Abstract
Objectives:
Disruption in the natural immune reaction due to SARS-CoV-2 infection can initiate a potent cytokine storm among COVID-19 patients. An elevated level of IL-6 and IL-10 during a hyperinflammatory state plays a vital role in increasing the risk of severity and mortality. In this study, we aimed to evaluate the potential of circulating IL-6 and IL-10 levels as biomarkers for detecting the severity and mortality of COVID-19.
Methods:
This study was conducted according to the Cochrane Handbook and PRISMA guidelines. Authorized databases were searched to extract suitable studies using specific search terms. RevMan 5.4 was applied for performing the meta-analysis. Mean differences in IL-6 and IL-10 levels were calculated among COVID-19 patients via a random-effects model. NOS scoring, publication bias and sensitivity analyses were checked to ensure study quality.
Results:
A total of 147 studies were selected, with 31 909 COVID-19 patients under investigation. In the severity analysis, the mean concentration of IL-6 was significantly higher in the severe COVID-19 cases than in the non-severe cases (MD: 19.98; P < .001; 95% CI: 17.56, 22.40). Similar result was observed for IL-10 mean concentration in severe COVID-19 cases (MD: 1.35; P < .001; 95% CI: 0.90, 1.80). In terms of mortality analysis, circulating IL-6 showed sharp elevation in the deceased patients (MD: 42.11; P < .001; 95% CI: 36.86, 47.36). IL-10 mean concentration was higher in the dead patients than in the survived patients (MD: 4.79; P < .001; 95% CI: 2.83, 6.75). Publication bias was not found except for comparing IL-6 levels with disease severity. Sensitivity analysis also reported no significant deviation from the pooled outcomes.
Conclusions:
Elevated levels of circulating IL-6 and IL-10 signifies worsening of COVID-19. To monitor the progression of SARS-CoV-2 infection, IL-6 and IL-10 should be considered as potential biomarkers for severity and mortality detection in COVID-19.
Systematic review registration:
INPLASY registration number: INPLASY202240046.
Keywords: COVID-19, interleukin-6, interleukin-10, meta-analysis, cytokine storm
Introduction
Novel coronavirus disease (COVID-19) prevalence was first commenced in 2019 by a highly variating virus “SARS-CoV-2” (severe acute respiratory syndrome coronavirus 2) infection. The infection spread rapidly, turning into a worldwide pandemic that caused nearly 5.6 million death in the last 2 years.1-3 A number of diagnostic and treatment approaches have been approved in the fight against COVID-19, although a concrete predictor of disease progression is yet to reveal.4,5 The highly unpredictable nature of this current pandemic has made it difficult to detect the severity of the condition in time. It is crucial to establish a reliable diagnostic marker to follow the pattern of disease development and to halt the process from getting severe, even fatal. Moreover, identifying sensitive and specific biomarkers would create an opportunity to promote stronger preventive and therapeutic strategies.6-8
The key negative prognostic factor of SARS-CoV-2 infection pathophysiology is cytokine storm, a hyperinflammatory process of cytokine releasing that causes acute systemic reactions. This specific immune reactive condition drives the disease state toward acute respiratory distress syndrome (ARDS). Inflammatory cytokines, more specifically interleukins, are found to be the main mediators involved in the cytokine storm development. 9 Although rapid innate immune system reaction following SARS-CoV-2 infection is the first-line defense against COVID-19, excessively active immune reaction generates severe complications. During SARS-CoV-2 infection, irreversibly critical damage occurs in the pulmonary system and lung tissues by higher plasma concentrations of circulating interleukins.9,10 Among the multifunctional proinflammatory cytokines, interleukin-6 (IL-6) and interleukin-10 (IL-10) are suspected to be strongly involved in the COVID-19 related cytokine storm for their potential roles in acute phase immune reactions.11-13
IL-6, an inflammatory mediator with pleiotropic nature, is highly produced during the initial stage of inflammation and rapidly activates multiple acute phases of inflammatory reactants. In COVID-19 patients, IL-6 is produced in response to antigens from several immune cell types, and a number of clinical investigative studies have reported that serum level of circulating IL-6 was critically higher among the COVID-19 patients from severe to the critical stage.11,14-16 Another cross-sectional study stated evidence that serum levels of IL-6 above 24.3 pg/ml might be associated with severe pneumonia in COVID-19 patients. 17 IL-10 exerts powerful anti-inflammatory actions that control severe host immune responses toward antigens by preventing multiple functions of T-cells and neutral killer (NK) cells. Again, dysregulation in IL-10 concentration may influence the immune response and severity of SARS-CoV-2 infected patients. 18 In this context, the serum level of IL-10 also showed significant elevation in severe and critical cases of COVID-19, commensurate with IL-6 serum level. Several studies showed evidence that both IL-6 and IL-10 are positively related to the severity and mortality of COVID-19.8,19-21 According to this evidence, alteration in the normal level of circulating IL-6 and IL-10 can act as potential biomarkers for COVID-19. 13
Although some previous meta-analyses attempted to evaluate the link between circulating IL-6 and IL-10 levels with severity and mortality of COVID-19, they recommended further investigation with a larger sample size to validate their findings. For this reason, we conducted this updated systematic review and meta-analysis with available literature to reveal the correlation between IL-6 and IL-10 elevation with COVID-19 and the effectiveness of testing serum IL-6 and IL-10 levels as clinical biomarkers.
Methods
The recommendations narrated in the Cochrane Handbook 22 and PRISMA (the Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines 23 were followed to conduct this systematic review and meta-analysis. The study is also registered in INPLASY (http://inplasy.com/), and the registration number is INPLASY202240046.
Literature searching strategy
The international scientific authorized databases such as Google Scholar, PubMed, Embase, CNKI, Cochrane Library, and Web of science were used as primary sources to identify and collect the eligible literature. Additional secondary databases were also comprehensively searched to extract more related studies. The specific search terms used for this study were: “COVID-19” OR “SARS-Cov-2”; “interleukin-6 “ OR “IL-6”; and “interleukin-10” OR “IL-10.” The search strategy for each database is enlisted in Supplemental Table 1. All the included literature was selected from December 2019 to December 2021 time period.
Study selection
Two authors individually screened titles and abstracts of the studies from different databases to avoid bias and shortlisted articles with eligibility potentials. The unrelated articles were eliminated from the list after full-text inspection based on the inclusion and exclusion criteria. Any difference of opinions among the authors was resolved via a logical argument with the assistance of the third researcher. The study selection process is outlined in Figure 1.
Figure 1.
Study flow chart representing the selection process of eligible studies.
Inclusion and exclusion criteria
Inclusion criteria: (1) clinical studies, case-control investigations or cohort studies; (2) articles representing severity and mortality in COVID-19 patients; (3) articles providing information on IL-6 and IL-10 level among mild-to-severe COVID-19 patients; (4) articles reporting IL-6 and IL-10 level in the COVID-19 survivors and deceased patients.
Critically ill patients, patients with severe dyspnea, critically low oxygen level, patients under mechanical ventilation, or admitted to the intensive care unit (ICU) were considered severe conditions of COVID-19.
Exclusion criteria: (1) meta-analysis, review articles, letters, or comments; (2) articles written in languages other than English or Chinese; (3) incomplete information required in this meta-analysis; (4) unavailability of full texts.
Quality assessment
The Newcastle-Ottawa Scale (NOS) can determine the quality range of studies by rating them from 0 to 10 stars based on some specific features (Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.). Articles that scored ⩾6 were considered high-quality ones. All the studies included in this meta-analysis were assessed with NOS for quality evaluation by 2 reviewers independently. Studies that scored less than 6 stars were excluded to maintain the quality range for the present analysis.
Data extraction
Data collection from the enlisted articles was conducted by 2 researchers. Basic information like author name, study period, study location, ethnicity, settings, study design, number of COVID-19 patients, age, mild state, severe to a critical state, number of deceased patients, IL-6, and IL-10 concentrations in patients were extracted.
Publication bias assessment
The risk of publication bias was determined by using Review Manager (RevMan 5.4) software for systematic review and meta-analysis. Egger regression test and Begg & Mazumdar test were performed to detect the presence of publication bias. Both the tests were used to verify the significance level of bias among the studies. The presence of asymmetry in the funnel plots also indicates a significant presence of publication bias.
Statistical analysis
The statistical analysis was performed by comparing the concentration level of both IL-6 and IL-10 among the COVID-19 patients according to the disease severity. Patients with mild symptoms or at the recovery stage were considered as the control population and patients with severe or critical conditions were termed as an experimental population. A secondary analysis was also conducted among the survived patients and deceased patients. The control arm showed the IL-6 and IL-10 concentrations among the survived patients, and the experimental arm showed peak concentration (last diagnostic count) levels among the dead patients. The name of the software used to carry out this meta-analysis was RevMan 5.4 from the Cochrane Collaboration, 2020. The unit of concentration measured as pg/ml. We used mean concentration with standard deviation for numerical presentation. The numerical data (mean and SD) was estimated using a validated equation. Estimation of the outcome was pooled as the mean difference with 95% CIs (confidence interval). Two analysis models were used for statistical calculation—the fixed-effects model and the random-effects model. In case of significant heterogeneity (chi-square I2 ⩾ 50 and P < .10), DerSimonian-Laird random-effects model was applied and, in the absence of the heterogeneity fixed-effects model (Mantel-Haenszel) was applied (chi-square I2 = 50 and P > .10). To evaluate the credibility of the results acquired from this study, we performed sensitivity analysis by omitting the studies one by one with the application of RevMan 5.4.
