Abstract
Objectives and background: Oral squamous cell carcinoma (OSCC) is a highly malignant disease with an increasing incidence. The need to improve therapeutic strategies for patients affected by OSCC is an urgent challenge. Currently, the advent of immunotherapy represents an important step toward this aim. Programmed cell death‐ligand 1 (PD‐L1), a membrane protein that can be expressed on tumor and inflammatory cells is a key biomarker whose expression is determined by means of immunohistochemistry and is necessary for selecting patients for immunotherapy. Methods: In this study, we review the methods of PD‐L1 assessment and outcomes achieved with immunotherapy in the treatment of OSCC patients. Results: Based on a meta‐analysis we demonstrate a lack of prognostic significance of PD‐L1 in OSCC. Conclusions: We also highlight unresolved issues including difficulties in standardizing PD‐L1 evaluation and discuss future opportunities such as leveraging digital pathology.
Keywords: CD274, meta‐analysis, oral cancer, oral squamous cell carcinoma, PD‐L1, programmed death‐ligand 1
1. INTRODUCTION
Oral squamous cell carcinoma (OSCC) is the most common tumor of the oral cavity, with an increasing incidence worldwide, with up to 370,000 new cases per year (Sung et al., 2021). Although recent therapeutic opportunities have improved, OSCC is still responsible for up to 177,000 deaths annually, with a 5‐year overall survival (OS) rate of around 60% (Adamski et al., 2021).
Histologically, OSCC is composed of polygonal atypical squamous cells, which may have abundant eosinophilic cytoplasm and contain irregular nuclei, often with prominent nucleoli. Based on cellular atypia and structural architecture, OSCC is graded into well, moderately, and poorly differentiated tumors. Tumor grading, as assessed by pathologists using light microscopy, has direct implications for prognostic stratification, with poorly differentiated tumors usually associated with an increased risk of disease recurrence and death.
Programmed cell death‐ligand 1 (PD‐L1) is a transmembrane protein that can be expressed on neoplastic cells and tumor‐infiltrating immune cells. Its interaction with programmed cell death protein 1 (PD‐1), which is usually expressed on activated T lymphocytes, causes the inactivation of the self/cell‐mediated immune response against tumor cells (Lenouvel et al., 2020). Recent evidence has pointed out that the PD‐1/PD‐L1 axis is typically altered in a significant proportion of OSCC cases, rendering this tumor type a promising candidate for immune checkpoint inhibitors, with the selective blockade of the PD‐L1/PD‐1 axis. Such targeted immunotherapy can be successfully achieved through the use of monoclonal antibodies directed either against PD‐L1 (e.g., Atezolizumab) or against PD‐1 (e.g., Pembrolizumab). By restoring antitumor adaptive immunity, immune checkpoint inhibitors have become one of the most important actors in the rapidly changing landscape of cancer immunotherapy (Paver et al., 2021).
The expression of PD‐L1 can be evaluated on both tumor cells and immune cells with immunohistochemistry (IHC), which is assessed by pathologists examining formalin‐fixed paraffin‐embedded tissues using light microscopy (Figure 1). Positive staining and the patterns of PD‐L1 immunoexpression represent one of the most important indicators for correctly selecting patients' eligibility for immunotherapy (Luchini et al., 2019). PD‐L1 is preferred to PD‐1 as a predictive biomarker of immunotherapy response since its expression is more reliably detected with IHC and unlike PD‐1, which is assessed only in inflammatory cells, PD‐L1 can be detected on both tumor and inflammatory cells.
Figure 1.

Forest plot showing the impact of PD‐L1 expression on overall survival in patients with oral squamous cell carcinoma. As shown by the direct contact of the summarizing box with the “1‐line,” statistical significance is not reached. PD‐L1, programmed cell death‐ligand 1.
In the present work, we review the fundamental role of PD‐L1 in OSCC, providing insight into recent advances and future perspectives of this crucial biomarker from the laboratory to the bedside. A meta‐analysis regarding the prognostic role of PD‐L1 in OSCC is also performed.
1.1. PD‐L1 assessment with IHC
The manual assessment of PD‐L1 via IHC remains a challenge for pathologists. There are several commercially available clones of the anti‐PD‐L1 antibody, each associated with different modalities of evaluation and thresholds of positivity. The evaluation of PD‐L1 in neoplastic cells must be performed in tumor areas comprising at least 100 viable cells and only a membranous staining pattern should be considered to represent positivity (Chen et al., 2019; Luchini et al., 2019). As for the evaluation of PD‐L1 in inflammatory cells, pathologists should only assess those inflammatory cells infiltrating tumor areas or in close relationship with tumor cells (so‐called “tumor‐infiltrating” immune cells). The inflammatory cell population is usually composed of lymphocytes (mainly cytotoxic CD8‐positive T lymphocytes) and macrophages. Unlike tumor cells, tumor‐infiltrating inflammatory cells are considered to be positive if any PD‐L1 staining is evident, independent of their staining pattern (Ventana PD‐L1 [SP142] Assay, 2019).
