the patients were evaluated. The diagnoses were reassesed based on the 2016 revision of World Health Organization classification. The patients who had active secondary primary malignancy and had received hypomethylating agent (HMA) or chemotherapy in last one year were excluded. The remaining 86 patients with de novo AML were enrolled in the study. Flow cytometry (FCM) analyses were performed on freshly collected bone marrow aspirate or blast rich peripheral blood samples by counting at least 10.000 events on the BD FACSCalibur (Becton Dickinson) instrument. Red cell lysis was performed with BD FACS Lysing Solution (Becton Dickinson). A forward scatter and side scatter (FSC/SSC) gate was applied to exclude debris. The presence of cellular aggregates was checked by determining the SSC area and the FSC width. After determining the blast gate in the cluster of differentiation (CD) 45/SSC plot, a monoclonal antibody panel containing CD34/117/33/13/64/14/41a and anti glycophorin A/HLA-DR/MPO was applied. CD274 (PD-L1) staining was performed with PE Mouse anti-human CD274 (Becton Dickinson). The quadrant cut-off was determined by isotype control to evaluate percent of blasts expressing PD-L1. The data were analysed with Cell Quest Pro software (BD Biosciences). Sequential measurements of 16 patients at the time of first relapse were available.
Time to relapse (TTR) was defined only for patients achieving complete remission (CR); measured from the date of CR until the date of relapse; patients not known to have relapsed at last follow-up were censored on the date they were last examined. Event free survival (EFS) was defined for all patients as the time from diagnosis to the date of primary refractory disease, or relapse from CR, or death from any cause; patients not known to have any of these events were censored on the date they were last examined. OS was defined as the time from diagnosis to death from any cause. Dates of death were determined through the central health record system. The study protocol was approved by the Faculty Research Ethical Committee.
All statistical analyses were performed using IBM SPSS for Windows version 20.0 (SPSS, Chicago, IL, USA). Kolmogorov–Smirnov and Shapiro–Wilk’s tests were used to assess the assumption of normality. Continuous variables were presented with either mean ± standard error of mean or median (minimum, maximum), whichever was appropriate. Categorical variables were summarized as counts (percentages). Comparisons of continuous variables between groups were carried out using Kruskal–Wallis and Mann–Whitney U tests. Dunn's test was used for multiple comparisons. The changes in variables between time periods were analyzed by related samples Wilcoxon signed rank test and Friedman’s two-way analysis of variance. Association between two continuous variables was examined by Spearman's correlation analysis. The Chi-square test or Fisher’s exact test, where appropriate, was used to compare proportions in different groups. Kaplan–Meier method with Log-Rank test was used for survival analysis. Potential prognostic factors for survival were evaluated in a multivariate analysis with the use of the Cox proportional hazards regression. All statistical analyses were carried out with 5% significance and a two-sided p-value < 0.05 was considered as statistically significant.
Results
Eighty-six patients with diagnosis of AML, were included in this study. Forty-six (53.4%) of the patients were male and the median age was 53 years (range, 18–83 years). The median follow-up period of the patients was 13 (0–73) months. Risk stratification could not be made due to insufficient cytogenetic data of the patients. Characteristics of the patients are shown in Table 1.
Table 1.
