ABSTRACT.
Substandard and falsified antimalarials contribute to the global malaria burden by increasing the risk of treatment failures, adverse events, unnecessary health expenditures, and avertable deaths. Yet no study has examined this impact in western francophone Africa to date. In Benin, where malaria remains endemic and is the leading cause of mortality among children under five years of age, there is a lack of robust data to combat the issue effectively and inform policy decisions. We adapted the Substandard and Falsified Antimalarial Research Impact (SAFARI) model to assess the health and economic impact of poor-quality antimalarials in this population. The model simulates population characteristics, malaria infection, care-seeking behavior, disease progression, treatment outcomes, and associated costs of malaria. We estimated approximately 1.8 million cases of malaria in Benin among children under five, which cost $193 million (95% CI, $192–$193 million) in treatment costs and productivity losses annually. Substandard and falsified antimalarials were responsible for 11% (nearly 700) of deaths and nearly $20.8 million in annual costs. Moreover, we found that replacing all antimalarials with quality-assured artemisinin combination therapies (ACTs) could result in $29.6 million in annual cost savings and prevent over 1,000 deaths per year. These results highlight the value of improving access to quality-assured ACTs for malaria treatment in Benin. Policy makers and key stakeholders should use these findings to advocate for increased access to quality-assured antimalarials and inform policies and interventions to improve health care access and quality to reduce the burden of malaria.
INTRODUCTION
In a recent meta-analysis, 13.6% of essential medicines were found to be substandard or falsified across low- and middle-income countries.1 The WHO defines substandard medicines as “authorized medical products that fail to meet either their quality standards or specifications or both,” whereas falsified medicines are defined as “medicinal products that deliberately or fraudulently misrepresent their identity, composition, or source.”2 Poor-quality medicines increase the risk of treatment failure, adverse events, and mortality.3,4 Patients may progress and develop sequelae that require more intensive treatment and admission to health care facilities, which takes time away from their ability to work. Substandard and falsified antimalarials come at the expense of increasing economic burden and can stunt national economic development by imposing additional health care costs and productivity losses.3,5
Antimalarials are the most commonly tested essential medicines found to be substandard or falsified in low- and middle-income countries.1–3 Regional rates of poor-quality antimalarials as high as 35% have been reported in Africa.6 Substandard and falsified antimalarials bring to light important health concerns such as drug resistance. Antimalarials with reduced levels of active pharmaceutical ingredients could contribute to the development of resistance.7
Malaria is a preventable parasitic disease that is commonly treated with artemisinin combination therapies (ACTs). These first-line antimalarials have been integral to the success of global malaria control.8 In addition to ACTs, the implementation of large-scale vector control campaigns and increasing medical access has reduced malaria cases around the world.9 However, the substantial progress made in curbing global malaria burden has recently stagnated, or started to reverse in many countries, posing a significant public health threat. Nearly 80% of the global malaria burden remains in sub-Saharan Africa.5,9 Moreover, children under five years of age are the most vulnerable population affected by this disease. Children account for 61% of all malaria deaths worldwide and are at the greatest risk to suffer from consequences of substandard and falsified antimalarials.5,9
Recent studies have used model simulations to evaluate the health and economic impact of substandard and falsified antimalarials on pediatric malaria.5,10–12 However, no study has examined this impact in western francophone Africa to date. This study focuses on Benin, where malaria is the leading cause of mortality among under five years of age.13 The private sector accounts for 70% of antimalarial distribution in Benin, and the country relies heavily on imported and sparsely regulated pharmaceuticals from neighboring countries such as Nigeria and Togo.13 As a result, Benin may be at risk of being impacted by substandard and falsified antimalarials. We applied our agent-based model in Benin to assess the impact of substandard and falsified antimalarials among pediatric patients to provide governments and policy makers with robust data to inform policy decisions and combat the issue effectively.
METHODS
Substandard and Falsified Antimalarial Research Impact (SAFARI) model.
We developed SAFARI, an agent-based model, to estimate the health and economic impact of poor-quality antimalarials in children under five years old.5,10 Agent-based models are good at capturing the heterogeneity of complex adaptive systems, such as patient care-seeking behavior and medicine supply chain processes that evolve over time.14 The SAFARI model has been used to estimate the burden of substandard and falsified antimalarials in the Democratic Republic of the Congo, Nigeria, Uganda, and Zambia.5,10–12 In our study, we adapted the SAFARI model to evaluate the burden of poor-quality antimalarials and simulate potential interventions in Benin. The model was developed in Python (version 3) to simulate population characteristics, malaria infection, care-seeking behavior, disease progression, treatment outcomes, and associated costs of malaria.11 We simulated 25,000 agents over a 1-year time horizon, broken into 5-day increments. Further details of the methods used by the SAFARI model are described in previous publications.5,10–12
Inputs.
