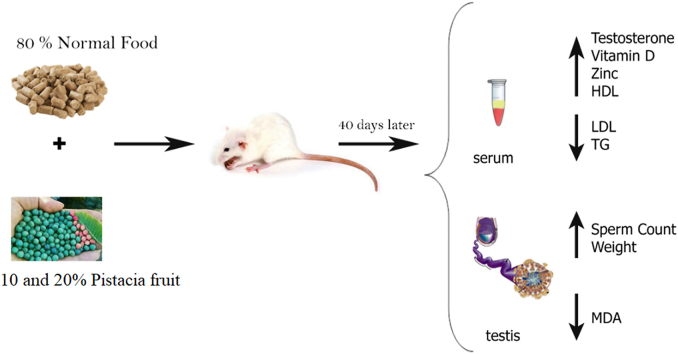
Abstract
Background and aim
Alongside the advancement of various modern treatments, attention has recently been focused on finding alternative infertility treatments based on diet and herbal medicine. Pistacia atlantica (wild pistachio; Baneh) is a plant species traditionally known as a libido booster and sexual enhancer.
Experimental procedure
The study aim was to evaluate the effects of a P. atlantica-enriched diet on the functions of the reproductive system of male rats.
Results and conclusion
Results revealed that the diet containing 20% P. atlantica doubled testosterone levels in the treatment group compared to the control. It decreased the total bodyweight, while significantly increased the ratios of the seminal vesicles, testis, and epididymis to the bodyweight of rats. The sperm count in the treatment group was 4.8 × 106/mL, which was significantly more than the control group (4.2 × 106/mL). Furthermore, the utilization of P. atlantica significantly raised HDL levels, though it reduced the LDL and TG levels and the concentration of testis MDA. Concerning other factors, the 20% of P. atlantica considerably increased the serum level of Zinc and vitamin D. Histomorphometric analysis revealed that the diet increased the testicular capsule thickness, whereas it did not cause any significant change in the diameter of the seminiferous tubule, the number of Leydig cells, and Sertoli cells. Additionally, the repopulation index, tubular differentiation index, and spermiogenesis index increased in the treatment group. Molecular analysis showed that the prescribed diet increased the expression of both oxytocin (OXT) and its receptor (OXTR) genes, improving the reproductive system function.
Keywords: Infertility; Oxytocin; Oxytocin receptor; Pistacia atlantica, reproductive system; Sex hormones; Testis
Graphical abstract
Highlights
-
•
Fertility and infertility are among the most complex issues in medical sciences.
-
•
Baneh (wild pistachio) traditionally is known as a sexual enhancer.
-
•
It enhances testosterone and oxytocin level in favour of increasing sperm counts.
-
•
Baneh improves lipid metabolism and increases the serum level of Zinc and vitamin D.
List of abbreviations
- OXT
oxytocin
- OXTR
oxytocin receptor
- DHT
dihydrotestosterone
- HDL
high-density lipoprotein cholesterol
- LDL
low-density lipoprotein cholesterol
- TG
triglyceride
- DEPC
diethyl-pyro carbonate
- H&E
Hematoxylin and eosin
- TDI
tubular differentiation indices
- SI
spermiogenesis indices
- RI
repopulation indices in males
- AST
Aspartate Aminotransferase
- ALT
Alanine Aminotransferase
- MDA
Malondialdehyde
- TBA
thiobarbituric acid
- ELISA
enzyme-linked immunosorbent assay
1. Introduction
Infertility is among the most prevalent issues worldwide, so that 15% of people in each society are infertile.1 In men, mainly, the most common cause of infertility is their inability to produce a sufficient number of healthy and active sperms.2 Hence, the normal functioning of testicles is one of the most important indexes of males’ reproductive health. Testosterone, as a primary sex hormone, plays a crucial role in this regard, so that testosterone deficiency may result in infertility, loss of libido, and muscle weakness.2,3 Toxic materials, diabetes, oxidative stress and ageing are among external or internal factors that interfere with normal testicular function.4,5 Ageing is mainly associated with decreasing sex hormones production, affecting many aspects of elderly health.6 Besides, recent studies have shown that nutritional factors and micronutrients play an essential role in the functioning of the reproductive system.7
Herbal medicines have been used to treat and prevent a wide range of diseases for a long time.8, 9, 10 Surprisingly, the majority of people worldwide still rely on local medications and traditional treatments that are mainly obtained from plant sources.11,12 Given that some plants are traditionally known as sexual enhancers,13,14 researchers, in recent years, have focused on the investigation of the effects of plant derivatives on the fertility of laboratory mammals that has resulted in invaluable achievements.
Wild pistachio, Pistacia atlantica, is a plant species that belongs to the Anacardiaceae family, which is called ‘Baneh’ in Iran. Different parts of P. atlantica (e.g., the gum, leaf, stem, and fruit) are used because of their therapeutic properties, including antihypertensive,15 anti-inflammatory,16 analgesic,16 anti-diabetic,15 antiepileptic, anti-hyperlipidemic,17 antitumor,18 and antimicrobial19 properties. The medicinal value of the plants depends on their chemical composition, such as alkaloids, phenolic compounds, fatty acids, and sterols.20 In addition, studies have revealed that the fruit of this plant consists of proteins, unsaturated fatty acids, and fibres, making it a valuable food21 prescribed to treat various disorders in traditional medicine. Also, it has been revealed that the extract of the plant is helpful in the treatment of eczema, renal stones, sexual arousal (aphrodisiac), as well as liver, urinary tract, and respiratory tract disorders.22
Recently, the phythochemicals of the oil of the plant fruits have been studied via gas chromatography mass spectrometry (GC-Mass).23 Wild pistachio oil contains significant amounts of tocopherol (some form of vitamin E), sterols, antioxidant polyphenols, essential fatty acids, essential and non-essential amino acids, and minerals such as iron, sodium, copper, and zinc.24 Omega-3, -6 and -9, oleic acid, palmitic acid, linoleic acid, and arachidonic acid are of most important fatty acids found in wild pistachio.25 Omega-3 and arachidonic acid are converted into prostaglandins in the body, which are involved in regulating sex hormones and blood pressure.25
Oxytocin (OT) is a well-known neuropeptide associate with animal and human reproductive and social behavior.26 The endocrine and paracrine roles that it plays in male reproduction has been determined. There is conclusive evidence that OT is synthesized within the mammalian testis, epididymis and prostate. The presence of OT receptors (OTRs) throughout the reproductive tract supports a local function for this peptide. It stimulates the seminiferous tubules, epididymis and prostate gland. OT has also been shown to modulate androgen levels in these tissues via stimulation of the testosterone conversion to dihydrotestosterone (DHT) by 5α-reductase.27,28
The objectives of this study were to assess the effect of the consumption of the fruit of the wild pistachio, P. atlantica, on the reproductive system of male rats. Toward this end, the animals were treated with diets containing 10 and 20% wild pistachio. Then, the testosterone level, the weight of reproductive organs and amounts of some micronutrients (zinc, vitamin D, and cholesterol), as well as the testis MDA concentration in animals were measured. Owning to OXT (structural gene for oxytocin) and OXTR (oxytocin receptor) frequently are involved in human social behavior, their expression was evaluated in this study too.
