Abstract
Background and aims
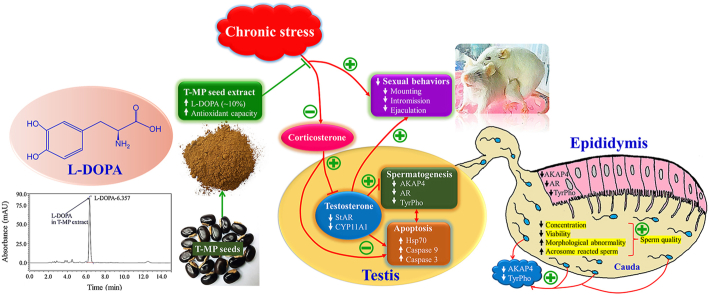
Chronic stress is a major common cause of male infertility. Many species of velvet beans are shown to be rich in l-DOPA. In Thai folklore medicine, seeds of Mucuna pruriens (L.) DC. var. pruriens (Thai Mhamui or T-MP) have been used for treating erectile dysfunction. This study aimed to determine l-DOPA levels in T-MP seed extract and investigate its preventive on sexual behaviors and reproductive parameter damages including essential proteins in chronic unpredictable mild stress (CUMS) mice.
Experimental procedure
Mice were divided into 4 groups: (I) control, (II) CUMS, (III) T-MP300 + CUMS, and (IV) T-MP600 + CUMS. Groups I and II received DW while groups III and IV were pretreated with the seed extracts (300 and 600 mg/kg BW) for 14 consecutive days before co-treatment with a randomly different CUMS/day (from 12 mild stressors) for 43 days.
Results and conclusion
T-MP seed extract contained l-DOPA approximately 10% of total dried weight. A dose of 600 mg/kg improved sexual performances and degenerative seminiferous epithelium in CUMS mice. Sperm qualities and testosterone level were elevated while corticosterone was decreased in co-treatment groups. T-MP-CUMS cotreated groups also improved expressions of AKAP4, AR, and TyrPho proteins in testis, epididymis, and sperm. T-MP increased StAR and CYP11A1 expressions in testis. It also suppressed testicular apoptosis via decreased expressions of Hsp70, caspases 3, and 9. T-MP seeds containing l-DOPA could improve sexual behaviors and essential reproductive proteins caused by CUMS.
Section
Natural Products;
Taxonomy (classification by evise)
Traditional Herbal Medicine; Animal Model; Histopathology.
Keywords: T-MP, CUMS, l-DOPA, AKAP4, TyrPho
Graphical abstract
Highlights
-
•
T-MP seed contains l-DOPA and improves sexual behaviors in CUMS mice.
-
•
TT-MP extract elevates testosterone and reduces corticosterone levels.
-
•
T-MP extract improves essential reproductive markers and sperm quality of CUMS mice.
-
•
T-MP extract suppressed testicular apoptosis under CUMS.
List of abbreviations
- AKAP4
A-kinase-anchoring protein 4
- AR
androgen receptor
- Apaf-1
protease activating factor
- Bax
Bcl-2-associated X
- Bcl-2
B-cell lymphoma 2
- BSA
bovine serum albumin
- BW
body weight
- cGMP
cyclic guanosine monophosphate
- CUMS
chronic unpredictable mild stress
- CYP11A1
cytochrome P450 family 11 subfamily A member 1
- DA
dopamine
- DW
distilled water
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- H&E
hematoxylin and eosin
- H2O2
hydrogen peroxide
- HPLC
high performance liquid chromatography
- HRP
horseradish peroxidase
- kDa
kilo daltons
- l-DOPA
L-3,4 dihydroxyphenyl alanine
- Hsp70
heat shock protein 70
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- Nrf2
nuclear factor erythroid 2-related factor 2
- RIPA
radioimmunoprecipitation assay
- ROS
reactive oxygen species
- RT
room temperature
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- StAR
steroidogenic acute regulatory
- TBST
Tris-buffered saline 0.1% Tween-20
- T-MP
Thai Mucuna pruriens
- TyrPho
tyrosine phosphorylated
1. Introduction
Chronic stress is known to be a psychological factor that can cause many neuropsychiatric disorders, metabolic syndromes, and infertility.1, 2, 3 In men, clinical studies have reported that chronic stress significantly increased serum cortisol level and reduced the sexual performances, androgen levels, and semen quality parameters.2, 4, 5 To explore more mechanism of male infertility caused by stress, the chronic unpredictable mild stress (CUMS) model has been widely used in experimental animals by randomly exposure to many unpredictable stressors to mimic the physiological symptoms of human depression.6,7 Previous studies reported that CUMS could impair male sexual behaviors and damaged the seminiferous and epididymal tissues in rodents.8,9 Recently, CUMS is shown to decrease the sperm qualities and essential testicular protein expressions including A-kinase-anchoring protein 4 (AKAP4, particularly involved in spermatogenesis and sperm motility), tyrosine phosphorylated (TyrPho), and androgen receptor (AR).8 Interestingly, decreased serum testosterone level induced by CUMS was improved by probiotic prevention via the increase of testicular cytochrome P450 family 11 subfamily A member 1 (CYP11A1) and steroidogenic acute regulatory (StAR) protein expressions.10 Indeed, CUMS induces testicular germ cell apoptosis by signaling changes of Bcl-2, Bax, and caspase 3 expressions.11 Although anxiolytic and antidepressant drugs have been used as combined pharmacotherapy in chronic stress patients, many adverse effects are still reported especially sexual dysfunctions.12,13 Hence, recent studies are being searched for novel effective herbal medicines to improve depression and reproductive damages from chronic stress.
Mucuna pruriens or itching bean is an effective medicinal plant traditionally used in tropical areas especially in India and China for the alternative treatment of parkinsonism, diabetes mellitus, and male infertility. For pharmacological properties, M. pruriens seed extract has been shown to have neuroprotective, anti-oxidative, anti-inflammatory, anti-hypertensive, anti-diabetic, anti-venom, and anti-cancer effects.14, 15, 16 Based on phytochemical profiles, the L-3,4 dihydroxyphenyl alanine (l-DOPA) is demonstrated to be a major active substance found in the seeds of M. pruriens with high antioxidant capacity.17, 18, 19 Moreover, M. pruriens seed has been proven to improve semen quality and antioxidant enzymes in seminal plasma of infertile men.5 In rodents, M. pruriens could reduce the damages of sperm DNA and testicular mitochondria.20,21 Mucuna pruriens (L.) DC. var. pruriens (T-MP) known as is commonly found in northeast Thailand. In Thai folk medicine, this plant is called Thai Mhamui (Mucuna pruriens (L.) DC. var. pruriens or T-MP) and used in treating erectile dysfunction since traditionally documented in 1998.22 Currently, T-MP seed extract was reported for the first time to have no vital toxicity and contain antioxidant activities with enhancing sperm parameters, testosterone level, and expressions of testicular AKAP4 and TyrPho proteins in rats.23 This study hypothesized that T-MP also contained l-DOPA like others and could improve male reproductive impairments caused from CUMS. Therefore, we aimed to investigate the protective effect of T-MP seed extract on sexual behaviors, sperm characteristics, and expressions of AKAP4 and TyrPho, steroidogenic, and apoptotic markers in reproductive tissues of CUMS mice.
2. Materials and methods
2.1. Plant extraction
The T-MP seed extract (% yield, 16.29) used in this study has been prepared and already proven to have antioxidant capacity from our laboratory as previously described.24 Briefly, the mature T-MP seeds were harvested from the ripe pods of trees (cultured in Surin province, Thailand) authenticated by Department of Biology, Faculty of Science, Khon Kaen University (specimen voucher code: S. Iamsaard 01). After grinding, the crushed seeds were extracted with distilled water (80 °C, 30 min). Subsequently, aqueous seed extract was filtered and lyophilized by spray dryer at Faculty of Pharmaceutical science, Khon Kaen, University.24
2.2. Quantification of l-DOPA by HPLC
Ultimate 3000 HPLC (Thermo Scientific) was used to quantify l-DOPA in the T-MP seed extract with following specifications: a reverse phase column C8 and C18 (250 × 4.6 mm, Phenomenex®, Sigma-Aldrich, Inc., USA) with a mobile phase of 10 mM hexanesulfonate (Fluka Chemie Ag, Switzerland) in sodium dihydrogen phosphate (20 mM, pH 2.5, Merck Millipore Corporation, USA) and methanol (CARLO ERBA, France). The peak of l-DOPA was detected by UV detector at wavelength of 280 nm with, injection rate (10 μl/min) and flow rate (1 ml/min). The concentration of sample solution (40 mg) was prepared in 10 mL of 1% formic acid (Merck Millipore Corporation, USA) or ethanol. This sample was analyzed with two replicates. The levodopa standard solution (5, 10, 20, and 100 μg/ml, Acros Organics®, UK) used as a specific control was prepared in 1% formic acid.25 Linear equation was plotted from the concentrations of levodopa (x-axis) against the area under the curve of each concentration (y-axis). The concentration of T-MP seed extract was calculated by using following formula: y = 0.2119x-0.2268 (R2 = 0.9998); y = area under the curve of each standard solution and x = the concentration of T-MP extract.
