Abstract
Purpose: The effect of our comprehensive strategy to reduce pain after minimally invasive mitral valve repair through a right mini-thoracotomy was assessed retrospectively.
Methods: Our comprehensive strategy constituted the following: planned rib cutting to avoid rib injury, sufficient intercostal muscle division to mobilize the cut rib, limiting the number of intercostal ports, avoiding nerve entrapment, continuous extra-pleural intercostal nerve block, and regular use of oral non-steroidal anti-inflammatory drugs. We compared patients treated with this comprehensive strategy (Group S, n = 13) and patients before this strategy was implemented (Group C, n = 13). We used a numerical rating scale (NRS) as a pain scale during the first 3 days postoperatively.
Results: The average NRS was significantly lower in Group S (0.82 ± 0.49) than in Group C (2.40 ± 1.46) (P <0.01). The maximum NRS was also significantly lower in Group S (3.23 ± 1.17) than in Group C (5.69 ± 2.43) (P <0.01). The number of patients using additional single-dose analgesic were significantly less in Group S (23.1%) than in Group C (84.6%) (P <0.01).
Conclusion: Our comprehensive pain control strategy effectively reduced postoperative pain in minimally invasive mitral valve repair.
Keywords: minimally invasive cardiac surgery, pain control, mitral valve repair
Introduction
Minimally invasive cardiac surgery for mitral valve repair through a right mini-thoracotomy (MICS-MVR) has several advantages, namely, a decreased incidence of wound infection and cosmetic benefits because of smaller incisions.1) However, despite the smaller incisions, patients can develop severe postoperative pain due to rib injury following rib retraction, pleural injury, and intercostal nerve injury.2) Rib injuries due to rib retraction is one of the major culprits of postoperative pain. Pleural injuries and intercostal nerve injuries may be caused by thoracotomy as well as intercostal port insertion and rib re-approximation. Because several factors are related to postoperative pain after MICS, a comprehensive strategy is necessary. For these reasons, we adopted the following comprehensive strategy for pain control in MICS: 1) planned rib cutting to avoid rib injury and sprain, 2) sufficient intercostal muscle division for easy mobilization of the cut rib, 3) limiting the number of intercostal ports, 4) avoiding nerve entrapment by the rib re-approximation, 5) placing a catheter for continuous extra-pleural intercostal nerve block, and 6) regular use of oral non-steroidal anti-inflammatory drugs after each meal. In this study, we retrospectively assessed the effect of our comprehensive strategy to reduce the pain after MICS.
Materials and Methods
Patients and assessment
We retrospectively reviewed 26 patients who underwent MICS-MVR in our institute until August 2020 and whose pain scales were recorded. Patients requiring postoperative prolonged ventilation were not included. We adopted this comprehensive strategy since May 2019. We compared patients treated with the comprehensive strategy (Group S, n = 13) and patients treated before this strategy was implemented (Group C, n = 13). This study was approved by the institutional ethics committee of Kansai Medical University. The patients’ characteristics in the two groups are summarized in Table 1. Continuous extra-pleural intercostal nerve block for 2 days was used in both groups. Regular non-steroidal anti-inflammatory drugs after each meal was used in 11 patients of Group C. A numerical rating scale (NRS) during the first 3 days postoperatively was used to assess postoperative pain. The NRS is an 11-point scale from number 0 to 10 (0 is no pain, and 10 is the worst imaginable pain), and the NRS was recorded routinely in each patient’s medical chart.