Results
Study selection
We conducted this meta-analysis on overall 31 909 COVID-19 patients from 147 studies.2,4-7,9-18,24-154 Among the recruited patients, 3137 were deceased, and the rest, 28 772 patients, showed mild to severe disease symptoms. The age range of the patients was between 6.25 ± 4.31 and 85.83 ± 7.61 years. All the studies had hospital-based settings; patients under investigation were admitted to the hospitals. No patients under self-quarantine or without hospital admission were included in the study. The recruited studies followed various designs, such as retrospective cohort, prospective cohort, observational cohort, single centered or multicentered cohort, case-control cohort, retrospective observational, prospective observational, prognostic cohort, retrospective longitudinal, non-randomized, cross-sectional observational, and clinical studies. From 147 studies, 107 studies reported IL-6 and IL-10 concentrations in COVID-19 patients and their association with disease severity. On the other hand, 49 studies reported an association of IL-6 and IL-10 serum levels with mortality in COVID-19 patients. Cytokine levels were measured using different biochemical assays—Enzyme-linked immunosorbent assay (ELISA); Electro-chemiluminescent immunoassay (ECLIA); Chemiluminescent immunoassay (CLIA); Online hemodiafiltration (OLHDF), Flow cytometry, Bio plex multiplex immunoassay, Automated immunoassay multiplex array system, and Enzyme-immune assay. The basic information is outlined in Table 1.
Table 1.
Baseline characteristics of the investigative studies reporting blood levels of IL-6 and IL-10 in COVID-19 patients.2,4-7,9-18,24-154.
| Study (reference) | Ethnicity | Location | Setting | Design | No. of participants | Age (mean ± SD) | Disease severity | Mortality | Cytokine assay | Biomarker study | NOS rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bergantini et al 2 | Caucasian | Italy | HB | Monocentric retrospective | 24 | Mild: 62.2 ± 15.6Severe: 65.2 ± 8 | Mild: 14Severe: 10 | NA | ECLIA | IL-6 | 7 |
| Burian et al 24 | Caucasian | Germany | HB | Retrospective cohort | 37 | 61.5 ± 17 | Mild: 25Severe: 12 | NA | Biochemical assay | IL-6 | 7 |
| Cai et al 25 | Asian | China | HB | Retrospective | 298 | 47.17 ± 20.86 | Mild: 240Severe: 58 | NA | Biochemical assay | IL-6 | 8 |
| Chang et al 26 | Asian | China | HB | Retrospective | 150 | NA | Mild: 93Severe: 57 | NA | Biochemical assay | IL-6 | 8 |
| Chen et al 4 | Asian | China | HB | Retrospective cohort | 660 | 52.33 ± 25.26 | NA | Dead: 82Survivors: 578 | Biochemical assay | IL-6 | 8 |
| Chen et al 27 | Asian | China | HB | Retrospective | 21 | 57 ± 11.93 | Mild: 10Severe: 11 | NA | CLIA | IL-6, IL-10 | 8 |
| Chen et al 28 | Asian | China | HB | Retrospective | 172 | 64 | NA | Dead: 87Survivors: 85 | Biochemical assay | IL-6 | 7 |
| Chen et al 29 | Asian | China | HB | Retrospective | 29 | NA | Mild: 15Severe: 14 | NA | ELISA | IL-6, IL-10 | 8 |
| Chen et al 30 | Asian | China | HB | Retrospective observational | 94 | 52.75 ± 16.09 | Mild: 69Severe: 25 | NA | CLIA | IL-6, IL-10 | 9 |
| Chen et al 31 | Asian | China | HB | Retrospective cohort | 548 | 56 ± 14.5 | Mild: 345Severe: 203 | Dead: 103Survivors: 445 | Biochemical assay | IL-6 | 8 |
| Chen et al 5 | Asian | China | HB | Retrospective cohort | 274 | 58.67 ± 19.38 | NA | Dead: 113Survivors: 161 | Biochemical assay | IL-6, IL-10 | 7 |
| Chen et al 32 | Asian | China | HB | Retrospective | 55 | 55 ± 54.05 | NA | Dead: 19Survivors: 36 | Biochemical assay | IL-6 | 6 |
| Chen et al 9 | Asian | China | HB | Retrospective | 48 | 64.6 ± 18.1 | Mild: 21Severe: 27 | NA | Biochemical assay | IL-6 | 6 |
| Chen et al 11 | Asian | China | HB | Retrospective | 1453 | NA | Mild: 962Severe: 491 | NA | ELISA | IL-6 | 8 |
| Chi et al 10 | Asian | China | HB | Retrospective cohort | 70 | Mild : 42 ± 10.98Severe: 43.24 ± 14.76 | Mild: 4Severe: 66 | NA | Multiplex biometric immunoassay | IL-6, IL-10 | 8 |
| Crespo et al 33 | Caucasian | Spain | HB | Prospective cohort | 16 | 73.6 ± 4.7 | NA | Dead: 8Survivors: 8 | Biochemical assay | IL-6 | 6 |
| De La Flor et al 34 | Caucasian | Spain | HB | Observational retrospective | 10 | 73.5 ± 9.46 | NA | Dead: 3Survivors: 7 | OLHDF | IL-6 | 7 |
| D’Alto et al 35 | Caucasian | Italy | HB | Prospective | 94 | Dead: 68 ± 12Survivors: 62 ± 13 | NA | Dead: 25Survivors: 69 | Biochemical assay | IL-6 | 7 |
| Ding et al 6 | Asian | China | HB | Prognostic | 104 | 62.82 ± 14.77 | Mild: 50Severe: 54 | Dead: 16Survivors: 88 | Biochemical assay | IL-6 | 7 |
| Ding et al 36 | Asian | China | HB | Retrospective | 32 | Mild: 54.9 ± 11.3Severe: 61.3 ± 17.9Critical: 73.5 ± 12.3 | Mild: 11Severe: 21 | NA | CLIA | IL-6 | 8 |
| El-Shabrawy et al 14 | African | Egypt | HB | Prognostic cohort | 116 | Mild : 44.67 ± 42.13Severe: 54.17 ± 54.97 | Mild: 99Severe: 17 | NA | ELISA | IL-6 | 8 |
| Fan et al 37 | Asian | China | HB | Retrospective | 73 | 58.36 ± 14.31 | NA | Dead: 47Survivors: 26 | Biochemical assay | IL-6 | 7 |
| Fan et al 38 | Asian | China | HB | Retrospective observational | 101 | 65.46 ± 9.74 | NA | Dead: 101Survivors: 0 | Biochemical assay | IL-6 | 8 |
| Fan et al 39 | Asian | China | HB | Retrospective longitudinal | 21 | 62.5 ± 12.6 | NA | Dead: 4Survivors: 17 | Biochemical assay | IL-6 | 6 |
| Fei et al 40 | Asian | China | HB | Retrospective | 72 | Mild: 55.7 ± 11.9Severe: 64 ± 16.8 | Mild: 52Severe: 20 | NA | ELISA | IL-6 | 7 |
| Fei et al 41 | Asian | China | HB | Retrospective cohort | 191 | 56.33 ± 15.69 | NA | Dead: 54Survivors: 137 | Biochemical assay | IL-6 | 7 |
| Feng et al 7 | Asian | China | HB | Single-centered, prospective, and observational | 114 | 63.96 ± 13.41 | Mild: 94Severe: 20 | NA | Biomarkers assay | IL-6, IL-10 | 7 |
| Gadotti et al 12 | Caucasian | Brazil | HB | Prospective cohort | 56 | 60.33 ± 19.78 | NA | Dead: 18Survivors: 38 | ELISA | IL-6, IL-10 | 8 |
| Gan et al 42 | Asian | China | HB | Retrospective case-control | 95 | 65.67 ± 15.06 | NA | Dead: 39Survivors: 56 | Biochemical assay | IL-6, IL-10 | 7 |
| Gao et al 43 | Asian | China | HB | Retrospective | 43 | 43.74 ± 12.12 | Mild: 28Severe: 15 | NA | ECLIA | IL-6 | 8 |
| Gil-Etayo et al 44 | Caucasian | Spain | HB | Prospective observational | 34 | 58.08 ± 23.79 | NA | Dead: 6Survivors: 28 | Flow cytometry | IL-6 | 8 |
| Guirao et al 15 | Caucasian | Spain | HB | Retrospective cohort | 50 | Mild: 56.2 ± 2.85Moderate: 65.7 ± 2.05Severe: 64.5 ± 2.26 | Mild: 10Severe: 40 | Dead: 14Survivor s: 36 | ECLIA | IL-6 | 9 |
| Guner et al 45 | Caucasian | Turkey | HB | Retrospective cohort | 222 | 50.6 ± 16.5 | Mild: 172Severe: 50 | NA | Biochemical assay | IL-6 | 8 |
| Han et al 13 | Asian | China | HB | Retrospective cohort | 102 | Mild: 58.3 ± 12.6Severe: 59.3 ± 14.4Critical:65.1 ± 14.4 | Mild: 42Severe: 60 | NA | Flow cytometry | IL-6, IL-10 | 9 |
| Yang et al 46 | Asian | China | HB | Single-centered, retrospective, and observational | 94 | Dead: 75.8 ± 12.9Survivors: 65.8 ± 10.2 | NA | Dead: 13Survivors: 81 | Biochemical assay | IL-6, IL-10 | 9 |
| Henry et al 18 | Caucasian | USA | HB | Prospective observational | 52 | 52 ± 20.59 | Mild: 36Severe: 16 | NA | ELISA | IL-6, IL-10 | 9 |
| He et al 47 | Asian | China | HB | Retrospective | 204 | 48.33 ± 20.91 | Mild: 135Severe: 69 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| He et al 48 | Asian | China | HB | Single-center retrospective | 93 | 47.9 ± 13.2 | Mild: 60Severe: 33 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Herold et al 16 | Caucasian | UK | HB | Prospective cohort | 89 | 54.33 ± 49.74 | Mild: 57Severe: 32 | NA | ELISA | IL-6 | 8 |
| Holt et al 49 | Caucasian | USA | HB | Retrospective cohort | 62 | Dead: 74.16 ± 11.4Survivors: 60.3 ± 12.5 | NA | Dead: 19Survivors: 43 | Biochemical assay | IL-6 | 6 |
| Hu et al 50 | Asian | China | HB | Retrospective cohort | 76 | 50.47 ± 3.10 | Mild: 63Severe: 13 | NA | Multiplex biometric immunoassay | IL-6 | 6 |
| Huang et al 51 | Asian | China | HB | Retrospective | 64 | 47.8 ± 18.5 | Mild: 43Severe: 21 | Dead: 4Survivors: 27 | Biochemical assay | IL-6, IL-10 | 6 |