Another unsettled issue pertains to the different scoring systems used for quantifying PD‐L1 staining patterns in different tumors. Along this line, two different scores have been recently introduced, and currently, they represent the gold standard for PD‐L1 evaluation in certain solid malignancies, including gastrointestinal cancer, non‐small cell lung cancer, as well as head and neck squamous cell carcinoma (Cohen et al., 2019; Paolino et al., 2021). These two scores are: (1) tumor proportion score (TPS), which represents the proportion (%) of PD‐L1 positive tumor cells relative to the total number of viable tumor cells × 100; and (2) combined positive score (CPS), which represents the number of all PD‐L1‐positive cells (neoplastic and inflammatory cells) relative to the number of all viable tumor cells × 100 (Paolino et al., 2021). Additional scores have been introduced for other solid tumors, including triple‐negative breast cancer and urothelial carcinoma. These extra scoring systems are: (1) immune cell score (IC), which only takes into account immune cells and is expressed as a percentage of the tissue area occupied by PD‐L1 positive immune cells to the total tissue area; and (2) tumor cell score (TC), which is a percentage of the PD‐L1‐positive tumor cells to the total number of tumor cells, similar to the TPS (Cohen et al., 2019; Crosta et al., 2021; Paolino et al., 2021). These different scores have been developed for the evaluation of PD‐L1 staining patterns with specific clones. For example, TPS and CPS are utilized in association with the clone 22C3 and IC/TC for the clone SP142. Currently, for immunotherapy eligibility of patients with OSCC, the score that is recommended in clinical practice is CPS for evaluating the 22C3 clone.
Given the cost of different platforms needed for preparation, not all PD‐L1 clones can be made simultaneously available in most hospitals, which may further impede IHC evaluation. Furthermore, subjectivity may exist when manually assessing PD‐L1 staining. Indeed, some recent studies have reported medium‐to‐low interobserver agreement in this regard among different pathologists (Crosta et al., 2021; Das et al., 2021; Dong et al., 2021; Pang et al., 2021). Of note, encouraging results showing moderate‐to‐high concordance have been recently reported for head and neck squamous cell carcinoma, based largely on the use of a standardized CPS score (Cerbelli et al., 2021; Girolami et al., 2021). Finally, the evaluation of PD‐L1 expression in small biopsy material can be very difficult and may generate false results, especially due to the heterogeneity of PD‐L1 expression in tissue samples (Kwon et al., 2018; Paolino et al., 2021). To overcome the aforementioned issues and have the field move toward more standardized evaluation, new approaches that leverage digital pathology (e.g., computer‐assisted image analysis) may better support pathologists when performing this difficult task.
1.2. PD‐L1 expression in precursor lesions and during OSCC tumor progression
PD‐L1 expression increases when oral precancerous lesions progress to invasive OSCC. The analysis of a cohort of 49 oral lesions by Dave et al. (including 20 nonprogressing dysplasia cases, 19 progressing dysplasia, and 10 invasive OSCC) showed a significant increase in PD‐L1 expression during OSCC progression (Girolami, Pantanowitz, Munari, et al., 2020). Similar findings were reported by other authors involving cases of oral leukoplakia with a high risk of malignant transformation (Girolami et al., 2020; Gurizzan et al., 2021; Kim et al., 2019). The increased expression of PD‐L1 during OSCC carcinogenesis is accompanied by profound modifications in the tumor microenvironment. Along this line, Das et al. (2021) described a crucial role of interferon receptor signaling in tumor growth by activating immune evasion mechanisms. Thus, PD‐L1 expression provides direct evidence of OSCC immune evasion toward malignant transformation and progression. All these findings suggest that PD‐L1 can be used as a helpful tool to recognize high‐risk progressing lesions, and have led to the development of in vitro and animal studies (Dong et al., 2021), followed by human clinical trials (Lee et al., 2021), to investigate the potential role of immunotherapy for preventing the malignant transformation of oral precancerous lesions. The overall results of these exploratory studies demonstrate the feasibility of immunotherapy in preventing the evolution of squamous dysplasia. Thus, in selected cases, restoring the activity of the immune system through the administration of checkpoint inhibitors may play an important role in preventing OSCC occurrence (Lee et al., 2021).
1.3. PD‐L1 is an unreliable marker for OSCC prognostic stratification
Given the increased expression of PD‐L1 during the malignant transformation of OSCC, several studies have tried to establish whether this biomarker was also an indicator of poor prognosis in this tumor type. An adverse prognostic value of PD‐L1 has already been demonstrated in other cancers, such as non‐small cell lung cancer, pancreatic undifferentiated carcinoma, thyroid, and prostate cancer (Lenouvel et al., 2020, 2021; Luchini et al., 2018; Shen et al., 2021). For OSCC, there are conflicting results, with some reports denoting PD‐L1 expression as an indicator of poor outcome and other studies supporting an opposing conclusion. While published meta‐analyses reveal the limited prognostic impact of PD‐L1, these pooled studies reported conflicting results (Lenouvel et al., 2020; Luchini et al., 2019).
To shed light on this complex scenario, we performed our own systematic review and meta‐analysis taking into account all published studies on this topic.