Model inputs for Benin were gathered through a PubMed literature search alongside a Google search for gray literature, including sources such as ACTwatch, the Benin Demographic and Health Survey, World Malaria Report, and the Worldwide Antimalarial Resistance Network database.9,15–19 Data inputs were chosen based on their quality, relevance, and generalizability, for the most recent year. Costs in West African Communauté Financière d’Afrique francs were converted to 2018 U.S. dollars using local inflation rates and exchange rates.20 All model inputs, including demographic, epidemiological, care-seeking, and cost data, are presented in Table 1.
Table 1.
Data inputs for the adapted Substandard and Falsified Antimalarial Research Impact (SAFARI) model
| Model inputs | Estimate | SD (range) | Source |
|---|---|---|---|
| Demographic and epidemiological data | |||
| Benin population < 5 y old | 1,909,000 | – | – |
| Malaria cases of children < 5 y old | 1,774,127 | – | WHO,9 Centers for Disease Control and Prevention22 |
| Case fatality rate for severe malaria in the community | 0.150 | 0.03* | Camponovo et al.33 |
| Case fatality rate, inpatient care | 0.097 | 0.02* | Camponovo et al.33 |
| Neurological sequelae rate (care sought) | 0.031 | (0.028–0.035) | Dondorp et al.36 |
| Neurological sequelae rate (care not sought) | 0.194 | 0.028 | Dondorp et al.36 |
| Probability of testing | Institut National de la Statistique et de l’Analyse Économique, ICF19 | ||
| Public facilities | 0.519 | – | |
| Private facilities | 0.296 | – | |
| Pharmacies | 0.085 | – | |
| Drug stores | 0.073 | – | |
| Cure rates | Abdulla et al.,17 Faucher et al.18 | ||
| ACT | 0.973 | 0.020 | |
| CQ | 0.444 | 0.112 | |
| Quinine | 0.710 | 0.087 | |
| Other treatment | 0.819 | 0.100 | |
| No treatment | 0.000 | – | |
| Caregiver length of care, d | 5 | – | Assumption |
| Length of illness, d | 5 | – | Assumption |
| Medication taken by facility | |||
| Public facilities | Institut National de la Statistique et de l’Analyse Économique, ICF19 | ||
| ACT | 52.3% | – | |
| CQ | 15.8% | – | |
| Quinine | 20.4% | – | |
| Other treatment | 11.6% | – | |
| Private facilities | |||
| ACT | 36.0% | – | |
| CQ | 2.9% | – | |
| Quinine | 30.9% | – | |
| Other treatment | 30.3% | – | |
| Pharmacies | |||
| ACT | 26.3% | – | |
| CQ | 36.9% | – | |
| Quinine | 24.0% | – | |
| Other treatment | 12.8% | – | |
| Drug stores | |||
| ACT | 29.8% | – | |
| CQ | 24.0% | – | |
| Quinine | 35.2% | – | |
| Other treatment | 11.0% | – | |
| Self-treatment | |||
| ACT | 33.8% | – | |
| CQ | 33.2% | – | |
| Quinine | 18.6% | – | |
| Other treatment | 14.2% | – | |
| Care-seeking behavior | |||
| Public facilities | 20.7% | – | Institut National de la Statistique et de l’Analyse Économique, ICF 19 |
| Private facilities | 8.4% | – | |
| Pharmacies | 9.2% | – | |
| Drug stores | 11.1% | – | |
| Self-treatment | 22.8% | – | |
| No treatment | 27.8% | – | |
| SF and treatment adherence proportions | |||
| ACT SF proportions | Baba-Moussa et al.24 | ||
| Not SF: API > 85% | 67.5% | – | |
| Category 1: API = 75–85% | 26.9% | – | |
| Category 2: API = 50–75% | 26.9% | – | |
| Category 3: API < 50% | 46.2% | – | |