2. Materials and methods
2.1. Preparation of food containing P. atlantica
The fruits of the wild pistachio (P. atlantica) tree, locally known as Baneh, were collected from the mountains near Fasa City, Fars Province, Iran, in October. After authentication of the plant samples by the Shahid Bahonar University of Kerman Herbarium (associate code: 2915), their fruits were washed and dried in the shade at room temperature. Next, the whole fruits were powdered using an electric mill and mixed with regular animal food to prepare food containing 10% and 20% (W/W) P. atlantica, and the final composites were stored to feeding rats. The regular animal food was purchased from Pars Animal Food Co. (Tehran, Iran). As represented in Table 1, the regular food components and estimated nutrient requirements for maintenance and growth of rats have been determined already.29
Table 1.
The components of regular food to maintenance and growth of rats.
| Nutrients | Amount | Nutrients | Amount |
|---|---|---|---|
| Protein | 24% | Zinc | 96 ppm |
| Fat | 6.7% | Biotin | 0.20 ppm |
| Fiber (Crude) | 4.2% | Choline | 1621 ppm |
| Calcium | 0.95% | Folic acid | 2.74 ppm |
| Chloride | 0.40% | Niacin | 57 ppm |
| Magnesium | 0.23% | Pantothenic acid | 15 ppm |
| Phosphorus | 0.65% | Pyridoxine | 7.6 ppm |
| Phosphorus (non-phytate) | 0.31% | Riboflavin | 6ppm |
| Potassium | 1.24% | Thiamin | 14.5 ppm |
| Sodium | 0.22% | Vitamin B12 | 47 μg/kg |
| Sulfur | 0.25% | Vitamin A, | 15742IU/kg |
| Copper | 16 ppm | Vitamin D3 | 1425 IU/kg |
| Iodine | 1.2 ppm | Vitamin E | 55IU/kg |
| Iron | 210 ppm | Vitamin K (as menadione) | 2.92 ppm |
| Manganese | 105 ppm | Beta-Carotene | 0.69 ppm |
| Selenium | 0.38 ppm |
2.2. Animals and treatment conditions
In this study, 24 healthy adult male Wistar rats (120–150 g, 6-8-week-old) were obtained from the Animal Center of Shahid Bahonar University of Kerman and adapted to the laboratory conditions for two weeks before the main experiments. They were kept in controlled conditions, including standard temperature (25 ± 1 °C), humidity (50 ± 10%), and a 12:12-h light-dark cycle. Then, the animals were randomly divided into three groups (n = 8); ⅰ) Control group, provided with a regular diet, ⅱ) fed with food containing 10% of P. atlantica, and ⅲ) fed with food containing 20% of P. atlantica. Next, the animals were treated for six weeks while they were housed in cages containing 4 rats per cage. All procedures were conducted according to the guidelines for caring and using laboratory animals in the Neuroscience Research Center of Kerman University of Medical Sciences and the Neuroscience Ethics Committee (EC/KNRC/95-8 A).
2.3. The effect of the diet on the animals’ body and organs weight
To evaluate the diet's impact on the weight of the body and reproductive organs of the animals, at the end of six weeks, 24 h after the last treatment day, the animals were weighed. Then, they were anesthetized using ether, sacrificed, and their testis, epididymis, seminal vesicle, and prostate glands were removed and weighed.
2.4. Determination of testosterone level in serum
At the end of 6 weeks, the rats were anesthetized, decapitated with a guillotine, and their trunk blood was collected and centrifuged at 2500 rpm for 10 min at 4 °C to obtain serum. Then, the serum level of testosterone was measured by using enzyme-linked immunosorbent assay (ELISA) kits (DiaSorin Inc., USA), according to manufacturer instructions.
2.5. Motility and count of epididymal sperm
To study the count and motility of sperms, the cauda epididymis were dissected. Then, the epididymis semen was squeezed and diluted with physiological saline (NaCl 0.9%). Next, two caudal epididymides were plunged into 10 ml 0.9% of NaCl, and the fatty tissue was taken. The number of spermatozoa was counted using a hemocytometer and a binocular microscope (M = 400 × ) and analyzed according to equation.1 Quantitative epididymal sperm motility was indicated as an index determined by counting both immotile and motile spermatozoa per unit area.30
| Spermatozoa concentration (million/ml) = N = conversion factor × 106 | (1) |
| N = number of spermatozoa in Neubauer column; Conversion factor = | 5 |
2.6. Malondialdehyde assay
As one of the most prevalent byproducts of lipid peroxidation during oxidative stress, the Malondialdehyde (MDA) level was measured in the animals. To this end, the testis was homogenized in a microtube and incubated with thiobarbituric acid (TBA) for 30 min at 95 °C. The samples were then centrifuged at 10,000 rpm for 10 min, and the absorbance of the clear pink supernatant was measured at 532 nm. Malonhydialdehyd bis-(dimethyl acetal) (Sigma-Aldrich, Germany) was utilized as the standard (control).31
2.7. Assessment of biochemical parameters and lipid profile
After scarification, the trunk blood was collected and centrifuged at 2500 rpm for 10 min at 4 °C to prepare serum to use in further assessments. Next, total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), triglyceride (TG), Zinc (Zn), Vitamin D, Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Urea and Creatine levels in serum were measured by using the kits and the Automatic Chemistry Analyzer (KONELAB 20XT, Finland) as previously described in the literature.32
2.8. Histomorphometric analysis
In order to make a more detailed study, attachment, appearance, and abnormal features of the capsule, interstitial tissue, and seminiferous tubules were histologically studied. Toward this end, the testis tissues were firstly embedded in paraffin, then they were dehydrated, blocked, cleared, and turned to sections with 5–7 μm thickness. Next, they were stained by using Hematoxylin and eosin (H&E) and mounted on slides. Finally, the diameter of the seminiferous tubule, the height of the epithelium of the seminiferous tubule, the number of Sertoli cells (in each seminiferous tubule), the number of Leydig cells, and the number of seminiferous tubules were examined. Furthermore, spermatogenesis and spermiogenesis studies, including tubular differentiation indices (TDI), spermiogenesis indices (SI), and repopulation indices in males (RI). were conducted, as previously described by Kalantari Hesari et al.33 Histomorphometric studies were carried out using the Dino-Lite lens digital camera and Dino-capture 2 Software.