| Huang et al 52 | Asian | China | HB | Single-center retrospective | 218 | 62.33 ± 13.43 | Mild: 116Severe: 102 | NA | ELISA, CLIA | IL-6 | 7 |
| Huang et al 53 | Asian | China | HB | Retrospective | 83 | 62 ± 12.31 | Mild: 21Severe: 62 | NA | Biochemical assay | IL-6 | 6 |
| Jain et al 54 | Asian | India | HB | Prospective observational | 154 | Mild: 42.34 ± 6.41Severe: 51.41 ± 9.12 | Mild: 91Severe: 63 | NA | ELISA | IL-6 | 7 |
| Ke et al 55 | Asian | China | HB | Single-centered, retrospective case-control | 194 | 63.08 ± 12.88 | NA | Dead: 46Survivors: 148 | Flow cytometry | IL-6, IL-10 | 7 |
| Keske et al 56 | Caucasian | Turkey | HB | Retrospective | 43 | 61.67 ± 51.39 | NA | Dead: 6Survivors: 37 | Biochemical assay | IL-6 | 6 |
| Kumar et al 57 | Asian | India | HB | Clinical study | 386 | Dead: 63.4 ± 14Survivors: 48.1 ± 16.3 | NA | Dead: 16Survivors: 370 | CLIA | IL-6 | 8 |
| Laguna-Goya et al 58 | Caucasian | Spain | HB | Prospective cohort | 454 | 52 ± 11.9 | NA | Dead: 33Survivors: 421 | Flow cytometry | IL-6 | 9 |
| Li et al 59 | Asian | China | HB | Retrospective | 476 | 61.33 ± 14.09 | NA | Dead: 183Survivors: 293 | Biochemical assay | IL-6, IL-10 | 7 |
| Li et al 60 | Asian | China | HB | Retrospective cohort | 102 | 57.33 ± 18.8 | NA | Dead: 15Survivors: 87 | Biochemical assay | IL-6, IL-10 | 6 |
| Li et al 61 | Asian | China | HB | Retrospective | 1449 | 55 ± 17.81 | NA | Dead: 122Survivors: 1327 | Biochemical assay | IL-6, IL-10 | 6 |
| Li et al 62 | Asian | China | HB | Single-center, retrospective | 215 | Mild: 42.67 ± 14.96Severe: 49.5 ± 39.57 | Mild: 159Severe: 56 | NA | Flow cytometry | IL-6, IL-10 | 7 |
| Liu et al 63 | Asian | China | HB | Multicenter, Retrospective cohort | 2044 | 61 ± 14.09 | Mild: 1087Severe: 957 | NA | CLIA | IL-6, IL-10 | 8 |
| Liu et al 64 | Asian | China | HB | Retrospective | 50 | 54 ± 20.86 | Mild: 24Severe: 26 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Liu et al 65 | Asian | China | HB | Single-center, retrospective | 51 | 43.33 ± 12.97 | Mild: 44Severe: 7 | NA | Biochemical assay | IL-6 | 6 |
| Liu et al 66 | Asian | China | HB | Retrospective cohort | 255 | 60 ± 50.7 | Mild: 214Severe: 41 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Liu et al 67 | Asian | China | HB | Retrospective cohort | 80 | 55 ± 43.3 | Mild: 11Severe: 69 | NA | ELISA | IL-6, IL-10 | 8 |
| Liu et al 68 | Asian | China | HB | Retrospective cohort | 124 | NA | Mild: 37Severe: 87 | NA | Biochemical assay | IL-6 | 7 |
| Liu et al 70 | Asian | China | HB | Retrospective cohort | 101 | 62.33 ± 17.3 | Mild: 47Severe: 54 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Liu et al 71 | Asian | China | HB | Retrospective | 76 | 47 ± 45.36 | Mild: 30Severe: 46 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Liu et al 72 | Asian | China | HB | Single-center, retrospective | 67 | Mild: 46 ± 22.79Severe: 64.77 ± 11.79Critical: 64 ± 11.21 | Mild: 10Severe: 57 | NA | Flow cytometry | IL-6, IL-10 | 6 |
| Luo et al 73 | Asian | China | HB | Multicenter, Retrospective | 1018 | 59.67 ± 14.85 | NA | Dead: 201Survivors: 817 | CLIA | IL-6, IL-10 | 9 |
| Lu et al 74 | Asian | China | HB | Single-center, retrospective | 121 | 6.25 ± 4.31 | Mild: 101Severe: 20 | NA | Flow cytometry | IL-6, IL-10 | 9 |
| Lv et al 75 | Asian | China | HB | Retrospective cohort | 354 | 58.33 ± 49.87 | Mild: 115Severe: 239 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Ma et al 76 | Asian | China | HB | Single-center, retrospective | 37 | 63.67 ± 8.48 | Mild: 17Severe: 20 | NA | Biochemical assay | IL-6 | 6 |
| Ma et al 77 | Asian | China | HB | Retrospective cohort | 84 | 50.93 ± 15.24 | Mild: 64Severe: 20 | NA | Flow cytometry | IL-6 | 7 |
| Maeda et al 78 | Caucasian | USA | HB | Single-center retrospective cohort | 224 | 63 ± 17 | Mild: 167Severe: 57 | NA | Biochemical assay | IL-6 | 8 |
| Mandel et al 79 | Caucasian | Israel | HB | Prospective non-randomized cohort | 71 | 62 ± 13.8 | NA | Dead: 12Survivors: 59 | ELISA | IL-6 | 8 |
| McElvaney et al 80 | Caucasian | Ireland | HB | Retrospective | 40 | 55.5 ± 17.7 | Mild: 20Severe: 20 | NA | ELISA | IL-6, IL-10 | 8 |
| Merza et al 81 | Caucasian | Iraq | HB | Retrospective | 56 | Mild: 35.7Severe: 51.75 | Mild: 41Severe: 15 | NA | ELISA | IL-6, IL-10 | 8 |
| Mikami et al 82 | Caucasian | USA | HB | Multicenter Retrospective cohort | 6493 | 58 ± 21.5 | Mild: 2785Severe: 3708 | Dead: 806Survivors: 2014 | Biochemical assay | IL-6 | 7 |
| Mo et al 83 | Asian | China | HB | Single-center, retrospective | 155 | 54 ± 17.96 | Mild: 70Severe: 85 | NA | Biochemical assay | IL-6 | 6 |
| Morisson et al 84 | Caucasian | USA | HB | Retrospective observational cohort | 81 | 64.33 ± 9.81 | NA | Dead: 35Survivors: 46 | Biochemical assay | IL-6 | 6 |
| Myhre et al 85 | Caucasian | Norway | HB | Prospective observational | 123 | Dead: 64.3 ± 10.7Survivors: 57.8 ± 16.3 | NA | Dead: 35Survivors: 88 | ECLIA | IL-6 | 7 |
| Nie et al 86 | Asian | China | HB | Retrospective | 97 | 43 ± 22.58 | Mild: 72Severe: 25 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Pandolfi et al 87 | Caucasian | Italy | HB | Prospective cohort | 33 | Mild: 62.67 ± 8.05Severe: 58 ± 11.33 | Mild: 5Severe: 28 | NA | ELISA | IL-6 | 8 |
| Qin et al 88 | Asian | China | HB | Single-center, retrospective | 452 | 57.33 ± 14.87 | Mild: 166Severe: 286 | NA | Flow cytometry | IL-6, IL-10 | 8 |
| Quartuccio et al 89 | Caucasian | Italy | HB | Retrospective | 24 | Dead: 68.8 ± 9.4Survivors: 65.8 ± 8.2 | NA | Dead: 6Survivors: 18 | ECLIA | IL-6 | 7 |
| Quiroga et al 90 | Caucasian | Spain | HB | Single-centered, prospective, and observational | 16 | 72 ± 15 | NA | Dead: 4Survivor s: 12 | Enzyme-immune assay | IL-6 | 7 |
| Rastrelli et al 91 | Caucasian | Italy | HB | Retrospective cohort | 31 | Mild: 61.5 ± 9.14Severe: 62.83 ± 48.15Dead: 73 ± 27.85 | Mild: 21Severe: 10 | NA | ECLIA | IL-6 | 8 |
| Ruan et al 92 | Asian | China | HB | Multicenter Retrospective cohort | 150 | Dead: 54.33 ± 49.99Survivors: 58.3 ± 27.9 | NA | Dead: 68Survivors: 82 | Biochemical assay | IL-6 | 6 |
| Sabaka et al 93 | Caucasian | Slovakia | HB | Retrospective | 45 | Mild: 80.33 ± 10.98Severe: 85.83 ± 7.61 | Mild: 26Severe: 19 | NA | ECLIA | IL-6 | 8 |
| Sarfaraz et al 94 | Asian | Pakistan | HB | Prospective cohort | 170 | Dead: 61 ± 12.57Survivors: 53 ± 13 | NA | Dead: 67Survivors: 103 | Biochemical assay | IL-6 | 6 |
| Sarhan et al 95 | African | Egypt | HB | Retrospective | 203 | 58.67 ± 23.89 | Mild: 26Severe: 19 | NA | ELISA | IL-6 | 8 |
| Shi et al 96 | Asian | China | HB | Multicenter Retrospective cohort | Zhao et al 148 | 50.58 ± 10.67 | Mild: 119Severe: 29 | NA | Biochemical assay | IL-6, IL-10 | 9 |
| Shi et al 97 | Asian | China | HB | Retrospective | 87 | 56.67 ± 49.75 | Mild: 51Severe: 36 | NA | Biochemical assay | IL-6 | 6 |
| Shi et al 98 | Asian | China | HB | Prospective observational | 45 | Mild: 40.23 ± 12.61Severe: 59.35 ± 18.07Critical: 66.9 ± 17.01 | Mild: 13Severe: 32 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Simioli et al 99 | Caucasian | Italy | HB | Single-center case-control | 29 | 64 ± 22.5 | Mild: 11Severe: 18 | NA | Biochemical assay | IL-6 | 7 |
| Song et al 100 | Asian | China | HB | Retrospective | 73 | Mild: 48 ± 17.1Severe: 55.93 ± 12.51 | Mild: 31Severe: 42 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Song et al 101 | Asian | China | HB | Single-center, retrospective cohort | 1172 | 59 ± 14.84 | Mild: 881Severe: 291 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Song et al 102 | Asian | China | HB | Cross sectional observational | 41 | 40.83 ± 12.68 | Mild: 29Severe: 12 | NA | Flow cytometry | IL-6 | 8 |
| Sun et al 103 | Asian | China | HB | Retrospective | 244 | Dead: 72 ± 9Survivors: 67.67 ± 6 | NA | Dead: 121Survivors: 123 | Biochemical assay | IL-6 | 7 |
| Sun et al 104 | Asian | China | HB | Prospective observational cohort | 99 | Mild: 52 ± 15.28Severe: 70.83 ± 14.88 | Mild: 49Severe: 50 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Sun et al 105 | Asian | China | HB | Prospective cohort | 63 | 45 ± 62.21 | Mild: 44Severe: 19 | NA | Biochemical assay | IL-6 | 6 |
