Abstract
Maximal oxygen uptake (V′O2max), assessed by cardiopulmonary exercise testing (CPET), is an important parameter for risk assessment in patients with pulmonary hypertension (PH). However, CPET may not be available for all PH patients. Thus, we aimed to test previously published predictive models of V′O2max from the 6-min walk distance (6MWD) for their accuracy and to create a new model.
We tested four models (two by Ross et al. (2010), one by Miyamoto et al. (2000) and one by Zapico et al. (2019)). To derive a new model, data were split into a training and testing dataset (70:30) and step-wise linear regression was performed. To compare the different models, the standard error of the estimate (SEE) was calculated and the models graphically compared by Bland–Altman plots. Sensitivity and specificity for correct prediction into low-risk classification (V′O2max >15 mL/min/kg) was calculated for all models.
A total of 276 observations were included in the analysis (194/82 training/testing dataset); 6MWD and V′O2max were significantly correlated (r=0.65, p<0.001). Linear regression showed significant correlation of 6MWD, weight and heart rate response (HRR) with V′O2max and the best fitting prediction equation was: V′O2max = 1.83 + 0.031 × 6MWD (m) – 0.023 × weight (kg) – 0.015 × HRR (bpm). SEEs for the different models were 3.03, 3.22, 4.36 and 3.08 mL/min/kg for the Ross et al., Miyamoto et al., Zapico et al. models and the new model, respectively. Predicted mean V′O2max was 16.5 mL/min/kg (versus observed 16.1 mL/min/kg).
6MWD and V′O2max reveal good correlation in all models. However, the accuracy of all models is inadequate for clinical use. Thus, CPET and 6MWD both remain valuable risk assessment tools in the management of PH.
Short abstract
Although maximal oxygen uptake and the 6-min walk distance show good correlation in pulmonary hypertension, all predictive models are of inadequate accuracy for clinical use and cardiopulmonary exercise testing remains an irreplaceable tool https://bit.ly/33HBQFk
Introduction
Pulmonary hypertension (PH) is a progressive disease, which may be idiopathic or associated with various conditions [1]. PH is characterised by progressive narrowing of the pulmonary vasculature with consecutive increase in pulmonary arterial pressure, eventually leading to right heart failure and premature death [2]. Patients with PH typically suffer from exercise limitation due to the inability to adequately increase the cardiac output, even at early stages of the disease. Measures of cardiorespiratory fitness can provide important information on the severity of functional impairment at diagnosis and are important to assess prognosis and response to therapies [3]. Measurements of maximal oxygen uptake (V′O2max), obtained from cardiopulmonary exercise testing (CPET), stand in direct correlation with cardiac performance and thus reflect cardiorespiratory fitness [4, 5]. V′O2max at physical exhaustion has been shown to be a valuable diagnostic and prognostic tool for a variety of diseases [4] including PH [6, 7], for which it acts as an independent predictor of survival [8]. However, correct measurement of V′O2max requires maximal effort of patients performing CPET; the performance of CPET may be impossible in everyday practice for some patients for logistical reasons, or due to patient factors or the contemporaneous assessment of other exercise parameters, such as the 6-min walk distance (6MWD). Furthermore, CPET requires expensive equipment, trained healthcare professionals for conduction and interpretation of the test and is a time-consuming procedure.
Estimation of V′O2max from a simpler and widely available test, such as the 6-min walk test (6MWT), has increasingly become of interest, as it would provide a useful alternative to relatively complex CPET. The 6MWT is well established for patients with PH, is inexpensive, easy to perform, safe, well tolerated by patients [9] and has been shown to be an important predictor of survival [10]. Several studies have found a correlation between 6MWD and V′O2max in a variety of diseases [11–15]. One of the first estimations of V′O2max from variables obtained in 6MWT in patients with advanced heart failure was proposed by Cahalin et al. [13] in 1996, who found that the distance ambulated in the 6MWT could not only predict V′O2max but also short-term survival. Miyamoto et al. [16] have shown that the 6MWD correlates well with maximal CPET assessments, such as V′O2max, and acts as a strong independent predictor of survival in PH. However, they did not suggest a prediction equation. Ross et al. [14] extracted regression equations from published scatterplots (e.g. from Miyamoto et al. [16]) and determined a generalised equation to predict V′O2max from mean 6MWD in a group of patients with diverse cardiovascular diseases. When looking specifically at PH, we only found one study, by Zapico et al. [17], that has suggested a predictive model for V′O2max from 6MWD. They distinguished between three equations for children, adolescents and adults with primary PH. Predicting individual V′O2max would provide a useful and cost-effective tool for health professionals and researchers in the assessment and follow-up of PH patients, allowing easy insight into functional exercise capacity.
The aim of this study was to apply the equations proposed by Ross et al. [14] (individual and generalised), by Miyamoto [16] (as determined by Ross et al. [14]), and by Zapico [17], to our own dataset and to compare them to a new multivariate predictive model for V′O2max based on our own set of patients with PH.
