Abstract
Background and aim
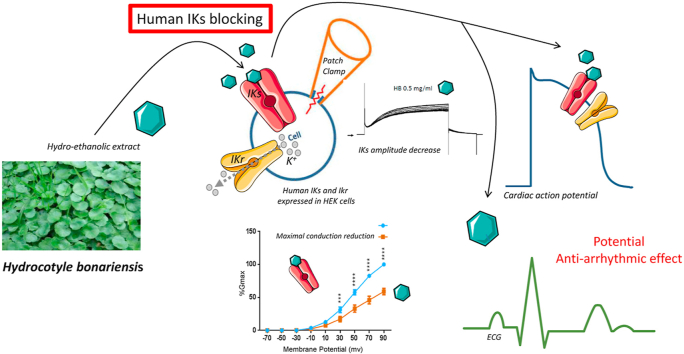
Hydrocotyle bonariensis Comm ex Lamm (Araliaceae) is one of these plants sufficiently exploited in traditional African medicine for its hypotensive effect. However, the pharmacological effects of those plants on cardiac functions are not well known. The potassium currents IKs and IKr, responsible for the repolarization of cardiac cell action potential, strongly influence the human cardiac rhythm. Therefore, modulators of these currents have a beneficial or undesirable medical importance in relation to cardiac arrhythmias. In order to optimize the therapeutic use of this medicinal plant, we studied the effects of hydro-ethanolic leaf extract of Hydrocotyle bonariensis on both potassium currents.
Experimental procedure
The patch clamp experiments for IK currents recording were performed on the HEK 293 (Human Embryonic Kidney 293) cell line, stably transfected with either KCNQ1 and KCNE1 genes encoding the channel responsible for the "IKs" current (HEK293 IKs), or with hERG (human ether-a-go-go related gene) gene encoding "IKr" current (HEK293 IKr).
Results and conclusion
This study revealed that the hydro-ethanolic leaf extract of H. bonariensis significantly inhibits the slow potassium component (IKs) without altering the fast potassium component (IKr). The extract at 0.5 mg/ml decreases IKs conductance by 24 ± 4.1% (n = 6) without modifying its activation threshold suggesting a direct blockade of the slow potassium channel. This selective action of the extract on the IKs current reflects a class III anti-arrhythmic effect.
Keywords: Hydrocotyle bonariensis, Electrophysiology, IKs and IKr currents, Anti-arrhymics
List of Abbreviations: HB, Hydrocotyle bonariensis; HEK, Human Embryonic Kidney; HERG, human ether-a-gogo related gene product
Graphical abstract
Highlights
-
•
Hydrocotyle bonariensis leaves's hydroalcoholic extract is pharmacologically active on the cardiac activity.
-
•
Hydrocotyle bonariensis leaves's hydroalcoholic extract selectively blocks the potassium current IKs.
-
•
Hydrocotyle bonariensis leaves's hydroalcoholic extract has a class III anti-arrhythmic effect.
1. Introduction
Hydrocotyle bonariensis Comm. ex Lam. is a herbaceous plant widely used by traditional Togolese medicine practitioners to treat mainly hypertension and diabetes and also many other diseases.1,2 Pharmacological studies of this plant have revealed its antioxidant and anti-inflammatory properties.3 However, all aspects of bioactive interactions of this plant, whether beneficial or risky, are not yet fully explored.
Cardiac arrhythmias induced by modulation of the QT interval via certain drugs including antiarrhythmics, antibiotics, antidepressants and neuroleptics, is a relatively frequent side effect of great clinical importance.4 Hydrocotyle bonariensis, like most medicinal plants used in sub-Saharan Africa in the traditional management of cardiovascular disease,5,6 has not been yet the subject of scientific studies evaluating its effect on the QT interval.
The slow cardiac delayed rectifier current (IKs) formed by co-assembly of the α-subunit KCNQ1 and the β-subunit KCNE17,8 and the fast potassium current (IKr) generated by the KCNH2 or hERG subunit (human Ether-à-go-go Related Gene),9 are the major repolarizing currents in the human heart. The slightest imbalance between current flows disrupts this repolarization phenomenon, making the heart more susceptible to the development of cardiac arrhythmias, specifically pathologies such as long QT syndrome, short QT syndrome and atrial fibrillation.10,11 Nevertheless, these channels are considered as targets of class III antiarrhythmic agents.12 Indeed, moderate IKs and IKr blockade may produce a beneficial class III antiarrhythmic effect.