2.9. Evaluation of OXT and OXTR gene expression
2.9.1. Tissue isolation and RNA extraction
Rats were decapitated the day after the end of the food schedule. The hypothalamus was quickly removed, frozen on dry ice and stored at −80 °C for further analysis. Total RNA was extracted from each tissue sample by the RNX-Plus reagent (CinnaGen Co), and treated with DNase (RNase-free DNase set from Roche, Manheim, Germany). The final RNA pellets were resuspended in 30 μl diethyl-pyrocarbonate-treated water (DEPC-treated water). The amount of the purified RNA was determined by Nanodrop (A260⁄A280 ≥ 1.9), and the integrity of RNA samples was analyzed on a 1.5% agarose gel. RNA was stored at −80 °C for further analysis.
2.9.2. cDNA synthesis
The cDNA synthesis reaction was performed using Oligo-dT primer and M-MuLV reverse transcriptase (Thermo Fisher Scientific, Germany) following the manufacturer's instruction. The reproducibility of single results was determined through a two-time measurement of cDNA aliquots and analysis of two different cDNA prepared from the same RNA extract.
2.9.3. Quantitative polymerase chain reaction (qPCR)
Quantification of relative RNA expression followed the established method using qPCR with the SYBR green reporter dye and protocol. The 2 × universal master mix (Ampliqun -Denmark) was used in the Real-time polymerase chain reaction (RT-PCR) reactions. The amplification program was as follows: a denaturation step of 15 min at 95 °C and 40 cycles for real-time PCR including 30 s at 95 °C (denaturation), 30 s at 60 °C (annealing), and 30s at 72 °C (polymerase elongation). A final melting curve of fluorescence vs temperature was generated to screen for primer dimers and to document single product formation. To confirm the amplification specificity of the PCR, products from each primer pair, in addition to melting curve analysis, were subjected to subsequent agarose gel (1.5%) electrophoresis. Thermal cycling utilized a Bio-Rad iQ5 detection system (Bio-Rad, Richmond, CA, USA). A final melting curve of fluorescence versus temperature was generated to screen for primer dimers and document single product formation. Primer sequences, RT-PCR fragment lengths, and NCBI accession numbers are mentioned in Table 2. All primer pairs produced a single band on agarose gel electrophoresis corresponding to the predicted size. The number of PCR products was normalized with housekeeping (B-actin) primers in separate reactions.
Table 2.
Primer sequences, RT-PCR fragment lengths, and NCBI accession numbers.
| Primer name | Primer sequence | Size of PCR product | NCBI accession number |
|---|---|---|---|
| actin-β | F: CTAGGCACCAGGGTGTGATG R: GGTTGGCCTTAGGGTTCAGAG |
234 | NM_031144.3 |
| OXT | F: CCTGGATATGCGCAAGTGTCTTC R: TCGGAGAAGGCAGACTCAGG |
267 | NM_012996.3 |
| OXTR | F: GGCTGCCGAGGGGAATGAC R:ATGGCAATGATGAAGGCAGAAGC |
222 | NM_012871.3 |
All samples were assayed in triplicate. Linearity and efficiency of PCR amplification were assessed using standard curves generated by decreasing amount of cDNA, using five points, diluted over a twofold range. In qPCR, the relative mRNA levels were calculated by the expression 2−ΔΔCT.34
2.10. Analysis of oxytocin levels in serum
At the end of the experiment, trunk blood was collected and its serum was aliquoted and kept at −80 °C until assays. The serum levels of Oxytocin was quantified by rat-specific ELISA Kit (ZellBio GmbH, Ulm, Germany) according to the manufacturer's instructions. The assay sensitivity of Oxytocin was 1 ng/L. Intra-assay and inter-assay coefficients of variability for Oxytocin were lower than 10% and 12%, respectively.
2.11. Statistical analysis
All of the results were expressed as mean ± SEM. Statistical comparison was performed using independent T-tests and One-way ANOVA using the SPSS 16.0. P-values less than 0.05 (P < 0.05) were considered as the significant level.
3. Results
3.1. Effects of P. atlantica on the weight of the body and organs
The results of the assessments, detailed in Table 3, showed that the food enriched with P. atlantica decreased the total body weight of animals compared to the control group that was fed the regular diet (P < 0.05). Although no significant difference was observed in the ratio of the prostate/body weight of these groups, the ratio of the testis and epididymis to the body weight in rats fed with diets containing both 10% and 20% P. atlantica was significantly higher than the control. In addition, the ratio of the seminal vesicles to the body weight of the rats had 20% P. atlantica in their food were substantially more than the control group.
Table 3.
Effects of the diet containing 10% and 20% of P. atlantica (treatment) on body weights and male reproductive organs (testis, epididymis, prostate, and seminal vesicle.
| groups | initial weight (g) | final weight (g) | body weight difference (g) | testis absolute weight (g) | epididymis absolute weight (g) | prostate absolute weight (g) | seminal vesicle absolute weight (g) | testis relative weight % | Epididymis relative weight % | Prostate relative weight % | Seminal relative weight % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| control | 142 ± 3.9 | 222.8 ± 4.5 | 80.8 | 2.38 ± 0.06 | 0.42 + .02 | 0.54 ± .02 | 0.72 ± .0.03 | 1.07 ± 0.04 | 0.18 ± .007 | 0.22 ± 0.01 | 0.32 ± .0.01 |
| 10% (w/w) of P. atlantica | 145 ± 1.84 | 198.8 ± 3.38∗ | 53.8 | 2.32 ± .1 | 0.462 ± .01 | 0.495 ± .01 | 0.745 ± .01 | 1.16 ± .03∗ | 0.23 ± .004∗ | 0.24 ± .005 | 0.37 ± .008 |
| 20% (w/w) of P. atlantica | 142 ± 4.7 |
204.5 ± 5.3 ∗ |
62.5 | 2.51 ± 0.07 ∗ |
0.57 + 0.01 ∗∗ |
0.56 ± 0.01 | 0.83 ± 0.06 ∗ |
1.19 ± 0.04∗ | 0.27 ± 0.007 ∗∗∗ |
0.25 ± 0.01 | 0.41 ± 0.03 ∗∗ |
Data have been expressed as mean ± SEM, ∗∗∗P < 0.001, ∗∗P < 0.01, ∗P < 0.05, significant differences compared to the control group.
3.2. Effects of P. atlantica on testosterone levels in serum
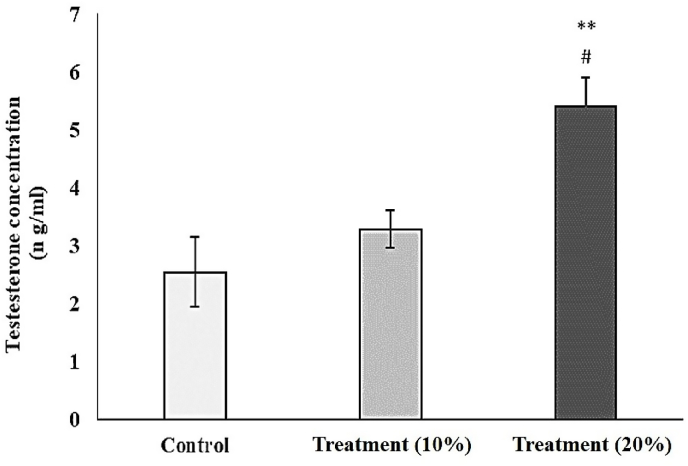
As Fig. 1 shows, although the diet containing 10% P. atlantica fruits did not show any considerable impact on the testosterone level in treated rats, the presence of 20% P. atlantica in their food could significantly increase this feature (P < 0.01). This was about 6 ng/ml in the group treated with 20% P. atlantica, which was twice as higher as the control.