| Taha et al 106 | African | Egypt | HB | Observational cohort | 85 | 54 ± 17.35 | Mild: 46Severe: 39 | Dead: 21Survivors: 64 | ELISA | IL-6 | 8 |
| Tang et al 107 | Asian | China | HB | Prospective | 120 | 58 ± 15.76 | Mild: 60Severe: 60 | NA | Flow cytometry | IL-6, IL-10 | 9 |
| Tian et al 108 | Asian | China | HB | Multicenter, retrospective, cohort | 751 | 63.33 ± 8.91 | Mild: 84Severe: 148 | NA | CLIA | IL-6, IL-10 | 9 |
| Toniati et al 109 | Caucasian | Italy | HB | Single-centered, prospective | 100 | 63.33 ± 10.53 | Mild: 77Severe: 23 | NA | Biochemical assay | IL-6 | 6 |
| Tu et al 110 | Asian | China | HB | Single-center, retrospective cohort | 174 | Dead: 71.33 ± 12.58Survivors: 50 ± 18.71 | NA | Dead: 25Survivors: 149 | Biochemical assay | IL-6 | 7 |
| Vultaggio et al 17 | Caucasian | Italy | HB | Retrospective observational cohort | 208 | 65.7 ± 15 | Mild: 145Severe: 63 | NA | ELISA | IL-6 | 8 |
| Wan et al 111 | Asian | China | HB | Prospective | 66 | Mild: 43.05 ± 13.12Severe: 61.29 ± 15.55 | Mild: 45Severe: 21 | NA | Flow cytometry | IL-6, IL-10 | 8 |
| Wang et al 112 | Asian | China | HB | Retrospective | 28 | 68.6 ± 9 | Mild: 14Severe: 14 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Wang et al 113 | Asian | China | HB | Multicenter, retrospective | 165 | 45.67 ± 11.97 | Mild: 115Severe: 50 | NA | Biochemical assay | IL-6 | 7 |
| Wang et al 114 | Asian | China | HB | Retrospective | 339 | 70 ± 8.19 | NA | Dead: 65Survivors: 274 | Biochemical assay | IL-6 | 6 |
| Wang et al 115 | Asian | China | HB | Single-center, retrospective, descriptive | 125 | 38.76 ± 13.799 | Mild: 100Severe: 25 | NA | Biochemical assay | IL-6 | 6 |
| Wang et al 116 | Asian | China | HB | Retrospective case-control | 43 | Mild: 43.05 ± 13.12Severe: 61.29 ± 15.55 | Mild: 35Severe: 8 | NA | Flow cytometry | IL-6. IL-10 | 8 |
| Wang et al 117 | Asian | China | HB | Retrospective cohort | 43 | 46.33 ± 20.44 | Mild: 36Severe: 7 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Wang et al 118 | Asian | China | HB | Retrospective | 59 | 67.4 ± 11.3 | NA | Dead: 41Survivors: 18 | CLIA | IL-6, IL-10 | 6 |
| Webb et al 119 | Caucasian | USA | HB | Prospective observational cohort | 72 | 55.67 ± 18.62 | Mild: 5Severe: 67 | NA | Biochemical assay | IL-6 | 6 |
| Wei et al 120 | Asian | China | HB | Retrospective | 252 | 64.8 ± 13.3 | Mild: 131Severe: 98 | NA | CLIA | IL-6, IL-10 | 9 |
| Wu et al 121 | Asian | China | HB | Retrospective cohort | 201 | 51.33 ± 12.69 | Mild: 117Severe: 84 | Dead: 44Survivor s: 40 | Biochemical assay | IL-6 | 6 |
| Wu et al 122 | Asian | China | HB | Single-center, retrospective cohort | Zhao et al 148 | 75 ± 78.61 | Mild: 60Severe: 88 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Wu et al 123 | Asian | China | HB | Retrospective | 71 | 57 ± 21.19 | Mild: 32Severe: 39 | NA | Flow cytometry | IL-6, IL-10 | 8 |
| Xiao et al 124 | Asian | China | HB | Retrospective | 143 | NA | Mild: 107Severe: 36 | NA | Biochemical assay | IL-6 | 6 |
| Xie et al 125 | Asian | China | HB | Retrospective | 29 | 64.1 ± 14.95 | Mild: 22Severe: 7 | NA | Biochemical assay | IL-6 | 7 |
| Xu et al 126 | Asian | China | HB | Single-centered, retrospective observational | 187 | 60.5 ± 16.81 | Mild: 80Severe: 107 | Dead: 28Survivors: 117 | Biochemical assay | IL-6, IL-10 | 7 |
| Xu et al 127 | Asian | China | HB | Multicenter Retrospective observational | 324 | 63.2 ± 14.5 | Mild: 177Severe: 147 | NA | Biochemical assay | IL-6 | 8 |
| Xu et al 128 | Asian | China | HB | Retrospective | 155 | Mild: 39.84 ± 15.09Severe: 50.97 ± 13.55 | Mild: 125Severe: 30 | NA | Biochemical assay | IL-6 | 6 |
| Xu et al 69 | Asian | China | HB | Single-center, retrospective cohort | 88 | 57.11 ± 15.39 | Mild: 47Severe: 41 | NA | Biochemical assay | IL-6 | 6 |
| Xu et al 129 | Asian | China | HB | Multicenter Retrospective | 69 | 56.33 ± 19.69 | Mild: 44Severe: 25 | NA | Flow cytometry | IL-6 | 9 |
| Yan et al 130 | Asian | China | HB | Single-centered, retrospective observational | 48 | 69.4 ± 9.9 | NA | Dead: 39Survivors: 9 | Biochemical assay | IL-6 | 6 |
| Yang et al 131 | Asian | China | HB | Single-center, retrospective | 93 | 46.4 ± 17.6 | Mild: 69Severe: 24 | NA | Flow cytometry | IL-6, IL-10 | 8 |
| Yang et al 132 | Asian | China | HB | Retrospective | 76 | NA | Mild: 42Severe: 34 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Yang et al 133 | Asian | China | HB | Retrospective observational | 55 | 44 ± 15.23 | Mild: 21Severe: 34 | NA | Biochemical assay | IL-6 | 6 |
| Yang et al 134 | Asian | China | HB | Retrospective case-control | 45 | 32 ± 29.87 | Mild: 23Severe: 22 | NA | Flow cytometry | IL-6 | 8 |
| Yuan et al 135 | Asian | China | HB | Retrospective | 189 | 60.33 ± 14.94 | Mild: 102Severe: 87 | NA | Biochemical assay | IL-6 | 6 |
| Yuan et al 136 | Asian | China | HB | Retrospective | 117 | 64.67 ± 10.51 | Mild: 53Severe: 54 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Zeng et al 137 | Asian | China | HB | Retrospective | 49 | Mild: 46 ± 19Severe: 60 ± 16Critical: 68 ± 20 | Mild: 28Severe: 21 | NA | Biochemical assay | IL-6, IL-10 | 6 |
| Zeng et al 138 | Asian | China | HB | Retrospective | 317 | 61 ± 14.15 | Mild: 93Severe: 224 | NA | CLIA | IL-6, IL-10 | 8 |
| Zhang et al 139 | Asian | China | HB | Retrospective | 222 | 61 ± 12.69 | Mild: 81Severe: 67 | NA | Automated immunoassay multiplex array system | IL-6, IL-10 | 8 |
| Zhang et al 140 | Asian | China | HB | Retrospective | 82 | 72.5 ± 11.32 | NA | Dead: 82Survivors: 0 | Automated immunoassay multiplex array system | IL-6 | 8 |
| Zhang et al 141 | Asian | China | HB | Retrospective case-series | 98 | 63.9 ± 1.4 | NA | Dead: 36Survivors: 62 | Biochemical assay | IL-6 | 7 |
| Zhang et al 142 | Asian | China | HB | Retrospective | 43 | Mild: 44.4 ± 15.9Severe: 61.9 ± 9.4 | Mild: 29Severe: 14 | NA | ELISA | IL-6, IL-10 | 8 |
| Zhang et al 143 | Asian | China | HB | Single-center, retrospective | 111 | 42.33 ± 18.78 | Mild: 93Severe: 18 | Dead: 18Survivors: 93 | Biochemical assay | IL-6, IL-10 | 7 |
| Zhang et al 144 | Asian | China | HB | Retrospective | 134 | 60.78 ± 12.98 | Mild: 33Severe: 101 | Dead: 101Survivors: 33 | Biochemical assay | IL-6 | 6 |
| Zhang et al 145 | Asian | China | HB | Single-center retrospective observational | 74 | 63.33 ± 12.10 | Mild: 47Severe: 27 | NA | Biochemical assay | IL-6 | 7 |
| Zhang et al 146 | Asian | China | HB | Retrospective | 38 | Dead: 37.7 ± 8.2Survivors: 35.8 ± 4.1 | NA | Dead: 18Survivors: 20 | Biochemical assay | IL-6 | 6 |
| Zhang et al 147 | Asian | China | HB | Retrospective | 326 | 51.33 ± 54.36 | Mild: 28Severe: 293 | NA | Flow cytometry | IL-6 | 8 |
| Zhao et al 149 | Asian | China | HB | Single-center, retrospective | 172 | 64.33 ± 10.47 | Mild: 112Severe: 60 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Zhao et al 150 | Asian | China | HB | Prospective | 71 | 49.33 ± 19.67 | Mild: 53Severe: 18 | NA | Bio plex multiplex immunoassay | IL-6, IL-10 | 8 |
| Zheng et al 150 | Asian | China | HB | Single-center, Retrospective cohort | 96 | 54.7 ± 15.43 | Mild: 22Severe: 74 | NA | Biochemical assay | IL-6, IL-10 | 7 |
| Zheng et al 151 | Asian | China | HB | Retrospective | 34 | 66.67 ± 13.93 | Mild: 19Severe: 15 | NA | ELISA | IL-6, IL-10 | 8 |
| Zhou et al 41 | Asian | China | HB | Multicenter, retrospective, cohort | 191 | 56.33 ± 15.69 | NA | Dead: 54Survivors: 137 | Biochemical assay | IL-6 | 7 |
| Zhou et al 152 | Asian | China | HB | Single-center, retrospective | 21 | 66.10 ± 13.94 | Mild: 8Severe: 13 | NA | Automatic biochemical analyzer | IL-6 | 9 |
| Zhu et al 153 | Asian | China | HB | Retrospective | 127 | 50.90 ± 15.26 | Mild: 111Severe: 16 | NA | Flow cytometry | IL-6, IL-10 | 8 |
| Zou et al 154 | Asian | China | HB | Retrospective | 121 | 63.83 ± 12.38 | Mild: 69Severe: 52 | Dead: 14Survivors: 107 | Biochemical assay | IL-6, IL-10 | 7 |
Abbreviations: CLIA, chemiluminescent immunoassay; ECLIA, electro-chemiluminescent immunoassay; ELISA, enzyme-linked immunosorbent assay; HB, hospital based; NA, not available; NOS, Newcastle Ottawa Scale; OLHDF, online hemodiafiltration.