2.3. Experimental animals and design
Ninety-six adult male (n = 48) and female (n = 48) ICR mice (10 weeks, weighing 35–40 g) were purchased from the Animal unit, Faculty of Medicine, Khon Kaen University, Thailand. All animals were accommodated under temperature (25 ± 2 °C), 12-h light/dark cycles, and humidity (40–60%). Commercial food pellet and distilled water were provided ad libitum. Forty-eight male mice were randomly separated into four groups (n = 12/group) including control (group I), CUMS (group II), T-MP300 + CUMS (group III), and T-MP600 + CUMS groups (group IV). In groups I-II, mice were received with DW whereas animals in groups III-IV were pre-treated with T-MP seed extracts at a dose of 300 and 600 mg/kg BW, respectively for 14 consecutive days (a preventive period or pre-CUMS) based on Iamsaard and coworkers (2020). For co-treatment period (or CUMS induction), mice (group I-II) were still fed with DW but those of groups III-IV were co-administered with T-MP extract (days 15–57 [or 43 days], based on a spermatogenesis cycle (36 days) and the sperm transit period (7 days) from testis into the cauda epididymis.26,27 After 1 h of treatment, mice in groups II-IV were induced by CUMS (described in Suppl. Table 1). Briefly, mice in CUMS and co-treated groups were induced by single stressor every day, randomized from different 12 stressors. Such stressors included (1) cold water forced swimming for 5 min, (2) 45° cage tilting for 12 h, (3) social isolation for 12 h, (4) immobilization stress for 6 h, (5) tail clamping for 1 min, (6) 95 dB noise for 6 h, (7) wet bedding for 12 h, (8) reversed light–dark cycle for 24 h, (9), food deprivation for 12 h, (10) flashing light for 12 h, (11) electric foot shock for 3 s, and (l2) water deprivation for 12 h (performed for 3 rounds). At the end of experiment, all animals were weighed to calculate the percentage of body weight change by using the final weight minus the initial weight X 100 and divided by the initial weight.28 The induction of CUMS has been previously described.8,29 Briefly, all animals (except the control group) were randomly induced by a stressor per day from different twelve stressors (4 rounds) as shown in Suppl. Table 1. On day 0 (baseline), day 14 (pre-CUMS), and day 50 (CUMS induction), mice were subjected to sexual behavior test. On day 57, animals were sacrificed to collect blood and reproductive organs for further analyses. This study was already approved by the Animal Ethics Research Committee of Khon Kaen University, based on the Ethics of Animal Experimentation of the National Research Council of Thailand (Rec. No. AEKKU 49/63).
2.4. Sexual behavior test
The sexual behavior test was performed as described in previous studies.8,30 To gain estrous animals, all adult female mice were subcutaneously injected with estradiol benzoate (10 mg/mouse; Sigma-Aldrich) at 48 h and progesterone (500 mg/mouse; Sigma-Aldrich) at 4 h before testing. In brief, a male mouse was placed onto the center of plexiglas box for acclimatization (10 min) and followed by gentle placing of an artificial estrous female. The sexual behavior parameters including latency and frequency of mounting, intromissions, and ejaculations were recorded within 30 min by using the video camera linked to notebook computer.
2.5. Serum hormone analyses
All mice were anesthetized by thiopental sodium injection (60 mg/kg BW, i.p.) and euthanized by cervical dislocation. Then, blood was rapidly collected by cardiac puncture and the serum was separated by centrifugation (13,000 g, 4 °C, 10 min). The levels of serum corticosterone and testosterone were evaluated by radioimmunoassay at the Radiology Unit, Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand.
2.6. Sperm preparation for counting
The left cauda epididymis was harvested to squeeze sperm fluid and dipped into 1 ml of phosphate buffer saline (PBS, pH 7.4, 37 °C). The sperm suspension was resuspended and fixed with 4% paraformaldehyde (20 μl). It was further diluted for 1:2 with PBS before dropping (20 μl) on Neubauer counting chamber (Paul Marienfeld GmbH & Co. KG, Germany). Total counted sperm on five squares of each counting chamber were used to calculate as sperm concentration (x106 cells/ml) as previously described.24,31
2.7. Sperm viability
After collection of fresh sperm within 30 min, sperm suspension (50 μl, pH 7.4, 37 °C) was pipetted and mixed with 50 μl of eosin–nigrosin (BASO diagnostics, Inc, China) at 37 °C to incubate for 30 s. Then the stained-sperm mixture (10 μl) was dropped onto a coated slide to be smeared and dried overnight. To quantify sperm viability, four hundred sperm in total were counted in each animal under light microscope. Live (viable) sperm is unstained with eosin at the head while the dead sperm is clearly stained with dark pink. Then, the numbers of the live sperm were calculated as the percentage of sperm viability.32,33
2.8. Sperm morphology
For the observation of sperm head and tail morphologies, the sperm samples were prepared as described previously.34,35 In brief, fixed sperm suspension was smeared on gelatin-coated slides. The dried sperm was stained with eosin for 30 min. Sperm morphologies were classified to be normal and abnormal sperm observed under light microscope.8 Four hundred sperms from each animal were examined and expressed as the percentage of abnormal sperm.
2.9. Acrosome reaction status
In brief, the smeared and dried sperm was stained with 0.22% Coomassie blue G-250 for 5 min and then washed with excessive DW. From each animal, two hundred sperm in total were investigated for acrosome status under light microscope. The acrosome intact (AI) shows the acrosome cap stained with Coomassie blue but acrosome reacted (AR) sperm head had no staining.24 The results were expressed as the percentage of AR.
2.10. Reproductive organ weights
After mice were sacrificed, the testes, epididymis plus vas deferens, and penis were dissected and collected out to remove fat pads surrounding before weighing of their absolute weights. The right side of such organs was rapidly fixed with 10% neutral buffered formalin for 48 h to be processed for H&E staining in histopathological observation. Additionally, left side of dissected tissues was further snap-freezed with liquid nitrogen before investigating the expression of targeted proteins by using Western blotting. For the calculation of the relative weight, the absolute weight of each organ was calculated by using following formula: the relative weight (g/kg BW) = the absolute organ weight (g) x 100 (g)/body weight (g) of animal.36
2.11. Histological observation
Right testis and epididymis fixed were histologically processed with gradual series of alcohol concentration, cleared by xylene, infiltrated and embedded with liquid paraffin (56–60 °C) to make the paraffinized tissue blocks. Then, the blocks were sectioned using semi-automatic rotary microtome (approximately 5–7 μm thickness, ERM 3100, Hestion, Australia). All tissue sections were further deparaffinized with xylene, subsequently rehydrated with serial alcohols and washed with tab water. Then, all sections were stained by hematoxylin and eosin (Bio-optica, Italy). After washing, the stained-tissue sections were dehydrated and mounted with dibutylphthalate polystyrene xylene (BDH Laboratory, UK). Before capturing the histological figures with a DXM1200 digital camera (Nikon), all sectioned tissues were observed under light microscope (Nikon Light ECLIPSE E200, Japan).33
2.12. Western blotting analysis
Left testis of each animal was cut and weighted to be 50 mg/sample whereas left epididymis was separated into three parts: caput, corpus, and cauda (approximately 20 mg/sample). Epididymal tissues were further minced into small pieces and then washed with chilled PBS (pH 7.4) to eliminate the stored sperm and residual contents including fluid within epididymal lumen. In each group, samples (n = 8/group) of testis or individual epididymis were pooled and homogenized using glass grinder with the cold lysis buffer containing 1X radioimmunoprecipitation assay (RIPA) buffer (Cell signaling Technology Inc., USA) and protease inhibitor cocktails (Sigma-Aldrich, Inc., USA) while pool sperm pellets (n = 8/group) were extracted in RIPA with cocktails of protease inhibitors and 4% SDS before incubation for 30 min. Subsequently, all samples were sonicated by using ultrasonicator (50 W, 20 times, 3 s) and further centrifuged by using microcentrifuge (12,000 g, 4 °C, 10 min) to collect the total protein lysate in supernatants. All supernatants were collected to measure the total protein concentration by using NANO drop ND-100 Spectrophotometer (NanoDrop Technologes Inc., USA). Then, the total proteins of each sample (300 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto the nitrocellulose membrane (0.45 μm, Merck Millipore Corporation, Billerica, USA). To block non-specific binding proteins, all membranes were incubated with 5% bovine serum albumin (BSA) in Tris-buffered saline, 0.1% Tween-20 (TBST) buffer at room temperature (RT) for 1 h before probing with rabbit monoclonal AKAP4, mouse monoclonal Hsp70 (Abcam, Cambridge, USA), rabbit polyclonal AR, rabbit polyclonal StAR (Santa Cruz Biotechnology, Inc., USA), mouse monoclonal phosphotyrosine, clone 4G10®, goat polyclonal CYP11A1, mouse monoclonal caspase 9, and mouse monoclonal caspase 3 (Merck Millipore corporation, Billerica, USA) diluted 1:1000 in TBST (overnight, 4 °C). The epidermal growth factor (EGF) was used as positive control whereas BSA was used as negative control for TyrPho protein detection. All membranes were washed with TBST (5 min, 3 times) and then probed with secondary antibodies conjugated with horseradish peroxidase (HRP) in TBST buffer (90 min, RT). After washing unbound antibodies (5 min, 3 times), the immunoreactive bands were detected by using enhance chemiluminescence (ECL) substrate reagent kit (Amersham™ ECL™ Prime, GE Healthcare Life Science, USA) and recorded under Gel Documentation 4 (ImageQuant 400, GH HealthCare, USA).