Fig. 1.
The effect of the diet containing 10% and 20% of P. atlantica (treatment) on the serum levels of testosterone in male rats. Data were expressed as mean ± SEM, ∗∗P < 0.01 significant differences than the control group. #P < 0.05, significant differences compared to the group fed with 10% P. atlantica.
3.3. Effects of P. atlantica on sperm counts and motility
The statistical analysis of the sperm counts and motility revealed a significant difference between the tested groups. According to the results, the sperm counts in rats fed with the diet containing 20% P. atlantica were 4.8 × 106/mL, while it was 4.2 × 106/mL in the control group. No significant difference was observed in the sperm motility of these groups (Table 4).
Table 4.
Effects of the diet containing 10% and 20% of P. atlantica (treatment) on sperm characteristics.
| Groups | Sperm Counts (106/ml) | Motility (%) |
|---|---|---|
| Control | 4.2 ± 0.1 | 78 ± 7.5 |
| 10% (w/w) of P. atlantica | 4.3 ± 0.6 | 80 + 5.57 |
| 20% (w/w) of P. atlantica | 4.8 ± 0.12 ∗∗ ## | 85 ± 6.5 |
Data have been expressed as mean ± SEM, ∗∗P < 0.01, significant differences compared to the control group. ##P < 0.01, significant differences compared to the group fed with 10% P. atlantica.
3.4. Effects of P. atlantica on lipid profile
The results of the assessment of the lipid profile of the male rats, including total cholesterol, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and triglyceride (TG), have been shown in Table 5. The results indicated that the utilization of P. atlantica caused a significant rise in HDL levels in the animals’ serum. Also, this diet significantly reduced the LDL and TG levels in the serum of the treated animals. These features decreased by 7.5 μg/dl and 25 μg/dl, respectively (Table 5).
Table 5.
Effects of the diet enriched with P. atlantica on the serum lipid profile of male rats.
| Groups | Cholesterol (μg/dl) | LDL (μg/dl) | HDL (μg/dl) | TG (μg/dl) |
|---|---|---|---|---|
| Control | 46.5 ± 3.6 | 37.8 ± 1.8 | 35.6 ± 1.1 | 77.12 ± 8.02 |
| Treatment 10% | 50.74 ± 2.63 | 31.54 ± 2.15 | 38.5 ± 1.79 | 64.6 ± 2.01∗ |
| Treatment 20% | 53.4 ± 3.4 | 30.4 ± 2.1∗ | 48.6 ± 3.7∗∗ | 52.4 ± 7.2∗∗ |
Data have been expressed as mean ± SEM, ∗∗P < 0.01, ∗P < 0.05, significant differences compared to the control group.
Given that the presence of 20% of P. atlantica in animal food significantly affected the level of testosterone, sperm count and serum lipid profile in adult male rats, the animals that were on this effective diet (containing 20% P. atlantica and six weeks treatment) were chosen for further assessments.
3.5. Effects of P. atlantica on malondialdehyde (MDA) and essential ingredients level
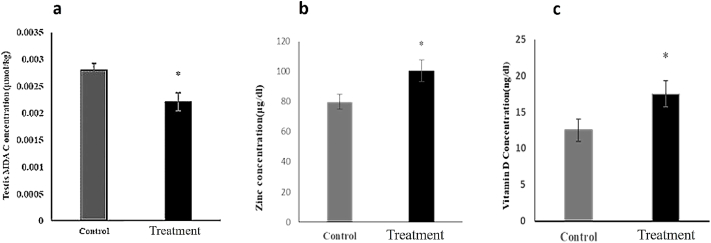
As Fig. 2a shows, feeding rats with P. atlantica resulted in a marked decrease in the concentration of the testis MDA from about 0.00279 μmol/kg in the control group to about 0.0022 μmol/kg in the treated group.
Fig. 2.
Effects of the diet containing 20% of P. atlantica (treatment) on testis MDA concentration (a), serum concentrations of Zinc (b), and vitamin D (c). Data have been expressed as mean ± SEM, ∗P < 0.05, significant differences compared to the control group.
Concerning further factors that influence the function of the reproductive system, such as the serum zinc and vitamin D concentration, 20% of P. atlantica significantly increased the serum zinc level (Fig. 2b). Likewise, the serum vitamin D level in this group was substantially higher than the control (P < 0.05) (Fig. 2c).
3.6. Effects of P. atlantica on the kidney and liver biochemical factors
Table 6 presents the effects of the P. atlantica on some important biochemical features related to the liver and kidney of male rats. According to the results, the P. atlantica reduced the AST level in the serum of animals to 186 IU/L, although this diet did not affect other factors considerably.
Table 6.
Effects of the diet containing P. atlantica on the kidney and liver features of male rats.
| Group | AST (IU/L) | ALT (IU/L) | Urea (mg/dl) | Creatine (mg/dl) |
|---|---|---|---|---|
| Control | 229.6 ± 9.8 | 95.4 ± 5.3 | 38.5 ± 2.2 | 0.37 ± .04 |
| Treatment 20% | 186.0 ± 16.0∗ | 83.4 ± 5.3 | 38.5 ± 2.2 | 0.46 ± .04 |
∗P < 0.05, significant differences compared to the control group.