2. METHODS
The review question was built upon a Population, Index, Comparator, Outcome (PICO) frame. The “population” is represented by patients with OSCC, “index” by the expression of PD‐L1 considered positive as defined in a single study in “comparison” to negative expression, and “outcome” incorporates survival measures of OS and disease‐free survival (DFS). The inclusion criteria derived from this framework were: (i) both prospective and retrospective studies investigating PD‐L1 expression in samples procured from primary and naive OSCC patients; and (ii) studies that reported survival indexes based on calculating multivariable hazard ratio (HR) and its 95% confidence interval (95% CI) for at least OS and/or DFS. Our meta‐analysis adhered to existing guidelines, including Meta‐analyses Of Observational Studies in Epidemiology (MOOSE) guidelines (Stroup, 2000), Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA), and the Newcastle‐Ottawa Scale (NOS) statement (Liberati et al., 2009; Mattox et al., 2017). Studies not dealing with OSCC, not reporting PD‐L1 positive and negative expression, or not reporting survival data in an extractable manner were excluded, as were studies represented by abstracts only or other article types, such as letters, reviews, animal and cell culture studies, and case reports. A literature search was performed using PubMed and Scopus without language restriction, from database inception until 23 October, 2021 with the following search strategy: (“PD‐L1” OR “PDL1” OR “CD274” OR “B7‐H1” OR “Programmed Death Ligand 1” OR “Programmed Death‐Ligand 1”) AND (“oral cancer” OR “oral squamous” OR “oral squamous cell” OR “oral carcinoma”). Two authors (R. N. and M. V.) separately screened the title and abstracts for potential inclusion; disagreement was resolved by consensus. The full text of potentially eligible articles was retrieved and evaluated by two authors to verify inclusion criteria and the quality of these studies. By applying the inclusion criteria, 12 studies were selected (Table 1) (Cho et al., 2011; de Vicente et al., 2019; Kogashiwa et al., 2017; Lenouvel et al., 2021; Mattox et al., 2017; Oliveira‐Costa et al., 2015; Pang et al., 2021; Quan et al., 2020; Satgunaseelan et al., 2016; Shen et al., 2021; Straub et al., 2016; Wu et al., 2021). Data extracted included: country and authors of each study, number of patients, clone of PD‐L1 used, cut‐off for positivity applied, and the HR for OS and DFS reported in the study. Pooled HR was calculated using DerSimonian‐Laird random‐effects models and heterogeneity across studies was assessed by the I2 metric.
Table 1.
Summarizing table of studies reporting HR data regarding the impact on survival indexes of PD‐L1 expression in oral squamous cell carcinoma
| Authors, years | Country | No. of patients | Detection method | Clone of PD‐L1 antibody | Cut‐off for positivity | HR overall survival (95% CI) | HR disease‐free survival (95% CI) |
|---|---|---|---|---|---|---|---|
| Cho (2011) | South Korea | 45 | IHC | ab82059 | Score >2 | 1.10 (043−2.82) | NA |
| Oliveira‐Costa (2015) | Brazil | 96 | IHC | ab28753 | >5% of tumor cells | 0.43 (0.29−0.63) | NA |
| Satgunaseelan (2016) | Australia | 217 | IHC | E1L3N | >5% of tumor cells | NA | 0.82 (0.41−1.63) |
| Straub (2016) | Germany | 80 | IHC | E1L3N | >5% of tumor cells | 2.94 (0.20−43.94) | 2.10 (0.38−11.53) |
| Mattox (2017) | USA | 53 | IHC | 5H1 | >1% of tumor and/or immune cells | 1.62 (0.22−11.73) | NA |
| Kogashiwa (2017) | Japan | 84 | IHC | SP142 | >5% of tumor cells | 0.26 (0.10−0.65) | 0.54 (0.28−0.89) |
| De Vicente (2019) | Spain | 125 | IHC | 22C3 | >10% | NA | 2.05 (1.02–4.11) |
| Quan (2020) | China | 88 | IHC | EPR19759 | >25% tumor cells; >10% immune cells | NA | 0.39 (0.16−0.99) |
| Adamski (2021) | Poland | 109 | IHC | E1L3N | >10% tumor cells; >20% immune cells | 2.51 (1.98−5.29)a | NA |
| Lenouvel (2021) | Spain | 55 | IHC | 22C3 | TPS >5% | 1.15 (0.48−2.73) | 0.58 (0.14−2.45) |
| Wu (2021) | China | 150 | TIMER | NA | NA | 1.67 (1.10−2.50) | NA |
| Subramaniam (2021) | India | 64 | IHC | NS | TILs >1% | 0.59 (0.10−1.04)b | NA |
Abbreviations: CI, confidence interval; HR, hazard ratio; IHC, immunohistochemistry; PD‐L1, programmed cell death‐ligand 1; TIMER, tumor immune estimation resource.
Specific data in squamous cell carcinoma of the tongue and the floor of the mouth.
Data on TILs only.
3. RESULTS AND DISCUSSION
The main result of this meta‐analysis, as also shown in Figure 2 (OS) and Figure 3 (DFS), is a definitive demonstration, using the most powerful survival indexes (HR from multivariable analysis), that PD‐L1 cannot be used as a significant prognosticator for OSCC patients (HR for OS: 0.97, 95% CI: 0.53−1.80; HR for DFS: 0.83, 95% CI: 0.47−1.46). Of interest, the study published by Adamski et al. (2021) indicated a potential significant association between PD‐L1 expression on tumor cells and a poor prognosis for squamous cell carcinoma of the tongue and the floor of the mouth, but not of other oral compartments (Shen et al., 2021). It remains to be determined if this conflicting literature is at all dependent on the aforementioned complexities about PD‐L1 assessment in OSCC (e.g., varying clones, scores, and thresholds for positive staining). For example, in the 12 manuscripts selected for our meta‐analysis, 7 different clones were used (Table 1). With the recent approval of Federal Drug Administration (FDA) and European Medicines Agencies (EMA) guidelines, there may be more standardization, which would in turn benefit the appropriate selection of immunotherapy.
Figure 2.
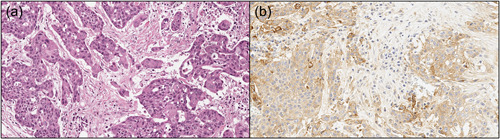
A classic example of a case of invasive oral squamous cell carcinoma, poorly differentiated, showing diffuse positivity for PD‐L1. (a) Hematoxylin & eosin stain, original magnification ×20; (b) PD‐L1 immunostaining with 22C3 antibody, 90% TPS, 90 CPS. CPS, combined positive score; PD‐L1, programmed cell death‐ligand 1; TPS, tumor proportion score.
