Abstract
Background
Carotid webs (CaWs) are associated with ischemic strokes in younger patients without degrees of stenosis that are traditionally considered clinically significant.
Objective
To compare the hemodynamic parameters in the internal carotid artery (ICA) bulbar segment in patients with CaW with those in patients with atherosclerotic lesions using time–density curve (TDC) analysis of digital subtraction angiography (DSA) images.
Methods
We retrospectively assessed DSA images of 47 carotid arteries in 41 adult patients who underwent ICA catheter angiography for evaluation after ischemic stroke. Hemodynamic parameters, including full width at half maximum (FWHM) and area under the time–density curve (AUC) as proxies for increased flow stasis, were calculated using TDC analyses of a region of interest (ROI) in the ICA bulb immediately rostral to the web/atherosclerotic plaque, relative to a standardized ROI in the ipsilateral distal common carotid artery (eg, relative FWHM (rFWHM)). Hemodynamic parameters were compared using non-parametric Kruskal-Wallis tests. Logistic regression was used to predict CaW versus mild/moderate atherosclerosis for each hemodynamic parameter, adjusting for degree of stenosis.
Results
Mean age of patients was 56.0±13 years, with 22 (53.7%) women. 17 CaWs, 22 atherosclerotic plaques (15 mild/moderate and 7 severe), and eight normal carotid arteries were assessed. Significant between-group differences were present in the relative total AUC (p<0.001), relative AUC at wash out (p=0.031), and relative FWHM (p=0.001). Logistic regression to predict CaW versus mild/moderate atherosclerosis showed that rAUC total had the highest predictive value (pAUC=0.96, 95% CI 0.90 to 1.00), followed by rFWHM (0.87, 95% CI 0.74 to 1.00), and rAUC WO (0.74, 95% CI (0.57 to 0.91).
Conclusion
CaW results in larger local hemodynamic disruption, characterized by flow stasis, than mild/moderate carotid atherosclerotic lesions, suggesting that CaWs may produce larger regions of thrombogenic flow stasis.
Keywords: atherosclerosis, blood flow, angiography, stroke
Introduction
Carotid webs (CaWs) are non-atheromatous intraluminal shelf-like fibrotic projections into the internal carotid artery (ICA) bulb, with an estimated population prevalence of 1–7%.1–3 Despite producing only mild luminal stenosis, CaWs are associated with up to 37% of cryptogenic ischemic strokes, particularly in younger patients without traditional risk factors2 4 5 and without significant intraluminal stenosis.6 The pathophysiology of CaW-associated stroke is not well understood. However, it is postulated that CaW causes alter thrombogenic hemodynamic patterns distal to the web, which can cause thrombus formation and eventual embolization resulting in stroke.6–8
CaWs appear on digital subtraction angiography (DSA) as distinct shelf-like filling defects in the bulbar segment. Moreover, contrast pooling, a surrogate for prothrombogenic flow stasis, may be observed into the venous phase on DSA in the distal aspect of the CaW, despite the mild degree of stenosis.6 This phenomenon can be quantitatively assessed through regional density measurements of a series of contrast injection images, which can be calculated to produce time–density curves (TDCs).9
TDC analyses enable calculation of parameters such as time to peak (TTP), relative TTP (rTTP), maximal upslope (MS), and angiographic full width at half maximum (FWHM), which have been used to assess flow characteristics of patients with atherosclerotic carotid stenosis.10 11 TDC analysis has not been applied to CaWs, which often produce stenoses similar to those of mild and moderate atherosclerotic plaques at the level of the carotid bulb. However, the different morphological and pathophysiologic features of CaWs may result in different distal hemodynamic patterns.
We aimed to compare DSA-based hemodynamic parameters in the ICA bulbar segment in patients with CaW with those in patients with atherosclerotic stenosis and normal ICA using DSA-based TDC analysis. We hypothesized that the CaW will produce altered hemodynamics distinct from atherosclerotic lesions with similar degrees of luminal narrowing.