3.7. Histomorphometric analysis
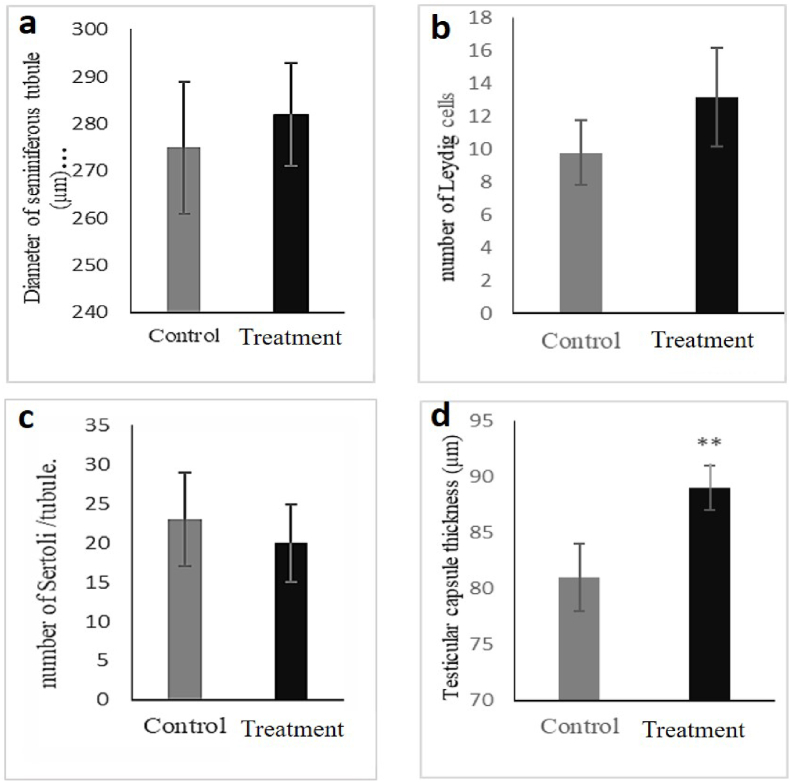
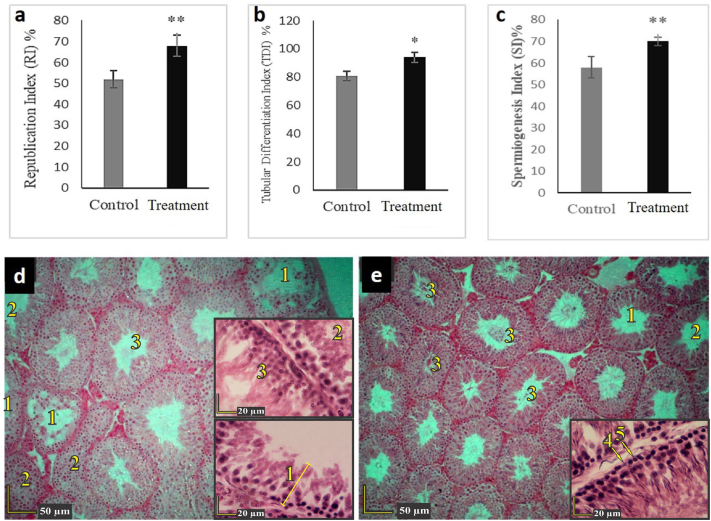
A detailed study on the sections provided from the testis of the animals revealed that the diet containing 20% of P. atlantica did not cause any significant change in the diameter of the seminiferous tubule, the number of Leydig cells, and Sertoli cells (Fig. 3a–c). At the same time, it could considerably increase the testicular capsule thickness from 82 μm in the control group to about 90 μm in the treatment group (Fig. 3, Fig. 4d,e). Furthermore, this particular diet significantly increased the republication index (RI), tubular differentiation index (TDI), and spermiogenesis index (SI) compared to the control group (Fig. 4 a-c).
Fig. 3.
Effects of the diet containing 20% of P. atlantica (treatment) on histomorphometric parameters of the testis in male rats. a) Diameter of the seminiferous tubule, b) the number of Leydig cells, c) the number of Sertoli cells, and d) testicular capsule thickness. Data have been expressed as mean ± SEM, ∗∗P < 0.01 ∗P < 0.05, significant differences compared to the control group.
Fig. 4.
Effects of the diet containing 20% of P. atlantica (treatment) on the republication index (a), tubular differentiation index (b), and spermiogenesis index (c) of rats in comparison with the control group. Also, (d) and (e) are the sections of the control and treatment groups, respectively. Number 1 indicates the seminiferous tubules with a negative tubular differentiation index (TDI). Number 2 shows the seminiferous tubules with a negative spermatogenesis index (SI), Number 3 shows the seminiferous tubules with a positive spermatogenesis index (SI), Number 4 shows spermatogonia type A and Number 5 shows spermatogonia type B.
3.8. Effects of P. atlantica on OXT and OXTR gene expression
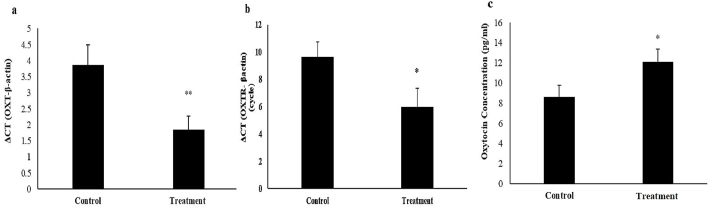
OXT and OXTR are two genes encoding the oxytocin and oxytocin receptors, respectively. According to the results shown in Fig. 5, the prescribed diet significantly increased the expression of both OXT and OXTR genes in the Rats’ hypothalamus and serum level of oxytocin hormone.
Fig. 5.
Effects of the diet containing 20% of P. atlantica (treatment) on the expression of OXT (a) and OXTR (b) genes in the Rats' hypothalamus and serum level of oxytocin hormone (c). Data have been expressed as mean ± SEM, ∗∗P < 0.01 ∗P < 0.05, significant differences compared to the control group.
4. Discussion
Infertility has long been one of the main concerns of humans, particularly in recent decades, and lots of efforts have been made to find an effective treatment for that. Diseases, injuries, chronic health problems, lifestyle and some other factors can negatively affect male fertility. Given that nutrition is one of the most influential factors on human health, it is expectable that the individuals' diet considerably affects their reproductive system. Wild pistachio (Pistacia atlantica), locally called Baneh, is traditionally consumed as a sexual enhancer food.35 To precisely study the effects of this food on the reproductive system, male rats were fed with food containing 10% and 20% powdered P. atlantica fruits and the main features related to their reproductive system were studied.
The results obtained in the present study showed that testosterone significantly increased in subjects fed with wild pistachio. Since this hormone is considered the primary sex hormone that plays a central role in the reproductive system, increasing its level undoubtedly affects the performance of this system. First of all, it should be explained how a P. atlantica-enriched diet may increase the testosterone level.
As recently reported, linolenic acid with about 27% is among the major fatty acids in P. atlantica.35 This fatty acid can be converted to dihomo-γ linolenic acid, then to arachidonic acid (AA), which is the precursor of prostaglandins such as PGE2.36,37 Studies show that AA and its metabolite PGE2 increase the production of adenylate cyclase (Cyclic AMP).38 They also increase the rate of cholesterol side-chain cleavage and stimulate the synthesis of testosterone.39 An investigation on mature rats showed that omega-6 fatty acids, such as linoleic acid, are essential for testicular growth.40 Furthermore, wild pistachio contains tocopherol and tocotrienol, which are antioxidants with similar functions to vitamin E in the body.41 It has been reported that vitamin E increases testosterone levels in the body.42 In turn, the increased level of testosterone can cause a decrease in body weight,43 as observed in this study.