Methods
For this study, we used retrospective data from the Swiss Pulmonary Hypertension Registry at the University Hospital in Zurich, Switzerland. All observations of V′O2max from January 2012 until May 2021 that coincided with a 6MWT within 3 months in stable PH patients of all diagnostic groups (mainly pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH)), without change in medication, were included. To account for outliers and a potential ceiling effect [18, 19], observations with a 6MWD <200 m or >650 m and V′O2max <5 mL/min/kg or >25 mL/min/kg were excluded. There was no limitation to the number of observations per patient. All patients gave written informed consent and the Cantonal Ethics Review Board of Zurich gave their approval (BASEC nr. 2021-01006).
Assessments
The 6MWT is routinely performed in our daily clinical setting, according to guidelines [9], walking back and forth in a hallway with 30 m markings. Standard procedures include measurements of blood pressure, heart rate and oxygen saturation before and at peak exercise. At the end of the test, a modified BORG scale (1 to 10) is used to determine the rating of perceived exhaustion.
CPET is performed in a sitting position on a cycle ergometer and metabolic measurements are collected using breath-by-breath analysis, which is then averaged in a 30 s range. End exercise variables are defined as the mean of the final 30 s of exercise. Depending on the individual patient's fitness, a ramp protocol with different Watt increments is used (10/15/20 Watts per min) to reach an approximate test duration of 10–12 min. All patients are instructed to cycle until exhaustion and measurements are done according to international recommendations [20]. As this is a retrospective clinical study in patients with chronic right heart failure, plateauing of V′O2max could not be verified in every patient and thus V′O2 at the end of exercise was taken as V′O2max in accordance with the American Thoracic Society (ATS) statement [20]. The exercise protocols remained the same over the whole observation period.
Statistical analysis
Data were statistically analysed using the program R (Version 4.0.1). All statistical tests were two-sided at the 5% statistical significance level with the corresponding confidence intervals of 95%. Normality distribution was assumed in a sample size of n>30 and data were graphically inspected.
Applying existing regression models
At first, the relationship between V′O2max and 6MWD was graphically inspected for the entire dataset. Correlation was analysed using the Pearson correlation coefficient (r). We tested the following four models for prediction of V′O2max from 6MWD:
Ross mean: mean V′O2max = 4.948 + 0.023 × mean 6MWD (m) [14]
Ross: V′O2max = 4.682 + 0.025 × 6MWD (m) [14]
Miyamoto: V′O2max = 4.213 + 0.026 × 6MWD (m); determined by Ross et al. [14] based on data published by Miyamoto et al. [16]
Zapico: V′O2max = −21.626 + 0.026 × 6MWD (m) + 4.103 × sex + 0.174 × height (cm) – 0.071 × weight [17]
Before further analysis, the data were randomly split at a 70:30 ratio into a training and a testing dataset [21], accounting for equal distribution of characteristics such as 6MWD, gender, diagnosis and age. The training dataset was used to determine a new predictive model, which was then, along with the other models, tested on the testing dataset.
Determining the multivariate regression model
Step-wise linear regression was used to determine the variables to be included in the multivariate regression model. Tested independent variables were: age, weight, height, heart rate response during 6MWT (HRR: maximal exercise heart rate – heart rate at rest), maximal heart rate and gender. If the slope of an independent variable was found to be significant (p<0.05), it was included in the multivariate regression model. Several models based on different methods were tested and compared, before choosing the best fitting model.
To compare the accuracy of the different models, the standard error of the estimate (SEE) and its proportion of mean V′O2max was calculated and data were graphically analysed. Bland–Altman graphs were used to visualise the difference between CPET results and the predictive models as two different approaches to determine V′O2max.
All models were tested on their ability to accurately classify patients into a low-risk profile (correct classification into V′O2max >15 mL/min/kg) using confusion matrixes, calculations of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Results
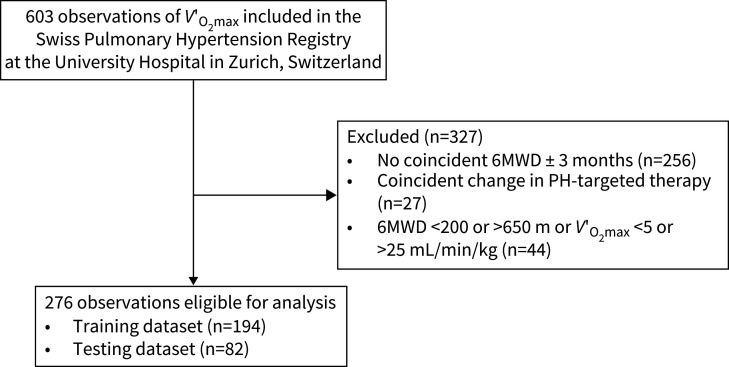
We were able to include 603 observations of V′O2max from the Swiss Pulmonary Hypertension Registry at the University Hospital in Zurich, Switzerland. Of these, 256 observations had to be excluded due to missing coincident observations of 6MWD, 27 due to coincident change in PH-targeted therapy and 44 because 6MWD or V′O2max were not within the predefined range (6MWD <200 or >650 m or V′O2max <5 or >25 mL/min/kg). This resulted in 276 observations eligible for analysis (fig. 1).