Table 1. Patients’ data.
| Group C (n = 13) | Group S (n = 13) | P-value | |
|---|---|---|---|
| Age* | 47.2 ± 10.9 | 57.8 ± 13.2 | 0.04 |
| Gender (male:female) | 8:5 | 9:4 | >0.99 |
| Body surface area (m2) | 1.70 ± 0.22 | 1.64 ± 0.19 | 0.59 |
| Body mass index | 21.8 ± 4.1 | 20.3 ± 3.3 | 0.40 |
| Hypertension | 4 (30.8) | 8 (61.5) | 0.24 |
| Dyslipidemia | 2 (15.4) | 5 (38.5) | 0.38 |
| Diabetes mellitus | 1 (7.7) | 0 | >0.99 |
| Smoking history | 8 (61.5) | 4 (30.8) | 0.24 |
| Chronic atrial fibrillation | 0 | 1 (7.7) | >0.99 |
| NYHA III, IV | 0 | 2 (15.4) | 0.48 |
| Left ventricular ejection fraction (%) | 69.2 ± 4.9 | 70.1 ± 6.9 | 0.68 |
| Tricuspid valve annuloplasty (+) | 1 (7.7) | 3 (23.1) | 0.59 |
| Pulmonary vein isolation (+) | 0 | 2 (15.4) | 0.48 |
| Atrial septal defect closure (+) | 0 | 1 (7.7) | >0.99 |
| Operation time (minutes) | 386.3 ± 59.6 | 380.4 ± 64.4 | 0.82 |
| Average NRS* | 2.40 ± 1.46 | 0.82 ± 0.49 | <0.01 |
| Maximum NRS* | 5.69 ± 2.43 | 3.23 ± 1.17 | <0.01 |
| Additional analgesics (+)* | 11 (84.6) | 3 (23.1) | <0.01 |
| Additional analgesics frequency (/patient)* | 2.38 ± 1.85 | 0.23 ± 0.44 | <0.01 |
Values are direct number or mean ± standard deviation, and values in parentheses are percentages
*Significant difference between Groups C and S
NYHA: New York Heart Association functional class; NRS: numerical rating scale
Surgical procedures
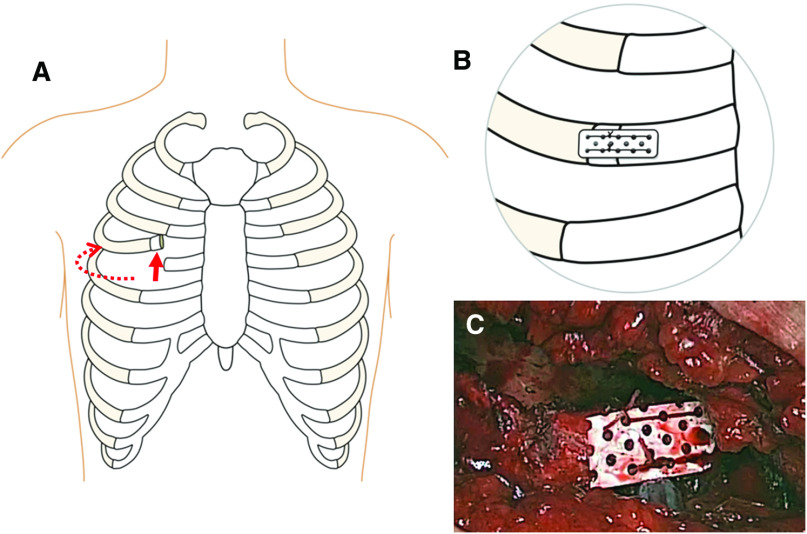
A right mini-thoracotomy was performed through an approximately 6-cm skin incision (right side from the nipple line), mainly at the fourth intercostal space. The fourth rib was intentionally cut as close to the sternum as possible (at the rib cartilage), and the intercostal muscles were divided much more widely than the skin incision toward the vertebra for easy mobilization of the cut rib (Fig. 1A). In case of bleeding from the intercostal artery, we ligated the artery. We used both a soft tissue retractor and an additional rib retractor. Because the rib was cut and the intercostal muscle was sufficiently divided, the risk of rib fractures was reduced. An arterial cannula was placed in the right femoral artery, and a venous cannula was placed in the right atrium via the right femoral vein. An additional venous cannula was inserted into the superior vena cava through the mini-thoracotomy. The ascending aorta was cross-clamped with a Cygnet clamp (Vitalitec, Plymouth, MA, USA), which was also placed through the mini-thoracotomy incision. We did not use a transthoracic clamp, to avoid the thoracic wall stab wound made by inserting the clamp. The endoscopic video camera port was placed in the fourth intercostal space on the right midaxillary line. Because we performed MICS-MVR through this incision and this one port, other intercostal space was not injured.