Association of IL-6 with the severity and mortality of COVID-19
We assessed 107 studies to verify fluctuation in serum IL-6 concentration among COVID-19 patients in response to disease severity. Comparatively, elderly patients showed severe to critical symptoms than younger patients, according to selected studies. The mean difference in serum IL-6 level was 19.98 higher in the severe patients than in the mild category patients. IL-6 level showed significant elevation in the severe COVID-19 cases (MD: 19.98; P < .001; 95% CI: 17.56, 22.40).
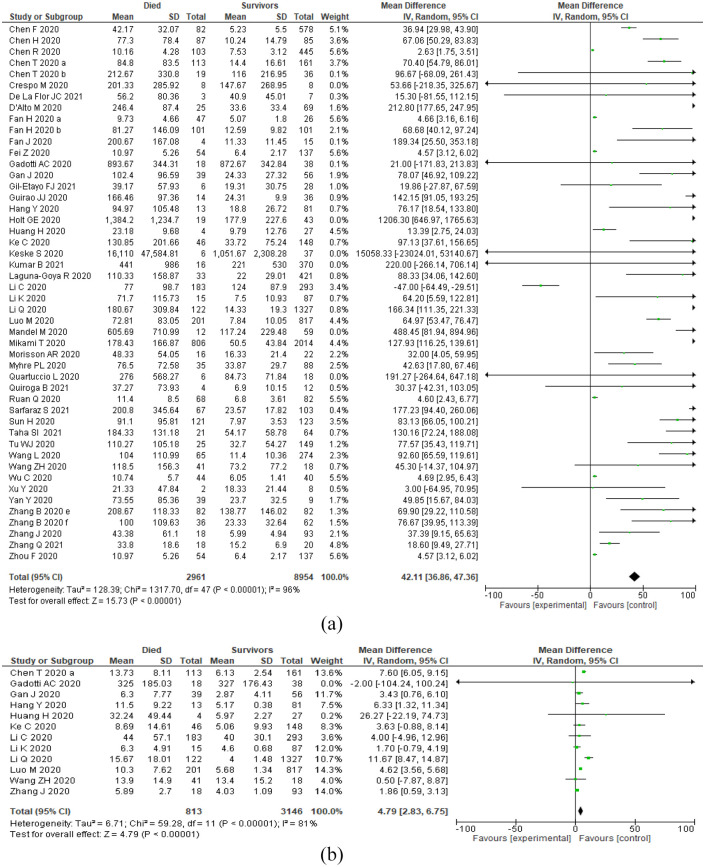
To evaluate the impact of IL-6 level on the mortality of COVID-19 patients, 49 studies of 147 included studies were assessed. The result showed that deceased COVID-19 patients had 42.11 times higher mean concentration than survived patients. IL-6 level was significantly increased in the dead patients (MD: 42.11; P < .001; 95% CI: 36.86, 47.36), and the fluctuation was highly noticeable (Table 2, Figures 2 and 3).
Table 2.
Effect of elevated IL-6 and IL-10 levels on disease severity and mortality in COVID-19 patients.
| Interleukin | Covariates | Test of association | Test of heterogeneity | Publication bias (P-value) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean difference | 95% CI | P-value | Model | P-value | I2 (%) | Egger’s test | Begg-Mazumdar’s test | ||
| IL-6 | Severity | 19.98 | 17.56, 22.40 | <.001 | Random | <.001 | 97 | 0.005 | 0.023 |
| Mortality | 42.11 | 36.86, 47.36 | <.001 | Random | <.001 | 98 | 0.718 | 0.716 | |
| IL-10 | Severity | 1.35 | 0.90, 1.80 | <.001 | Random | <.001 | 91 | 0.091 | 0.455 |
| Mortality | 4.79 | 2.83, 6.75 | <.001 | Random | <.001 | 81 | 0.669 | 1.00 | |
Figure 2.
Forest plots showing IL-6 and IL-10 levels in COVID-19 patients based on disease severity index: (a) IL-6 levels in severe and non-severe COVID-19 cases and (b) IL-10 levels in severe and non-severe COVID-19 cases.
Figure 3.
Forest plots showing IL-6 and IL-10 levels in COVID-19 patients based on mortality index: (a) IL-6 levels in dead and survivors COVID-19 cases and (b) IL-10 levels in dead and survivors COVID-19 cases.
Association of IL-10 with the severity and mortality of COVID-19
Fifty-two studies were assessed to identify fluctuation in serum IL-10 concentration according to disease severity among COVID-19 patients. The mean difference in serum IL-10 level was 1.35 between severe and mild COVID-19 patients. IL-10 level showed significantly high concentration in the severe COVID-19 cases (MD: 1.35; P < .001; 95% CI: 0.90, 1.80).
To evaluate the impact of elevated IL-10 level on the mortality of COVID-19 patients, 12 studies of 147 included studies were assessed. The outcome showed that dead COVID-19 patients had an increased IL-10 to a mean concentration of 4.79 than survived patients. IL-10 level was significantly increased in the deceased patients (MD: 4.79; P < .001; 95% CI: 2.83, 6.75), and the fluctuation indicated that IL-10 might be associated with mortality in COVID-19 (Table 2, Figures 2 and 3).
Sensitivity analysis and publication bias
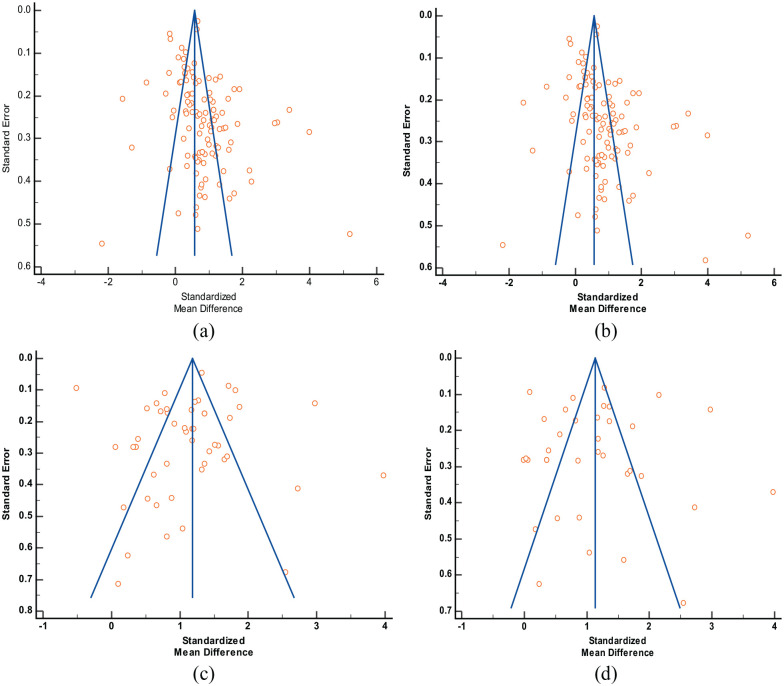
No visual asymmetry was observed during analyzing funnel plots indicating the absence of publication bias (Figure 4). Egger’s regression test showed a significant outcome in IL-6 versus COVID-19 severity model (P = .005). Other analyses did not show any significant publication bias (IL-6 vs COVID-19 mortality: P = .652; IL-10 vs COVID-19 severity: P = .091; IL-10 vs COVID-19 mortality: P = .669). Begg-Mazumdar’s test also showed similar results (IL-6 vs COVID-19 severity: P = .023; IL-6 vs COVID-19 mortality: P = .730; IL-10 vs COVID-19 severity: P = .455; IL-10 vs COVID-19 mortality: P = 1.00). The results are shown in Table 2. Sensitivity analysis was performed by excluding the studies one by one to verify the stability of the final outcome. The final outcome was stable, and none of the studies interfered with the core results (not shown).