2.13. Immunofluorescence staining
The paraffinized testicular sections were incubated with 10 mM sodium citrate buffer (pH 6.0) under a microwave (560 W, 10 min) as previously described.8,37 Then the endogenous peroxidases activities on tissue section were blocked with 3% hydrogen peroxide (H2O2) for 30 min. After washed with PBS, tissue sections were permeabilized with 0.2% Triton X-100 for 10 min. Subsequently, the nonspecific binding proteins were blocked using 3% BSA (Merck Millipore) in PBS for 1 h and followed by incubation with mouse monoclonal antibody against caspase 3 (diluted 1:200 in PBS, Merck Millipore) for overnight (4 °C). After washed unbound antibodies with PBS, each section was probed with goat anti-mouse IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor® 488 (diluted 1:300 in PBS, Invitrogen) for 1 h. All sections were washed with PBS and then counterstained with Hoechst 33342 for 10 min (diluted 1:10,000 in PBS, Abcam). The specific antigen-antibody complexes were observed and photographed under fluorescence microscope using a fluorescein isothiocyanate (FITC) filter (Nikon ECLIPSE 80i, Japan).
2.14. Statistical analysis
In this study, all data are shown as mean ± SEM. The difference of data was analyzed by one-way analysis of variance (ANOVA) followed by Tukey's multiple range test to compare the difference between each pair of means by using the SPSS software (Statistical package for the social science, version 19.0, Armonk, New York, USA). The P < 0.05 is considered as significantly statistical difference.
3. Results
3.1. Determination of l-DOPA in T-MP seed extract
The HPLC result showed a symmetric peak of l-DOPA in the aqueous seed extract of T-MP with a retention time of 6.357 min (purified l-DOPA standard, 6.430 min) as shown in Suppl. Fig. 1A and B. The amounts of l-DOPA (% w/w) detected in T-MP seed extract were approximately 10.16 ± 0.01% (extracted with formic acid) and 10.30 ± 0.05% (extracted with ethanol) as shown in Suppl. Table 2.
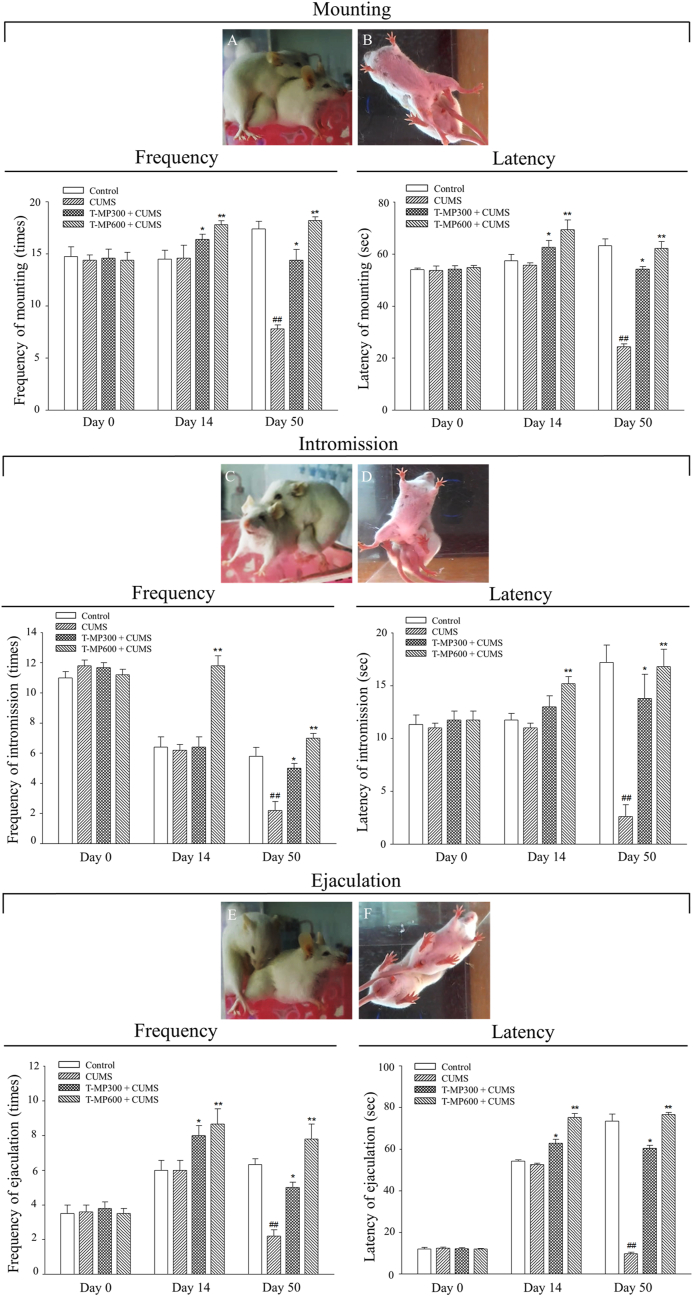
3.2. T-MP extract improved sexual behaviors of CUMS mice
Sexual performances including frequencies and latencies of mounting, intromission, and ejaculation of the control, CUMS, and T-MP treated animals were shown in Fig. 1. The baseline data (on day 0) showed that all parameters observed in sexual behaviors were comparable among the control and treated groups. After treatment with T-MP extract for 14 consecutive days (pre-CUMS), significantly increased in latencies and frequencies of all sexual behaviors were observed. On day 50 (CUMS induction period), all parameters were significantly decreased in CUMS animals when compared with control mice. Interestingly, T-MP extract (300 and 600 mg/kg) could significantly improve sexual parameters in a dose-dependent manner as compared to untreated-CUMS group (Fig. 1A–F).
Fig. 1.
Showing frequency and latency of (A, B) mounting, (C, D) intromission, and (E, F) ejaculation behaviors observed both lateral and inferior aspects. Such behaviors were compared among control, CUMS, and treated groups within 30 min after couple with estrous female at day 0 (baseline), day 14 (pre-CUMS), day 50 (post-CUMS) of experiment. All data are represented as mean ± SEM (n = 6, each group). Significant difference (#P < 0.05, # #P < 0.01) vs. the control group. Significant difference (∗P < 0.05, ∗ ∗P < 0.01) vs. the CUMS group.
3.3. Effect of T-MP extract on body and reproductive organ weights of CUMS mice
After administration with T-MP extracts, the body weight before CUMS induction tended to be slightly increased but not statistically different as compared to that of untreated animals (Table 1). For post-CUMS, the BW of CUMS group was significantly lower than that of control and was improved after treating with T-MP extracts (Table 1). Additionally, it was found that T-MP extract could significantly increase the percentage change of BW in CUMS group when compared with control group (Table 1). Corroborated with the size of their reproductive organs (Suppl. Fig. 2), the weights of testis, epididymis plus vas deferens, and penis of CUMS group were significantly lower than those of control and they were improved after co-treatment with T-MP (Table 1). However, only relative weight of penis among groups was not significantly different.