FIGURE 1.
Patient flow chart. 6MWD: 6-min walk distance; PH: pulmonary hypertension; V′O2max: maximal oxygen uptake.
Characteristics for the training (n=194, 61% female) and testing (n=82, 71% female) dataset are shown in table 1. Both datasets showed a similar distribution of different diagnoses (PAH 60% versus 61%, CTEPH 33% versus 34% for training and testing dataset, respectively). Age (mean±sd) was 59±14 years for the training and 58±15 years for the testing dataset. 6MWD (mean±sd) and V′O2max (mean±sd) were 504±85 m and 15.3±3.8 mL/min/kg for the training, and 503±90 m and 16.1±3.7 mL/min/kg for the testing dataset.
TABLE 1.
Characteristics of the training and testing datasets
| Training dataset | Testing dataset | |
| Observations n | 194 | 82 |
| Female | 118 (61) | 58 (71) |
| Diagnosis | ||
| PAH | 117 (60) | 50 (61) |
| PH due to left heart disease | 1 (1) | |
| PH due to lung disease | 4 (2) | 1 (1) |
| CTEPH | 64 (33) | 28 (34) |
| Miscellaneous | 8 (4) | 3 (4) |
| Haemodynamics at baseline | ||
| mPAP, mmHg | 42±4.1 | 39±13.0 |
| PVR, mmHg | 12±63 | 6±4 |
| PAWP, mmHg | 11±4 | 11±4 |
| CI, mL/min/kg | 2.8±0.8 | 2.9±0.7 |
| Characteristics | ||
| Age, years | 59±14 | 58±15 |
| Height, cm | 169±11 | 169±10 |
| Weight, cm | 76±19 | 73±17 |
| BMI, kg/m2 | 27±5 | 25±5 |
| NYHA functional class | 2.2±0.7 | 2.2±0.6 |
| 6-min walk test | ||
| 6-min walk distance, m | 504±85 | 503±90 |
| At rest | ||
| Systolic BP, mmHg | 125±18 | 120±14 |
| Diastolic BP, mmHg | 79±12 | 78±12 |
| Heart rate, bpm | 84±14 | 84±12 |
| Peripheral oxygen saturation, % | 96±3 | 96±3 |
| At peak exercise | ||
| Systolic BP, mmHg | 145±25 | 142±26 |
| Diastolic BP, mmHg | 83±14 | 82±13 |
| Heart rate, bpm | 121±21 | 121±22 |
| Heart rate response, bpm | 37±19 | 37±20 |
| Peripheral oxygen saturation, % | 89±9.0 | 90±7.8 |
| BORG CR10, score | 4.7±2 | 4.6±2 |
| Cardiopulmonary exercise test | ||
| V′O2max, mL/min/kg | 15.3±3.8 | 16.1±3.7 |
| Maximal workload, Watts | 93±33 | 96±36 |
Data are presented as n (%) or mean±sd, unless otherwise stated. BMI: body mass index; BP: blood pressure; CI: cardiac index; CTEPH: chronic thromboembolic pulmonary hypertension; mPAP: mean pulmonary arterial pressure; NYHA: New York Heart Association; PAH: pulmonary arterial hypertension; PAWP: pulmonary arterial wedge pressure; PH: pulmonary hypertension; PVR: pulmonary vascular resistance; V′O2max: maximal oxygen uptake.
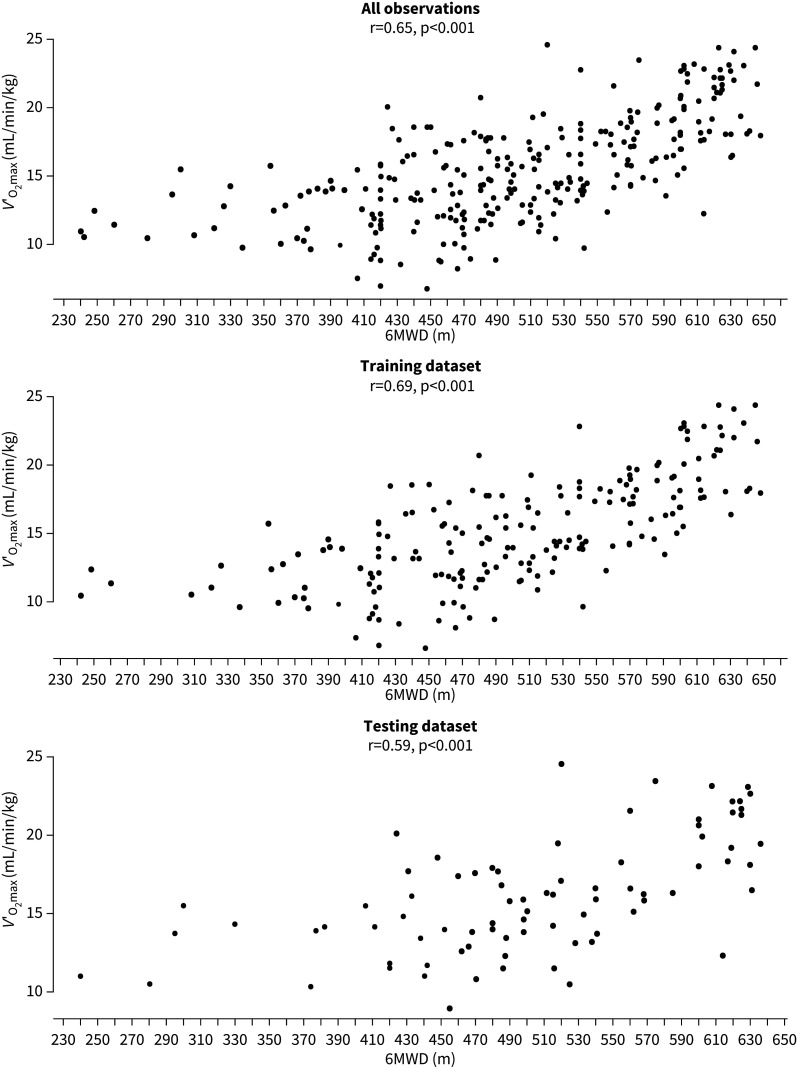
6MWD and V′O2max showed a significant correlation for the dataset overall (r=0.65, p<0.001), which remained true for the training and the testing dataset (r=0.69 and 0.59, respectively, both p<0.001) (fig. 2). Application of Ross's model to predict mean V′O2max for the whole dataset resulted in 16.5 mL/min/kg (versus 15.5 mL/min/kg observed). The predictive equations by Ross et al. [14], Miyamoto et al. [16] and Zapico et al. [17] for individual V′O2max resulted in an SEE of 6.27, 6.30 and 7.62 mL/min/kg (40%, 41% and 49% of mean V′O2max), respectively when applied on the whole dataset.