Figure 3.
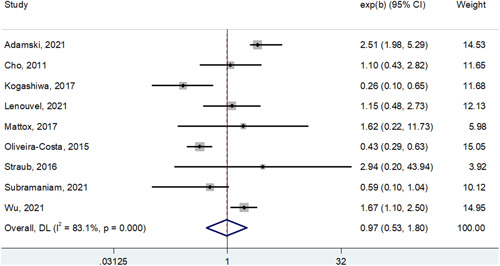
Forest plot showing the impact of PD‐L1 expression on disease‐free survival in patients with oral squamous cell carcinoma. As shown by the direct contact of the summarizing box with the “1‐line,” statistical significance is not reached. PD‐L1, programmed cell death‐ligand 1.
3.1. PD‐L1 for OSSC in clinical practice
Immunotherapy‐based approaches for the treatment of OSCC are already playing an important role in clinical practice. The PD‐1 inhibitor nivolumab received FDA approval for patients progressing with the disease after first‐line platinum‐based therapy, based on findings of the “Checkmate‐141” trial (Ferris et al., 2018). Based on the published findings of two distinct clinical trials, named “KEYNOTE‐012” and “KEYNOTE‐040,” the PD‐1 inhibitor pembrolizumab was officially approved by the FDA and EMA for the treatment of recurrent and metastatic head and neck squamous cell carcinoma, in those cases with a TPS >50% and evaluated with the 22C3 clone (pharmDx/Agilent Technologies, Inc.) at IHC (Cohen et al., 2019; Mehra et al., 2018).
A subsequent randomized, open‐label, Phase 3 clinical trial named “KEYNOTE‐048” demonstrated that pembrolizumab was associated with improved OS compared to the alternate standard of care for patients with head and neck squamous cell carcinoma (Burtness et al., 2019), thereby indicating that pembrolizumab monotherapy is an appropriate first‐line treatment for PD‐L1‐positive (i.e., CPS > 1) recurrent and metastatic tumors (Borcoman et al., 2021). Subsequent analysis demonstrated that the CPS score has increased sensitivity compared to the TPS score (de Vicente et al., 2019). These trials have also shown that PD‐L1 expression is associated with an increased objective response rate in patients with CPS ≥ 1 and with a better response for CPS value ≥ 20 (Burtness et al., 2019). Indeed, the EMA approved pembrolizumab to be used both as monotherapy or in combination with chemotherapy, as first‐line treatment for recurrent and metastatic head and neck squamous cell carcinoma in patients whose tumors express PD‐L1 with a CPS ≥ 1, regardless of the test (antibody and IHC platform) used. Therefore, a predictive role of PD‐L1 expression has been established, since CPS categorization has clinical and therapeutic implications, and a proportion of negative patients (CPS ≤ 1) of around 15% is expected (Burtness et al., 2019; de Vicente et al., 2019). Moreover, at CPS ≥ 20 an enhanced therapeutic response is expected, thus reinforcing the predictive role of the CPS value.
Recently, the important oncology concept referred to as hyperprogressive disease (HD) was introduced, based on the observation of different response patterns to immunotherapy during cancer treatment (specifically, rapid tumor progression after the initiation of immunotherapy) (Kim et al., 2019). HD has been observed across various types of cancers, including OSCC, and has been associated with poor survival (Kim et al., 2019). Of note, IHC positivity for PD‐L1 is inversely correlated with HD, further highlighting the importance of striving for a standardized assessment of this biomarker.
4. CONCLUSION AND FUTURE PERSPECTIVES
The main highlights gathered in this study are summarized in Table 2. The most important theme to emerge is the need for standardized guidelines for PD‐L1 evaluation that clearly elucidate how best to handle intratumor heterogeneity of PD‐L1 expression, subjectivity in IHC evaluation, and the utilization of different clones with different scores. Future perspectives for advancing the management of OSCC patients based on PD‐L1 assessment could benefit from advances in digital pathology‐based tools, such as automated computer‐assisted quantitative image analysis, for supporting IHC evaluation and thereby improving the selection of patients for immunotherapy. Interestingly, a recent study has indicated that high PD‐L1 expression in OSCC may even be predicted with a similar analysis of radiology images (Tekiki et al., 2021). Finally, based on the finding of PD‐L1 expression in precancerous lesions and invasive OSCC, new therapeutic strategies based on immune checkpoint inhibitors may begin to be applied in selected cases with a high risk of malignant transformation.
Table 2.
Summary of the main findings of the current study
| Evidence | Main findings |
|---|---|
| Prognostic value of PD‐L1 | Current evidence does not support the role of PD‐L1 expression as a significant prognosticator for OSCC patients (HR for OS: 0.97, 95% CI: 0.53−1.80; HR for DFS: 0.83, 95% CI: 0.47−1.46). |
| Predictive value of PD‐L1 | Trials support that PD‐L1 expression is associated with an increased objective response rate in patients with CPS ≥1, with better response with CPS value ≥20. |
| Limitations of studies available in the literature | High heterogeneity of studies in terms of PD‐L1 clone and platform used. |
| Different scoring systems for defining positivity. | |
| Suboptimal investigation of effects of previous therapy on PD‐L1 expression. | |
| Future directions | Standardization of clones and scoring systems to have more homogeneous data. |
| Best selection of patients. | |
| Aid coming from artificial intelligence tools on digital slides to evaluate PD‐L1 expression. |
Abbreviations: CI, confidence intervals; CPS, combined positive score; DFS, disease‐free survival; HR, hazard ratio; OS, overall survival; OSCC, oral squamous cell carcinoma; PD‐L1, programmed cell death‐ligand 1.