Methods
Study design
We retrospectively analyzed consecutive DSA images of 47 carotid arteries in a cohort of 41 adult patients who underwent ICA catheter angiography evaluation for ischemic stroke at a comprehensive stroke center between December 2013 and August 2020. This study was approved by the institutional review board and compliant with the Health Insurance Probability and Accountability Act.
Inclusion and exclusion criteria
We included adult patients with symptomatic CaW or atherosclerotic plaque in the carotid bulb who underwent DSA as part of a standard, routine clinical protocol for the evaluation of acute stroke between December 2013 and August 2020. Sixty-four patients with CaW and atherosclerotic plaques were identified by searching the institutional electronic medical record database (EPIC Systems Corporation, Madison, Wisconsin, USA) for carotid DSA reports that mentioned terms including ‘carotid web’, ‘web’, ‘shelf’, and permutations of ‘mild/moderate/severe’ and ‘narrowing/stenosis/atherosclerosis/plaque’. Symptomatic lesion was defined as a CaW or atherosclerotic plaque that was considered to be a cause of ipsilateral stroke. If imaging was obtained, the contralateral normal carotid arteries in the patients with CaW and atherosclerosis were also included for analysis. Each carotid artery was classified as CaW, atherosclerosis, or normal by consensus between two neurointerventionalists with 8 and 19 years of post-fellowship experience, based on morphology on DSA characterized by a focal shelf-like projection into the lumen without any findings suggestive of atherosclerosis on DSA and CT angiography. Patients with CaW and superimposed atherosclerotic calcifications were not included in the cohort.
Exclusion criteria included DSA images with incomplete inclusion of the common carotid artery (CCA) bifurcation and the carotid bulb (n=4), poor contrast bolus or vessel opacification (n=5), suboptimal subtraction (n=6), superimposed devices such as stents (n=2), images terminating too early (eg, before full wash out; n=4), and excessive patient motion (n=2). Among the 41 remaining patients, six had a DSA scan of the contralateral vessel that met the inclusion criteria.
Image analysis
The degree of stenosis in each atherosclerotic lesion was determined according to the North American Symptomatic Carotid Endarterectomy Trial criteria, which classifies stenosis as mild (0–49%), moderate (50–69%), or severe (70–99%).12 13 Degree of stenosis produced by CaW was similarly estimated according to previously published methods.6
ROI placement
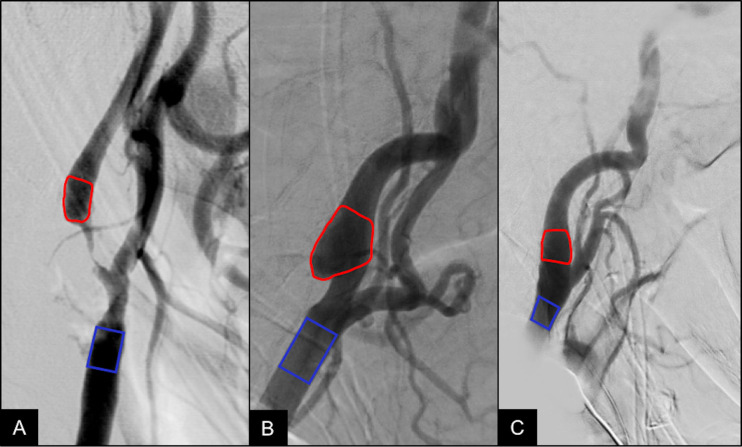
A region of interest (ROI) was placed in the carotid bulb just rostral to the CaW or atherosclerotic lesion while avoiding overlapping anatomic structures and external support devices. Each ROI was placed in a standardized location and measured approximately 1 cm along the longitudinal axis of the vessel to adequately cover the carotid bulb, with diameter spanning the entire caliber of the targeted vessel segment (figure 1). To account for differences in contrast injection rate, bolus volume, and catheter tip distance from bifurcation in each DSA examination, a second ROI was placed in the ipsilateral distal CCA to normalize the hemodynamic parameters for each vessel. Oblique lateral DSA images that best depicted the carotid bifurcation were used for ROI placement and TDC analysis.
Figure 1.