FIGURE 2.
Correlation between 6-min walk distance (6MWD) and maximal oxygen uptake (VO2max). The three panels show the correlation for all observations, the training dataset and the testing dataset. Correlation coefficients (r) and p-values are given for all three datasets separately.
Table 2 shows a comparison of the different prediction models. Coefficients, values for correlation (r) and p-values for Ross et al. [14], Miyamoto et al. [16] and Zapico et al. [17] were copied from the original publications. Based on our own data, we created a new model (R2=0.48) with the regression equation 1.83 + 0.031 × 6MWD (m) – 0.023 × weight (kg) – 0.015 × HRR (bpm). When applied to the testing dataset SEE was 3.03, 3.22, 4.36 and 3.08 mL/min/kg (19%, 20%, 27% and 19% of mean V′O2max) for Ross et al. [14], Miyamoto et al. [16], Zapico et al. [17] and the new model, respectively. Predicted mean V′O2max using the generalised equation suggested by Ross et al. [14] in the testing dataset was 16.5 mL/min/kg (versus 16.1±3.7 mL/min/kg observed).
TABLE 2.
Coefficients of different linear regression models and application to testing data
| Ross et al. [ 14 ] mean (r=0.82) | p-value | Ross et al. [ 14 ] (r=0.59) | p-value | Miyamoto et al. [ 16 ] (r=0.68) | p-value | Zapico et al. [ 17 ] (R2=0.48) | p-value | New model (R2=0.48) | p-value | |
| Observations n | 1038 | 1083 | 27 | 82 | 194 | |||||
| Intercept | 4.948 | 4.682 | 4.213 | −21.626 | 1.83 | 0.248 | ||||
| 6MWD, m | 0.023 | 0.025 | 0.026 | 0.026 | <0.001 | 0.031 | <0.001 | |||
| Sex | 4.103 | 0.002 | ||||||||
| Height, cm | 0.174 | 0.043 | ||||||||
| Weight, kg | −0.071 | 0.02 | −0.023 | 0.22 | ||||||
| HRR, bpm | −0.015 | 0.047 | ||||||||
| Applied to testing dataset | ||||||||||
| SEE, mL/min/kg | 3.03 | 3.22 | 4.36 | 3.08 | ||||||
| SEE% mean | 19% | 20% | 27% | 19% | ||||||
| Ability to classify to low risk (V′O2 max >15 mL/min/kg) | ||||||||||
| Sensitivity | 0.96 | 0.96 | 0.91 | 0.69 | ||||||
| Specificity | 0.23 | 0.23 | 0.22 | 0.78 | ||||||
| PPV | 0.63 | 0.63 | 0.63 | 0.82 | ||||||
| NPV | 0.80 | 0.80 | 0.64 | 0.64 |
r from correlation between maxima oxygen uptake (V′O2max) and 6-min walk distance (6MWD); R2 from linear regression of V′O2max against 6MWD. HRR: heart rate response in 6-min walk test (heart rate at maximal exercise – heart rate at rest); NPV: negative predictive value; PPV: positive predictive value; SEE: standard error of the estimate; SEE% mean: SEE as percentage of mean peak oxygen uptake. All coefficients, p-values and R2 for Ross et al. [14], Miyamoto et al. [16] and Zapico et al. [17] are copied from the original publications.