AUTHOR CONTRIBUTIONS
All authors have participated in the conception and design or analysis and interpretation of the data. All authors contributed to the drafting of the manuscript and approved the final version of the manuscript. The study was supported by a fund from Barone Rossi and Community of Albaredo d'Adige.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Nocini, R. , Vianini, M. , Girolami, I. , Calabrese, L. , Scarpa, A. , Martini, M. , Morbini, P. , Marletta, S. , Brunelli, M. , Molteni, G. , Parwani, A. , Pantanowitz, L. , & Eccher, A. (2022). PD‐L1 in oral squamous cell carcinoma: A key biomarker from the laboratory to the bedside. Clinical and Experimental Dental Research, 8, 690–698. 10.1002/cre2.590
Riccardo Nocini and Matteo Vianini contributed equally to this study and should be considered joint co‐first author.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Adamski, Ł. J. , Starzyńska, A. , Adamska, P. , Kunc, M. , Sakowicz‐Burkiewicz, M. , Marvaso, G. , Alterio, D. , Korwat, A. , Jereczek‐Fossa, B. A. , & Pęksa, R. (2021). High PD‐L1 expression on tumor cells indicates worse overall survival in advanced oral squamous cell carcinomas of the tongue and the floor of the mouth but not in other oral compartments. Biomedicines, 9, 1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borcoman, E. , Marret, G. , & Le Tourneau, C. (2021). Paradigm change in first‐line treatment of recurrent and/or metastatic head and neck squamous cell carcinoma. Cancers (Basel), 13 (11), 2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness, B. , Harrington, K. J. , Greil, R. , Soulières, D. , Tahara, M. , de Castro, G. Jr , Psyrri, A. , Basté, N. , Neupane, P. , Bratland, Å. , Fuereder, T. , Hughes, B. , Mesía, R. , Ngamphaiboon, N. , Rordorf, T. , Wan Ishak, W. Z. , Hong, R. L. , González Mendoza, R. , Roy, A. , … KEYNOTE‐048 Investigators . (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): A randomised, open‐label, phase 3 study. Lancet, 394, 1915–1928. [DOI] [PubMed] [Google Scholar]
- Cerbelli, B. , Girolami, I. , Eccher, A. , Costarelli, L. , Taccogna, S. , Scialpi, R. , Benevolo, M. , Lucante, T. , Luigi Alò, P. , Stella, F. , Gemma Pignataro, M. , Fadda, G. , Perrone, G. , D'Amati, G. , & Martini, M. (2021). Evaluating PD‐L1 in head & neck squamous cell carcinoma: Concordance between 22C3 PharmaDx and SP263 assays on whole sections from a multicenter study. Histopathology, 80(2), 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. J. , Tan, Y. Q. , Zhang, N. , He, M. J. , & Zhou, G. (2019). Expression of programmed cell death‐ligand 1 in oral squamous cell carcinoma and oral leukoplakia is associated with disease progress and CD8+ tumor‐infiltrating lymphocytes. Pathology Research and Practice, 215, 152418. [DOI] [PubMed] [Google Scholar]
- Cho, Y. A. , Yoon, H. J. , Lee, J. I. , Hong, S. P. , & Hong, S. D. (2011). Relationship between the expressions of PD‐L1 and tumor‐infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncology, 47, 1148–1153. [DOI] [PubMed] [Google Scholar]
- Cohen, E. E. W. , Soulières, D. , Le Tourneau, C. , Dinis, J. , Licitra, L. , Ahn, M. J. , Soria, A. , Machiels, J. P. , Mach, N. , Mehra, R. , Burtness, B. , Zhang, P. , Cheng, J. , Swaby, R. F. , Harrington, K. J. , & KEYNOTE‐040 Investigators . (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): A randomised, open‐label, phase 3 study. Lancet, 393, 156–167. [DOI] [PubMed] [Google Scholar]
- Cramer, J. D. , Burtness, B. , & Ferris, R. L. (2019). Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncology, 99, 104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosta, S. , Boldorini, R. , Bono, F. , Brambilla, V. , Dainese, E. , Fusco, N. , Gianatti, A. , L'Imperio, V. , Morbini, P. , & Pagni, F. (2021). PD‐L1 testing and squamous cell carcinoma of the head and neck: A multicenter study on the diagnostic reproducibility of different protocols. Cancers (Basel), 13, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, D. , Maitra, A. , Panda, C. K. , Ghose, S. , Roy, B. , Sarin, R. , & Majumder, P. P. (2021). Genes and pathways monotonically dysregulated during progression from normal through leukoplakia to gingivo‐buccal oral cancer. NPJ Genomic Medicine, 6, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave, K. , Ali, A. , & Magalhaes, M. (2020). Increased expression of PD‐1 and PD‐L1 in oral lesions progressing to oral squamous cell carcinoma: A pilot study. Scientific Reports, 10, 9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y. , Wang, Z. , Mao, F. , Cai, L. , Dan, H. , Jiang, L. , Zeng, X. , Li, T. , Zhou, Y. , & Chen, Q. (2021). PD‐1 blockade prevents the progression of oral carcinogenesis. Carcinogenesis, 42, 891–902. [DOI] [PubMed] [Google Scholar]