Then, we would like to evaluate the effects of the hydro-ethanolic leaf extract of this hypotensive plant on the potassium currents involved in the regulation of the cardiac rhythm, by targeting the human IKs current and the IKr current. The aim of this study is to detect a potential side effect of H. bonariensis related to the cardiac rhythm disorder, and in a second time any beneficial effect of this plant for the cardiac function.
2. Materials and methods
2.1. Biological materials
2.1.1. Plant extract
The leaves of Hydrocotyle bonariensis (Araliacea) collected in Togo, have been identified in the Laboratory of Botany and Plant Ecology of the Faculty of Sciences of the University of Lomé and deposited in the herbarium under the identification number TG 15183. The hydro-ethanolic extract was obtained by classical isolation and solubilization procedures (see supplementary).13
2.1.2. HEK 293 IKs and HEK 293 hERG stable cell lines
The patch clamp experiments for IKs current recording were performed on the HEK 293 (Human Embryonic Kidney 293) cell line stably transfected with the KCNQ1 and KCNE1 genes encoding the channel responsible for the "IKs" current (HEK293 IKs). After selection in 1.5 mg·mL-1 geneticin (Sigma Aldrich) for 2 weeks, colonies were picked using cloning cylinders (Sigma Aldrich). The current carried by the hERG channel will be called IKr even if the regulatory subunit of IKr is lacking. Complementary deoxyribonucleic acid (cDNA) encoding for the human ether-a-go-go related gene product (HERG; GenBank Acc. No. U04270) was cloned into pcDNA3.1-Zeo (Invitrogen, Carlsbad, CA, USA) vector.14,15 IKs and IKr were examined for channel expression by whole-cell configuration of patch clamp technique.
2.1.3. Cell culture
HEK 293 IKs and HEK 293 hERG cells are stored in cryotubes placed in liquid nitrogen at −180 °C. For culturing, cryotubes are thawed under a laminar flow hood and seeded in 75 cm 2 culture flasks at 37 °C in a humidified atmosphere enriched with 5% CO2. Cells are grown in Dulbecco's modified Eagle's medium DMEM supplemented with 10% (v/v) fetal bovine serum (Cambrex Bio Science Verviers, Belgium) and 1% antibiotic (streptomycin, 50 IU/mL, Cambrex). The culture media are replaced after 24 h. When the cells are confluent, a transplantation is performed. The medium is changed every 48 h during the transplantation of the cells. The cells are seeded in 35 mm Petri dishes to obtain a confluence of about 50% on the day of the experiment.14,15
2.2. Electrophysiology
2.2.1. Patch clamp technique
The ionic potassium currents were recorded in the whole-cell configuration using an Axopatch 200A amplifier with a CV 202AU headstage (Molecular Devices, CA, USA). Voltage clamp were generated by a personal computer equipped with an analog-digital converter (Digidata 1322a, Molecular Devices) using pClamp software v10 (Molecular Devices). Analyses were performed using Clampfit 10 software.15
2.2.2. IKs recording protocol
Cells were held at a membrane potential of −80 mV. Currents were triggered by 3s test pulses at +100 mV applied every 15 s until steady state was reached. Current-voltage relationships (IKs) were obtained by stepping the membrane voltage for 5.5 s at voltages between −70 and + 90 mV in 20 mV increments, and tail currents were generated by the following repolarization to −40 mV (for 3 s).15 Cells were superfused with vehicle until stabilization, then with H. bonariensis extract at 0.5 mg/ml (n = 5) until steady state, followed by a washout period with vehicle. Note that preliminary results obtained in our laboratory indicate that 0.5 mg/ml reduces blood pressure in anesthetized rabbit by more than 30% (not shown).
2.2.3. IKhERG recording protocol
Cells were held at a membrane potential of −80 mV. Currents were triggered by 5 s test pulses at +20 mV applied every 10 s until steady state was reached. Current-voltage (IV) relationships were obtained by varying the membrane voltage for 4.25 s at voltages between −70 and + 50 mV in 20 mV increments. Deactivation tail currents were recorded at −50 mV14.
2.3. Statistics
Results are expressed as mean ± standard error on the mean (n = 6). They are processed with GraphPad Prism 7.0 software. Analysis of variance (ANOVA) and T-test are used to compare the means. The results are significant if the p-value < 0.05.