Table 2 also provides information on sensitivity and specificity for the ability to correctly classify patients into a low-risk profile (V′O2max >15 mL/min/kg) in all models. Sensitivity ranged from 91 to 96% for the existing models and was 69% for our new model, while specificity ranged from 22 to 23% for the existing models and was 78% for our new model. The PPV was 63% for all existing models and 82% for our new model.
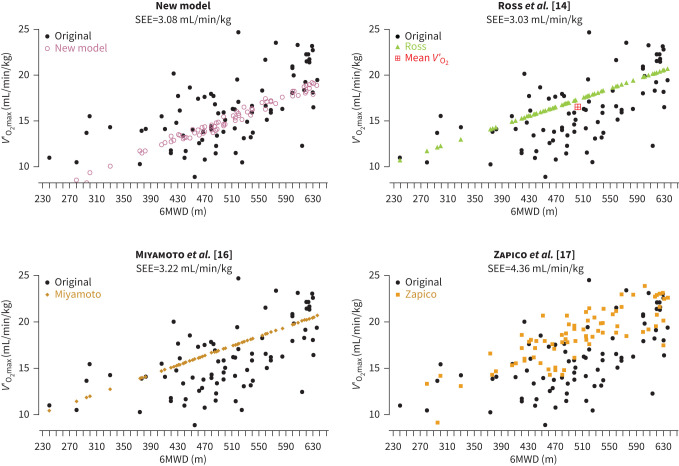
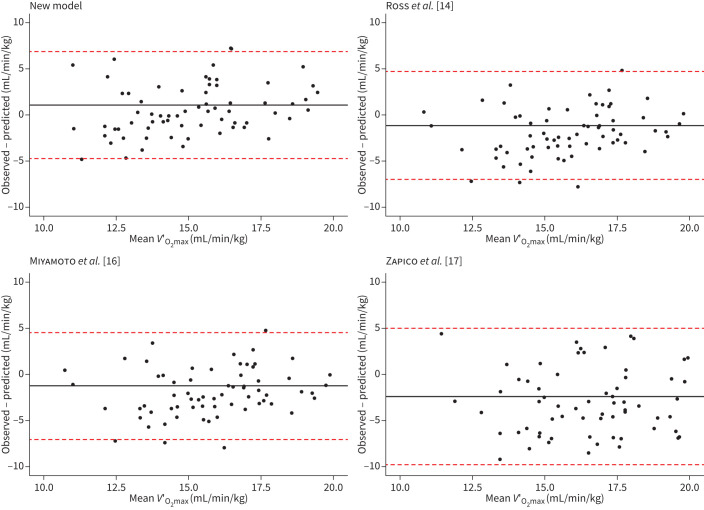
Figure 3 shows the graphical comparison between observed V′O2max in the testing dataset and predicted V′O2max for our new model and the models of Ross et al. [14], Miyamoto et al. [16] and Zapico et al. [17]. Bland–Altman graphs comparing CPET and the predictive models as two approaches to determine V′O2max are shown in figure 4. For the new model, bias (95% CI) was 1.08 (0.40 to 1.75) mL/min/kg with an upper limit of agreement (ULoA) (95% CI) of 6.89 (5.73 to 8.04) and a lower limit of agreement (LLoA) of −4.72 (−5.89 to −3.58). Bias, ULoA and LLoA were −1.12 (−1.77 to 0.46) mL/min/kg, 4.73 (3.60 to 5.85), −6.96 (−8.09 to −5.84) for the model of Ross et al. [14], −1.15 (−1.80 to −0.49) mL/min/kg, 4.7 (3.58 to 5.83), −7.00 (−8.13 to −5.88) for Miyamoto et al. [16] and −2.34 (−3.18 to −1.49) mL/min/kg, 5.06 (3.60 to 6.52) and −9.74 (−11.20 to −8.28) for Zapico et al. [17].
FIGURE 3.
Comparison of predictive models. The panels show the predicted values for maximal oxygen uptake (VO2max) against 6-min walk distance (6MWD) for each model separately. In each panel, the original data from the testing dataset is depicted for comparison; standard error of the estimate (SEE) for each model is shown below the panel headers. Mean oxygen uptake (V′O2) as determined by the generalised equation by Ross et al. [14] is shown in the corresponding panel.
FIGURE 4.
Bland–Altman graphs for the comparison of maximal oxygen uptake (V′O2max) determined by cardiopulmonary exercise testing versus using a predictive model based on 6-min walk test. The x-axis shows the calculated mean between the observed and the predicted values for V′O2max while the y-axis shows the difference between the two methods. The line corresponds to the mean difference in V′O2max and the two dashed lines show the 95% confidence interval. Each panel shows a different model as indicated by the panel's headers.