Table 1.
Comparisons of body and male reproductive organ weights, sperm quality parameter, and hormones among control, CUMS, and treated groups. All data are represented as mean ± SEM (n = 12, each group). Significant difference (#P < 0.05,##P < 0.01) vs. the control group. Significant difference (∗P < 0.05, ∗ ∗P < 0.01) vs. the CUMS group.
| Parameters | Groups |
|||
|---|---|---|---|---|
| Control | CUMS | T-MP300 + CUMS | T-MP600 + CUMS | |
| Baseline BW |
36.33 ± 0.64 |
36.50 ± 0.50 |
36.50 ± 0.50 |
36.50 ± 0.50 |
| Pre-CUMS BW |
37.75 ± 0.71 |
37.75 ± 0.59 |
38.33 ± 0.31 |
38.58 ± 0.65 |
| Post-CUMS BW |
39.67 ± 0.40 |
37.43 ± 0.48## |
39.29 ± 0.47∗ |
39.57 ± 0.81∗∗ |
| Percentage change of BW |
8.55 ± 1.39 |
4.09 ± 1.04# |
7.15 ± 1.61 |
8.40 ± 1.52∗ |
| Testis | ||||
| Absolute weights (g) | 0.17 ± 0.00 | 0.15 ± 0.00## | 0.16 ± 0.00∗ | 0.17 ± 0.00∗∗ |
| Relative weights (g/kg BW) |
0.42 ± 0.01 |
0.39 ± 0.00## |
0.42 ± 0.00∗ |
0.43 ± 0.01∗∗ |
| Epididymis plus vas deferens | ||||
| Absolute weights (g) | 0.08 ± 0.00 | 0.07 ± 0.00## | 0.08 ± 0.00∗ | 0.08 ± 0.00∗ |
| Relative weights (g/kg BW) |
0.19 ± 0.00 |
0.18 ± 0.00# |
0.20 ± 0.00∗∗ |
0.21 ± 0.00∗∗ |
| Penis | ||||
| Absolute weights (g) | 0.04 ± 0.00 | 0.03 ± 0.00# | 0.04 ± 0.00∗ | 0.04 ± 0.00∗∗ |
| Relative weights (g/kg BW) |
0.10 ± 0.00 |
0.10 ± 0.00 |
0.10 ± 0.00 |
0.10 ± 0.00 |
| Sperm quality | ||||
| Sperm concentration (106 cells/ml) | 5.25 ± 0.67 | 2.54 ± 0.70## | 4.37 ± 0.58∗ | 5.92 ± 0.62∗∗ |
| Sperm head abnormality (%) | 4.15 ± 0.46 | 10.35 ± 1.03## | 5.20 ± 0.51∗ | 4.40 ± 0.33∗∗ |
| Sperm tail abnormality (%) | 3.05 ± 0.24 | 25.07 ± 1.13## | 6.20 ± 0.43∗ | 3.48 ± 0.51∗∗ |
| Acrosome reaction (%) |
2.25 ± 0.15 |
11.33 ± 0.33## |
4.56 ± 0.29∗ |
1.63 ± 0.30∗∗ |
| Hormones | ||||
| Serum corticosterone (μg/ml) | 0.28 ± 0.04 | 0.75 ± 0.09## | 0.36 ± 0.01∗∗ | 0.33 ± 0.03∗ |
| Serum testosterone (ng/ml) | 0.66 ± 0.07 | 0.42 ± 0.07# | 0.62 ± 0.12∗ | 2.32 ± 0.14∗∗ |
3.4. T-MP extract attenuated sperm parameter damages in CUMS mice
The result showed that T-MP extract significantly increased the percentage of sperm viability (Suppl. Fig. 3A and B) and sperm concentration (Table 1) in a dose-dependent manner in CUMS mice. Additionally, it was observed that CUMS caused both sperm head (Suppl. Fig. 4B-F) and tail abnormalities (Suppl. Fig. 4G-K). As quantified, the result showed that both doses of T-MP extract could significantly improve the sperm abnormalities as compared to those of untreated-CUMS animals (Table 1). All doses of T-MP treatment significantly decreased the percentage of acrosome reaction in a dose-dependent manner as compared to that of untreated-CUMS group (Table 1). Moreover, T-MP extract could significantly improve the decreased testosterone level in a dose-dependent manner compared to untreated-CUMS mice. Significantly, the corticosterone levels were reduced in co-treated group when compared to CUMS alone (Table 1).
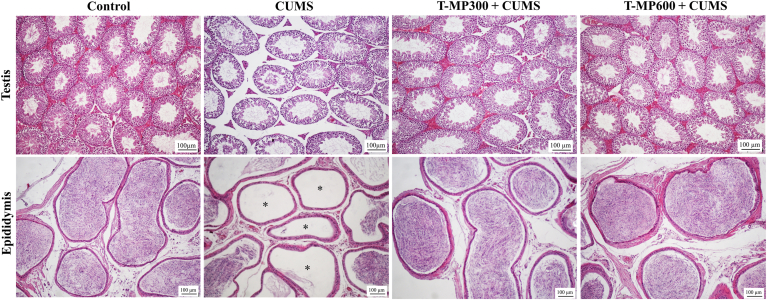
3.5. T-MP extract prevented damages of testis and epididymis in CUMS mice
In control group, the seminiferous epithelium was properly organized while some seminiferous atrophies were observed in CUMS group (Fig. 2). In contrast to control (Suppl. Fig. 5A), the histopathological features of seminiferous tubules were observed in CUMS group as demonstrated in Suppl. Fig. 5B-I, including exfoliated germ cell, vacuolization within Leydig cells, spermatogonia, and primary spermatocytes, sloughing of spermatogenic cells, depletion of elongated spermatids, giant cell, pyknotic nuclei of spermatogonial cells, and necrotic seminiferous epithelium. It was found that T-MP extracts could improve such testicular damages as compared to those of untreated-CUMS group (Fig. 2). Moreover, the cauda segment of ductus epididymis in CUMS group were found to have the loss of sperm mass compared to control (Fig. 2). Obviously, the T-MP extracts could also improve epididymal damages caused by CUMS (Fig. 2).
Fig. 2.
Representative photomicrographs showing histopathology of testis and epididymis strained by H&E, compared among control, CUMS, and treated groups. Asterisks; reduction of sperm mass within the caudal epididymal lumen.
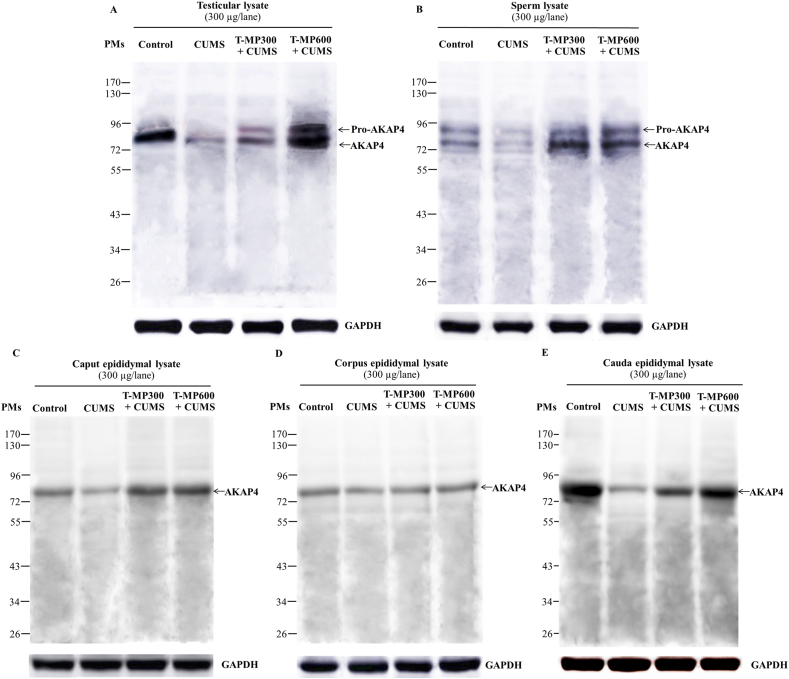
3.6. Effect of T-MP extract on AKAP4 expression in testis, epididymis, and sperm of CUMS mice
The results demonstrated that the pro-form (94 kDa) and mature form (82 kDa) of AKAP4 were only expressed in the testicular and sperm lysate (Fig. 3A and B). Such expressions were obviously decreased in CUMS mice. In contrast, only mature AKAP4 form was expressed in the epididymal tissues (caput, corpus, and cauda segments) while its expression was decreased in CUMS groups (Fig. 3C–E). Interestingly, T-MP extract could increase the expressions of both AKAP4 forms in all tissue lysates when compared to untreated CUMS mice (Fig. 3).