3. Results
3.1. Effects of H. bonariensis extract on IKs potassium current
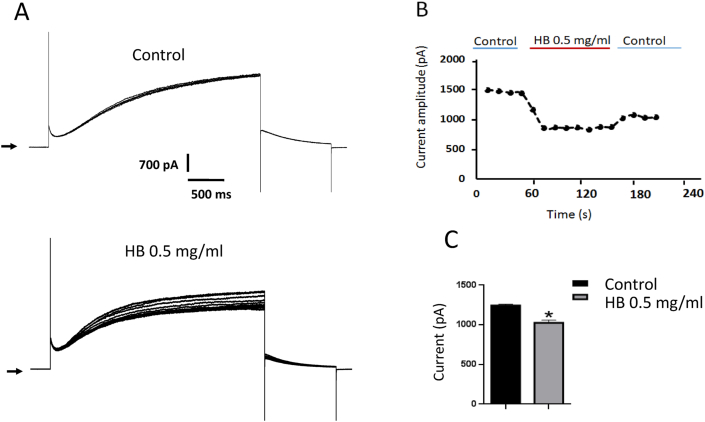
We first examined the effect of the Hydrocotyle bonariensis hydro-ethanolic leaves extract (0.5 mg/ml) on the IKs currents, with HEK 293 cell lines stably transfected with KCNQ1/KCNE1 subunits (Fig. 1). During the application of voltage clamp protocols (see supplement) cells were superfused with vehicle until stabilization (Fig. 1 A up panel), then H. bonariensis extract at 0.5 mg/ml until steady state, followed by a washout period with vehicle (Fig. 1A down panel). As show in Fig. 1 B, H. bonariensis extract at 0.5 mg/ml inhibits IKs to reach a stable state. This effect is partially reversible. The mean inhibition value obtained is estimated at 21.88 ± 3.69% (p < 0.05, n = 6) (Fig. 1C).
Fig. 1.
Currents recorded on HEK 293-IKs cells by imposing the stimulation protocol (n = 6). A: IKs current amplitude recording in control condition (upper panel) and in treated condition (H. bonariensis extract at 0.5 mg/kg, lower panel). Arrows correspond to current I = 0. B: Time-course representing maximal IKs current before, during and after H. bonariensis 0.5 mg/ml perfusion. C: IKs amplitude variation showed by T-test + mann-whitney test (∗P < 0,05).
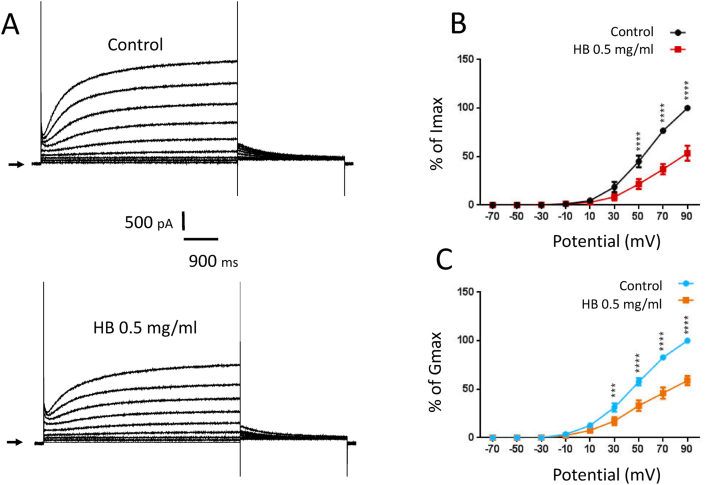
The effect of H. bonariensis extract on the current-voltage relationship of the IKs channel was estimated by normalizing current values plotted against the test potential in the absence (Fig. 2 A up panel) and presence (Fig. 1 A down panel) of the extract. Normalized currents were obtained from the peak current amplitudes in response to depolarizing pulses, in the range of −70 to +90 mV (Fig. 2 A and B). The analyze of activation curves indicates a significant decrease in maximal conductance without induced activation range shift. Indeed, Gmax is reduced by 24 ± 4.1% (p < 0.01, n = 6) percent while V1/2 is not modified (43 ± 1.6 mV vs 45 ± 2.3 mV p > 0.5, n = 6).
Fig. 2.
Currents recorded on HEK 293-IKs cells by imposing the I/V stimulation protocol (n = 6). A: IV curve recording in control condition (upper panel) and in treated condition (H. bonariensis extract at 0.5 mg/kg, lower panel). Arrows correspond to current I = 0. B: Normalized IKs IV curve amplitude variation showed by ANOVA + sidak's multiple comparisons test. C: Normalized activation curve obtained from tail current traces as a function of pre-potentials (ANOVA + sidak's multiple comparisons test). ∗∗∗p < 0.001 & ∗∗∗∗p < 0.0001.