Fig. 1. (A) Schema of the planned rib cutting. The intercostal muscle was sufficiently divided (dotted line), and the intercostal space was easily spread wide. (B and C) The cut rib was reconstructed using a mesh plate.
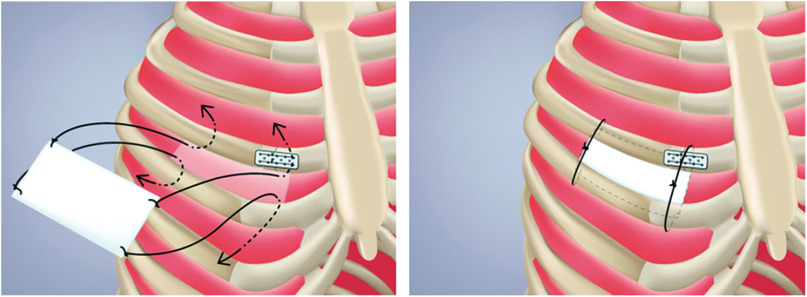
After cessation of cardiopulmonary bypass and establishment of hemostasis, two 19-Fr chest tubes were inserted through the fourth intercostal space and placed in the pericardial and the pleural cavities. The pericardium was closed with interrupted sutures. A catheter for a continuous extra-pleural intercostal nerve block to relieve post-thoracotomy pain was placed under the parietal pleura at the fourth intercostal space. The cut rib was reconstructed using Super-Fixsorb MX40 Mesh (Takiron Co., Ltd., Osaka, Japan) (Fig. 1B and 1C). No sutures were used for re-approximation of the intercostal space. An expanded polytetrafluoroethylene membrane patch was placed at the fourth intercostal space to prevent subsequent lung herniation (Fig. 2).
Fig. 2. Placing an expanded polytetrafluoroethylene membrane patch at the fourth intercostal space to prevent subsequent lung herniation. A braided silk suture was tied on each of the four corners of an expanded polytetrafluoroethylene membrane patch. These sutures were passed through the 3rd and 5th intercostal muscles from inside to outside the pleural cavity and were then tied loosely to avoid intercostal nerve entrapment.
Statistics
Continuous variables were expressed as means and standard deviations, and categorical variables were expressed as frequencies and percentages, where appropriate. Differences between the groups were assessed by the Wilcoxon rank-sum test for continuous variables or by the chi-square test for categorical variables using the JMP 11 software package (SAS Institute Inc., Cary, NC, USA). P-values <0.05 were considered statistically significant.
Results
There were no significant differences in patients’ characteristics and operative data except for the mean age (C:S = 47.2 ± 10.9:57.8 ± 13.2, P = 0.04) (Table 1). Operation through right mini-thoracotomy was completed in all patients, and there were no cases of conversion to the mitral valve replacement. Re-exploration for bleeding and cardiac tamponade were not observed in either group, and there were no major complications (cerebral infarction, infection, major organs failure) and no hospital deaths in both groups.
The average and maximum NRS scores during the first 3 days and the frequency of an additional single-dose analgesic use during the first 7 days were compared between the groups; the results are summarized in Table 1. The average NRS score during the first 3 days was significantly lower in Group S (0.82 ± 0.49) than in Group C (2.40 ± 1.46) (P <0.01). The maximum NRS score was also significantly lower in Group S (3.23 ± 1.17) than in Group C (5.69 ± 2.43) (P <0.01). In 22 patients (Group C: 12, Group S: 10), NRS scores were recorded until postoperative day 6. The average NRS score during the first 6 days was also significantly lower in Group S (0.70 ± 0.19) than in Group C (1.86 ± 1.10) (P = 0.01). Although regular use of oral non-steroidal anti-inflammatory drugs after each meal was used, additional single-dose analgesics were necessary in some patients. The number of patients using additional single-dose analgesics were significantly lower in Group S (23.1%) than in Group C (84.6%) (P <0.01). In Group S, an additional single dose of analgesics was used only 0.23 ± 0.44 times per patient during the first 7 days in contrast to 2.38 ± 1.85 per patients in Group C (P <0.01).