Discussion
The current analysis shows that the distance ambulated in the 6MWT and V′O2max were significantly correlated. The newly generated predictive equation and three previously published ones [14, 16, 17] were all able to predict V′O2max from demographics and the 6MWT with varying accuracy (SEE ranging from 3.03 to 4.36 mL/min/kg). Unfortunately, we judge this inaccuracy in the prediction of an individual's V′O2max as insufficient for clinical use. With the new prediction model derived from our own dataset, we could markedly increase the specificity and PPV to predict a favourable V′O2max >15 mL/min/kg from 6MWD compared to published equations.
Our new predictive model for determination of V′O2max from 6MWD and demographics revealed an SEE of 3.08 mL/min/kg and thus showed similar accuracy to the published model of Ross et al. [14] with a SEE of 3.03 mL/min/kg, which was the most accurate from the tested equations. It seems that the model of Ross et al. [14] generally overestimates V′O2max in our population (see fig. 3). Ross et al. [14] based their equation on a set of 11 populations with a mean 6MWD of 392 m and mean V′O2max of 13.9 mL/min/kg. In our dataset mean 6MWD is surprisingly high at 504 m, corresponding to 94% of predicted reference values for 6MWD [22], while mean V′O2max (15.3 mL/min/kg) still shows a relevant impairment of respiratory capacity. The large difference in walking distance compared to the smaller difference in V′O2max could explain why the models of Ross et al. [14] and Miyamoto et al. [16], although having similar SEE (approximately 20% of mean V′O2max), overestimate an individual's V′O2max in our dataset. The model of Zapico et al. [17], although being multivariate, shows less accurate prediction of V′O2max (SEE 4.36 mL/min/kg, or 27% of mean V′O2max). They based their equation on a substantially younger population than our own, with a mean age of 27±6.7 years (versus 59±14 years). However, despite similar mean V′O2max (16.03±6 mL/min/kg), mean 6MWD was again lower than in our own population (459±115 m) [17]. Also compared to the French Pulmonary Hypertension Registry, where the mean 6MWD was 329±109 m [23], or the UK Registry where the mean 6MWD was 292.4±123 m [24], our study population showed a higher mean 6MWD, which has already been previously shown in the Swiss Registry [25]. It is known that 6MWD shows a ceiling effect at higher values due to a general limitation in walking speed. Some studies suggest that this ceiling effect in PH patients can be detected starting from 450 m onwards [18, 26]. A potential ceiling effect would suggest that variability in V′O2max would increase with increasing 6MWD. However, contrary to patients with chronic heart failure [15], where the 6MWD could not accurately predict V′O2max in patients with 6MWD >500 m, in our population the correlation between V′O2max and 6MWD was moderate to good in observations with 6MWD >500 m and only fair for <500 m (r=0.64, p<0.001 versus r=0.26, p=0.001) and showed less variability. This has already been established in patients with mild chronic heart failure [19], yet the reason remains unclear.
All of the models showed a SEE of over 3 mL/min/kg. While there is still little information about the minimal clinically important difference (MCID) for V′O2max in PH, rehabilitation was shown to improve V′O2max by 1.5 mL/min/kg [27]. Accordingly, the SEE of over 3 mL/min/kg appears to be clearly above a MCID level and thus relevant, which suggests that the variation of all models for the individual V′O2max, as depicted by SEE, is too large for clinical use, and meaningful changes in true V′O2max might not be detected. As for 6MWD, the MCID is around 25–33 m [27, 28], which would correspond to an increase in V′O2max of 0.83 mL/min/kg as predicted by the model of Ross et al. [14]. In the Bland–Altman analysis all the models showed only a small bias, ranging from −2.34 (−3.18 to −1.49) to 1.08 (0.40 to 1.75) mL/min/kg. Most of the measurements remain within the limits of agreement, which were defined as two times the sd of mean differences, showing good agreement of the two models. However, as already established above, one can see that the models generally overestimate mean V′O2max. In addition, the upper and lower limits of agreement are too large, showing inadequate precision of the models.
We further examined whether the models, despite being unable to assess an individual's V′O2max accurately, were able to classify patients correctly to low- or high-risk groups as defined by the PH risk stratification model, where a V′O2max of >15 mL/min/kg classifies as low risk, and corresponds to an estimated 1-year mortality of <5% [29]. The models of Ross et al. [14], Miyamoto et al. [16] and Zapico et al. [17] all showed high sensitivity (91–96%) but very low specificity (22–23%), supporting the claim that these models overestimate V′O2max in our data, resulting in many falsely positive classifications to the low-risk group, which would be very unfavourable for clinical use. Our own model had a sensitivity of 69% and specificity of 78%, suggesting a poorer predictive ability, but fewer false-positive results than the other models, meaning fewer patients wrongly classified to the low-risk group.
Previous studies in patients with cardiac disease have shown that V′O2max cannot accurately be predicted from the 6MWT [30, 31]. Ross et al. [14] found a SEE of 3.82 mL/min/kg over a variety of diseases and exercise protocols, suggesting that predicting an individual's V′O2max shows poor accuracy independent of underlying disease. Our results are in line with these findings and extend them to PH patients. When we applied the generalised equation to predict the mean V′O2max of a population suggested by Ross et al. [14] to our own data, the resulting mean was 16.5 mL/min/kg, which is only 0.4 mL/min/kg higher than the actual mean of 16.1 mL/min/kg. Thus, this population mean equation is substantially more accurate than the prediction for individual V′O2max but has less clinical use.