3.2. Effects of H. bonariensis extract on IKr potassium current
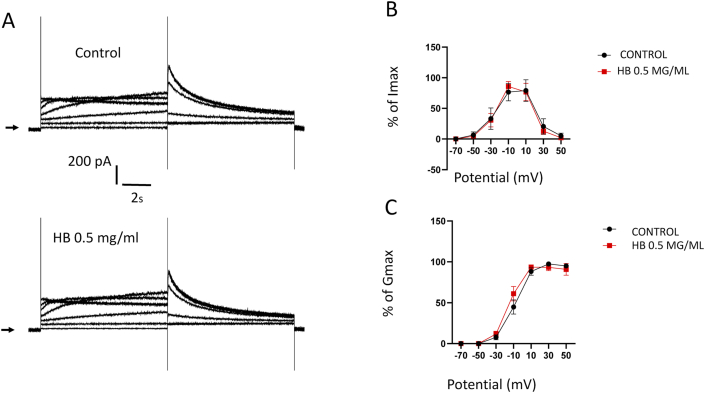
We examined next the effect of the Hydrocotyle bonariensis hydro-ethanolic leaves extract on IKr at 0.5 mg/ml. Unlike IKs channels, the extract did not induce any change of amplitude and kinetic of IKr (Fig. 3 A). In the same way, no significant effect of the extract is detected on both the I/V (Fig. 3 B) and activation curves (Fig. 3C) properties compared to the control.
Fig. 3.
Current recorded on HEK 293-IKr cells by imposing the IV curve stimulation protocol (n = 6). A: IV curve recording in control condition (upper panel) and in treated condition (H. bonariensis extract at 0.5 mg/kg, lower panel). Arrows correspond to current I = 0. B: Normalized IKr IV curve amplitude variation showed by ANOVA + sidak's multiple comparisons test. C: Normalized activation curve obtained from tail current traces as a function of pre-potentials (ANOVA + sidak's multiple comparisons test). No significant difference is observed.
4. Discussion
In this study, we investigated the impact of the hydro-ethanolic leaves extract of the hypotensive medicinal plant Hydrocotyle bonariensis on cardiac rhythmicity, by evaluating its effects on human potassium currents IKs and IKr.
The IKs and IKr currents are the major potassium components which contribute to repolarization of human heart.11 They have been recognized as potential targets for class III antiarrhythmic drugs that prolong action potential duration (APD) and refractoriness; they have been shown to be effective in preventing/suppressing cardiac arrhythmias.11,12,16
The present study showed that the extract of H. bonariensis at 0.5 mg/ml, significantly decreases the amplitude of IKs and the IV curve. The extract reduces the overall conductance of IKs by about 24% without modifying the activation threshold. The absence of a shift of the activation curve indicates that the extract modifies the channel conductance without altering their kinetics. In consequence, it is likely that the active molecules present in the extract block the channel pore.
On the other hand, we have observed that the effect of the extract is partially reversible. A possible explanation for this partial reversibility would be the binding of the bioactive compounds on different sites of one or more subunits of the slow component (IKs) of the potassium channel. In addition, it is plausible that these sites have a variable affinity toward the various components present in the extract, explaining the partial reversibility. Sites with slower interactions would put the channel in under-conducting states. These results are similar to other studies performed on the pharmacology of IKs potassium channels.17, 18, 19 In contrast, no significant change of both amplitude and kinetics of IKr has been induced by the extract.
Several previous studies have also highlighted the anti-arrhythmic properties of certain plants or plant-based compounds indicating their effects on the slow and fast potassium current. For example, in guinea pig ventricular myocytes, dauricin, a bioactive component of Menispermum dauricum inhibited both IKs and IKr currents.20 Further examinations on the cardiac potassium channels expresssed on HEK cells showed that grape flavonoids decrease HERG channels. In the same way, glycyrrhetinic acid, a component of licorice root (Glycyrrhiza glabra) dose-dependently blocked IKr and IKs in guinea pig ventricular myocytes.21,22 The peculiarity of the effects of H. bonariensis leaf extract that emerges from our work is its selective inhibition of the IKs current. In human myocardium, in the absence of β-adrenergic stimulation, inhibition of IKs induces little or no effect on the duration of action potential repolarization and on the QT interval.23 In contrast, in guinea pig papillary muscle, the inhibition of IKs causes a prolongation of action potential duration.24 The increase in repolarization and QT interval by blocking IKs will be greater in the presence of an already increased repolarization duration, either pharmacologically with inhibitors of IKr or IK1 currents, or pathologically, in the presence of long QT syndrome or heart disease. Furthermore, pharmacological inhibition of IKs which plays an important role of repolarization reserve, has been shown to enhance the susceptibility of heart to develop torsade de pointes in the presence of an IKr block.23,25 Moreover, specific IKs inhibitors (HMR1556) have not been associated with QT lengthening and torsade de pointes in humans under normal physiological conditions.24 In the present study, the reduction of IKs conductance by the plant extract is relatively small suggesting that proarrhythmic properties of flavonoids appear unlikely.