Discussion
Patients can experience severe postoperative pain after MICS-MVR through a right mini-thoracotomy. Several factors are related to postoperative pain after thoracotomy. As mentioned earlier, rib, pleura, and intercostal nerve injuries may be caused by thoracotomy as well as port insertion and rib re-approximation in MICS. Because postoperative pain is multifactorial, we adopted the comprehensive strategy to overcome postoperative pain in MICS-MVR. From our results, we believe that this strategy effectively reduced postoperative pain in MICS-MVR through a right mini-thoracotomy. A previous study revealed the efficacy of postoperative maintenance analgesia using non-steroidal anti-inflammatory drug and continuous extrapleural intercostal nerve block regarding lung function after thoracotomy.3) According to this report, we also adopted a continuous extrapleural intercostal nerve block for 2 days after surgery and regular use of oral non-steroidal anti-inflammatory drugs after each meal during the hospital stay. Both of these types of analgesia were used in almost all patients of both groups. Therefore, it can be said that pain control was improved owing to the other surgical procedures in the comprehensive strategy except for the both types of analgesia.
Planned rib cutting was implemented to avoid rib fractures and sprains. As mentioned earlier, although rib fracture can be a crucial cause, several factors are related to postoperative pain. Damage to the intercostal nerve is also an important factor.2) Rogers et al.4) demonstrated that intercostal nerve injury occurs routinely because of rib retraction during thoracotomy. Cerfolio et al.5) reported the technique of harvesting of an intercostal muscle flap and leaving the muscle intact so that it dangles under the chest retractor to prevent trauma to the intercostal nerves by the retractor in pulmonary operations. Because the cut rib easily moves in our procedure, which reduces compression of the intercostal nerves above the incision by the chest retractor, the procedure can decrease severity of postoperative pain. Intercostal nerve damage can also be caused in rib re-approximation. Intracostal suturing in rib re-approximation reportedly frees the intercostal nerve below the incision from entrapment.6) In a comparative study between intercostal and intracostal sutures in rib approximation, intracostal sutures significantly decreased post-thoracotomy pain.6) Thus, avoiding intercostal nerve compression in rib re-approximation is also important.7) According to these findings, we only reconstructed the cut rib using a mesh plate, avoided excessive rib approximation, and did not use both intra- and intercostal sutures. However, because no sutures in rib re-approximation might cause lug herniation, we placed an expanded polytetrafluoroethylene membrane patch at the fourth intercostal space.
Limiting the number of intercostal ports may also reduce acute postoperative pain.2) Because the intercostal space can be easily spread by using intentional rib cutting, instrument manoeuvers are less inhibited; therefore, the number of additional port can be decreased. Furthermore, drainage tubes were also placed through the fourth intercostal space. Thus, planned rib cutting to prevent rib injuries, rib reconstruction to avoid intercostal nerve compression caused by rib re-approximation, and limiting the number of intercostal ports to avoid thoracic wall injury were all related to reducing pain after MICS-MVR.
Different pain scales have been reported, such as the visual analogue scale, the verbal rating scale, or the NRS.8) The NRS and visual analogue scale are commonly used to assess pain intensity for acute pain after surgery. Because the NRS was routinely recorded in our hospital, we used this scale in the present study. In the previous reports, NRS ≥4 was identified as the pain treatment threshold or cut-off point for moderate pain.9,10) In the present study, the mean maximum NRS of the patients with comprehensive pain strategy (3.23 ± 1.17) was below this value, and the frequency of additional single-dose analgesic use was low.