| Treatment adherence proportions | Proportions, Bruxvoort et al.;34 | ||
| Good: completes 5–6 doses | 74.7% | 1† | coefficients, assumption |
| Okay: completes 4 doses | 10.9% | 0.75† | |
| Bad: completes 3 doses | 7.3% | 0.5† | |
| Very bad: completes 2 doses | 3.1% | 0.25† | |
| Does not adhere: completes 0–1 dose | 3.9% | 0† | |
| Patient costs for care-seeking | |||
| Public, private (urban) | $0.73 | 0.18‡ | Rashed et al.37 |
| Public, private (rural) | $0.57 | 0.14‡ | |
| Pharmacy, drug store | $0.37 | 0.09‡ | |
| Pharmacy, drug store (rural) | $0.28 | 0.07‡ | |
| Miscellaneous cost when receiving treatment (e.g., special foods in hospital) | $1.00 | 0.25‡ | Hansen et al.38 |
| Cost of supplemental medicines | $1.00 | 0.25‡ | Batwala et al.39 |
| Average testing cost, private | $1.79 | 0.20 | ACTwatch Group15 |
| Average testing cost, public | $0.00 | – | |
| Additional cost for care-seeking, private | $0.79 | 0.20 | Jimoh et al.,40 Salawu et al.41 |
| Cost of hospitalization | $1.17 | – | Rashed et al.37 |
| Productivity loss per sick day | $2.55 | – | World Bank21 |
| Productivity losses from death | $23,281.21 | – | |
| Neurological sequelae disability productivity losses | $9,731.74 | – | World Bank,21 Dundorp et al.36 |
| Neurological sequelae disability productivity losses (addition for severe cases) | $3,015.91 | – | |
| Patient drug costs | |||
| Public facilities | ACTwatch Group15 | ||
| Average cost of ACTs | $0.00 | – | |
| Average cost of quinine | $0.00 | – | |
| Private facilities | |||
| Average cost of ACTs | $1.82 | ($1.36–$3.41) | |
| Average cost of CQ | $0.42 | ($0.41–$1.06) | |
| Average cost of quinine | $4.30 | ($3.58–$5.37) | |
| Pharmacies | |||
| Average cost of ACTs | $4.97 | ($3.97–$6.56) | |
| Average cost of CQ, “Assumption same as private” | $0.42 | ($0.41–$1.06) | |
| Average cost of quinine | $19.31 | ($5.89–$26.06) | |
| Drug stores | |||
| Average cost of ACTs | $1.36 | ($1.02–$1.70) | |
| Average cost of CQ | $0.41 | ($0.41–$0.42) | |
| Average cost of quinine | $3.58 | ($2.86–$4.30) | |
| Self-treatment | Assumption | ||
| Average cost of ACTs | $0.00 | – | |
| Average cost of CQ | $0.00 | – | |
| Average cost of Quinine | $0.00 | – | |
| Public facility costs | |||
| Cost per test | $1.27 | $0.29 | Uzochukwu et al.,42 Onwujekwe et al.43 |
| Cost of supplemental drugs (antibiotics, analgesics, etc.) | $1.90 | $0.36 | Management Sciences for Health,44 WHO35 |
| Cost per ACT | $1.28 | $0.05 | |
| Cost per CQ | $0.05 | $0.01 | |
| Cost per quinine | $0.05 | $0.03 | |
| Cost per case (without testing or drugs) | $2.96 | $2.19 | WHO45 |
| Cost per pediatric malaria hospitalization | $69.30 | $31.70 | |
| Stockout probabilities of any ACT | |||
| Public facilities | 5.0% | – | ACTwatch Group15 |
| Private facilities | 52.5% | – | |
| Pharmacies | 0.0% | – | |
| Drug stores | 61.5% | – | |
| Self-treatment | 0.0% | – | Assumption |
ACT = artemisinin-based combination therapy; API = active pharmaceutical ingredient; Coeff = coefficient; CQ = chloroquine; SD = standard deviation; SF = substandard or falsified; WHO = World Health Organization.
Assumed SD of 20%.
Coefficient in model.
Assumed SD of 25%.
Model simulation.