This selective inhibitory action of the extract of Hydrocotyle bonariensis leaves on the IKs current may contribute to its efficacy similar of class III antiarrhythmics, in addition to its hypotensive virtues. Indeed, class III antiarrhythmic drugs that block potassium channels and particularly IKs, are thought to prolong cardiac repolarization but not the conduction velocity. Prolongation of the action potential and refractory period of potassium channel-rich tissues, combined with maintenance of normal conduction velocity are known to prevent arrhythmic phenomena.26
5. Conclusion and perspectives
In this study, we evaluated the pharmacological effects on the potassium channels IKs and IKr of the hydro-ethanolic extract of the leaves of Hydrocotyle bonariensis; we found a selective inhibitory effect of the IKs current. This effect is similar to the effects of class III anti-arrhythmics suggesting the medical potential of this plant in the management of cardiac arrythmias. In vivo arrhythmia studies will be conducted to confirm the active and selective blocking of IK channels by the hydro-ethanolic extract and to assess its antiarrhythmic effect. Moreover, as the extract has not been yet characterized, bioactive compounds identification will be performed by classical chemical procedures.
Author contributions
K. Kaboua, A. Mouzou, P. Bois, T. Pakoussi and A. Diallo conceived the project and defined research methodology; K. Kaboua, A. Mouzou, P. Bois, T. Pakoussi, M. Assih and J. Bescond performed the experiments; K. Kaboua, P. Bois, Aurelien Chatelier, J. Bescond and A. Mouzou wrote the paper.
Declaration of competing interest
None.
Acknowledgement
We would like to thank C. Magaud, C-A Chapotte-Baldacci, S Mirval and E. Bere for their expertise. The work is supported by Campus France and La Fondation Pierre Fabre.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.09.004.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Monyn E.D., Bakayoko A., Bi F.H.T., Yao K., Kone M.W. Niveau de connaissance et composition minérale d'Hydrocotyle bonariensis Lam. (Araliaceae), une plante utilisée dans les ménages du District d'Abidjan (Côte d'Ivoire) Int J Brain Cognit Sci. 2016;10(5):2046–2061. [Google Scholar]
- 2.Ouviña A.G., Flores M.L. Hydrocotyle bonariensis Comm. ex Lam.. In: Máthé Á., Bandoni A. (eds) Medicinal and Aromatic Plants of South America, 2021 ; Vol. 2. Medicinal and Aromatic Plants of the World, vol vol. 7. Springer, Cham.