Characteristics of the patients
| AML (n:86) n (%) | |
|---|---|
| Diagnosis | |
| AML with recurrent genetic abnormalities | |
| t(8,21) | 7 (8.1) |
| inv(16) | 2 (2.3) |
| t(15,17) | 13 (15.1) |
| t(9,11) | 3 (3.5) |
| t(9,22) | 1 (1.2) |
| NPM1mut | 2 (2.3) |
| CEBPAmut | 1 (1.2) |
| AML with myelodysplasia-related changes | 9 (10.5) |
| Therapy-related myeloid neoplasms | 2 (2.3) |
| AML not otherwise specified | |
| AML with minimal differentiation | 6 (7.0) |
| AML without maturation | 8 (9.3) |
| AML with maturation | 5 (5.8) |
| Acute myelomonocytic leukemia | 12 (14) |
| Acute monoblastic/monocytic leukemia | 14 (16.3) |
| Pure erythroid leukemia | 1 (1.2) |
| Comorbidity ( ±) | 43/43 |
| Secondary primary malignancy | 3 (3.5) |
| CAD | 5 (5.8) |
| DM | 5 (5.8) |
| HT | 11 (12.8) |
| DM + HT | 2 (2.3) |
| DM + HT + CAD | 4 (4.7) |
| CKD | 2 (2.3) |
| Others * | 11 (12.8) |
| Presence of lymphadenopathy | 11 (12.8) |
| Presence of splenomegaly | 15 (17.4) |
| Extramedullary involvement ** | 4 (4.7) |
| t(9,22) (n***:82) | 1 (1.2) |
| t(15,17) (n:82) | 13 (15.8) |
| FLT3-ITD (n:33) | 5 (15.1) |
| t(8,21) (n:38) | 7 (18.4) |
| inv(16) (n:32) | 2 (6.2) |
| NPM1 mutations (n:15) | 3 (20) |
| CEBPA biallelic mutations (n:15) | 1 (6.7) |
| t(9,11) (n:35) | 3 (8.5) |
CAD coronary artery disease; HT hypertension; DM diabetes mellitus; CKD chronic kidney disease
*Chronic obstructive pulmonary disease, rheumatoid arthritis, turner syndrome, chron’s disease, hypothyroidism, psoriasis,evans syndrome, cirrhosis
**1 leukemia cutis,1 pericardial, 1 gingival, 1 lymph node involvement
***the number of patients tested
Two patients died before starting treatment. There were 3 patients who had primary refractory disease after two courses of intensive induction regimen. One of them responded the third induction therapy and achieved CR. In total 60 patients achieved CR and 30 had relapse. The details of treatments and responses can be seen in the supplementary data 1. The median TTR, EFS and OS was 19 months [95% Confidence Interval (CI);15.17–22.82], 13 months [95% CI; 10.49–15.50] and 17 months [95% CI; 8.82–25.17] respectively.
PD-L1 expression was analyzed on leukemic blast cells and the percentage of positive cells was used for the expression level quantification (Fig. 1). The mean of expression level was 3.22 ± 0.47 at diagnosis and ranged from 0 to 28% (< 5% in 66 (76.7%), 5–10% in 13 (15.1%), 10–30% in 7 (8.1%) of the patients). PD-L1 expression tended to be lower in patients with acute promyelocytic leukemia (APL) (2.47 ± 1.08, p = 0.09) but there was no significant difference between neither diagnostic nor cytogenetic subgroups (Table 2) Again there was no difference in PD-L1 levels between the patients who achieved CR (3.4 ± 0.61) and those who did not (2.91 ± 0.72, p = 0.94).
Fig. 1.
a AML blasts showing high PD-L1 expression, b AML blasts showing low PD-L1 expression
Table 2.
PD-L1 expressions at diagnosis in subgroups
| The Percentages of PD-L1 positive blasts (mean ± S.ED) | p | |
|---|---|---|
| t(8,21) | ||
| Positive (n:7) | 4.44 ± 5.22 1.97 | 0.282 |