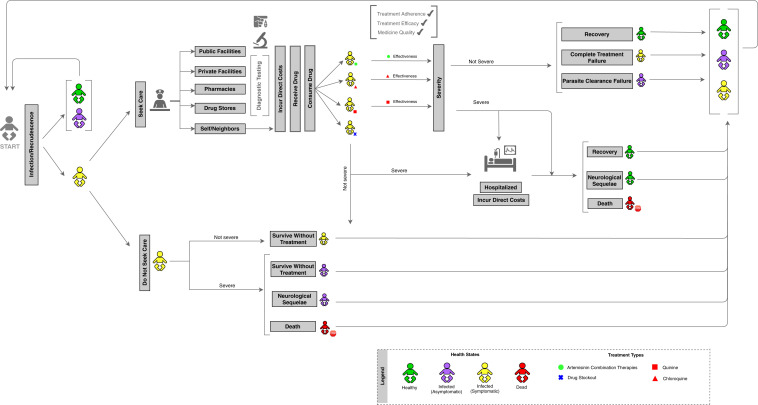
The flow diagram found in Figure 1 depicts the movement of agents through the adapted SAFARI model.5 Agents represent hypothetical children in Benin. Each agent was assigned five demographic characteristics—age, geographic region, socio-economic status, residence type (urban/rural), and maternal education—based on distributions from the latest Benin Demographic and Health Survey.19 Agents moved through the disease simulation according to probabilities calculated from individual characteristics among children under five in Benin.9,22 When agents became sick and symptomatic, they were simulated to seek care at one of five locations: public facilities, private facilities, pharmacies, drug stores, or at home (self-treatment). Some agents did not seek malaria treatment within a 5-day period, which meant that they faced a greater chance of their malaria becoming severe.23 Agents who sought care received antimalarial treatment based on reported availability of four treatment options at each facility: ACTs, chloroquine, quinine, or other antimalarial treatments.19 Stockout probabilities, or the probability of a facility running out of antimalarials, were obtained from national stock data.15
Figure 1.
Substandard and Falsified Antimalarial Research Impact (SAFARI) model flow diagram. This figure appears in color at www.ajtmh.org.
Some agents in the model received substandard and falsified antimalarials, based on a study evaluating antimalarial quality in Benin.24 In our baseline simulation, the prevalence of substandard and falsified ACTs was set to 32.5%.23 This prevalence was based on the proportion of samples that were found to be under-dosed or contained no active pharmaceutical ingredient.23 To account for adverse outcomes caused by substandard and falsified antimalarials, agents who received poor-quality antimalarials faced a lower treatment efficacy compared to those who received quality medicines. Here, a reduced treatment efficacy is assumed as the therapeutic implication of consuming an antimalarial with substandard amounts of the active pharmaceutical ingredient.9,15,25–32 As a result of reduced efficacy, these agents were at an increased risk for disease progression to severe malaria.9 Based on medication efficacy, adherence, and quality, agents either recovered from malaria in a 5-day period or remained sick into the following week, returning to the start of the model.33,34 Some cases of malaria became severe, in which case agents were hospitalized and faced the probability of death based on case fatality rates, or suffered from malaria-induced neurological complications.17,18,35,36
Outputs.
Primary model outputs are estimates of the health impact, direct costs, and productivity losses attributable to substandard and falsified antimalarials taken by children under five in Benin. The health impact is presented as the number of hospitalizations, neurological sequelae, and deaths. Economic outputs assessed direct costs to the patient for medications, testing, and treatment, as well as costs incurred by public facilities for providing treatments at no cost.15,37–45 Out-of-pocket costs included medical consultation costs from seeking treatment at a health facility, and transportation costs incurred by the caretaker and patient.37 Productivity losses included short-term lost caretaker time caring for sick children, as well as long-term productivity losses from malaria-induced disability or premature death.
Sensitivity and scenario analyses.
To account for natural variations in model inputs, we conducted sensitivity analyses in which key data inputs were ranged probabilistically across 10,000 model runs. Epidemiological data were varied based on beta distributions, whereas cost data were ranged using gamma distributions.
We simulated five scenarios by altering model inputs or assumptions. The baseline model was run first to estimate the annual health and economic burden of malaria among children in Benin. This baseline result was compared with two main scenarios: 1) if all antimalarials were of adequate quality (i.e., absence of substandard and falsified antimalarials); and 2) if all antimalarials were quality-assured ACTs (i.e., replaced chloroquine, quinine, and other antimalarial treatments with quality-assured ACTs). These scenarios were used to assess the impact of improving the quality of antimalarials, and the additional benefit of increasing access to ACTs along with improved quality. Three additional scenarios were simulated to represent various supply chain, antimalarial treatment policies, and caregiver education interventions. These scenarios included: 1) encouraging 20% more patients to seek care for malaria treatment (i.e., increase in care-seeking habits); 2) having no antimalarial medication stockouts across all sectors; and 3) replacing all antimalarials with ACTs (i.e., maintaining the current mix of quality-assured and nonquality-assured ACTs).