- Ferris, R. L. , Blumenschein, G. Jr , Fayette, J. , Guigay, J. , Colevas, A. D. , Licitra, L. , Harrington, K. J. , Kasper, S. , Vokes, E. E. , Even, C. , Worden, F. , Saba, N. F. , Docampo, L. , Haddad, R. , Rordorf, T. , Kiyota, N. , Tahara, M. , Lynch, M. , Jayaprakash, V. , … Gillison, M. L. (2018). Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2‐year long‐term survival update of CheckMate 141 with analyses by tumor PD‐L1 expression. Oral Oncology, 81, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolami, I. , Pantanowitz, L. , Barberis, M. , Paolino, G. , Brunelli, M. , Vigliar, E. , Munari, E. , Satturwar, S. , Troncone, G. , & Eccher, A. (2021). Challenges facing pathologists evaluating PD‐L1 in head & neck squamous cell carcinoma. Journal of Oral Pathology and Medicine, 50(9), 864–873. [DOI] [PubMed] [Google Scholar]
- Girolami, I. , Pantanowitz, L. , Mete, O. , Brunelli, M. , Marletta, S. , Colato, C. , Trimboli, P. , Crescenzi, A. , Bongiovanni, M. , Barbareschi, M. , & Eccher, A. (2020). Programmed death‐ligand 1 (PD‐L1) is a potential biomarker of disease‐free survival in papillary thyroid carcinoma: A systematic review and meta‐analysis of PD‐L1 immunoexpression in follicular epithelial derived thyroid carcinoma. Endocrine Pathology, 31, 291–300. [DOI] [PubMed] [Google Scholar]
- Girolami, I. , Pantanowitz, L. , Munari, E. , Martini, M. , Nocini, R. , Bisi, N. , Molteni, G. , Marchioni, D. , Ghimenton, C. , Brunelli, M. , & Eccher, A. (2020). Prevalence of PD‐L1 expression in head and neck squamous precancerous lesions: A systematic review and meta‐analysis. Head and Neck, 42, 3018–3030. [DOI] [PubMed] [Google Scholar]
- Gurizzan, C. , Lorini, L. , Paderno, A. , Tomasoni, M. , Zigliani, G. , Bozzola, A. , Ardighieri, L. , Battocchio, S. , Bignotti, E. , Ravaggi, A. , Romani, C. , De Cecco, L. , Serafini, M. S. , Miceli, R. , Bardellini, E. , Majorana, A. , Piazza, C. , & Bossi, P. (2021). Immunotherapy for the prevention of high‐risk oral disorders malignant transformation: The IMPEDE trial. BMC Cancer, 21, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. Y. , Lee, K. H. , Kang, J. , Borcoman, E. , Saada‐Bouzid, E. , Kronbichler, A. , Hong, S. H. , de Rezende L., Ogino, S. , Keum, N. , Song, M. , Luchini, C. , van der Vliet H. J., Shin, J. I. , & Gamerith, G. (2019). Hyperprogressive disease during anti‐PD‐1 (PDCD1)/PD‐L1 (CD274) therapy: A systematic review and meta‐analysis. Cancers (Basel), 11, 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogashiwa, Y. , Yasuda, M. , Sakurai, H. , Nakahira, M. , Sano, Y. , Gonda, K. , Ikeda, T. , Inoue, S. , Kuba, K. , Oba, S. , Ishikawa, J. , Enoki, Y. , Matsumura, S. , Minami, K. , Ebihara, Y. , & Sugasawa, M. (2017). PD‐L1 expression confers better prognosis in locally advanced oral squamous cell carcinoma. Anticancer Research, 37, 1417–1424. 10.21873/anticanres.11465 [DOI] [PubMed] [Google Scholar]
- Kulangara, K. , Zhang, N. , Corigliano, E. , Guerrero, L. , Waldroup, S. , Jaiswal, D. , Ms, M. J. , Shah, S. , Hanks, D. , Wang, J. , Lunceford, J. , Savage, M. J. , Juco, J. , & Emancipator, K. (2019). Clinical utility of the combined positive score for programmed death ligand‐1 expression and the approval of pembrolizumab for treatment of gastric cancer. Archives of Pathology and Laboratory Medicine, 143, 330–337. [DOI] [PubMed] [Google Scholar]
- Kwon, M. J. , Rho, Y. S. , Nam, E. S. , Cho, S. J. , Park, H. R. , Min, S. K. , Seo, J. , Choe, J. Y. , Kim, E. S. , Park, B. , Hong, M. , & Min, K. W. (2018). Clinical implication of programmed cell death‐1 ligand‐1 expression in tonsillar squamous cell carcinoma in association with intratumoral heterogeneity, human papillomavirus, and epithelial‐to‐mesenchymal transition. Human Pathology, 80, 28–39. [DOI] [PubMed] [Google Scholar]
- Lee, J. B. , Hong, M. H. , Park, S. Y. , Chae, S. , Hwang, D. , Ha, S. J. , Shim, H. S. , & Kim, H. R. (2021). Overexpression of PVR and PD‐L1 and its association with prognosis in surgically resected squamous cell lung carcinoma. Scientific Reports, 11, 8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenouvel, D. , González‐Moles, M. Á. , Ruiz‐Ávila, I. , Gonzalez‐Ruiz, L. , Gonzalez‐Ruiz, I. , & Ramos‐García, P. (2020). Prognostic and clinicopathological significance of PD‐L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta‐analysis. Oral Oncology, 106, 104722. [DOI] [PubMed] [Google Scholar]
- Lenouvel, D. , González‐Moles, M. Á. , Ruiz‐Ávila, I. , Chamorro‐Santos, C. , González‐Ruiz, L. , González‐Ruiz, I. , & Ramos‐García, P. (2021). Clinicopathological and prognostic significance of PD‐L1 in oral cancer: A preliminary retrospective immunohistochemistry study. Oral Diseases, 27, 173–182. [DOI] [PubMed] [Google Scholar]