| Negative (n:31) | 2.18 ± 2.82 0.51 | |
| inv(16) | ||
| Positive (n:2) | 0.92 ± 0.67 0.47 | 0.629 |
| Negative (n:30) | 2.89 ± 3.62 0.66 | |
| t(15,17) | ||
| Positive (n:13) | 2.47 ± 3.88 1.08 | 0.09 |
| Negative (n:69) | 3.38 ± 4.56 0.55 | |
| t(9,11) | ||
| Positive (n:3) | 2.3 ± 2.65 1.53 | 0.934 |
| Negative (n:32) | 3.51 ± 5.57 0.98 | |
| NPM1mut | ||
| Positive (n:3) | 5.43 ± 4.91 2.84 | 0.448 |
| Negative (n:12) | 2.96 ± 3.7 1.07 | |
| FLT3-ITD | ||
| Positive (n:5) | 3.35 ± 3.92 1.75 | 0.981 |
| Negative (n:28) | 3.83 ± 6 1.13 | |
| AML with myelodysplasia-related changes (n:9) | 2.96 ± 2.99 1.0 | 0.73 |
| AML without myelodysplasia-related changes (n:77) | 3.25 ± 4.5 0.51 | |
| AML with minimal differentiation (n:6) | 5.15 ± 3.77 1.54 | 0.408 |
| AML without maturation (n:8) | 2.71 ± 3.52 1.25 | |
| AML with maturation (n:5) | 2.02 ± 0.82 0.37 | |
| Acute myelomonocytic leukemia (n:12) | 1.94 ± 2.06 0.59 | |
| Acute monoblastic/monocytic leukemia (n:14) | 3.73 ± 7.28 1.95 | |
| Lymphadenopathy | ||
| Exist (n:11) | 6.34 ± 7.98 2.41 | 0.092 |
| Not exist (n:75) | 2.77 ± 3.39 0.39 | |
| Splenomegaly | ||
| Exist (n:15) | 3.84 ± 7.44 1.92 | 0.357 |
| Not exist (n:71) | 3.09 ± 3.44 0.41 | |
| Extramedullary involvement | ||
| Exist (n:4) | 4.08 ± 2.59 1.30 | 0.136 |
| Not exist (n:82) | 3.15 ± 4.42 0.49 | |
| CRP | ||
| > ULT (n:59) | 3.54 ± 4.84 0.63 | 0.564 |
| < ULT (n:27) | 2.53 ± 3 0.58 |
ULT upper limit of normal
For further characterization of PD-L1 expression in AML clinical course, PD-L1 expression was analysed in 16 patients who had the data of first diagnosis (4.52 ± 0.93), and relapse (2.04 ± 0.79). A significant decrease was detected at relapse stage (p = 0.049). There was no difference for PD-L1 expression at relapse between the patients who had relapsed before 12 months (2.39 ± 1.20) and after (1.44 ± 0.74, p = 0.95). On the other hand the patients who had OS longer than 1 year showed lower PD-L1 expression (0.66 ± 0.28) at relapse compared to who had shorter survival (5.06 ± 1.92, p = 0.052).
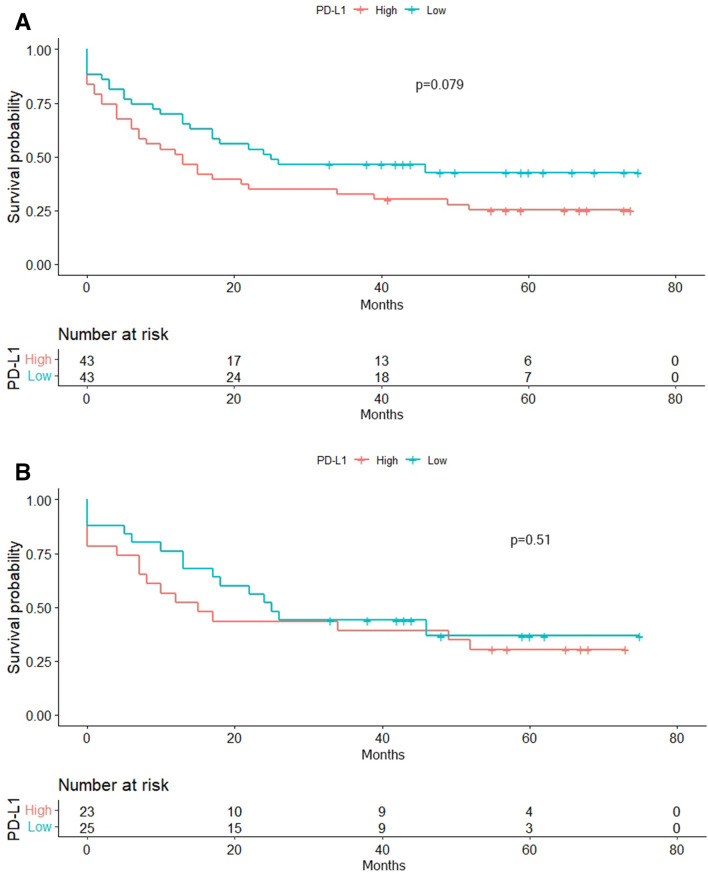
The median expression level of PD-L1 (1.48) at the time of diagnosis was used as a cut-off point for survival analyses. The Kaplan–Meier analyses for the TTR and EFS in groups with high (TTR; median 19 months [95% CI;12.95–25.04], EFS; 7 mo [95% CI; 1.63–12.36]) and low surface expression of PD-L1 (TTR; median 21 mo [95% CI;15.11–26.88], EFS; 14 mo [95% CI; 8.99–19]) revealed no statistically significant difference (p = 0.98, p = 0.45 respectively). However 53.5% of patients with low PD-L1 at diagnosis had an EFS above one year, this rate was 34.9 in those with high PD-L1 (p = 0.082). There was a tendency in patients with low PD-L1 expression levels (median 25 mo [95% CI; 0–56.7]) to have a longer OS compared with high PD-L1 expressors (median 13 mo [95% CI; 5.52–25.17], p = 0.079) (Fig. 2). Additionally in a model that examined the effect of clinical characteristics on OS, low white blood cell count and low PD-L1 were found to be associated with improved survival. (Table 3).