Recent discussions have brought up the question of whether it is clinically important to measure V′O2max in PH as drug studies showed contradicting results. Clinical trials leading to regulatory approval of most drugs to treat PH used the 6MWD as the main outcome, whereas contemporary sequential, add-on drug combination therapy trials mainly used composite end-points [32]. V′O2max as main outcome used in the Stride-1 study did not result in a significant difference between PAH patients treated with sitaxsentan versus placebo, whereas the 6MWD as secondary outcome was different [33]. Part of this discrepancy was attributed to the centre's experience with CPET [11]. In a recent multicentre randomised study, a standardised training programme in PH resulted in significant improvements of both 6MWD and V′O2max [34]. Some of the discrepancy between 6MWD and CPET measures may be attributed to the experience of investigators and patients, time lag between tests and other logistics; others may simply reflect that these two tests do not measure the same physiobiological process. However, due to the correlation between these two measurements, the 6MWD may still be used as a simple surrogate to V′O2max [35], even if its prediction has been shown to be inaccurate.
A limitation of our study is that our population included very few severely limited patients, with a presumably very low V′O2max, for whom a prediction equation would be more important because they may not be able to perform CPET due to the need for oxygen supplementation. Another limitation is that we included coincident observation ranging up to a 3 month time difference, rather than only including observations obtained on the same day. However, in clinical practice, performing several exercise tests contemporaneously might not be possible, and due to the retrospective nature of our data, we could not guarantee that the observations were actually from the same day. Further, we see a limitation in the fact that some patients had several observations that were included in the analysis. It would have been interesting to include more CPET variables in the analysis, but our registry did not provide more detailed data.
Conclusion
Although the good correlation between 6MWD and V′O2max allows for the development of a predictive model based on regression equations, all proposed models show inadequate accuracy for clinical use in individual patients or classification into the low-risk group. The prediction of mean V′O2max with the generalised equation proposed by Ross et al. [14] is highly accurate, in the present study also, which supports their statement that the equation can be used to predict mean V′O2max in a population of patients from its mean 6MWD. While the 6MWT remains an important tool to assess prognosis and survival in PH, the 6MWD cannot be used to predict V′O2max for individual PH patients and thus cannot replace CPET. Besides V′O2max, CPET allows for deeper insights into the underlying pathophysiological mechanisms of exercise limitation and can play a greater role in the diagnosis and management of PH.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: P. Appenzeller and S. Ulrich contributed to the conception of the design of the study, data acquisition, analysis and interpretation, and drafting of the manuscript. E.I. Schwarz, F. Gautschi, J. Müller, M. Lichtblau, S. Sazer, S.R. Schneider and S. Ulrich contributed to the critical revision of the manuscript and provided final approval before publishing.
Conflict of interest: The authors have no conflicts of interest in relation to the present work. S. Ulrich has received research grants outside this work from the Swiss National Science Foundation, Swiss and Zurich Lung Leagues, and Orpha Swiss; and honoraria for lectures and support for attending meetings from Actelion/Janssen SA, MSD SA and Orpha Swiss.
References
- 1.Galie N, Humbert M, Vachiery JL, et al. . 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Montani D, Celermajer DS, et al. . Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53: 1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weatherald J, Farina S, Bruno N, et al. . Cardiopulmonary exercise testing in pulmonary hypertension. Ann Am Thorac Soc 2017; 14: Suppl. 1, S84–S92. doi: 10.1513/AnnalsATS.201610-788FR [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Williams MA, et al. . Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 2007; 116: 329–343. doi: 10.1161/CIRCULATIONAHA.106.184461 [DOI] [PubMed] [Google Scholar]
- 5.Sartor F, Vernillo G, de Morree HM, et al. . Estimation of maximal oxygen uptake via submaximal exercise testing in sports, clinical, and home settings. Sports Med 2013; 43: 865–873. doi: 10.1007/s40279-013-0068-3 [DOI] [PubMed] [Google Scholar]
- 6.Laveneziana P, Weatherald J. Pulmonary vascular disease and cardiopulmonary exercise testing. Front Physiol 2020; 11: 964. doi: 10.3389/fphys.2020.00964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farina S, Correale M, Bruno N, et al. . The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev 2018; 27: 170134. doi: 10.1183/16000617.0134-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wensel R, Opitz CF, Anker SD, et al. . Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation 2002; 106: 319–324. doi: 10.1161/01.CIR.0000022687.18568.2A [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society . ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med 2002; 166: 111–117. doi: 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 10.Heresi GA, Rao Y. Follow-up functional class and 6-minute walk distance identify long-term survival in pulmonary arterial hypertension. Lung 2020; 198: 933–938. doi: 10.1007/s00408-020-00402-w [DOI] [PubMed] [Google Scholar]