Region of interest placement: representative lateral digital subtraction angiography image of a patient with severe carotid atherosclerosis (A) and carotid web (B) with color-coded red region of interest (ROI) distal to the lesion and a second blue ROI placed at the distal common carotid artery bifurcation for calculation of normalized hemodynamic parameters. A normal carotid bifurcation (C) and ROI placement is shown for comparison.
Hemodynamic parameters
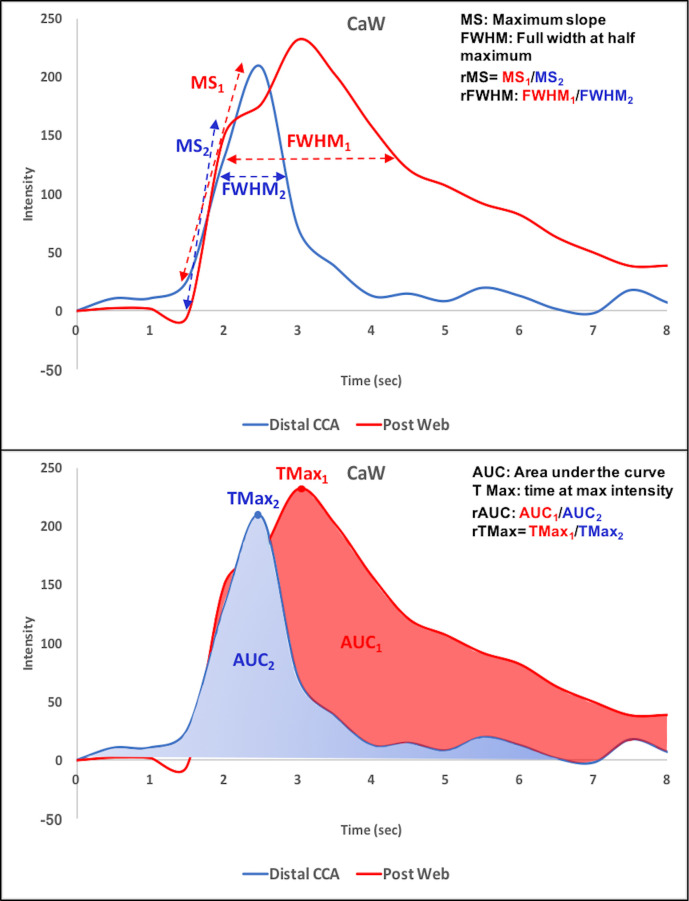
Hemodynamic parameters were estimated using TDC analyses (figure 2) of a ROI in the ICA bulb immediately distal to the web or atherosclerotic plaque, relative to a second ROI placed in the ipsilateral distal CCA. For each carotid artery, the following hemodynamic parameters were calculated: relative time at maximal intensity (rTMax), defined as the ratio of the differences in time of maximal intensity between these two target ROIs12 14; total relative area under the curve (rAUC total), defined as the ratios of the AUC between the two ROIs from beginning of wash in to the end of wash out; rAUC during wash in (rAUC WI) and wash out (rAUC WO); relative full width at half maximum (rFWHM), defined as the ratio of the FWHM of the curve15; relative time to peak (rTTP), defined as the ratio of the time difference in the time of maximum of the curve and bolus arrival, and relative maximal slope (rMS), defined as the maximum slope between the time of the contrast medium arrival and the time of maximal signal intensity of an ROI.
Figure 2.
Time–density curve-based hemodynamic parameters. Representative time-density curve of a patient with carotid webs from the color-coded regions of interest and representation of hemodynamic parameters including (A) maximal slope and full width at half maximum; (B) area under the curve and time at maximal intensity (TMax). CaW, carotid web; CCA, common carotid artery.
Statistical analyses
Statistical analyses were performed using SPSS v27 (SPSS Inc., Chicago, Illinois, USA). Significance was defined as a two-tailed p value of ≤0.05. Patient characteristics were summarized as means and SD for continuous variables and as numbers and percentages for categorical variables.