According to the results, triglyceride and LDL levels have decreased, while HDL levels have increased in rats fed with wild pistachio, which agrees with other reports.44,45 It has been proved that an increase in serum or gonadal levels of cholesterol through herbal medicines can enhance sexual activity.46 Cholesterol is a precursor of predominant physiologic steroids, including bile acids, steroid hormones, and vitamin D47,48; therefore, increased cholesterol levels may lead to increased levels of testosterone through steroidogenesis, which should generally improve reproductive performance and libido.47
The increasing of the weight of testicles, epididymis, and seminal vesicles and the ratio of these organs’ weight to total body weight was another striking result. The seminal vesicle epithelium thickness, tubular differentiation index (TDI), repopulation index (RI), and spermatogenesis index (SI) also increased in the tested group. The present study findings also indicated that feeding with 20% of wild pistachio increased sperm density. Since androgens (testosterone and androstenedione) are essential for the growth and normal functioning of the sexual organs, and there is a direct relationship between testosterone level and the weight of testicle and epididymis, the significant increase in the weight of testicle and epididymis can be attributed to the substantial increase in serum levels of testosterone in rats. Moreover, the changes in testosterone level may cause an increase in the weight of all auxiliary organs.
It is worth mentioning that testosterone alters sperm production by directly affecting Sertoli cells, released tubal fluid, and several proteins, including growth factors and transferrin playing a role in feeding and dividing sexual cells. Thus, considering the important role of testosterone in spermatogenesis, the increase of this hormone will increase the number of sperms,49 which follows what was observed in this study.
According to the results, body weight decreased significantly under wild pistachio treatment. A similar study has previously investigated the effects of consumption of different amounts of P. atlantica and showed it could remarkably reduce the weight of rats.50 The decreased body weight may be due to the high fibre content of pistachio and its fast digestion.51,52 Besides, pistachio reduced levels of LDL and triglyceride. Studies show that excessive consumption of plant sterols can reduce serum LDL levels.53,54 According to Wang et al., pistachio consumption can be useful in decreasing plasma triglyceride levels and body weight of obese people.55 Another study investigated the effect of wild pistachio powder on phosphatidate phosphatase (PAP) and lipids profiles of male rats.44 It was found that the decrease observed in the enzymatic activity was partly due to oleic and linoleic acids in wild pistachio, which reduces the PAP enzyme levels, and ultimately, liver triglyceride levels.56
Evaluation of the effects of wild pistachio on MDA (an oxidative stress indicator) concentrations in the testicle revealed that this feature considerably decreased in the treatment group. This effect can be related to the antioxidant properties of wild pistachio.57 The fruits of this plant contain flavonoids, and phenolic compounds, which are potent antioxidants neutralizing reactive oxygen species (ROS) produced during metabolic activities and reduce sperm counts and sperm mobility.58 Therefore, the increasing sperm counts observed in this study can be due to the presence of antioxidant compounds. Previous studies also showed the prescription of P. atlantica extract reduces MDA levels and increases the level of antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase in the blood.57
Vitamin D is an essential micronutrient that its level considerably increased in the rats fed with P. atlantica. The role of vitamin D in the reproductive function of different animals has been demonstrated and various studies have also proved this important role in humans.39,59
The results indicated an increase in zinc levels in the P. atlantica-fed animals. Obviously, the changes in the concentration of some elements such as iron, zinc, magnesium, and potassium can affect the functioning of the reproductive system.60 Although no study has so far investigated the effect of P. atlantica on serum zinc levels, it has been reported that consumption of another type of pistachio (Pistacia lentiscus) could increase zinc levels in the prostate.61 Zinc may promote cell division and can also facilitate cell division processes. Furthermore, it has been revealed that zinc deficiency increases the risk of oxidative damage and reproductive weakness in both sexes.62
In this study, feeding with pistachio decreased AST levels in males. It has recently been reported that Pistacia lentiscus oil caused changes in transaminase levels in rats, which may be due primarily to tocopherol, phytosterols and unsaturated fatty acid compounds.63
The level of testosterone has been shown to have a direct relationship with the expression of the oxytocin and oxytocin receptor genes in the hypothalamus.64 It seems that testosterone and its metabolites, such as dihydrotestosterone and estrogen, control the expression of these genes.65 In the present study, in line with what was mentioned, these features increased in the hypothalamus which can be attributed to the increase observed in the testosterone levels of treated Rats. Furthermore, a study previously carried out by our group showed that treatment with P. atlantica reduced the stress of gonadectomized rats under stress.66 Since oxytocin is a well-known stress-reducing factor, the decrease observed in the stress level can be attributed to the increase of oxytocin caused by P. atlantica.
5. Conclusion
The results of this study revealed that how P. atlantica (wild pistachio, Baneh), as a traditional libido booster and sexual enhancer, affects the reproductive physiology and anatomy of rats. This food through enhancing two key hormones level, testosterone and oxytocin, causes a significant increase in the ratio of the reproductive organs' weight to the body weight and affects testis morphology in favour of increasing sperm counts. It also improves lipid metabolism and considerably increases the serum level of Zinc and vitamin D. Concluding, the foods containing P. atlantica deserve to be more considered by both reproductive physiology and infertility researchers and food industries.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors appreciate the contribution of Dr Seyed Mansour Mirtadzadini from the Department of Biology, Shahid Bahonar University of Kerman, for the identification and confirmation of P. atlantica. This study was financially supported by a fund from Shahid Bahonar University, Kerman, Iran (Grant No. 8/95).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
References