- 3.Obaseki O.E., Adesegun O.I., Anyasor G.N., Abebawo O.O. Evaluation of the anti-inflammatory properties of the hexane extract of Hydrocotyle bonariensis Comm. Ex Lam. leaves. Afr J Biotechnol. 2016;15(49):2759–2771. [Google Scholar]
- 4.Kannankeril P., Roden D.M., Darbar D. Drug-induced long QT syndrome. Pharmacol Rev. 2010 Dec;62(4):760–781. doi: 10.1124/pr.110.003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbosa C.C., Rodrigues T.C., Ataídes C.F.S., et al. Protective effects of Hydrocotyle umbellata var. bonariensis Lam. (Araliaceae) on memory in sleep-impaired female mice. J Ethnopharmacol. 2019;245:112183. doi: 10.1016/j.jep.2019.112183. [DOI] [PubMed] [Google Scholar]
- 6.Florinsiah L., Zuraina M.F., Shahirah N.N., Norfazlina M., Zaila C.S., Rajab N. Mutagenicity effect of hydrocotyle bonariensis extracts in salmonella/microsome assay. Int J Pharmaceut Sci Rev Res. 2013;20(2):47–50. [Google Scholar]
- 7.Sanguinetti M.C., Curran M.E., Zou A., et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 8.Warmke J.W., Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91(8):3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy C.E., Rudy Y. Cellular consequences of HERG mutations in the long QT syndrome: precursors to sudden cardiac death. Cardiovasc Res. 2001;50(2):301–313. doi: 10.1016/s0008-6363(00)00293-5. [DOI] [PubMed] [Google Scholar]
- 10.Hedley P.L., Jørgensen P., Schlamowitz S., et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30(11):1486–1511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- 11.Tamargo J. Drug-induced torsade de pointes: from molecular biology to bedside. Jpn J Pharmacol. 2000;83(1):1–19. doi: 10.1254/jjp.83.1. [DOI] [PubMed] [Google Scholar]
- 12.Tamargo J., Caballero R., Gómez R., Valenzuela C., Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62(1):9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Pakoussi T., Mouzou A.P., Metowogo K., Aklikokou K.A., Gbeassor M. How do Spondias mombin L (Anacardiaceae) leaves extract increase uterine smooth muscle contractions to facilitate child birth in parturient women? Afr Health Sci. 2018;18(2):235–243. doi: 10.4314/ahs.v18i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferro F., Ouillé A., Tran T.A., et al. Long-chain acylcarnitines regulate the hERG channel. PloS One. 2012;7(7) doi: 10.1371/journal.pone.0041686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Printemps R., Salvetat C., Faivre J.F., Le Grand M., Bois P., ou Maati H.M. Role of cardiac IKs current in repolarization reserve process during late sodium current (INaL) activation. Cardiology and Cardiovascular Medicine. 2019;3(4):168–185. [Google Scholar]
- 16.Clancy C.E., Kurokawa J., Tateyama M., Wehrens X.H., Kass R.S. K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annu Rev Pharmacol Toxicol. 2003;43:441–461. doi: 10.1146/annurev.pharmtox.43.100901.140245. [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Sesti F., Goldstein S.A. Pore- and state-dependent cadmium block of I(Ks) channels formed with MinK-55C and wild-type KCNQ1 subunits. Biophys J. 2003;84(6):3679–3689. doi: 10.1016/S0006-3495(03)75097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K.W., Goldstein S.A. Subunit composition of minK potassium channels. Neuron. 1995;14(6):1303–1309. doi: 10.1016/0896-6273(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 19.Tai K.K., Goldstein S.A. The conduction pore of a cardiac potassium channel. Nature. 1998;391(6667):605–608. doi: 10.1038/35416. [DOI] [PubMed] [Google Scholar]
- 20.Xia J.S., Guo D.L., Zhang Y., Zhou Z.N., Zeng F.D., Hu C.J. Inhibitory effects of dauricine on potassium currents in Guinea pig ventricular myocytes. Acta Pharmacol Sin. 2000;21(1):60–64. [PubMed] [Google Scholar]
- 21.Zitron E., Scholz E., Owen R.W., et al. QTc prolongation by grapefruit juice and its potential pharmacological basis: HERG channel blockade by flavonoids. Circulation. 2005;111(7):835–838. doi: 10.1161/01.CIR.0000155617.54749.09. [DOI] [PubMed] [Google Scholar]
- 22.Wu D., Jiang L., Wu H., et al. Inhibitory effects of glycyrrhetinic Acid on the delayed rectifier potassium current in Guinea pig ventricular myocytes and HERG channel. Evid Based Complement Alternat Med. 2013:481830. doi: 10.1155/2013/481830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jost N., Papp J.G., Varró A. Slow delayed rectifier potassium current (IKs) and the repolarization reserve. Ann Noninvasive Electrocardiol. 2007 Jan;12(1):64–78. doi: 10.1111/j.1542-474X.2007.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gögelein H., Brüggemann A., Gerlach U., Brendel J., Busch A.E. Inhibition of I Ks channels by HMR 1556. N Schmied Arch Pharmacol. 2000;362(6):480–488. doi: 10.1007/s002100000284. [DOI] [PubMed] [Google Scholar]
- 25.Jost N., Virág L., Bitay M., et al. Restricting excessive cardiac action potential and QT prolongation: a vital role for IKs in human ventricular muscle. Circulation. 2005;112(10):1392–1399. doi: 10.1161/CIRCULATIONAHA.105.550111. [DOI] [PubMed] [Google Scholar]
- 26.Wyse D.G., Waldo A.L., DiMarco J.P., et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.