χ2 tests were used to compare the frequency of medical comorbidities among patients with CaW, atherosclerosis, and normal carotid arteries. Wilcoxon rank sum tests were used to compare the degrees of stenosis between CaW and groups with mild and moderate atherosclerosis. Non-parametric statistics using Kruskal-Wallis tests were used to compare hemodynamic parameters across the following groups: CaW, mild, moderate, and severe atherosclerosis, and normal carotid. Pairwise comparisons using Wilcoxon rank sum tests with the Holm correction for multiple comparisons16 was performed to further clarify the hemodynamic parameters that were significantly different among groups in the Kruskal-Wallis tests. For the subset of patients with mild atherosclerosis, moderate atherosclerosis, or CaW, a separate logistic regression was used to predict CaW for each hemodynamic parameter, including degree of stenosis as a covariate. Area under the curve (AUC) analysis from the receiver operating characteristic (ROC) was conducted using R 4.0.3 and the pROC package14 to calculate the predictive AUC (pAUC) of each hemodynamic parameter to distinguish between CaW and mild/moderate atherosclerosis groups.
Results
Patient characteristics
Patient characteristics are summarized for each carotid artery in table 1. Among the included 47 carotid arteries, classification breakdown was as follows: CaW (n=17), mild atherosclerosis (n=8), moderate atherosclerosis (n=7), severe atherosclerosis (n=7), and normal (n=8). The mean age overall was 56.0±13 years, and 53% of the patients were women. χ2 tests showed differences in the rates of comorbidities including hypertension (p=0.021) and diabetes (p=0.017), but not dyslipidemia (p=0.177) among patient groups.
Table 1.
Patient and vessel characteristics
| Characteristics | n=41 |
| Age (years)* | 56.0±13.0 (31–81) |
| Female | 22 (53.7) |
| Male | 19 (46.3) |
| Ipsilateral stroke | 33 (80.5) |
| Ipsilateral LVO | 18 (43.9) |
| Baseline NIHSS score* | 5 (2–13) |
| Endovascular carotid treatment† | 18 (43.9) |
| Medical history | |
| Hypertension | 32 (78.0) |
| Dyslipidemia | 17 (41.5) |
| Diabetes | 13 (31.7) |
| Chronic kidney disease | 7 (17.1) |
| Smoking | 16 (39.0) |
| Cocaine use | 2 (4.9) |
| Carotid classification | n=47 |
| Normal | 8 (17.0) |
| Mild atherosclerosis | 8 (17.0) |
| Moderate atherosclerosis | 7 (14.9) |
| Severe atherosclerosis | 7 (14.9) |
| Carotid web | 17 (36.2) |
*Age reported as mean±SD (range). Baseline NIHSS reported as median (IQR). All other variables reported as number (percentage).
†Undergoing ipsilateral carotid artery treatment with thrombectomy, angioplasty, and/or stent.
LVO, large vessel occlusion; NIHSS, National Institutes of Health Stroke Scale.
DSA image assessment
The mean degree of stenosis in the CaW group was 30.8% (range 15.9–58.3%). Groups with mild, moderate, and severe atherosclerosis had mean stenosis of 20.4% (range 5.9–46.3%), 61.8% (range 50.9–69.0%), and 83.1% (range 71.4–90.2%), respectively. Wilcoxon rank sum test demonstrated differences in the degrees of stenosis between CaW and mild atherosclerosis (p=0.04) and between CaW and moderate atherosclerosis groups (p<0.001). Contrast pooling immediately distal to the web was observed in 14 of the 17 cases. Of the 14 cases, definite pooling to the venous phase was seen in nine cases. In the remaining five, the contrast pooling was observed until the end of the cine image sequence, which terminated at the late arterial phase. Contrast pooling was absent in three of the 17 cases.