Fig. 3.
Expression of AKAP4 compared among control, CUMS, and treated groups by Western blotting in (A) testicular, (B) sperm, (C) caput, (D) corpus, and (E) cauda epididymal lysates. GAPDH: glyceraldehyde-3-phosphate dehydrogenase used as an internal control. CUMS: chronic unpredictable mild stress. PMs: pre-stained markers. Pro-AKAP4: proform of A-kinase anchor protein 4. T-MP: Thai mucuna pruriens. n = 8 per group.
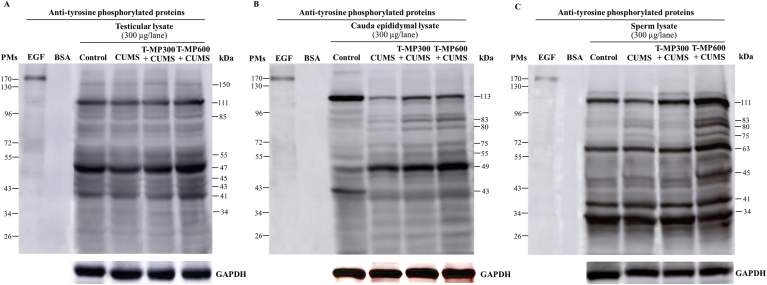
3.7. T-MP extract improved TyrPho protein expressions in reproductive tissue lysates of CUMS mice
The expressions of TyrPho proteins were obviously changed in testis, cauda epididymis, and sperm of CUMS mice as compared to the control group (Fig. 4). As compared between control and CUMS groups (Fig. 4A–C), the decreased expressions of TyrPho proteins were investigated in testis (150, 111, 55, 47, and 41 kDas), cauda epididymis (113 kDa except 49 kDa), and sperm (111 kDa except 83, 80, and 75 kDas). It was found that the high dose of T-MP extract could improve such protein expressions in testis (150, 111, 85, 55, 47, 45, 43 and 41 kDas), cauda epididymis (113, 83, 80, 75, 55, and 49 kDas), and sperm (111, 83, 80, 75, 63, and 45 kDas), respectively, when compared to untreated CUMS group (Fig. 4A–C).
Fig. 4.
Expression of tyrosine phosphorylated (TyrPho) proteins observed in (A) testicular, (B) cauda epididymal, and (C) sperm lysates compared among groups of control, CUMS, and treated groups. EGF, epidermal growth factor used as positive control; BSA, bovine serum albumin used as negative control. n = 8 per group.
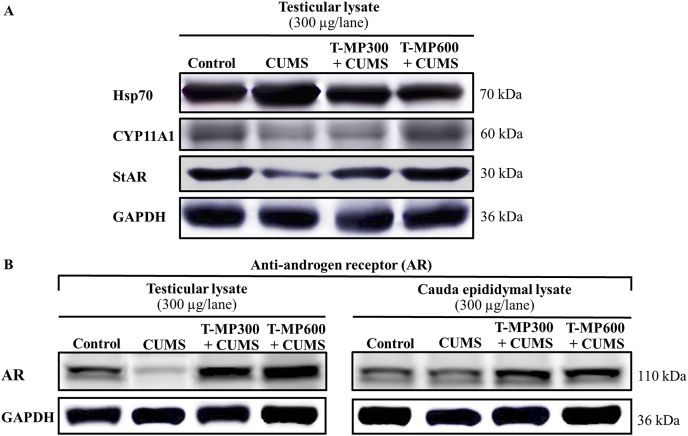
3.8. T-MP extract improved functional protein expressions in CUMS testis
It was revealed that the steroidogenic acute regulatory (StAR), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), and androgen receptor (AR) proteins were obviously decreased in CUMS testis (Fig. 5A and B), but the heat shock protein 70 (Hsp70) expression was increased when compared to control (Fig. 5A). Moreover, the expression of AR in cauda epididymal lysate of CUMS mice was decreased as shown in Fig. 5B. The T-MP extract could improve all testicular StAR, CYP11A1, AR, and Hsp70 in a dose-dependent manner as compared to untreated-CUMS group (Fig. 5A and B). Additionally, T-MP extract could also increase AR expression in cauda epididymis of CUMS mice (Fig. 5B).
Fig. 5.
Expressions of heat shock protein 70 (Hsp70), cytochrome P450 family 11 subfamily A member 1 (CYP11A1), steroidogenic acute regulatory (StAR) in (A) testis as well as androgen receptor (AR) in (B) testicular and cauda epididymal lysates compared among control, CUMS, and treated groups. n = 8 per group.
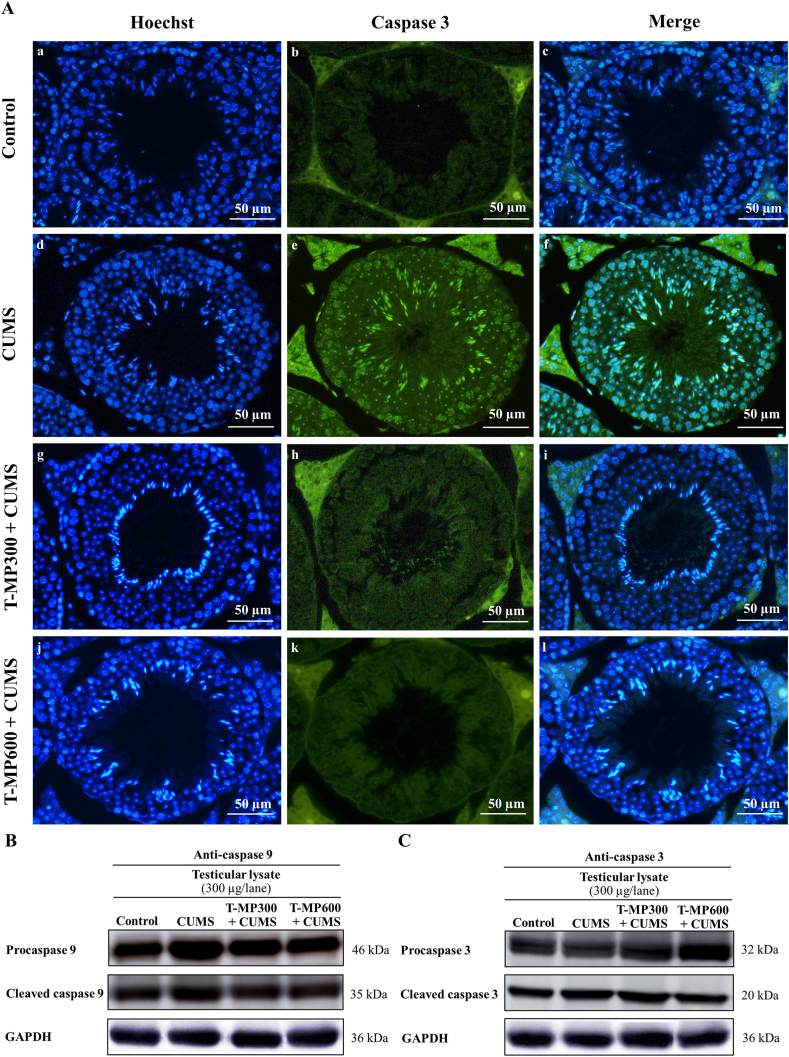
3.9. T-MP extract inhibited apoptosis via suppression of testicular caspase 9 and 3
The expressions of caspase 9 and 3 (both pro and cleaved forms) were increased in CUMS testis compared to those of control (Fig. 6B and C). It was found that T-MP could decrease the caspase expressions in all doses of T-MP extract as compared with untreated-CUMS group (Fig. 6B and C). In addition, the intensity of caspase-3 immunostaining shows more intense in nuclei and cytoplasm of both spermatogenic and Leydig cells of CUMS group, indicating apoptosis of those cells (Fig. 6Ae-f). After treatment with T-MP extract, such intense positivity in testicular tissue was obviously decreased, suggesting suppression of apoptosis induced by CUMS (Fig. 6Ah-i and 6Ak-l).
Fig. 6.