- 11.Oudiz RJ, Barst RJ, Hansen JE, et al. . Cardiopulmonary exercise testing and six-minute walk correlations in pulmonary arterial hypertension. Am J Cardiol 2006; 97: 123–126. doi: 10.1016/j.amjcard.2005.07.129 [DOI] [PubMed] [Google Scholar]
- 12.Guazzi M, Dickstein K, Vicenzi M, et al. . Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail 2009; 2: 549–555. doi: 10.1161/CIRCHEARTFAILURE.109.881326 [DOI] [PubMed] [Google Scholar]
- 13.Cahalin LP, Mathier MA, Semigran MJ, et al. . The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest 1996; 110: 325–332. doi: 10.1378/chest.110.2.325 [DOI] [PubMed] [Google Scholar]
- 14.Ross RM, Murthy JN, Wollak ID, et al. . The six minute walk test accurately estimates mean peak oxygen uptake. BMC Pulm Med 2010; 10: 31. doi: 10.1186/1471-2466-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du H, Wonggom P, Tongpeth J, et al. . Six-minute walk test for assessing physical functional capacity in chronic heart failure. Curr Heart Fail Rep 2017; 14: 158–166. doi: 10.1007/s11897-017-0330-3 [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto S, Nagaya N, Satoh T, et al. . Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161: 487–492. doi: 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 17.Zapico AG, Fuentes D, Rojo-Tirado MA, et al. . Predicting peak oxygen uptake from the 6-minute walk test in patients with pulmonary hypertension. J Cardiopulm Rehabil Prev 2016; 36: 203–208. doi: 10.1097/HCR.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 18.Frost AE, Langleben D, Oudiz R, et al. . The 6-min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol 2005; 43: 36–39. doi: 10.1016/j.vph.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 19.Lipkin DP, Scriven AJ, Crake T, et al. . Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed) 1986; 292: 653–655. doi: 10.1136/bmj.292.6521.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Thoracic Society, American College of Chest Physicians . ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am J Respir Crit Care Med 2003; 167: 211–277. doi: 10.1164/rccm.167.2.211 [DOI] [PubMed] [Google Scholar]
- 21.Kuhn M, Johnson K. Applied Predictive Modeling. New York, Springer, 2018. [Google Scholar]
- 22.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998; 158: 1384–1387. doi: 10.1164/ajrccm.158.5.9710086 [DOI] [PubMed] [Google Scholar]
- 23.Humbert M, Sitbon O, Chaouat A, et al. . Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. doi: 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 24.Ling Y, Johnson MK, Kiely DG, et al. . Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. doi: 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 25.Mueller-Mottet S, Stricker H, Domeninghetti G, et al. . Long-term data from the Swiss pulmonary hypertension registry. Respiration 2015; 89: 127–140. doi: 10.1159/000370125 [DOI] [PubMed] [Google Scholar]
- 26.Rasekaba T, Lee AL, Naughton MT, et al. . The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J 2009; 39: 495–501. doi: 10.1111/j.1445-5994.2008.01880.x [DOI] [PubMed] [Google Scholar]
- 27.Puente-Maestu L, Stringer WW, Casaburi R. Exercise testing to evaluate therapeutic interventions in chronic respiratory diseases. Barcelona Respir Netw Rev 2018; 4: 274–286. [Google Scholar]
- 28.Mathai SC, Puhan MA, Lam D, et al. . The minimal important difference in the 6-minute walk test for patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 428–433. doi: 10.1164/rccm.201203-0480OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raina A, Humbert M. Risk assessment in pulmonary arterial hypertension. Eur Respir Rev 2016; 25: 390–398. doi: 10.1183/16000617.0077-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doutreleau S, Di Marco P, Talha S, et al. . Can the six-minute walk test predict peak oxygen uptake in men with heart transplant? Arch Phys Med Rehabil 2009; 90: 51–57. doi: 10.1016/j.apmr.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 31.Ingle L, Goode K, Rigby AS, et al. . Predicting peak oxygen uptake from 6-min walk test performance in male patients with left ventricular systolic dysfunction. Eur J Heart Fail 2006; 8: 198–202. doi: 10.1016/j.ejheart.2005.07.008 [DOI] [PubMed] [Google Scholar]
- 32.Sitbon O, Gomberg-Maitland M, Granton J, et al. . Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801908. doi: 10.1183/13993003.01908-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barst RJ, Langleben D, Frost A, et al. . Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med 2004; 169: 441–447. doi: 10.1164/rccm.200307-957OC [DOI] [PubMed] [Google Scholar]
- 34.Grunig E, MacKenzie A, Peacock AJ, et al. . Standardized exercise training is feasible, safe, and effective in pulmonary arterial and chronic thromboembolic pulmonary hypertension: results from a large European multicentre randomized controlled trial. Eur Heart J 2021; 42: 2284–2295. doi: 10.1093/eurheartj/ehaa696 [DOI] [PubMed] [Google Scholar]
- 35.Systrom D, Warren A, Naeije R. The role of exercise testing in pulmonary vascular disease: diagnosis and management. Clin Chest Med 2021; 42: 113–123. doi: 10.1016/j.ccm.2020.11.003 [DOI] [PubMed] [Google Scholar]