- Lenouvel, D. , González‐Moles, M. Á. , Talbaoui, A. , Ramos‐García, P. , González‐Ruiz, L. , Ruiz‐Ávila, I. , & Gil‐Montoya, J. A. (2020). An update of knowledge on PD‐L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral Diseases, 26, 511–526. [DOI] [PubMed] [Google Scholar]
- Liberati, A. , Altman, D. G. , Tetzlaff, J. , Mulrow, C. , Gøtzsche, P. C. , Ioannidis, J. P. , Clarke, M. , Devereaux, P. J. , Kleijnen, J. , & Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ, 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchini, C. , Bibeau, F. , Ligtenberg, M. J. L. , Singh, N. , Nottegar, A. , Bosse, T. , Miller, R. , Riaz, N. , Douillard, J. Y. , Andre, F. , & Scarpa, A. (2019). ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD‐1/PD‐L1 expression and tumour mutational burden: A systematic review‐based approach. Annals of Oncology, 30, 1232–1243. [DOI] [PubMed] [Google Scholar]
- Luchini, C. , Cros, J. , Pea, A. , Pilati, C. , Veronese, N. , Rusev, B. , Capelli, P. , Mafficini, A. , Nottegar, A. , Brosens, L. , Noë, M. , Offerhaus, G. , Chianchiano, P. , Riva, G. , Piccoli, P. , Parolini, C. , Malleo, G. , Lawlor, R. T. , Corbo, V. , … Scarpa, A. (2018). PD‐1, PD‐L1, and CD163 in pancreatic undifferentiated carcinoma with osteoclast‐like giant cells: Expression patterns and clinical implications. Human Pathology, 81, 157–165. [DOI] [PubMed] [Google Scholar]
- Luchini, C. , Stubbs, B. , Solmi, M. , & Veronese, N. (2017). Assessing the quality of studies in meta‐analyses: Advantages and limitations of the Newcastle Ottawa Scale. World Journal of Meta‐Analysis, 5, 80. [Google Scholar]
- Madera, M. , Tirado Amador, L. , & Leal Acosta, C. (2021). Therapeutic options in unresectable oral squamous cell carcinoma: A systematic review. Cancer Management and Research, 13, 6705–6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattox, A. K. , Lee, J. , Westra, W. H. , Pierce, R. H. , Ghossein, R. , Faquin, W. C. , Diefenbach, T. J. , Morris, L. G. , Lin, D. T. , Wirth, L. J. , Lefranc‐Torres, A. , Ishida, E. , Chakravarty, P. D. , Johnson, L. , Zeng, Y. C. , Chen, H. , Poznansky, M. C. , Iyengar, N. M. , & Pai, S. I. (2017). PD‐1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4+ TILs in the presence of PD‐L1+ TAMs. Cancer Research, 77, 6365–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra, R. , Seiwert, T. Y. , Gupta, S. , Weiss, J. , Gluck, I. , Eder, J. P. , Burtness, B. , Tahara, M. , Keam, B. , Kang, H. , Muro, K. , Geva, R. , Chung, H. C. , Lin, C. C. , Aurora‐Garg, D. , Ray, A. , Pathiraja, K. , Cheng, J. , Chow, L. , & Haddad, R. (2018). Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long‐term follow‐up in KEYNOTE‐012. British Journal of Cancer, 119, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito, T. , Udagawa, H. , Sato, J. , Horinouchi, H. , Murakami, S. , Goto, Y. , Kanda, S. , Fujiwara, Y. , Yamamoto, N. , Zenke, Y. , Kirita, K. , Matsumoto, S. , Yoh, K. , Niho, S. , Motoi, N. , Ohe, Y. , Ishii, G. , & Goto, K. (2019). A minimum of 100 tumor cells in a single biopsy sample is required to assess programmed cell death ligand 1 expression in predicting patient response to nivolumab treatment in nonsquamous non‐small cell lung carcinoma. Journal of Thoracic Oncology, 14, 1818–1827. [DOI] [PubMed] [Google Scholar]
- Oliveira‐Costa, J. P. , de Carvalho, A. F. , da Silveira da, G. G. , Amaya, P. , Wu, Y. , Park, K. J. , Gigliola, M. P. , Lustberg, M. , Buim, M. E. , Ferreira, E. N. , Kowalski, L. P. , Chalmers, J. J. , Soares, F. A. , Carraro, D. M. , & Ribeiro‐Silva, A. (2015). Gene expression patterns through oral squamous cell carcinoma development: PD‐L1 expression in primary tumor and circulating tumor cells. Oncotarget, 6, 20902–20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, J. B. , Castles, B. , Byrne, D. J. , Button, P. , Hendry, S. , Lakhani, S. R. , Sivasubramaniam, V. , Cooper, W. A. , Armes, J. , Millar, E. , Raymond, W. , Roberts‐Thomson, S. , Kumar, B. , Burr, M. , Selinger, C. , Harvey, K. , Chan, C. , Beith, J. , Clouston, D. , … kConFab . (2021). SP142 PD‐L1 scoring shows high interobserver and intraobserver agreement in triple‐negative breast carcinoma but overall low percentage agreement with other PD‐L1 clones SP263 and 22C3. American Journal of Surgical Pathology, 45, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolino, G. , Pantanowitz, L. , Barresi, V. , Pagni, F. , Munari, E. , Moretta, L. , Brunelli, M. , Bariani, E. , Vigliar, E. , Pisapia, P. , Malapelle, U. , Troncone, G. , Girolami, I. , & Eccher, A. (2021). PD‐L1 evaluation in head and neck squamous cell carcinoma: Insights regarding specimens, heterogeneity and therapy. Pathology Research and Practice, 226, 153605. [DOI] [PubMed] [Google Scholar]