Immunofluorescence staining against caspase 3 antibody of testicular A(b–c) control, A(e–f) CUMS, A(h–i) T-MP300 + CUMS, A(k–l) T-MP600 + CUMS groups. Nuclei stained by Hoechst 33342 (blue fluorescence). The intensity of caspase 3 immunostaining (green fluorescence) is a prominent on spermatogenic and Leydig cells of CUMS group. Expressions of (B) caspase 9 and (C) caspase 3 including pre and cleaved forms compared among control, CUMS, and treated groups in testicular lysates. n = 8 per group.
4. Discussion
The T-MP used in this study has been identified in the Fabaceae family which is commonly found in Northeast Thailand.23,38 Its seed has been documented in Thai traditional medicine textbook named “Paet-Saat-Song-Kroh” (Thai word) to has aphrodisiac property and be used in treating of erectile dysfunction.22 However, the specific biostimulant of T-MP seed has not been fully reported. In general, the Mucuna bean especially Indian species was screened to contain various amino acids particularly l-DOPA, phenolic compounds (quercetin and myricetin), alkaloids (mucunine, mucunadine, prurienidine, and nicotine), fatty acids (palmitic, stearic, oleic, and linoleic acids), and several microelements (potassium, phosphorus, and magnesium), respectively.19, 39 Such Indian bean has been demonstrated to improve male infertility in both men and animal models.40,41 By weight of total dried extract, this study showed approximately 10% of l-DOPA levels in T-MP seeds which was previously reported to contain phenolic compounds and antioxidant capacity.23 It has been reported that purified levodopa has adverse effects on gastrointestinal system with nausea/vomiting, anorexia, and weight loss. Usually, the typical treatment regimen is ranged from 150 to 1000 mg of levodopa total/day (divided 3–4 times). In our study, the highest dose (T-MP, 600 mg/KgBW) relatively contained l-DOPA approximately 60 mg because the quantified HPLC result shows 10.3% of l-DOPA in total dried weight (as shown in supplement data Table 2). Therefore, such amount of l-DOPA may have no side effects to our experimental animals.
Recent study demonstrated for the first time that T-MP seed extract could enhance and improve sexual behaviors in CUMS mice. It is possible that the antioxidant and aphrodisiac potentials like l-DOPA in T-MP seeds facilitate the stimulation of nitric oxide (NO) to maintain the level of cyclic guanosine monophosphate (cGMP), resulting penile erection via Ca2+ and K+ channel regulations.42,43 Additionally, l-DOPA may activate the dopaminergic neurons responsible for male sexual behaviors to increase dopamine (DA) productions as previously described.44 Lampariello and coworkers (2012) reported that l-DOPA in seed extract may play an essential role in the aphrodisiac activity in CUMS mice. Moreover, of the increased testosterone level in CUMS mice treated with T-MP may promote the copulation by improvement of eNOS level and DA releasing.45
In this study, the improvements of epididymal sperm qualities in all T-MP extract treated CUMS mice may be resulted from high antioxidant activities.23 Indeed, it was revealed that l-DOPA has strong antioxidant capacity, free radical scavenging activity, and chelating capabilities.46 Previously, M. pruriens seed has been shown to improve sperm qualities by increasing of nuclear factor erythroid 2-related factor 2 (Nrf2) and inhibiting of the nuclear factor-kappa B (NF-κB) protein expressions in testis and epididymis.47 It might ameliorate damages of spermatogenic cells from CUMS by increasing antioxidant enzymes and decreasing ROS.20, 48 Such bean could also protect the lipid peroxidation on sperm membrane, DNA damage, and mitochondrial membrane permeability.20,49 In this study, T-MP seed suppressed the expressions of Hsp70, caspase 9 and 3 in CUMS testis, suggesting its preventive effects on germ cell apoptosis. In addition, Hsp70 was shown to suppress apoptosis by preventing recruitment of procaspase 9 to produce apoptotic protease activating factor 1 (Apaf-1) oligomers called apoptosome.50 It could inhibit Bcl-2-associated X (Bax) activation leading to preventing the release of cytochrome C from mitochondria.51 We assumed that T-MP extract could protect mitochondrial membrane injury in CUMS via-apoptotic pathway of procaspases 9 and 3.
Consequence of increased testosterone levels and antioxidant capacity of T-MP extract may be involved in improvement of seminiferous and epididymal epithelium damages as shown in Fig. 3. It was demonstrated that the loss of spermatogenic cells in CUMS mice was related to the decrease of androgen receptor (AR) expression.8,52 Possibly, the T-MP extract may facilitate the transcription and translation of AR in the spermatogenic and Sertoli cells.23 A study reported that quercetin is found in M. pruriens seed and could improve junctional proteins and the integrity of the blood-testis barrier in mouse testis.53 Such study even revealed the significant decrease of StAR and CYP11A1 expressions in mouse CUMS testis. It was in agreement with previous reports demonstrating that CUMS decreased the StAR and CYP11A1 expressions with increased corticosterone level.54,55 Indeed, our study significantly showed the improvement of those protein expressions in CUMS testis administered with T-MP extracts. It was suggested that flavonoids in T-MP extract could increase the StAR expression in Leydig cells.56 The increase of body and reproductive weights in T-MP treated CUMS mice in our recent study may result from stimulation of l-DOPA and dopaminergic neurons in the dorsal striatum, responsible for eating behaviors.57 Additionally, the increased testosterone could stimulate AR receptors in muscle mass to induce myoblast proliferation and suppress the ubiquitin ligase-mediated atrophy pathways.58 Moreover, l-DOPA was shown to increase the testicular and epididymal masses that are associated with the unregulated AR protein expressions.59
Previously, Choowong-In and coworkers (2021) demonstrated that CUMS not only decreased sexual behaviors but also affected the expressions of AKAP4 and TyrPho proteins testis, epididymis, and sperm in mouse model. Herein, such impairments were improved in CUMS mice treated with T-MP. It indicated that the extract could somehow facilitate the expressions of essential proteins involved in sexual behaviors and spermatogenesis. It has been shown that increase of testicular AKAP4 expression could complete spermiogenesis including organization and integrity of the sperm fibrous sheath and flagellum as previously described.60,61 Additionally, the improved expression of epididymal AKAP4 in T-MP treated CUMS may be associated with secreting of the functional epididymosomes onto the sperm surface membrane. Indeed, such process is a major process of sperm maturation particularly facilitating motility within ductus epididymis.62 Moreover, increased AKAP4 in sperm may enhance the progressive motility called sperm capacitation during transport with in the female reproductive tract. Similarly, T-MP seed could increase the AKAP4 expression in the caudally epididymal sperm of normal adult rats.23 It is known that AKAP4 playing roles in both testicular sperm production and ejaculated sperm motility is activated by tyrosine phosphorylation. The TyrPho proteins have been localized and identified in male reproductive tissues, assumed to play roles in spermatogenesis and sperm physiology. The T-MP administration could increase the expressions of testicular TyrPho proteins in CUMS mice, similar to a previous study showing such improvement effects of Phyllanthus emblica L. (PE) extract in chronic stress rats.63 Possibly, the increased TyrPho protein expressions (150, 111, 85, 55, 47, 45, 43, and 41 kDa) in CUMS animals treated T-MP are involved in AKAP4 expression, testosterone synthesis, and spermatogenesis. In cauda epididymis, T-MP extract increased the expressions of many TyrPho proteins in CUMS animals, facilitating epididymosome biogenesis and physiological sperm maturation.64 Moreover, T-MP increased such protein expressions (111, 83, 80, 75, 63, and 45 kDas) in sperm lysate of CUMS mice, indicating motility initiation prompted for capacitation and acrosome reaction in early process of fertilization.65
The limitations of this study were not able to perform the functional tests of sperm including sperm motility, capacitation, and acrosome reaction in vitro to confirm the associations and mechanism between AKAP4 and TyrPho proteins. However, some TyrPho proteins have been characterized for their functions particularly involved in progressive motility.66,67 The possible underlying mechanisms of sexual enhancing effect of T-MP extract need to be further improved via dopaminergic signaling system. This study showed that T-MP seed extract containing l-DOPA with high levels and could improve the sexual performances and reproductive parameters especially functional proteins in testis, epididymis, and sperm of CUMS mice.
5. Conclusion
This study showed that T-MP seed extract containing l-DOPA with high levels and could improve the sexual performances and reproductive parameters especially functional proteins in testis, epididymis, and sperm of CUMS mice.
Author contributions
SI conceived and supervised the project; PB screened the compound; PC performed the pharmacologic experiments; PC and NT did the animal experiments; PC analyzed the data; PC and SI wrote the manuscript; JS and AW reviewed and modified the paper. PC, CP, TS, SA, and NU provided the critical idea and discussion. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of competing interest
The authors state that there are no conflicts of interest.