Fig. 2.
Overall survival by the PD-L1 expression level A. In all cohort, B. In 7 + 3 subcohort
Table 3.
Multivariate analysis of factors affecting overall survival
| Relative risk of death (95% CI) | p | PHA | |
|---|---|---|---|
| WBC < 20,000 | 1.00 (reference) | 0.43 | |
| WBC 20,000–50,000 | 2.17 (1.13–4.15) | 0.019 | |
| WBC > 50,000 | 3.71 (1.63–8.45) | 0.002 | |
| LDH < ULT | 1.00 (reference) | 0.50 | |
| LDH ULT-500 | 2.11 (0.85–5.21) | 0.104 | |
| LDH > 500 | 1.07 (0.34–3.33) | 0.904 | |
| CRP < ULT | 1.00 (reference) | 0.49 | |
| CRP > ULT | 1.63 (0.86–3.07) | 0.108 | |
| PD-L1 < 1.48 | 1.00 (reference) | 0.92 | |
| PD-L1 > 1.48 | 2.09 (1.12–3.89) | 0.019 |
WBC White blood cell; LDH lactate dehydrogenase; ULT upper limit of normal; CRP C reactive protein; PHA Proportinal hazard assumption
There was no statistically significant difference in TTR, EFS and OS in the groups with high (TTR; median 11 months [95% CI;9.61–12.38], EFS; 12 mo [95% CI; 4.75–19.24], OS; 15 mo [95% CI; 4.04–25.95]) and low surface expression of PD-L1 (TTR; median 10 mo [95% CI;7.25–12.75], EFS; 13 mo [95% CI; 7.24–18.75], OS; 25 mo [95% CI; 18.47–31.52]) in patients who received 7 + 3 as induction regimen (p = 0.43, p = 0.97, p = 0.51 respectively). (Fig. 2.) There was no correlation between age, percentage of blasts, white blood cell and platelet count, hemoglobin, C reactive protein, creatinine, lactate dehydrogenase levels and the expression of PD-L1. However a negative correlation between CD33 and PD-L1 (r = − 0.303, p = 0.005) was detected.
Discussion
Although marked expression of PD-L1 has been reported in several hematological malignancies, the results on AML are still conflicting.[9, 10]. If we examine the studies performed by flow cytometric method closely, it is seen that cut-off points for positivity are varying between studies. In a study conducted by Berthon et al. [11] expression of PD-L1 was detected as > 30% of blast cells in 18% of patients. In another study PD-L1 was considered positive if expression is present on more than 10% of total AML cells and positivity was detected in 9 of 30 patients [12]. Tamura et al. [13] analyzed the PD-L1 expression on leukemic cells of 36 de novo AML patients and PD-L1 positive leukemic cells were determined as < 5% in 32 patients. In consistent with Tamura et al., low expression levels were detected in the present study. All of these findings suggest that PD-L1 expression is limited in AML.
In the literature, there are studies detected higher PD-L1 expression in French-American-British (FAB) M5 type, as well as studies with no difference between FAB groups [11, 14–16]. The common point of studies detecting differences is that they include all kinds of acute leukemias [14, 15]. While no increased expression was observed in AML M5 in the present study, a tendency for decreased expression in APL was detected. Since APL shows high CD33 expression, the negative correlation determined between PD-L1 and CD33 also supports this result. Additionally in a study tested the cytotoxicity of AMG 330 (a CD33/CD3 bispecific T-cell engagerantibody construct) on primary AML cells, it was shown that AMG330 exposure induced a significant upregulation of PD-L1 on primary AML cells. Also, it was demonstrated that the blockage of the PD-1/PD-L1 interaction resulted in a significant increase in AMG 330 mediated lysis. Authors attributed this result to the production of proinflammatory cytokines as a result of potent T cell activation and hypothesized that tumor cells employ immunosuppressive strategies to escape cytokine-mediated immune response [17]. Through these findings we speculate that combination of PD-1 and PD-L1 inhibitors with CD33 targeted immunotherapies will be an effective treatment approach like HMA combinations in AML. After PD-L1 upregulation by epigenetic therapy was shown, the combinations of HMAs with immune checkpoint inhibitors have been evaluated in clinical trials and encouraging early results have been revealed [18–20].