- Paver, E. C. , Cooper, W. A. , Colebatch, A. J. , Ferguson, P. M. , Hill, S. K. , Lum, T. , Shin, J. S. , O'Toole, S. , Anderson, L. , Scolyer, R. A. , & Gupta, R. (2021). Programmed death ligand‐1 (PD‐L1) as a predictive marker for immunotherapy in solid tumours: A guide to immunohistochemistry implementation and interpretation. Pathology, 53, 141–156. [DOI] [PubMed] [Google Scholar]
- Pereira, M. A. , Ramos, M. F. K. P. , Dias, A. R. , Ribeiro, R. , Cardili, L. , Zilberstein, B. , Cecconello, I. , Jr Ribeiro, U. , de Mello, E. S. , de Castria, T. B. (2021). Scoring systems for PD‐L1 expression and their prognostic impact in patients with resectable gastric cancer. Virchows Archive, 478, 1039–1048. [DOI] [PubMed] [Google Scholar]
- Quan, H. , Liu, S. , Shan, Z. , Liu, Z. , Chen, T. , Hu, Y. , Yao, Z. , & Fang, L. (2020). Differential expression of programmed death‐1 and its ligand, programmed death ligand‐1 in oral squamous cell carcinoma with and without oral submucous fibrosis. Archives of Oral Biology, 119, 104916. [DOI] [PubMed] [Google Scholar]
- Ries, J. , Agaimy, A. , Wehrhan, F. , Baran, C. , Bolze, S. , Danzer, E. , Frey, S. , Jantsch, J. , Möst, T. , Büttner‐Herold, M. , Wickenhauser, C. , Kesting, M. , & Weber, M. (2021). Importance of the PD‐1/PD‐L1 axis for malignant transformation and risk assessment of oral leukoplakia. Biomedicines, 9, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satgunaseelan, L. , Gupta, R. , Madore, J. , Chia, N. , Lum, T. , Palme, C. E. , Boyer, M. , Scolyer, R. A. , & Clark, J. R. (2016). Programmed cell death‐ligand 1 expression in oral squamous cell carcinoma is associated with an inflammatory phenotype. Pathology, 48, 574–580. [DOI] [PubMed] [Google Scholar]
- Shen, H. , Liu, J. , Sun, G. , Yan, L. , Li, Q. , Wang, Z. , & Xie, L. (2021). The clinicopathological significance and prognostic value of programmed death‐ligand 1 in prostate cancer: A meta‐analysis of 3133 patients. Aging (Albany NY), 13, 2279–2293. 10.18632/aging.202248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub, M. , Drecoll, E. , Pfarr, N. , Weichert, W. , Langer, R. , Hapfelmeier, A. , Götz, C. , Wolff, K. D. , Kolk, A. , & Specht, K. (2016). CD274/PD‐L1 gene amplification and PD‐L1 protein expression are common events in squamous cell carcinoma of the oral cavity. Oncotarget, 7, 12024–12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup, D. F. (2000). Meta‐analysis of observational studies in epidemiology. A proposal for reporting. Journal of the American Medical Association, 283, 2008–2016. [DOI] [PubMed] [Google Scholar]
- Subramaniam, N. , Nambiar, A. , Dhar, S. , Thankappan, K. , Koyakutty, M. , Balasubramanian, D. , Das, M. , & Iyer, S. (2021). Low PDL1 expression in tumour infiltrating lymphocytes predicts local recurrence in oral squamous cell carcinoma. Indian Journal of Surgical Oncology, 12, 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, H. , Ferlay, J. , Siegel, R. L. , Laversanne, M. , Soerjomataram, I. , Jemal, A. , & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. [DOI] [PubMed] [Google Scholar]
- Tekiki, N. , Fujita, M. , Okui, T. , Kawai, H. , Oo, M. W. , Kawazu, T. , Hisatomi, M. , Okada, S. , Takeshita, Y. , Barham, M. , Nagatsuka, H. , Yanagi, Y. , & Asaumi, J. I. (2021). Dynamic contrast‑enhanced MRI as a predictor of programmed death ligand‑1 expression in patients with oral squamous cell carcinoma. Oncology Letters, 22, 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiano, G. , Caponio, V. C. A. , Zhurakivska, K. , Arena, C. , Pannone, G. , Mascitti, M. , Santarelli, A. , & Lo Muzio L. (2019). High PD‐L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: A meta‐analysis of the literature. Cell Proliferation, 52, e12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventana PD‐L1 (SP142) Assay . (2019). P160002/S009, FDA. https://www.fda.gov/medical-devices/recently-approved-devices/ventana-pd-l1-sp142-assay-p160002s009
- de Vicente, J. C. , Rodríguez‐Santamarta, T. , Rodrigo, J. P. , Blanco‐Lorenzo, V. , Allonca, E. , & García‐Pedrero, J. M. (2019). PD‐L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiology, Biomarkers and Prevention, 28, 546–554. [DOI] [PubMed] [Google Scholar]
- Wu, T. , Tang, C. , Tao, R. , Yong, X. , Jiang, Q. , & Feng, C. (2021). PD‐L1‐mediated immunosuppression in oral squamous cell carcinoma: Relationship with macrophage infiltration and epithelial to mesenchymal transition markers. Frontiers in Immunology, 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, P. , Guo, C. , Li, L. , Guo, L. , Zhang, F. , & Ying, J. (2021). The reproducibility of histopathologic assessments of programmed cell death‐ligand 1 using companion diagnostics in NSCLC. JTO Clinical and Research Reports, 2, 100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