Acknowledgments
This study was supported by a grant from Faculty of Medicine, Khon Kaen University (Grant Number IN63327), Research Institute for Human High Performance and Health Promotion (HHP&HP), and Postgraduate Study Support Grant of Faculty of Medicine, Khon Kaen University, Thailand.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2021.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bergmann N., Gyntelberg F., Faber J. The appraisal of chronic stress and the development of the metabolic syndrome: a systematic review of prospective cohort studies. Endocr Connect. 2014;3(2):55–80. doi: 10.1530/EC-14-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilacqua A., Izzo G., Emerenziani G.P., Baldari C., Aversa A. Lifestyle and fertility: the influence of stress and quality of life on male fertility. Reprod Biol Endocrinol. 2018;16(1):1–11. doi: 10.1186/s12958-018-0436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Negrón-Oyarzo I., Aboitiz F., Fuentealba P. Impaired functional connectivity in the prefrontal cortex: a mechanism for chronic stress-induced neuropsychiatric disorders. Neural Plast. 2016;2016:1–16. doi: 10.1155/2016/7539065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byun J.S., Lyu S.W., Seok H.H., Kim W.J., Shim S.H., Bak C.W. Sexual dysfunctions induced by stress of timed intercourse and medical treatment. BJU Int. 2013;111(4):227–234. doi: 10.1111/j.1464-410X.2012.11577.x. [DOI] [PubMed] [Google Scholar]
- 5.Shukla K.K., Mahdi A.A., Ahmad M.K., Jaiswar S.P., Shankwar S.N., Tiwari S.C. Mucuna pruriens reduces stress and improves the quality of semen in infertile men. Evid Based Complementary Altern Med. 2010;7(1):137–144. doi: 10.1093/ecam/nem171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Willner P. The chronic mild stress (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2016;6:78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choowong-In P., Sattayasai J., Poodendaen C., Iamsaard S. Decreased expression of AKAP4 and TyrPho proteins in testis, epididymis, and spermatozoa with low sexual performance of mice induced by modified CUMS. Andrologia. 2021;53(3) doi: 10.1111/and.13977. [DOI] [PubMed] [Google Scholar]
- 9.Grønli J., Murison R., Fiske E., et al. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84(4):571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y., Du X., Bian Y., Wang S. Chronic unpredictable stress-induced reproductive deficits were prevented by probiotics. Reprod Biol. 2020;20(2):175–183. doi: 10.1016/j.repbio.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Zou P., Wang X., Yang W., et al. Mechanisms of stress-induced spermatogenesis impairment in male rats following unpredictable chronic mild stress (uCMS) Int J Mol Sci. 2019;20(18):1–17. doi: 10.3390/ijms20184470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballenger J.C. Anxiety and depression: optimizing treatments. Prim Care Companion J Clin Psychiatry. 2000;2(3):71–79. doi: 10.4088/pcc.v02n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright C., Gibson K., Read J., Cowan O., Dehar T. Long-term antidepressant use: patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401–1407. doi: 10.2147/PPA.S110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan M.Y., Kumar V. Mechanism of antihypertensive effect of Mucuna pruriens L. seed extract and its isolated compounds. J Compl Integr Med. 2017;14(4):1–11. doi: 10.1515/jcim-2017-0014. [DOI] [PubMed] [Google Scholar]
- 15.Lampariello L.R., Cortelazzo A., Guerranti R., Sticozzi C., Valacchi G. The magic velvet bean of. Mucuna pruriens. J Tradit Complement Med. 2012;2(4):331–339. doi: 10.1016/s2225-4110(16)30119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha S., Sharma S., Vora J., Shah H., Srivastava A., Shrivastava N. Mucuna pruriens (L.) DC chemo sensitize human breast cancer cells via downregulation of prolactin-mediated JAK2/STAT5A signaling. J Ethnopharmacol. 2018;217:23–35. doi: 10.1016/j.jep.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Pastor I., Luque-Muñoz A., Rivas F., et al. Quantitative NMR analysis of L-Dopa in seeds from two varieties of Mucuna pruriens. Phytochem Anal. 2019;30(1):89–94. doi: 10.1002/pca.2793. [DOI] [PubMed] [Google Scholar]
- 18.Longhi J.G., Perez E., Lima J.J., Cândido L.M.B. In vitro evaluation of Mucuna pruriens (L.) DC. antioxidant activity. Braz J Pharm Sci. 2011;47(3):535–544. [Google Scholar]
- 19.Misra L., Wagner H. Extraction of bioactive principles from Mucuna pruriens seeds. Indian J Biochem Biophys. 2007;44(1):56–60. [PubMed] [Google Scholar]
- 20.Singh A.P., Sarkar S., Tripathi M., Rajender S. Mucuna pruriens and its major constituent L-DOPA recover spermatogenic loss by combating ROS, loss of mitochondrial membrane potential and apoptosis. PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0054655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suresh S., Prithiviraj E., Lakshmi N.V., Ganesh M.K., Ganesh L., Prakash S. Effect of Mucuna pruriens (Linn.) on mitochondrial dysfunction and DNA damage in epididymal sperm of streptozotocin induced diabetic rat. J Ethnopharmacol. 2013;45(1):32–41. doi: 10.1016/j.jep.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Ayuraved-Wittayarai Foundation . first ed. Ministry of Public Health; Thailand: 1998. Thai Traditional Medicine Textbook (Paet-Saat-Song-Kror) [Google Scholar]
- 23.Iamsaard S., Arun S., Burawat J., et al. Evaluation of antioxidant capacity and reproductive toxicity of aqueous extract of Thai Mucuna pruriens seeds. J Integr Med. 2020;18(3):265–273. doi: 10.1016/j.joim.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Iamsaard S., Prabsattroo T., Sukhorum W., et al. Anethum graveolens Linn. (dill) extract enhances the mounting frequency and level of testicular tyrosine protein phosphorylation in rats. J Zhejiang Univ - Sci B. 2013;14(3):247–252. doi: 10.1631/jzus.B1200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boonchoong P., Juengmunkong Z., Saohin W., Chanluang S., Kaiyafai K., Tapkeaw C. Quantitative analysis of L-DOPA in Mucuna pruriens seeds by high performance liquid chromatography. Thai J Pharm Sci. 2018;13(4):187–191. [Google Scholar]
- 26.Dadoune J.P., Alfonsi M.F. Autoradiographic investigation of sperm transit through the male mouse genital tract after tritiated thymidine incorporation. Reprod Nutr Dev. 1984;24(6):927–935. doi: 10.1051/rnd:19840709. [DOI] [PubMed] [Google Scholar]
- 27.Ray D., Pitts P.B., Hogarth C.A., Whitmore L.S., Griswold M.D., Ye P. Computer simulations of the mouse spermatogenic cycle. Biol Open. 2014;4(1):1–12. doi: 10.1242/bio.20149068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paiz R.C., Juárez-Flores B., Rivera J., et al. Glucose-lowering effect of xoconostle (Opuntia joconostle A. Web., Cactaceae) in diabetic rats. J Med Plants Res. 2010;4:2326–2333. [Google Scholar]
- 29.López López A.L., Escobar Villanueva M.C., Brianza Padilla M., Bonilla Jaime H., Alarcón Aguilar F.J. Chronic unpredictable mild stress progressively disturbs glucose metabolism and appetite hormones in. Acta Endocrinol. 2018;14(1):16–23. doi: 10.4183/aeb.2018.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novati A., Yu-Taeger L., Gonzalez Menendez I., Quintanilla Martinez L., Nguyen H.P. Sexual behavior and testis morphology in the BACHD rat model. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dogani M., Askari N., Kalantari-Hesari A., Rahbar F.H. The effects of P. atlantica as a libido booster and sexual enhancer on the reproductive system of male rats. J Tradit Complement Med. 2021:1–9. doi: 10.1016/j.jtcme.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agarwal A., Gupta S., Sharma R. Springer; Switzerland, AG: 2016. Andrological Evaluation of Male Infertility. [Google Scholar]
- 33.Mousa A.A., Elweza A.E., Elbaz H.T., et al. Eucalyptus Globulus protects against diclofenac sodium induced hepatorenal and testicular toxicity in male rats. J Tradit Complement Med. 2019;10(6):521–528. doi: 10.1016/j.jtcme.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tangsrisakda N., Iamsaard S. Effect of ethanol on the changes in testicular protein expression in adult male rats. Andrologia. 2020;52(10) doi: 10.1111/and.13784. [DOI] [PubMed] [Google Scholar]