Figure 4.
Funnel plots for publication bias analysis in different meta-analysis models: (a) IL-6 and severity of COVID-19, (b) IL-10 and severity of COVID-19, (c) IL-6 and mortality of COVID-19, and (d) IL-10 and mortality of COVID-19.
Discussion
The wave of COVID-19 is still ongoing, with full rhythm failing a number of attempts to control this pandemic situation. Most of the COVID-19 cases remain mild, and patients get their recovery from the fully active natural immune system, but 14% of patients face severe symptoms that lead to ARDS, septic shock and multiple organ failure. 13 The ultimate outcome of severe SARS-CoV-2 infection becomes life-threatening, which is undeniable. Moreover, the rate of survival is minimal in severe to critical cases. The burden of emergency COVID-19 cases is uprising drastically worldwide. SARS-CoV-2 infection has become a threat to human race, and researchers are still struggling to improve this overwhelming situation. Early detection of the severe stage of infection could cease the disease progression toward the critical stage. Checkpoint of severity will also reduce the risk of mortality from COVID-19. 8
In our study, we have accumulated a number of evidence suggesting that cytokine storms developed during SARS-CoV-2 infection intensify the damage rapidly. Elderly patients, children, or patients with a previous disease condition with a weak immunity system mostly show a severe immune reaction. Anti-inflammatory treatments could not instantly reduce the sharp elevation of cytokines in the human body. As a result, the consequences of acute tissue damage and critical lung inflammation become challenging to control. 155 IL-6 and IL-10 show a significant elevation in COVID-19 patients with mild conditions, and the concentration sharply increases manyfold when the condition gets worsens. These 2 biomarkers should be observed as a primary prognostic indicator in COVID-19 patients to understand the disease state.155,156
Efficient immune activity is essential in the fight against any infection, although overproduction and unnecessary activation of active immune cells may cause much more irreversible damage than the actual infection. In COVID-19 cases, cytokine storm in the risk population reduces the lung capacity by flooding lung surfaces with inflammatory cells. The oversensitive immune activity becomes ineffective and fills the air sacks of the lungs with fluid limiting their oxygen uptake ratio, which leads to inevitable deaths.157-159 IL-6 and IL-10 are major pleiotropic interleukins involved in potent inflammatory reactions observed in human body during any infection. Among these 2 cytokines, IL-6 helps to conduct acute phase immune reactions by recruiting immune cells in the infected area. But the excess level of IL-6 is responsible for anaphylactic shock or cytokine storm. This phenomenon will cause additional damage rather than wiping out infectious agents. On the other hand, IL-10 is responsible for maintaining homeostatic balance in the immune system by exerting anti-inflammatory actions. Human immune system can control or inhibit severe inflammation itself when the body starts healing by a homeostatic mechanism. Both IL-6 and IL-10 are closely involved in COVID-19 pathogenesis.160-162 IL-6 is one of the critical inflammatory mediators in patients severely suffering from COVID-19. The level of IL-6 is elevated in these subjects and has been considered an important choice for COVID-19 targeting. Therapeutic agents (eg, sarilumab, tocilizumab) that suppress the IL-6 signaling mechanism have been reported to be effective against COVID-19.163-165
Many studies attempted to find out immune-inflammatory predictors for disease severity in COVID-19. A recent systematic review and meta-analysis that included 19 studies with 3115 participants found that IL-6 and IL-10 level was higher in the severe COVID-19 cases than in the non-severe cases. 166 Another study performed on 24 articles with 6212 participants recommended both IL-6 and IL-10 as potential biomarkers for COVID-19 severity and mortality. 155 Bao et al 167 reported that severe patients had increased levels of IL-6 (1.93-fold) and IL-10 (1.55-fold) serum concentration in a study involving 35 articles (5912 patients). Zawawi et al 168 showed that both the interleukins are associated with the severity of COVID-19 in their recent meta-analysis. In another network meta-analysis with 71 eligible studies involving 8647 patients, a rise in the IL-6 and IL-10 count was observed with worsening of the COVID-19 infection. 169 Other studies with a limited sample size also conducted a similar assessment and reported similar findings.8,19-21 The evidence from these studies was sub-optimal and significant heterogeneity was observed due to the limited sample size. To create valid evidence that sharp elevation in IL-6 and IL-10 levels should be considered as a checkpoint of COVID-19 severity and mortality, we carried out this large-scale updated meta-analysis.
The findings from our present meta-analysis revealed that the mean IL-6 and IL-10 serum level was significantly higher in the COVID-19 patients. Severe category patients faced a sharp increase in the serum level compared to non-severe category patients. A similar result was also observed in the case of mortality. The deceased patients showed abnormally high serum concentrations of IL-6 and IL-10 than the survived patients. Moreover, the concentration of serum interleukins in the dead patients was significantly higher than the severe cases of COVID-19.
The current meta-analysis had some drawbacks that should be mentioned. Most of the included studies were retrospective cohort studies with smaller sample sizes. As the number of studies was huge, some detailed and basic information like—sex, treatment, duration of infection, smoking habit, and body mass index (BMI) could not be added to the meta-analysis. The presence of heterogeneity was another limitation of the study. The heterogeneity may be due to the different ethnic groups, sample size variation, different interventions to treat the symptoms of COVID-19 and variation in the inclusion and exclusion criteria for mild and severe groups selection. In spite of the limitations, our study is methodologically strong. According to our understanding, this is the most comprehensive and updated systematic review and meta-analysis on the association between circulating levels of IL-6 and IL-10 and the severity and mortality of COVID-19.
Conclusion
In summary, this investigative meta-analysis confirmed that sharp elevation in serum IL-6 and IL-10 worsens COVID-19 clinical outcomes. IL-6 and IL-10 are associated with the severity and mortality of COVID-19. The circulating level of both interleukins can act as potential biomarkers for the disease severity and mortality in SARS-CoV-2 infected patients.
Supplemental Material
Supplemental material, sj-docx-1-bmi-10.1177_11772719221106600 for Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies by Sarah Jafrin, Md. Abdul Aziz and Mohammad Safiqul Islam in Biomarker Insights
Acknowledgments
This meta-analysis was supported and assisted by the Department of Pharmacy, Noakhali Science and Technology University, Sonapur, Noakhali.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: MSI conceptualized this meta-analysis; SJ and MAA wrote the primary draft; MSI carried out the statistical analyses; MSI critically reviewed and revised the manuscript; Before submission, all authors read and approved the final version of the manuscript.
Data Availability Statement: All data generated or analyzed during the present meta-analysis are available from the corresponding author on reasonable request.
ORCID iD: Mohammad Safiqul Islam  https://orcid.org/0000-0003-4924-5319
https://orcid.org/0000-0003-4924-5319
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergantini L, Bargagli E, d’Alessandro M, et al. Prognostic bioindicators in severe COVID-19 patients. Cytokine. 2021;141:155455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO. COVID-19 Weekly Epidemiological Update. World Health Organization; 2021. Accessed December 20, 2021. https://www.who.int/publications/m/item/covid-19-weekly-epidemiological-update [Google Scholar]
- 4. Chen F, Sun W, Sun S, Li Z, Wang Z, Yu L. Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China. Clin Transl Med. 2020;10:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding L, Zhang W, Zhang F, et al. Prognostic role and diagnostic power of seven indicators in COVID-19 patients. Front Med. 2021;8:733274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feng X, Li P, Ma L, et al. Clinical characteristics and short-term outcomes of severe patients with COVID-19 in Wuhan, China. Front Med. 2020;7:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [DOI] [PubMed] [Google Scholar]
- 9. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chi Y, Ge Y, Wu B, et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis. 2020;222:746-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Zhou J, Chen C, et al. Consecutive monitoring of interleukin-6 is needed for COVID-19 patients. Virol Sin. 2021;36:1093-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gadotti AC, de Castro Deus M, Telles JP, et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020;289:198171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9:1123-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El-Shabrawy M, Alsadik ME, El-Shafei M, et al. Interleukin-6 and C-reactive protein/albumin ratio as predictors of COVID-19 severity and mortality. Egypt J Bronchol. 2021;15:5. [Google Scholar]
- 15. Guirao JJ, Cabrera CM, Jiménez N, Rincón L, Urra JM. High serum IL-6 values increase the risk of mortality and the severity of pneumonia in patients diagnosed with COVID-19. Mol Immunol. 2020;128:64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146:128-136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vultaggio A, Vivarelli E, Virgili G, et al. Prompt predicting of early clinical deterioration of moderate-to-severe COVID-19 patients: usefulness of a combined score using IL-6 in a preliminary study. J Allergy Clin Immunol Pract. 2020;8:2575-2581.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henry BM, Benoit SW, Vikse J, et al. (Covid-19). Clin Chem Lab Med. 2020;59:599-607. [DOI] [PubMed] [Google Scholar]
- 19. Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51:e13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8:1233-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coomes EA, Haghbayan H. Interleukin-6 in covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019). Cochrane; 2019. https://training.cochrane.org/handbook/current [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burian E, Jungmann F, Kaissis GA, et al. Intensive care risk estimation in COVID-19 pneumonia based on clinical and imaging parameters: experiences from the Munich cohort. J Clin Med. 2020;9:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [DOI] [PubMed] [Google Scholar]
- 26. Chang Z, Yang W, Wang Q, Liao G, et al. Clinical significance of serum hs-CRP, IL-16, and PCT in diagnosis and prognosis of patients with COVID-19. Drugs Clin. 2020;35:417-420. [Google Scholar]
- 27. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig. 2020;130:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen H, Wang J, Su N, Bao X, Li Y, Jin J. Simplified immune-dysregulation index: a novel marker predicts 28-day mortality of intensive care patients with COVID-19. Intensive Care Med. 2020;46:1645-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Huiguo L, Wei L, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:203-208. [DOI] [PubMed] [Google Scholar]