Assessment of hemodynamic parameters
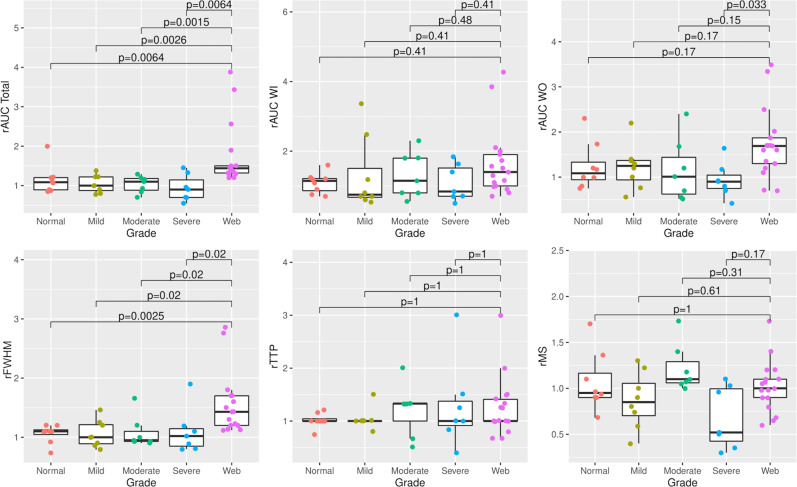
Kruskal-Wallis group-level comparisons of relative hemodynamic parameters in the carotid bulb are summarized in table 2. There were significant overall differences in rAUC total (p<0.001), rAUC WO (p=0.031), and rFWHM (p=0.001) among patient groups. No differences were observed for rTTP (p=0.882) or rMS (p=0.105). Although rFWHM differed across patient groups, secondary analysis showed that this might have been partially due to differences in the absolute FWHM in the CCA (online supplemental material). That said, post hoc pairwise comparisons with the Bonferroni-Holm correction showed that there was no significant difference in absolute FWHM in the moderate atherosclerosis and web groups (p=1.00), while rFWHM differed significantly between these two groups (p=0.02). We also conducted an analysis in which we randomly selected one observation from the seven patients with two observations. rAUC total and rFWHM were still significant (p<0.001), while rAUC WO was not (p=0.18).
Table 2.
Hemodynamic parameters: summary statistics
| Parameter* | Normal (n=8) | Mild atherosclerosis (n=8) | Moderate atherosclerosis (n=7) | Severe atherosclerosis (n=7) | Carotid web (n=17) | P value† |
| rAUC total | 1.08 (0.33) | 1.00 (0.42) | 1.10 (0.36) | 0.90 (0.63) | 1.44 (0.39) | <0.001 |
| rAUC WI | 1.15 (0.44) | 0.80 (1.7) | 1.15 (1.1) | 0.84 (0.93) | 1.4 (0.96) | 0.368 |
| rAUC WO | 1.09 (0.75) | 1.25 (0.89) | 1.01 (0.83) | 0.90 (0.47) | 1.69 (0.70) | 0.031 |
| rFWHM | 1.10 (0.21) | 0.97 (0.32) | 1.00 (0.49) | 1.02 (0.38) | 1.43 (0.50) | 0.001 |
| rTTP | 1.00 (0.13) | 1.00 (0.37) | 1.33 (0.75) | 1.00 (0.67) | 1.00 (0.46) | 0.882 |
| rMS | 0.95 (0.40) | 0.95 (0.61) | 1.10 (0.23) | 0.52 (0.68) | 1.00 (0.30) | 0.105 |
*All hemodynamic parameters are reported as median (IQR).
†P values: Kruskal-Wallis test, r: relative (normalized to distal common carotid).
AUC, area under the curve; FWHM, full width at half maximum; MS, maximum slope during wash in; TTP, time to peak; WI, wash in; WO, wash out.
neurintsurg-2021-017588supp001.pdf (31.3KB, pdf)
Pairwise comparisons using Holm correction for multiple comparisons (figure 3) showed higher rAUC total in the CaW group compared with the mild atherosclerosis (p=0.003), moderate atherosclerosis (p=0.002), severe atherosclerosis (p=0.006), and normal carotids (p=0.006). Similarly, rFWHM was higher in CaW than in mild atherosclerosis (p=0.02), moderate atherosclerosis (p=0.02), severe atherosclerosis (p=0.02), and normal carotids (p=0.003). Comparison of absolute parameters in the ICA and CCA are listed in the online supplemental material.