Studies on unpaired samples with small cohorts have demonstrated higher PD-L1 levels at relapse [14, 21]. Also in another study 5 out of 9 patients showed higher expression at relapse in sequential analysis [11]. Contrary to these results, it was detected that PD-L1 expression levels decreased during relapse in the current study. Thus, further investigations on paired samples with larger cohorts are necessary to clarify this relationship.
Recently the negative impact of high PD-L1 expression on patients survival was shown in a study conducted by Chen et al. [22] Likewise there are reports showing that high PD-L1 is associated with worse survival in AML, specifically for patients with concomitant FLT3-ITD and NPM1 mutation and AML M5 [14, 23]. However these results were not confirmed in a study that included 90 AML patients [16]. The present study demonstrated a statistically insignificant but clinically substantially significant negative effect of high PD-L1 on overall survival in both at diagnosis and at relapse. The mechanism underlying the negative prognostic effect of high PD-L1 has not been completely explained yet. In our study and in the literature, the relationship between PD-L1 expression and treatment response could not be demonstrated [16, 21]. In addition, as discussed above, its relationship with relapsed disease has not been clearly demonstrated. However PD-L1 coats blast cells as a molecular protector and is a strong weapon to counteract host immune attacks. Although we could not make comments due to the lack of basic cytogenetic and molecular data in our study, the association of PD-L1 with TP53 mutation and advance cytogenetic risk has been shown in the literature [24, 25]. That may lead to an unfavorable clinical course of the disease. Eventually despite its low expression levels, PD-L1 appears to be a clinically important prognostic factor.
The limitations of current study can be listed as insufficient genetic data, lack of specific fluorescence indices and minimal residual disease evaluations. Additionally there was not a control group.
Conclusion
In summary, low expression level of PD-L1 on blast cells was demonstrated in the present study. Also, a possible positive prognostic effect of low PD-L1 expression on overall survival was observed. A negative correlation was determined between PD-L1 and CD33 which supports the combination approach of PD-L1 inhibitors and CD33 targeted immunotherapies. As a result, as we learn more about the immune profile of AML, the information obtained could guide treatments and potentially allow personalized selection of immune checkpoint pathways to be targeted.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
No funds, grants, or other support was received.
Data availability
Available.
Declarations
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee of University Kocaeli approved this study. Ethics Committee Approval: GOKAEK-2020/12.30.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019 doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O'Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. KEYNOTE-024 Investigators. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016 doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018 doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 4.Ramchandren R, Domingo-Domènech E, Rueda A, Trněný M, Feldman TA, Lee HJ, Provencio M, Sillaber C, Cohen JB, Savage KJ, Willenbacher W, Ligon AH, Ouyang J, Redd R, Rodig SJ, Shipp MA, Sacchi M, Sumbul A, Armand P, Ansell SM. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin Lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J Clin Oncol. 2019 doi: 10.1200/JCO.19.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase II CheckMate 205 trial. J Clin Oncol. 2018 doi: 10.1200/JCO.2017.76.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao D, Wang M, Liao Y, Li J, Niu T. A review of efficacy and safety of checkpoint ınhibitor for the treatment of acute myeloid leukemia. Front Pharmacol. 2019 doi: 10.3389/fphar.2019.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong Y, Han Y, Huang Y, Jiang S, Huang Z, Chen R, Yu Z, Yu K, Zhang S. PD-L1 ıs expressed and promotes the expansion of regulatory t cells in acute myeloid leukemia. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA, Rodig SJ. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7–H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007 doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 11.Berthon C, Driss V, Liu J, Kuranda K, Leleu X, Jouy N, Hetuin D, Quesnel B. In acute myeloid leukemia, B7–H1 (PD-L1) protection of blasts from cytotoxic T cells is induced by TLR ligands and interferon-gamma and can be reversed using MEK inhibitors. Cancer Immunol Immunother. 2010 doi: 10.1007/s00262-010-0909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antohe I, Dǎscǎlescu A, Dǎnǎilǎ C, Titieanu A, Zlei M, Ivanov I, Sireteanu A, Pavel M, Cianga P. B7-Positive and B7-Negative acute myeloid leukemias display distinct T cell maturation profiles, ımmune checkpoint receptor expression, and European Leukemia net risk profiles. Front Oncol. 2020 doi: 10.3389/fonc.2020.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamura H, Dan K, Tamada K, Nakamura K, Shioi Y, Hyodo H, Wang SD, Dong H, Chen L, Ogata K. Expression of functional B7–H2 and B7.2 costimulatory molecules and their prognostic implications in de novo acute myeloid leukemia. Clin Cancer Res. 2005 doi: 10.1158/1078-0432.CCR-04-2672. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Liu S, Wang L, Zhang W, Ji Y, Ma X. Clinical significance of B7–H1 (PD-L1) expression in human acute leukemia. Cancer Biol Ther. 2008 doi: 10.4161/cbt.7.5.5689. [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZF, Zhang QT, Xin HZ, Gan SL, Ma J, Liu YF, Xie XS, Sun H. Expression of Programmed Death Ligand-1 (PD-L1) in Human Acute Leukemia and Its Clinical Significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2015 doi: 10.7534/j.issn.1009-2137.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Schmohl JU, Nuebling T, Wild J, Kroell T, Kanz L, Salih HR, Schmetzer H. Expression of RANK-L and in part of PD-1 on blasts in patients with acute myeloid leukemia correlates with prognosis. Eur J Haematol. 2016 doi: 10.1111/ejh.12762. [DOI] [PubMed] [Google Scholar]
- 17.Krupka C, Kufer P, Kischel R, Zugmaier G, Lichtenegger FS, Köhnke T, Vick B, Jeremias I, Metzeler KH, Altmann T, Schneider S, Fiegl M, Spiekermann K, Bauerle PA, Hiddemann W, Riethmüller G, Subklewe M. Blockade of the PD-1/PD-L1 axis augments lysis of AML cells by the CD33/CD3 BiTE antibody construct AMG 330: reversing a T-cell-induced immune escape mechanism. Leukemia. 2016 doi: 10.1038/leu.2015.214. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, Garcia-Manero G. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014 doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daver N, Basu S, Garcia-Manero G, Cortes JE, Ravandi F, Jabbour E, Hendrickson S, Brandt M, Pierce S, Gordon T, Pemmaraju N, Andreeff M, Ning J, Kornblau S, Kadia TM, Dinardo CD, Konopleva M, Allison JP, Kantarjian HM, Sharma P. Phase IB/II study of nivolumab in combination with 5-azacytidine (Aza) in patients (pts) with relapsed acute myeloid leukemia (AML) J Clinic Oncol. 2017 doi: 10.1200/JCO.2017.35.15_suppl.7026. [DOI] [Google Scholar]
- 20.Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, Kantarjian H. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018 doi: 10.1038/s41375-018-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang CY, Zha XF, Wen WR (2019) Expression and Clinical Significance of PD-L1 and MicroRNA-138-5p in Patients with Acute Myeloid Leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 10.19746/j.cnki.issn.1009-2137.2019.02.010 [DOI] [PubMed]
- 22.Chen C, Liang C, Wang S, Chio CL, Zhang Y, Zeng C, Chen S, Wang C, Li Y. Expression patterns of immune checkpoints in acute myeloid leukemia. J Hematol Oncol. 2020 doi: 10.1186/s13045-020-00853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodská B, Otevřelová P, Šálek C, Fuchs O, Gašová Z, Kuželová K. High PD-L1 expression predicts for worse outcome of leukemia patients with concomitant NPM1 and FLT3 mutations. Int J Mol Sci. 2019 doi: 10.3390/ijms20112823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zajac M, Zaleska J, Dolnik A, Bullinger L, Giannopoulos K. Expression of CD274 (PD-L1) is associated with unfavourable recurrent mutations in AML. Br J Haematol. 2018 doi: 10.1111/bjh.15040. [DOI] [PubMed] [Google Scholar]
- 25.Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, Ravandi F, Jabbour EJ, Al-Hamal Z, Konopleva M, Ning J, Xiao L, Hidalgo Lopez J, Kornblau SM, Andreeff M, Flores W, Bueso-Ramos C, Blando J, Galera P, Calvo KR, Al-Atrash G, Allison JP, Kantarjian HM, Sharma P, Daver NG. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2019 doi: 10.1002/cncr.31896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available.