- 30. Chen LD, Zhang ZY, Wei XJ, et al. Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir Res. 2020;21:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146:89-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75:1788-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20:2883-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De La Flor JC, Valga F, Marschall A, et al. Targeting cytokine storm in COVID-19: a role of online hemodiafiltration with asymmetric cellulose triacetate in maintenance hemodialysis patients-a report of 10 cases. Case Rep Nephrol. 2021;2021:5575928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. D’Alto M, Marra AM, Severino S, et al. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit Care. 2020;24:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding M, Zhang Q, Li Q, Wu T, Huang YZ. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir Med. 2020;167:105981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan H, Zhang L, Huang B, et al. Cardiac injuries in patients with coronavirus disease 2019: not to be ignored. Internet J Infect Dis. 2020;96:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol Infect. 2020;148:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fan J, Wang H, Ye G, et al. Letter to the editor: Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fei M, Tong F, Tao X, Wang J. Value of neutrophil-to-lymphocyte ratio in the classification diagnosis of coronavirus disease 2019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:554-558. [DOI] [PubMed] [Google Scholar]
- 41. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gan J, Li J, Li S, Yang C. Leucocyte subsets effectively predict the clinical outcome of patients with COVID-19 pneumonia: a retrospective case-control study. Front Public Health. 2020;8:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gil-Etayo FJ, Suàrez-Fernández P, Cabrera-Marante O, et al. T-Helper cell subset response is a determining factor in COVID-19 progression. Front Cell Infect Microbiol. 2021;11:624483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Güner R, Hasanoğlu I, Kayaaslan B, et al. COVID-19 experience of the major pandemic response center in the capital: results of the pandemic’s first month in Turkey. Turk J Med Sci. 2020;50:1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang H, Yang Lc, Zhang RT, Ling YP, Ge QG. Risks factors for death among COVID-19 patients combined with hypertension, coronary heart disease or diabetes. Beijing Da Xue Xue Bao. 2020;52:420-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID-19 pneumonia. J Clin Virol. 2020;127:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He S, Zhou C, Lu D, et al. Relationship between chest CT manifestations and immune response in COVID-19 patients. Internet J Infect Dis. 2020;98:125-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Holt GE, Batra M, Murthi M, et al. Lack of tocilizumab effect on mortality in COVID19 patients. Sci Rep. 2020;10:17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hu ZJ, Xu J, Yin JM, et al. Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Front Immunol. 2020;11:585647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang H, Song B, Xu Z, et al. Predictors of coronavirus disease 2019 severity: a retrospective study of 64 cases. Jpn J Infect Dis. 2021;74:54-60. [DOI] [PubMed] [Google Scholar]
- 52. Huang W, Li M, Luo G, et al. The inflammatory factors associated with disease severity to predict COVID-19 progression. J Immunol. 2021;206:1597-1608. [DOI] [PubMed] [Google Scholar]
- 53. Huang Z, Xu S, Chen J, et al. Predictive value of laboratory tests on severity of newly hospitalized patients with COVID-19. Chin J Lab Med. 2020;43:973-977. [Google Scholar]
- 54. Jain A, Chaurasia R, Sengar NS, Singh M, Mahor S, Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10:20191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ke C, Yu C, Yue D, Zeng X, Hu Z, Yang C. Clinical characteristics of confirmed and clinically diagnosed patients with 2019 novel coronavirus pneumonia: a single-center, retrospective, case-control study. Med Clin. 2020;155:327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Keske Ş, Tekin S, Sait B, et al. Appropriate use of tocilizumab in COVID-19 infection. Internet J Infect Dis. 2020;99:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kumar B, Mittal M, Gopalakrishnan M, Garg MK, Misra S. Effect of plasma glucose at admission on COVID-19 mortality: experience from a tertiary hospital. Endocr Connect. 2021;10:589-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Laguna-Goya R, Utrero-Rico A, Talayero P, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:799-807.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li K, Chen D, Chen S, et al. Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res. 2020;21:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Q, Cao Y, Chen L, et al. Hematological features of persons with COVID-19. Leukemia. 2020;34:2163-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li X, Liu Y, Li J, et al. Immune characteristics distinguish patients with severe disease associated with SARS-CoV-2. Immunol Res. 2020;68:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu D, Cui P, Zeng S, et al. Risk factors for developing into critical COVID-19 patients in Wuhan, China: a multicenter, retrospective, cohort study. EClinicalMedicine. 2020;25:100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liu L, Zheng Y, Cai L, et al. Neutrophil-to-lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID-19. Int J Lab Hematol. 2021;43:329-335. [DOI] [PubMed] [Google Scholar]
- 65. Liu L, Gao JY, Hu WM, et al. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. MedRxiv. Published online February 23, 2020. doi: 10.1101/2020.02.20.20025536 [DOI] [Google Scholar]
- 66. Liu SP, Zhang Q, Wang W, et al. Hyperglycemia is a strong predictor of poor prognosis in COVID-19. Diabetes Res Clin Pract. 2020;167:108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12:e12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu X, Li Z, Liu S, et al. Potential therapeutic effects of dipyridamole in the severely ill patients with COVID-19. Acta Pharm Sin B. 2020;10:1205-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xu X, Yu MQ, Shen Q, et al. Analysis of inflammatory parameters and disease severity for 88 hospitalized COVID-19 patients in Wuhan, China. Int J Med Sci. 2020;17:2052-2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu XQ, Xue S, Xu JB, et al. Clinical characteristics and related risk factors of disease severity in 101 COVID-19 patients hospitalized in Wuhan, China. Acta Pharmacol Sin. 2022;43:64-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Y, Liao W, Wan L, Xiang T, Zhang W. Correlation between relative nasopharyngeal virus RNA load and lymphocyte count disease severity in patients with COVID-19. Viral Immunol. 2021;34:330-335. [DOI] [PubMed] [Google Scholar]
- 72. Liu Y, Tan W, Chen H, et al. Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect Dis. 2021;21:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Luo M, Liu J, Jiang W, Yue S, Liu H, Wei S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight. 2020;5:e139024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lu W, Yang L, Li X, et al. Early immune responses and prognostic factors in children with COVID-19: a single-center retrospective analysis. BMC Pediatr. 2021;21:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lv Z, Cheng S, Le J, et al. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microbes Infect. 2020;22:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ma J, Yin J, Qian Y, Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J Infect. 2020;81:318-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ma KL, Liu ZH, Cao CF, et al. COVID-19 myocarditis and severity factors: an adult cohort study. MedRxiv. Published online March 23, 2020. doi: 10.1101/2020.03.19.20034124 [DOI] [Google Scholar]
- 78. Maeda T, Obata R, Rizk Do D, Kuno T. The association of interleukin-6 value, interleukin inhibitors, and outcomes of patients with COVID-19 in New York City. J Med Virol. 2021;93:463-471. [DOI] [PubMed] [Google Scholar]
- 79. Mandel M, Harari G, Gurevich M, Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine. 2020;134:155190. doi: 10.1016/j.cyto.2020.155190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202:812-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Merza MY, Hwaiz RA, Hamad BK, Mohammad KA, Hama HA, Karim AY. Analysis of cytokines in SARS-CoV-2 or COVID-19 patients in Erbil city, Kurdistan Region of Iraq. PLoS One. 2021;16:e0250330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mikami T, Miyashita H, Yamada T, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2021;36:17-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73:e4208-e4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Morrison AR, Johnson JM, Griebe KM, et al. Clinical characteristics and predictors of survival in adults with coronavirus disease 2019 receiving tocilizumab. J Autoimmun. 2020;114:102512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Myhre PL, Prebensen C, Strand H, et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation. 2020;142:2128-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nie S, Zhao X, Zhao K, et al. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. MedRxiv. Published online March 26, 2020. doi: 10.1101/2020.03.24.20042283 [DOI] [Google Scholar]
- 87. Pandolfi L, Fossali T, Frangipane V, et al. Broncho-alveolar inflammation in COVID-19 patients: a correlation with clinical outcome. BMC Pulm Med. 2020;20:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Quartuccio L, Sonaglia A, Pecori D, et al. Higher levels of IL-6 early after tocilizumab distinguish survivors from nonsurvivors in COVID-19 pneumonia: a possible indication for deeper targeting of IL-6. J Med Virol. 2020;92:2852-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Quiroga B, Muñoz Ramos P, Giorgi M, et al. Dynamic assessment of interleukin-6 during hemodialysis and mortality in coronavirus disease-19. Ther Apher Dial. 2021;25:908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rastrelli G, Di Stasi V, Inglese F, et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. 2021;9:88-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sabaka P, Koščálová A, Straka I, et al. Role of interleukin 6 as a predictive factor for a severe course of covid-19: retrospective data analysis of patients from a long-term care facility during covid-19 outbreak. BMC Infect Dis. 2021;21:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sarfaraz S, Shaikh Q, Saleem SG, et al. Determinants of in-hospital mortality in COVID-19; a prospective cohort study from Pakistan. PLoS One. 2021;16:e0251754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sarhan W, Shalaby SM, Zidan N, et al. Cytokine storm in COVID-19 patients: association between cytokines and disease severity. Zagazig Univ Med J. 2021. doi:10.21608/ZUMJ.2021.76794.2237. [Google Scholar]
- 96. Shi H, He L, Sun W, et al. Clinical characteristics and prognostic factors of 148 COVID-19 cases in a secondary epidemic area. SSRN Electron J. Published online April 23, 2020. doi: 10.2139/ssrn.3572911 [DOI] [Google Scholar]
- 97. Shi S, Liu X, Xiao J, et al. Prediction of adverse clinical outcomes in patients with coronavirus disease 2019. J Clin Lab Anal. 2021;35:e23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shi X, Qin L, Yang L, Bai W, Jing L, Mei K. Value of interleukin-6 and CD4. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:1165-1170. [DOI] [PubMed] [Google Scholar]