Figure 3.
Distribution of hemodynamic parameters with significant differences per Kruskal-Wallis tests by patient groups. Adjusted p values obtained from pairwise comparisons using Holm correction for multiple comparisons between patients with CaW and other groups. CaW demonstrated higher rAUC total and rFWHM than groups with mild/moderate/severe atherosclerosis and normal carotid arteries. CaW, carotid webs; CCA, common carotid artery; rAUC, relative area under the curve; rFWHM, relative full width at half maximum; rMS, relative maximal slope; rTTP.relative time to peak; WI, wash in; WO, wash out.
ROC analyses are summarized in table 3. Using logistic regression to classify CaW versus mild/moderate atherosclerosis while accounting for the degree of stenosis, rAUC total had the highest predictive value (pAUC=0.96, 95% CI 0.90 to 1.00), followed by rFWHM (0.87, 95% CI 0.74 to 1.00), and rAUC WO (0.74, 95% CI 0.57 to 0.91). The rAUC WI, rMS, and rTTP had the lowest predictive values, with 95% CI that included 0.50.
Table 3.
Receiver operating characteristic analysis of hemodynamic parameters to diagnose carotid web versus mild/moderate atherosclerotic stenosis based on logistic regression adjusted for degree of stenosis
| Variable model | pAUC | 95% CI |
| rAUC total | 0.957 | 0.897 to 1.000 |
| rAUC WI | 0.631 | 0.422 to 0.841 |
| rAUC WO | 0.741 | 0.568 to 0.914 |
| rFWHM | 0.867 | 0.737 to 0.996 |
| rTTP | 0.631 | 0.427 to 0.836 |
| rMS | 0.600 | 0.364 to 0.836 |
AUC, area under the curve; FWHM, full width at half maximum; MS, maximum slope during wash in; pAUC, predictive area under the curve; r, relative (normalized to distal common carotid); TTP, time to peak; WI, wash in; WO, wash out.
Discussion
The major finding of this study was that local hemodynamic parameters which characterize the amount of flow stasis were significantly elevated in CaW compared with mild and moderate carotid atherosclerosis, despite having degrees of stenosis that were below the range generally used to consider patients for endovascular treatments.17 Altered hemodynamics induced by CaW are probably related to the phenomenon of contrast pooling that continues into the venous phase on DSA in the ICA bulb, which is commonly observed in patients with CaW, but not with atherosclerotic plaques. The contrast pooling is due to increased particle residence time that is reflected in the increased rAUC total values that were seen in this study.
Current understanding of CaW and resultant altered flow dynamics is limited.18 Compagne et al, recently used computational fluid dynamics modeling to show that CaWs demonstrate a larger recirculation zone distal to the web, characterized by higher transverse wall shear stress and oscillatory shear index, compared with the contralateral normal carotid bifurcation.8 The study, however, was limited to computational flow modeling with multiple assumptions and did not account for patient-specific flow patterns.
In patients with CaWs, the intraluminal shelf-like projection causes a flow acceleration in the proximal side of the web. Distal to the web, the lumen area expands significantly, which creates an even larger unstable, separated, flow pattern that gives rise to a large recirculation region and potentially a flow stagnation region, probably represented by the larger AUC total demonstrated in our patients with CaWs. This sudden area expansion distal to the carotid web might result in an extreme form of flow disturbance; thus we expect that advance modalities such as 4D flow MRI19 20 may demonstrate larger recirculation zones and altered hemodynamic parameters distal to a CaW in comparison with a healthy bulb.
In the meantime, DSA remains the gold standard for carotid artery evaluation owing to its temporal and spatial resolution, and the possibility of effective therapeutic interventions.6 21 In some cases, there may be overlap between the appearance of carotid webs, particularly those that have a pyramidal morphology, and atherosclerotic plaques that can make the distinction difficult based on morphology alone. Our results suggest that adding TDC analysis to DSA to assess quantitative flow parameters may be useful when the distinction between CaW and mild and moderate atherosclerotic plaque is not clear. In addition, the altered hemodynamics associated with CaW compared with atherosclerosis suggest that the etiology of thrombogenesis in CaW may be distinct from atherosclerosis.