- 1.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13(1):1–9. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karagiannis A., Harsoulis F. Gonadal dysfunction in systemic diseases. Eur J Endocrinol. 2005;152(4):501–513. doi: 10.1530/eje.1.01886. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Fernandez R., Martini A., Navarro V., et al. Novel signals for the integration of energy balance and reproduction. Mol Cell Endocrinol. 2006;254:127–132. doi: 10.1016/j.mce.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 4.Ali Z.Y. Neurotoxic effect of lambda-cyhalothrin, a synthetic pyrethroid pesticide: involvement of oxidative stress and protective role of antioxidant mixture. New York Sci J. 2012;9(5):93–103. [Google Scholar]
- 5.Nunes K.P., Webb R.C. Erectile Dysfunction-Disease-Associated Mechanisms and Novel Insights into Therapy. InTech; 2012. Mechanisms in erectile function and dysfunction: an overview. [Google Scholar]
- 6.Joshi D., Van Schoor N.M., De Ronde W., et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol. 2010;72(2):232–240. doi: 10.1111/j.1365-2265.2009.03641.x. [DOI] [PubMed] [Google Scholar]
- 7.Cemile M., Cigdem E. The effects of oxidative stress and some of the popular antioxidants on reproductive system: a mini review. J Nutr Food Sci. 2016;6(2):2–3. [Google Scholar]
- 8.Fereidooni F., Komeili G., Fanaei H., Safari T., Khorrami S., Feizabad A.K. ☆ Protective effects of ginseng on memory and learning and prevention of hippocampal oxidative damage in streptozotocin-induced Alzheimer's in a rat model. Neurol Psychiatr Brain Res. 2020;37:116–122. [Google Scholar]
- 9.Chandrasekara A., Shahidi F. Herbal beverages: bioactive compounds and their role in disease risk reduction-A review. Journal of traditional and complementary medicine. 2018;8(4):451–458. doi: 10.1016/j.jtcme.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamidpour M., Hamidpour R., Hamidpour S., Shahlari M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. Journal of traditional and complementary medicine. 2014;4(2):82–88. doi: 10.4103/2225-4110.130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karunamoorthi K., Jegajeevanram K., Vijayalakshmi J., Mengistie E. Traditional medicinal plants: a source of phytotherapeutic modality in resource-constrained health care settings. Journal of Evidence-Based Complementary & Alternative Medicine. 2013;18(1):67–74. [Google Scholar]
- 12.Saggu S., Divekar H., Gupta V., Sawhney R., Banerjee P., Kumar R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: a dose dependent study. Food Chem Toxicol. 2007;45(4):609–617. doi: 10.1016/j.fct.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Ebrahimpour N., Khazaneha M., Mehrbani M., Rayegan P., Raeiszadeh M. Efficacy of Herbal Based Syrup on male sexual experiences: a double-blind randomized clinical trial. Journal of Traditional and Complementary Medicine. 2021;11(2):103–108. doi: 10.1016/j.jtcme.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indurwade N., Kawtikwar P., Kosalge S., Janbandhie N. Herbal plants with aphrodisiac activity. Indian Drugs. 2005;42(2):67–72. [Google Scholar]
- 15.Ahmed Z.B., Yousfi M., Viaene J., et al. Potentially antidiabetic and antihypertensive compounds identified from Pistacia atlantica leaf extracts by LC fingerprinting. J Pharmaceut Biomed Anal. 2018;149:547–556. doi: 10.1016/j.jpba.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 16.Nadri S., Mahmoudvand H., Mahmoudvand H., Rashnoo M., Khaksarian M. Chemical composition, antinociceptive and acute toxicity of Pistacia atlantica fruit extract. Entomol Appl Sci Lett. 2018;5(3):8–12. [Google Scholar]
- 17.Hosseini S., Nili-Ahmadabadi A., Nachvak S.M., et al. Antihyperlipidemic and antioxidative properties of Pistacia atlantica subsp. kurdica in streptozotocin-induced diabetic mice. Diabetes, Metab Syndrome Obes Targets Ther. 2020;13:1231. doi: 10.2147/DMSO.S250417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasban-Aliabadi H. Effects of Baneh (Pistacia atlantica) gum on human breast cancer cell line (MCF-7) and its interaction with anticancer drug doxorubicin. Iran J Pharm Res (IJPR): Int J Phys Res. 2019;18(4):1959. doi: 10.22037/ijpr.2019.1100853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fathollahi M., Aminzare M., Mohseni M., Hassanzadazar H., editors. Urmia University; Urmia, Iran: 2019. Antioxidant capacity, antimicrobial activities and chemical composition of Pistacia atlantica subsp. kurdica essential oil. Veterinary Research Forum. (Faculty of Veterinary Medicine). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahmani M., Saki K., Asadbeygi M., et al. The effects of nutritional and medicinal mastic herb (Pistacia atlantica) J Chem Pharmaceut Res. 2015;(1):646–653. [Google Scholar]
- 21.Benhassaini H., Bendahmane M., Benchalgo N. The chemical composition of fruits of Pistacia atlantica desf. subsp. atlantica from Algeria. Chem Nat Compd. 2007;43(2):121. [Google Scholar]
- 22.Bozorgi M., Memariani Z., Mobli M., Salehi Surmaghi M.H., Shams-Ardekani M.R., Rahimi R., vera P., atlantica P., terebinthus P., khinjuk P., lentiscus P. A Review of Their Traditional Uses, Phytochemistry, and Pharmacology. The Scientific World Journal; 2013. Five Pistacia species. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asadollahzadeh H., Shamspur T. Chemical composition of the extracts of fruits of Pistacia atlantica desf. From kerman Province in Iran. Journal of Essential Oil Bearing Plants. 2013;16(2):243–246. [Google Scholar]
- 24.Soleiman-Beigi M., Arzehgar Z. A review study on chemical properties and food indexes of mastic Oil compared with Olive, sunflower and canola oils. The Ilamian traditional uses of mastic. scientific journal of ilam university of medical sciences. 2013;21(5):1–13. [Google Scholar]
- 25.Arena E., Campisi S., Fallico B., Maccarone E. Distribution of fatty acids and phytosterols as a criterion to discriminate geographic origin of pistachio seeds. Food Chem. 2007;104(1):403–408. [Google Scholar]
- 26.Quintana D.S., Rokicki J., van der Meer D., et al. Oxytocin pathway gene networks in the human brain. Nat Commun. 2019;10(1):1–12. doi: 10.1038/s41467-019-08503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stadler B., Whittaker M.R., Exintaris B., Middendorff R. Oxytocin in the male reproductive tract; the therapeutic potential of oxytocin-agonists and-antagonists. Front Endocrinol. 2020;11:753. doi: 10.3389/fendo.2020.565731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alley J.C., Diamond L.M. Oxytocin and human sexuality: recent developments. Curr Sex Health Rep. 2020:1–4. [Google Scholar]
- 29.Council N.R. 1995. Nutrient Requirements of Laboratory Animals: 1995. [Google Scholar]
- 30.Overstreet J., Katz D. Semen analysis. Urol Clin. 1987;14(3):441–449. [PubMed] [Google Scholar]
- 31.Auer T., Khoschsorur G., Rabl H., Iberer F., Petutschnigg B., editors. Transplantation Proceedings. 1995. Detection of lipid peroxidation products by malondialdehyde (MDA-TBA reaction) in organ transplantation. [PubMed] [Google Scholar]
- 32.Stavlienic-Flukavinaz A. Evaluation of the Konelab 20XT Clinical Chemistry Analyzer. [DOI] [PubMed]