- 99. Simioli F, Annunziata A, Langella G, Martino M, Musella S, Fiorentino G. Early prone positioning and non-invasive ventilation in a critical COVID-19 subset. A single centre experience in Southern Italy. Turk Thorac J. 2021;22:57-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. MedRxiv. Published online March 8, 2020. doi: 10.1101/2020.03.05.20031906 [DOI] [Google Scholar]
- 101. Song J, Deng YK, Wang H, et al. Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. Curr Med Sci. 2021;41:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Song JW, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11:3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sun H, Ning R, Tao Y, et al. Risk factors for mortality in 244 older adults with COVID-19 in Wuhan, China: a retrospective study. J Am Geriatr Soc. 2020;68:E19-E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sun JT, Chen Z, Nie P, et al. Lipid profile features and their associations with disease severity and mortality in patients with COVID-19. Front Cardiovasc Med. 2020;7:584987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sun Y, Dong Y, Wang L, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun. 2020;112:102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Taha S, Shata A, El-Sehsah E, et al. Evaluation of the predictive value of C-reactive protein, interleukin-6 and their derived immune-inflammatory indices in COVID-19 Egyptian patients. Microbes Infect Dis. 2022; 3(1):13-23. doi: 10.21608/mid.2021.103331.1207. [DOI] [Google Scholar]
- 107. Tang Y, Li Y, Sun J, Pan H, Yao F, Jiao X. Selection of an optimal combination panel to better triage COVID-19 hospitalized patients. J Inflamm Res. 2020;13:773-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. 2020;19:102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tu WJ, Cao J, Yu L, Hu X, Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. 2020;46:1117-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br J Haematol. 2020;189:428-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang F, Yang Y, Dong K, et al. Clinical characteristics of 28 patients with diabetes and covid-19 in Wuhan, China. Endocr Pract. 2020;26:668-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Wang H, Luo S, Shen Y, et al. Multiple enzyme release, inflammation storm and hypercoagulability are prominent indicators for disease progression in COVID-19: a multi-centered, correlation study with CT imaging score. Lancet. 2020. Accessed December 20, 2021. https://ssrn.com/abstract=3544837
- 114. Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. Int J Infect. 2020;80:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Internet J Infect Dis. 2020;95:421-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wang Y, Zhu F, Wang C, et al. Children hospitalized with severe COVID-19 in Wuhan. Pediatr Infect Dis J. 2020;39:e91-e94. [DOI] [PubMed] [Google Scholar]
- 117. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang ZH, Shu C, Ran X, Xie CH, Zhang L. Critically ill patients with coronavirus disease 2019 in a designated ICU: clinical features and predictors for mortality. Risk Manag Healthc Policy. 2020;13:833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Webb BJ, Peltan ID, Jensen P, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2:e754-e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wei X, Su J, Yang K, et al. Elevations of serum cancer biomarkers correlate with severity of COVID-19. J Med Virol. 2020;92:2036-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3:e2010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Wu Y, Huang X, Sun J, et al. Clinical characteristics and immune injury mechanisms in 71 patients with COVID-19. mSphere. 2020;5:e00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Xiao K, Lili S, Xiaohua P, et al. The clinical features of the 143 patients with COVID-19 in north-east of Chongqing. J Third Mil Med Univ. 2020;6:549-554. [Google Scholar]
- 125. Xie Y, You Q, Wu C, et al. Impact of cardiovascular disease on clinical characteristics and outcomes of coronavirus disease 2019 (COVID-19). Circ J. 2020;84:1277-1283. [DOI] [PubMed] [Google Scholar]
- 126. Xu B, Fan CY, Wang AL, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. 2020;81:e51-e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Xu J, Yang X, Huang C, et al. A novel risk-stratification models of the high-flow nasal cannula therapy in COVID-19 patients with hypoxemic respiratory failure. Front Med. 2020;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xu J, Zhao F, Han M, Ma L, Zhang T. Analysis of the clinical characteristics and early warning model construction of severe/critical coronavirus disease 2019 patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:401-406. [DOI] [PubMed] [Google Scholar]
- 129. Xu Y, Li YR, Zeng Q, et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. MedRxiv. Published online March 10, 2020. doi: 10.1101/2020.03.08.20031658 [DOI] [Google Scholar]
- 130. Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yang AP, Li HM, Tao WQ, et al. Infection with SARS-CoV-2 causes abnormal laboratory results of multiple organs in patients. Aging. 2020;12:10059-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yang J, Wang X, Tao Y. Analysis of clinical features of 76 cured patients of COVID-19. Acta Med Univ Sci Technol Huazhong. 2020;49:609-613. [Google Scholar]
- 133. Yang P, Ding Y, Xu Z, et al. Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China. MedRxiv. Published online March 3, 2020. doi: 10.1101/2020.02.28.20028068 [DOI] [Google Scholar]
- 134. Yang Q, Wen Y, Qi F, et al. Suppressive monocytes impair MAIT cells response via IL-10 in patients with severe COVID-19. J Immunol. 2021;207:1848-1856. [DOI] [PubMed] [Google Scholar]
- 135. Yuan X, Tong X, Wang Y, Wang H, Wang L, Xu X. Coagulopathy in elderly patients with coronavirus disease 2019. Aging Med (Milton). 2020;3:260-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Yuan X, Huang W, Ye B, et al. Changes of hematological and immunological parameters in COVID-19 patients. Int J Hematol. 2020;112:553-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zeng YL, Zhang C, Gao F, et al. Analysis of clinical characteristics of 49 cases of COVID-19. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:654-658. [DOI] [PubMed] [Google Scholar]
- 138. Zeng Z, Yu H, Chen H, et al. Longitudinal changes of inflammatory parameters and their correlation with disease severity and outcomes in patients with COVID-19 from Wuhan, China. Crit Care. 2020;24:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang B, Dong C, Li S, Song X, Wei W, Liu L. Triglyceride to high-density lipoprotein cholesterol ratio is an important determinant of cardiovascular risk and poor prognosis in coronavirus disease-19: a retrospective case series study. Diabetes Metab Syndr Obes. 2020;13:3925-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang H, Wang X, Fu Z, et al. Potential factors for prediction of disease severity of COVID-19 patients. MedRxiv. Published online March 23, 2020. doi: 10.1101/2020.03.20.20039818 [DOI] [Google Scholar]
- 143. Zhang J, Yu M, Tong S, Liu LY, Tang LV. Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J Clin Virol. 2020;127:104392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang L, Huang B, Xia H, et al. Retrospective analysis of clinical features in 134 coronavirus disease 2019 cases. Epidemiol Infect. 2020;148:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhang Q, Wei Y, Chen M, Wan Q, Chen X. Clinical analysis of risk factors for severe COVID-19 patients with type 2 diabetes. J Diabetes Complications. 2020;34:107666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang Q, Xiong Y, Wu T, Zhu W. Very fast-progressive pulmonary opacities and high inflammatory factors levels are associated with decease of young coronavirus disease 2019 patients. Medicine. 2021;100:e24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437-440. [DOI] [PubMed] [Google Scholar]
- 148. Zhao C, Bai Y, Wang C, et al. Risk factors related to the severity of COVID-19 in Wuhan. Int J Med Sci. 2021;18:120-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhao Y, Qin L, Zhang P, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 2020;5:e139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zheng Y, Sun LJ, Xu M, et al. Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China. J Zhejiang Univ Sci B. 2020;21:378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhou Y, Han T, Chen J, et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin Transl Sci. 2020;13:1077-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Internet J Infect Dis. 2020;95:332-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zou L, Dai L, Zhang Y, et al. Clinical characteristics and risk factors for disease severity and death in patients with coronavirus disease 2019 in Wuhan, China. Front Med. 2020;7:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Udomsinprasert W, Jittikoon J, Sangroongruangsri S, Chaikledkaew U. Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-analysis. J Clin Immunol. 2021;41:11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Brábek J, Jakubek M, Vellieux F, et al. Interleukin-6: molecule in the intersection of cancer, ageing and COVID-19. Int J Mol Sci. 2020;21:7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. 2019;33:869-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A. The Middle East respiratory syndrome (MERS). Infect Dis Clin North Am. 2019;33:891-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fattori E, Cappelletti M, Costa P, et al. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Stüber F, Wrigge H, Schroeder S, et al. Kinetic and reversibility of mechanical ventilation-associated pulmonary and systemic inflammatory response in patients with acute lung injury. Intensive Care Med. 2002;28:834-841. [DOI] [PubMed] [Google Scholar]
- 162. Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1-6. [DOI] [PubMed] [Google Scholar]
- 163. Yonas E, Alwi I, Pranata R, et al. Elevated interleukin levels are associated with higher severity and mortality in COVID 19 - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:2219-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Villaescusa L, Zaragozá F, Gayo-Abeleira I, Zaragozá C. A new approach to the management of COVID-19. Antagonists of IL-6: Siltuximab. Adv Ther. 2022;39:1126-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Calabrese C, Rajendram P, Sacha GL, Calabrese L. Practical aspects of targeting IL-6 in COVID-19 disease. Cleve Clin J Med. Published online March 14, 2020. doi: 10.3949/ccjm.87a.ccc018 [DOI] [PubMed] [Google Scholar]
- 166. Panda S, Nanda R, Tripathy PK, Mangaraj M. Immuno-inflammatory predictors of disease severity in COVID-19: a systematic review and meta-analysis. J Fam Med Prim Care. 2021;10:1102-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bao J, Li C, Zhang K, Kang H, Chen W, Gu B. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin Chim Acta. 2020;509:180-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Zawawi A, Naser AY, Alwafi H, Minshawi F. Profile of circulatory cytokines and chemokines in human coronaviruses: a systematic review and meta-analysis. Front Immunol. 2021;12:666223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Yan W, Chen D, Bigambo FM, Wei H, Wang X, Xia Y. Differences of blood cells, lymphocyte subsets and cytokines in COVID-19 patients with different clinical stages: a network meta-analysis. BMC Infect Dis. 2021;21:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-bmi-10.1177_11772719221106600 for Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies by Sarah Jafrin, Md. Abdul Aziz and Mohammad Safiqul Islam in Biomarker Insights