Limitations of this study are primarily due to its retrospective nature. There were technical differences in DSA acquisition that included injector rates, contrast bolus volumes, and catheter tip distances from carotid bifurcation. We attempted to mitigate these factors by calculating hemodynamic parameters relative to a standardized ROI within the distal CCA. Owing to the plane, positioning, and relative early origins of different branch vessels in the external carotid artery (ECA), it was not possible to place a uniformed standardized ROI in the ECA as opposed to the CCA, which was acquired in plane with the ICA. Furthermore, as subjects were identified by current or prior strokes, it was not possible to assess the predictive value of DSA-based parameters for the likelihood of developing stroke. Moreover, most of the patients underwent DSA following reperfusion. This retrospective selection criterion also limited the number of normal carotid DSA examinations as most of the patients with CaWs had either bilateral webs or unilateral webs that did not undergo imaging of the contralateral side. Lastly, there was an inherent age and sex bias towards young, female patients in the CaW group consistent with prior studies on CaW.5 22 Age has been shown to be associated with differences in flow and vessel wall parameters.23 Although using age- and sex-matched control subjects would be ideal, this was limited by the invasive nature of DSA. Thus, it might be better to investigate and quantify hemodynamic parameters in patients with carotid vessel disease using non-invasive methods.
Conclusion
CaW produces altered hemodynamic disturbances compared with mild and moderate carotid atherosclerosis, despite having similar degrees of luminal narrowing. These differences in hemodynamic parameters suggest that the anatomical shape and location of CaWs produce altered flow that suggests prothrombotic flow stasis compared with atherosclerotic lesions. Future studies are needed to further clarify the relationship between flow disturbance and the pathogenesis/risk of ischemic strokes in patients with CaW, which may lead to improved risk stratification and clinical management.
Footnotes
Twitter: @diogohaussen
Contributors: CCP, JNO, and JWA conceived and planned the study. DCH, RGN, and JWA contributed to interpretation of the results. CCP and JWA took lead in manuscript writing, with all other authors providing critical feedback that resulted in the final manuscript.
Funding: CCP received funding from the RSNA Research Resident Grant (RR2056) to conduct this study.
Competing interests: JNO receives research project funding from Siemens Medical Solutions.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Ethical approval to conduct this study was obtained from the Emory University Institutional Review Board (approval ID IRB 00091421). This is a non-interventional study with retrospective analysis of radiology images, which were obtained from PACS without direct contribution from the studied population. Therefore, the data obtained for evaluation did not require individual consent per the IRB approval.
References
- 1. Coutinho JM, Derkatch S, Potvin ARJ, et al. Carotid artery web and ischemic stroke: a case-control study. Neurology 2017;88:65–9. 10.1212/WNL.0000000000003464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joux J, Boulanger M, Jeannin S, et al. Association between carotid bulb diaphragm and ischemic stroke in young Afro-Caribbean patients: a population-based case-control study. Stroke 2016;47:2641–4. 10.1161/STROKEAHA.116.013918 [DOI] [PubMed] [Google Scholar]
- 3. Sajedi PI, Gonzalez JN, Cronin CA, et al. Carotid bulb webs as a cause of "cryptogenic" ischemic stroke. AJNR Am J Neuroradiol 2017;38:1399–404. 10.3174/ajnr.A5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SJ, Allen JW, Bouslama M, et al. Carotid webs in cryptogenic ischemic strokes: a matched case-control study. J Stroke Cerebrovasc Dis 2019;28:104402. 10.1016/j.jstrokecerebrovasdis.2019.104402 [DOI] [PubMed] [Google Scholar]