- 33.Hesari A.K., Shahrooz R., Ahmadi A., Malekinejad H., Saboory E. Crocin prevention of anemia-induced changes in structural and functional parameters of mice testes. J Appl Biomed. 2015;13(3):213–223. [Google Scholar]
- 34.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Farhoosh R., Khodaparast M.H.H., Sharif A. Bene hull oil as a highly stable and antioxidative vegetable oil. Eur J Lipid Sci Technol. 2009;111(12):1259–1265. [Google Scholar]
- 36.Kapoor M., Kojima F., Crofford L.J. Arachidonic acid-derived eicosanoids in rheumatoid arthritis: implications and future targets. Int J Clin Rheumatol. 2006;1(3):323. [Google Scholar]
- 37.Ruan K.-H., Cervantes V., So S.-P. Engineering of a novel hybrid enzyme: an anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Engineering. Design & Selection. 2009;22(12):733–740. doi: 10.1093/protein/gzp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelletier S., Dubé J., Villeneuve A., et al. Prostaglandin E2 increases cyclic AMP and inhibits endothelin-1 production/secretion by Guinea-pig tracheal epithelial cells through EP4 receptors. Br J Pharmacol. 2001;132(5):999–1008. doi: 10.1038/sj.bjp.0703886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen M.B. Vitamin D and male reproduction. Nat Rev Endocrinol. 2014;10(3):175. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- 40.Wade M.G., Van Der Kraak G. Arachidonic acid and prostaglandin E2 stimulate testosterone production by goldfish testis in vitro. Gen Comp Endocrinol. 1993;90(1):109–118. doi: 10.1006/gcen.1993.1065. [DOI] [PubMed] [Google Scholar]
- 41.Awad A.B., Hartati M.S., Fink C.S. Phytosterol feeding induces alteration in testosterone metabolism in rat tissues. J Nutr Biochem. 1998;9(12):712–717. [Google Scholar]
- 42.Umeda F., Kato K.-I., Muta K., Ibayashi H. Effect of vitamin E on function of pituitary-gonadal axis in male rats and human subjects. Endocrinol Jpn. 1982;29(3):287–292. doi: 10.1507/endocrj1954.29.287. [DOI] [PubMed] [Google Scholar]
- 43.Fui M.N.T., Dupuis P., Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16(2):223–231. doi: 10.4103/1008-682X.122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nazifi S., Saeb M., Yavari M., Jalaee J. Studies on the effects of turpentine powder on the serum concentration of lipids and lipoproteins of male rabbits. Iranian Journal of Endocrinology and Metabolism. 2005;7(1):73–78. [Google Scholar]
- 45.Saeb M.N.S., Mirzaee A., Jalaee J. Studies on the effects of turpentine powder on the serum concentration of lipids and lipoproteins of female rabbits. Iranian Journal of Endocrinology and Metabolism. 2006;60(4) [Google Scholar]
- 46.Chauhan N.S., Sharma V., Dixit V., Thakur M. A review on plants used for improvement of sexual performance and virility. BioMed Res Int. 2014;2014 doi: 10.1155/2014/868062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watcho P., Kamtchouing P., Sokeng S.D., et al. Androgenic effect of Mondia whitei roots in male rats. Asian J Androl. 2004;6(3):269–272. [PubMed] [Google Scholar]
- 48.Mehraban F., Jafari M., Toori M.A., et al. Effects of date palm pollen (Phoenix dactylifera L.) and Astragalus ovinus on sperm parameters and sex hormones in adult male rats. Iran J Reproductive Med. 2014;12(10):705. [PMC free article] [PubMed] [Google Scholar]
- 49.Carlson B.M. Elsevier Health Sciences; 2012. Human Embryology and Developmental Biology E-Book: With STUDENT CONSULT Online Access. [Google Scholar]
- 50.Wang X., Li Z., Liu Y., Lv X., Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr J. 2012;11(1):1–6. doi: 10.1186/1475-2891-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards K., Kwaw I., Matud J., Kurtz I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. J Am Coll Nutr. 1999;18(3):229–232. doi: 10.1080/07315724.1999.10718856. [DOI] [PubMed] [Google Scholar]
- 52.Ghaseminasab P.M., Ahmadi A., Mazloomi S.M. 2015. A Review on Pistachio: Its Composition and Benefits Regarding the Prevention or Treatment of Diseases. [Google Scholar]
- 53.Katan M.B., Grundy S.M., Jones P., Law M., Miettinen T., Paoletti R., editors. Mayo Clinic Proceedings. Elsevier; 2003. Efficacy and safety of plant stanols and sterols in the management of blood cholesterol levels. [DOI] [PubMed] [Google Scholar]
- 54.Berger A., Jones P.J., Abumweis S.S. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3(1):5. doi: 10.1186/1476-511X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Li Z., Liu Y., Lv X., Yang W. Effects of pistachios on body weight in Chinese subjects with metabolic syndrome. Nutr J. 2012;11(1):20. doi: 10.1186/1475-2891-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berger A., Jones P.J., Abumweis S.S. Plant sterols: factors affecting their efficacy and safety as functional food ingredients. Lipids Health Dis. 2004;3(1):1–19. doi: 10.1186/1476-511X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fakour S., Heydari S., Akradi L., Rahymi Bane R. Effect of Pistacia atlantica mastic extract on experimental wound healing and various biochemical parameters of blood serum in rabbit models. J. Med. Plants. 2017;16(63):78–91. [Google Scholar]
- 58.Agarwal A., Ikemoto I., Loughlin K.R. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J Urol. 1994;152(1):107–110. doi: 10.1016/s0022-5347(17)32829-x. [DOI] [PubMed] [Google Scholar]
- 59.Blomberg Jensen M., Bjerrum P.J., Jessen T.E., et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26(6):1307–1317. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 60.Yakubu M., Akanji M., Oladiji A. Aphrodisiac potentials of the aqueous extract of Fadogia agrestis (Schweinf. Ex Hiern) stem in male albino rats. Asian J Androl. 2005;7(4):399–404. doi: 10.1111/j.1745-7262.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 61.Sawidis T., Yurukova L., Askitis T. Chios mastic, a natural supplement for zinc to enhance male sexuality and prostate function. Pharmaceut Biol. 2010;48(1):48–54. doi: 10.3109/13880200903029399. [DOI] [PubMed] [Google Scholar]
- 62.Ebisch I., Thomas C., Peters W., Braat D., Steegers-Theunissen R. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2006;13(2):163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- 63.Djerroua Z., Hamdi-Pacha Y., Belkhiri A., et al. Evaluation of Pistacia lentiscus fatty oil effects on glycemic index, liver functions and kidney functions of New Zealand rabbits. Afr J Tradit, Complementary Altern Med. 2011;8(5S) doi: 10.4314/ajtcam.v8i5S.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amico J., Crowley R., Insel T., Thomas A., O'keefe J. Effect of gonadal steroids upon hypothalamic oxytocin expression. Adv Exp Med Biol. 1995;395:23–35. [PubMed] [Google Scholar]
- 65.Jirikowski G.F., Ochs S.D., Caldwell J.D. Oxytocin and steroid actions. Behavioral Pharmacology of Neuropeptides: Oxytocin. 2017:77–95. doi: 10.1007/7854_2017_9. [DOI] [PubMed] [Google Scholar]
- 66.Rashidi S., Askari N., Abbasnejad M. Anxiolytic-like effect of Pistacia atlantica fruit in intact and gonadectomized rats subjected to chronic stress. Journal of Occupational Health and Epidemiology. 2014;3(3):152–159. [Google Scholar]