- 35.Awodele O., Kale O.E., Odewabi A.O., Ekor M., Salau B.A., Adefule-Ositelu A.O. Safety evaluation of Bon-santé cleanser® polyherbal in male Wistar rats: further investigations on androgenic and toxicological profile. J Tradit Complement Med. 2017;8(1):212–219. doi: 10.1016/j.jtcme.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aniagu S.O., Nwinyi F.C., Akumka D.D., et al. Toxicity studies in rats fed nature cure bitters. Afr J Biotechnol. 2005;4(1):72–78. [Google Scholar]
- 37.Park H.J., Zhang M., Lee W.Y., et al. Toxic effects of nonylphenol on neonatal testicular development in mouse organ culture. Int J Mol Sci. 2020;21(10):1–15. doi: 10.3390/ijms21103491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav S.K., Rai S.N., Singh S.P. Mucuna pruriens reduces inducible nitric oxide synthase expression in Parkinsonian mice model. J Chem Neuroanat. 2017;80:1–10. doi: 10.1016/j.jchemneu.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad M.K., Mahdi A.A., Shukla K.K., Islam N., Jaiswar S.P., Ahmad S. Effect of Mucuna pruriens on semen profile and biochemical parameters in seminal plasma of infertile men. Fertil Steril. 2008;90(3):627–635. doi: 10.1016/j.fertnstert.2007.07.1314. [DOI] [PubMed] [Google Scholar]
- 40.Abarikwu S.O., Onuah C.L., Singh S.K. Plants in the management of male infertility. Andrologia. 2020;52(3) doi: 10.1111/and.13509. [DOI] [PubMed] [Google Scholar]
- 41.Mahajan G.K., Mahajan A.Y., Mahajan R.T. Efficacy of aphrodisiac plants towards improvement in semen quality and motility in infertile males. J Compl Integr Med. 2012;9(1):1–12. doi: 10.1515/1553-3840.1520. [DOI] [PubMed] [Google Scholar]
- 42.Chen L., Shi G.R., Huang D.D., et al. Male sexual dysfunction: a review of literature on its pathological mechanisms, potential risk factors, and herbal drug intervention. Biomed Pharmacother. 2019;112:108585. doi: 10.1016/j.biopha.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 43.Duangnin N., Phitak T., Pothacharoen P., Kongtawelert P. In vitro and in vivo investigation of natural compounds from seed extract of Mucuna pruriens lacking l-DOPA for the treatment of erectile dysfunction. Asian Pac J Trop Med. 2017;10(3):238–252. doi: 10.1016/j.apjtm.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 44.Dominguez J.M., Hull E.M. Dopamine, the medial preoptic area, and male sexual behavior. Physiol Behav. 2005;86(3):356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Hull E.M., Muschamp J.W., Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiol Behav. 2004;83(2):291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Cacciatore I., Marinelli L., Di Stefano A., et al. Chelating and antioxidant properties of l-Dopa containing tetrapeptide for the treatment of neurodegenerative diseases. Neuropeptides. 2018;71:11–20. doi: 10.1016/j.npep.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Sahin K., Orhan C., Akdemir F., et al. Comparative evaluation of the sexual functions and NF-κB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Compl Alternative Med. 2016;16(1):1–11. doi: 10.1186/s12906-016-1303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashidi J.S., Owagboriaye F.O., Yaya F.B., Payne D.E., Lawal O.I., Owa S.O. Assessment of reproductive function in male albino rat fed dietary meal supplemented with Mucuna pruriens seed powder. Heliyon. 2019;5(10) doi: 10.1016/j.heliyon.2019.e02716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suresh S., Prithiviraj E., Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33(1):22–32. doi: 10.1111/j.1365-2605.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 50.Beere H.M., Wolf B.B., Cain K., et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2(8):469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 51.Stankiewicz A.R., Lachapelle G., Foo C.P., Radicioni S.M., Mosser D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280(46):38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 52.Li T., Yao J., Zhang Q., et al. Chronic stress impairs male spermatogenesis function and Nectin-3 protein expression in the testis. Physiol Res. 2020;69(2):297–306. doi: 10.33549/physiolres.934287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dolati P., Khodabandeh Z., Zamiri M.J., Jamhiri I., Mehrabani D. The effect of lead acetate and quercetin on the tight and gap junctions in the mouse testis. Biol Trace Elem Res. 2020;198(2):535–543. doi: 10.1007/s12011-020-02079-x. [DOI] [PubMed] [Google Scholar]
- 54.Dhanabalan S., Mathur P.P., Latha P. TCDD and corticosterone on testicular steroidogenesis and antioxidant system of epididymal sperm in rats. Toxicol Ind Health. 2015;31(9):811–822. doi: 10.1177/0748233713475501. [DOI] [PubMed] [Google Scholar]
- 55.Sakr H.F., Abbas A.M., Elsamanoudy A.Z., Ghoneim F.M. Effect of fluoxetine and resveratrol on testicular functions and oxidative stress in a rat model of chronic mild stress-induced depression. J Physiol Pharmacol. 2015;66(4):515–527. [PubMed] [Google Scholar]
- 56.Martin L.J., Touaibia M. Improvement of testicular steroidogenesis using flavonoids and isoflavonoids for prevention of late-onset male hypogonadism. Antioxidants. 2020;9(3):237. doi: 10.3390/antiox9030237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reinholz J., Skopp O., Breitenstein C., Bohr I., Winterhoff H., Knecht S. Compensatory weight gain due to dopaminergic hypofunction: new evidence and own incidental observations. Nutr Metab. 2008;5(35):1–4. doi: 10.1186/1743-7075-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rana K., Lee N.K., Zajac J.D., MacLean H.E. Expression of androgen receptor target genes in skeletal muscle. Asian J Androl. 2014;16(5):675–683. doi: 10.4103/1008-682X.122861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singh V.P., Chaturvedi C.M. Interrelationship between NO and androgenic activity in mice, Mus musculus, following temporal phase relation of serotonergic and dopaminergic neural oscillations. Endocrine. 2014;46(3):624–633. doi: 10.1007/s12020-013-0148-z. [DOI] [PubMed] [Google Scholar]
- 60.Fang X., Huang L.L., Xu J., et al. Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Dev Biol. 2019;454(2):118–127. doi: 10.1016/j.ydbio.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Miki K., Willis W.D., Brown P.R., Goulding E.H., Fulcher K.D., Eddy E.M. Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Dev Biol. 2002;248(2):331–342. doi: 10.1006/dbio.2002.0728. [DOI] [PubMed] [Google Scholar]
- 62.James E.R., Carrell D.T., Aston K.I., Jenkins T.G., Yeste M., Salas-Huetos A. The role of the epididymis and the contribution of epididymosomes to mammalian reproduction. Int J Mol Sci. 2020;21(15):1–17. doi: 10.3390/ijms21155377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arun S., Burawat J., Yannasithinon S., Sukhorum W., Limpongsa A., Iamsaard S. Phyllanthus emblica leaf extract ameliorates testicular damage in rats with chronic stress. J Zhejiang Univ - Sci B. 2018;19(12):948–959. doi: 10.1631/jzus.B1800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arun S., Chaiyamoon A., Lapyuneyong N., Bunsueb S., Wu A.T., Iamsaard S. Chronic stress affects tyrosine phosphorylated protein expression and secretion of male rat epididymis. Andrologia. 2021;53(3) doi: 10.1111/and.13981. [DOI] [PubMed] [Google Scholar]
- 65.Sati L., Cayli S., Delpiano E., Sakkas D., Huszar G. The pattern of tyrosine phosphorylation in human sperm in response to binding to zona pellucida or hyaluronic acid. Reprod Sci. 2014;21(5):573–581. doi: 10.1177/1933719113504467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buffone M.G., Calamera J.C., Verstraeten S.V., Doncel G.F. Capacitation-associated protein tyrosine phosphorylation and membrane fluidity changes are impaired in the spermatozoa of asthenozoospermic patients. Reproduction. 2005;129(6):697–705. doi: 10.1530/rep.1.00584. [DOI] [PubMed] [Google Scholar]
- 67.González-Fernández L., Ortega-Ferrusola C., Macias-Garcia B., Salido G.M., Peña F.J., Tapia J.A. Identification of protein tyrosine phosphatases and dual-specificity phosphatases in mammalian spermatozoa and their role in sperm motility and protein tyrosine phosphorylation. Biol Reprod. 2009;80(6):1239–1252. doi: 10.1095/biolreprod.108.073486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.