- 5. Zhang AJ, Dhruv P, Choi P, et al. A systematic literature review of patients with carotid web and acute ischemic stroke. Stroke 2018;49:2872–6. 10.1161/STROKEAHA.118.021907 [DOI] [PubMed] [Google Scholar]
- 6. Haussen DC, Grossberg JA, Bouslama M, et al. Carotid web (intimal fibromuscular dysplasia) has high stroke recurrence risk and is amenable to stenting. Stroke 2017;48:3134–7. 10.1161/STROKEAHA.117.019020 [DOI] [PubMed] [Google Scholar]
- 7. Choi PMC, Singh D, Trivedi A, et al. Carotid webs and recurrent ischemic strokes in the era of CT angiography. AJNR Am J Neuroradiol 2015;36:2134–9. 10.3174/ajnr.A4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Compagne KCJ, Dilba K, Postema EJ, et al. Flow patterns in carotid webs: a patient-based computational fluid dynamics study. AJNR Am J Neuroradiol 2019;40:703–8. 10.3174/ajnr.A6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hesselink JR, Chang KH, Chung KJ, et al. Flow analysis with digital subtraction angiography: 2. acquisition and accuracy of transit-flow measurements. AJNR Am J Neuroradiol 1986;7:427–31. [PMC free article] [PubMed] [Google Scholar]
- 10. Lin C-J, Chang F-C, Guo W-Y, et al. Changes of time-attenuation curve blood flow parameters in patients with and without carotid stenosis. AJNR Am J Neuroradiol 2015;36:1176–81. 10.3174/ajnr.A4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Teng MMH, Chang F-C, Lin C-J, et al. Peritherapeutic hemodynamic changes of carotid stenting evaluated with quantitative DSA in patients with carotid stenosis. AJNR Am J Neuroradiol 2016;37:1883–8. 10.3174/ajnr.A4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnett HJM, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med Overseas Ed 1998;339:1415–25. 10.1056/NEJM199811123392002 [DOI] [PubMed] [Google Scholar]
- 13. Chang Y-J, Golby AJ, Albers GW. Detection of carotid stenosis. Stroke 1995;26:1325–8. 10.1161/01.STR.26.8.1325 [DOI] [PubMed] [Google Scholar]
- 14. Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. 10.1186/1471-2105-12-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee H-J, Hong J-S, Lin C-J, et al. Automatic flow analysis of digital subtraction angiography using independent component analysis in patients with carotid stenosis. PLoS One 2017;12:e0185330. 10.1371/journal.pone.0185330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stats 1979;6:65–70. [Google Scholar]
- 17. International Carotid Stenting Study investigators, Ederle J, Dobson J, et al. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International carotid stenting study): an interim analysis of a randomised controlled trial. Lancet 2010;375:985–97. 10.1016/S0140-6736(10)60239-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SJ, Nogueira RG, Haussen DC. Current understanding and gaps in research of carotid webs in ischemic strokes: a review. JAMA Neurol 2019;76:355–61. 10.1001/jamaneurol.2018.3366 [DOI] [PubMed] [Google Scholar]
- 19. Harloff A, Zech T, Wegent F, et al. Comparison of blood flow velocity quantification by 4D flow MR imaging with ultrasound at the carotid bifurcation. AJNR Am J Neuroradiol 2013;34:1407–13. 10.3174/ajnr.A3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Markl M, Frydrychowicz A, Kozerke S, et al. 4D flow MRI. J Magn Reson Imaging 2012;36:1015–36. 10.1002/jmri.23632 [DOI] [PubMed] [Google Scholar]
- 21. Hill MD, Demchuk AM, Frayne R. Noninvasive imaging is improving but digital subtraction angiography remains the gold standard. Neurology 2007;68:2057–8. 10.1212/01.wnl.0000268580.86336.af [DOI] [PubMed] [Google Scholar]
- 22. Madaelil TP, Grossberg JA, Nogueira RG, et al. Multimodality imaging in carotid web. Front Neurol 2019;10:220. 10.3389/fneur.2019.00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jarvis K, Soulat G, Scott M, et al. Investigation of aortic wall thickness, stiffness and flow reversal in patients with cryptogenic stroke: a 4D flow MRI study. J Magn Reson Imaging 2021;53:942–52. 10.1002/jmri.27345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
neurintsurg-2021-017588supp001.pdf (31.3KB, pdf)
Data Availability Statement
The data that supports the findings of this study are available from the corresponding author upon reasonable request.