Other alternative ways to reduce postoperative pain in MICS are a robotic-assisted procedure and a conventional MICS through smaller rib spreading with some ports. However, these procedures are technically difficult in some cases and require experiences. Using many ports can be a cause of bleeding, intercostal nerve injury, and postoperative pain. Furthermore, minimally invasive thoracic surgery causes significant postoperative pain in some cases even with robotic-assisted procedure.11,12) As a result, multimodal analgesic techniques for MICS have been reported and evaluated in cardiothoracic anesthesiology area.11,12) Thus, the pain control is still an important issue in MICS. Although all elements of our comprehensive strategy are not mandatory, we believe some elements are useful to reduce postoperative pain even in robotic-assisted or smaller rib-spreading MICS-MVR.
The following were limitations of the present study. In addition to the retrospective design, the number of cases was small. Because the pain score was significantly decreased in Group S, we could not ethically increase the number of Group C patients (without this strategy). As another limitation, because we adopted several procedures and methods simultaneously in our strategy, we could not determine which factor was the most effective. Further study is necessary to clarify this issue.
In conclusion, our comprehensive pain control strategy was effective, with good operability, and reduced postoperative pain in MICS-MVR through a right mini-thoracotomy.
Disclosure Statement
None of the authors have a conflict of interest concerning this study.
References
- 1). Iribarne A, Easterwood R, Chan EYH, et al. The golden age of minimally invasive cardiothoracic surgery: current and future perspectives. Future Cardiol 2011; 7: 333– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Mesbah A, Yeung J, Gao F. Pain after thoracotomy. BJA Educ 2016; 16: 1– 7. [Google Scholar]
- 3). Richardson J, Sabanathan S, Mearns AJ, et al. Efficacy of pre-emptive analgesia and continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. J Cardiovasc Surg (Torino) 1994; 35: 219– 28. [PubMed] [Google Scholar]
- 4). Rogers ML, Henderson L, Mahajan RP, et al. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg 2002; 21: 298– 301. [DOI] [PubMed] [Google Scholar]
- 5). Cerfolio RJ, Bryant AS, Maniscalco LM. A nondivided intercostal muscle flap further reduces pain of thoracotomy: a prospective randomized trial. Ann Thorac Surg 2008; 85: 1901– 6; discussion 1906–7 . [DOI] [PubMed] [Google Scholar]
- 6). Cerfolio RJ, Price TN, Bryant AS, et al. Intracostal sutures decrease the pain of thoracotomy. Ann Thorac Surg 2003; 76: 407– 11; discussion 411–2. [DOI] [PubMed] [Google Scholar]
- 7). Bayram AS, Ozcan M, Kaya FN, et al. Rib approximation without intercostal nerve compression reduces post-thoracotomy pain: a prospective randomized study. Eur J Cardiothorac Surg 2011; 39: 570– 4. [DOI] [PubMed] [Google Scholar]
- 8). Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs 2005; 14: 798– 804. [DOI] [PubMed] [Google Scholar]
- 9). Gerbershagen HJ, Rothaug J, Kalkman CJ, et al. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth 2011; 107: 619– 26. [DOI] [PubMed] [Google Scholar]
- 10). Mei W, Seeling M, Franck M, et al. Independent risk factors for postoperative pain in need of intervention early after awakening from general anaesthesia. Eur J Pain 2010; 14: 149.e1– 7. [DOI] [PubMed] [Google Scholar]
- 11). Finnerty DT, McMahon A, McNamara JR, et al. Comparing erector spinae plane block with serratus anterior plane block for minimally invasive thoracic surgery: a randomised clinical trial. Br J Anaesth 2020; 125: 802– 10. [DOI] [PubMed] [Google Scholar]
- 12). Ha B, Usman AA, Augoustides JG. Minimally invasive cardiac surgery-identifying opportunities for further improvement in the quality of postoperative patient recovery. J Cardiothorac Vasc Anesth 2020; 34: 3231– 3. [DOI] [PMC free article] [PubMed] [Google Scholar]