RESULTS
The SAFARI model simulated nearly 1.8 million cases of malaria among children under five in Benin. Agents who progressed to severe malaria resulted in around 16,000 hospitalizations and 6,500 deaths annually. The overall economic impact of malaria was estimated to be $193 million (95% CI, $191.6–$193.8 million), with $184 million (95% CI, $182.7–$184.9 million) in total productivity losses, which included $27.4 million in short-term productivity losses and $156 million in lifetime productivity losses. Total direct costs of seeking malaria treatment amounted to $8.9 million, or 4.6% of the total economic impact (95% CI, $8.9–$9.0 million), including $3.9 million in medication costs, $3.7 million in costs incurred by public facilities, and $1.2 million in nonmedication out-of-pocket costs. The health and economic burden of malaria in Benin is summarized in Table 2.
Table 2.
Potential impact of simulated scenarios compared with the current burden of malaria in Benin
| Type of impact | Burden of malaria | No SF ACTs | All ACTs + no SF ACTs | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 95% CI | Potential impact | % Diff | P value | Potential impact | % Diff | P value | |
| Health | ||||||||
| Average no. of malaria cases | 1,774,237 | 1,774,015–1,774,459 | – | – | – | – | – | – |
| Average no. of hospitalizations | 15,984 | 15,918–16,049 | 2,242 | –14% | < 0.001* | 3,033 | –19% | < 0.001* |
| Average no. of deaths | 6,526 | 6,479–6,572 | 693 | –11% | < 0.001* | 1,038 | –16% | < 0.001* |
| Economic | ||||||||
| Medication costs | $3,987,083 | $3,981,462–$3,992,704 | 465,156 | –12% | < 0.001* | 809,508 | –20% | < 0.001* |
| Out-of-pocket costs | $1,192,992 | $1,184,693–$1,201,291 | 138,136 | –12% | < 0.001* | 144,115 | –12% | < 0.001* |
| Facility costs | $3,744,985 | $3,718,230–$3,771,740 | 429,793 | –11% | < 0.001* | 467,716 | –12% | < 0.001* |
| Total direct costs | $8,925,060 | $8,896,570–$8,953,550 | 1,033,085 | –12% | < 0.001* | 1,421,339 | –16% | < 0.001* |
| Short-term productivity losses | $27,378,493 | $27,374,635–$27,382,351 | 3,069,029 | –11% | < 0.001* | 3,211,167 | –12% | < 0.001* |
| Lifetime productivity losses | $156,399,212 | $155,306,467–$157,491,958 | 16,718,318 | –11% | < 0.001* | 25,004,364 | –16% | < 0.001* |
| Total productivity losses | $183,777,705 | $182,685,025–$184,870,385 | 19,787,347 | –11% | < 0.001* | 28,215,531 | –15% | < 0.001* |
| Total economic impact | $192,702,765 | $191,608,898–$193,796,632 | 20,820,432 | –11% | < 0.001* | 29,636,870 | –15% | < 0.001* |
ACTs = artemisinin-based combination therapies; CI = confidence interval; % Diff = percentage difference of the scenario compared to baseline; SF = substandard or falsified.
P < 0.05.
Substandard and falsified antimalarials were responsible for approximately 2,200 (14%) hospitalizations and 700 (11%) deaths (P < 0.001). The economic impact of poor-quality antimalarials taken by children in Benin was estimated at $20.8 million annually, which included $19.8 million in total productivity losses. Annual direct costs resulting from poor-quality antimalarials were estimated at $1,033,000, which included $465,000 in medication costs, $430,000 in costs incurred by public facilities, and $138,000 in nonmedication out-of-pocket costs. Short-term productivity losses were estimated at $3.1 million, and $16.7 million were attributed to lifetime productivity losses.
It has been reported that only 52.3% of antimalarials that were stocked in public facilities in Benin were ACTs.19 Availability of ACTs was even lower in other health facilities, where ACTs consisted of 36.0% of antimalarials in private facilities, 26.3% at pharmacies, 29.8% at drug stores, and 33.8% at home.19 We simulated replacing all antimalarials with quality-assured ACTs, in which children would have increased access to ACTs and benefit from improved medication quality, This scenario reduced around 3,000 (19%) hospitalizations and 1,000 (16%) deaths annually compared with the status quo (P < 0.001). This resulted in annual cost savings of $29.6 million, including $28.2 million in total productivity savings. Direct costs savings amounted to $1,421,000 annually, including $810,000 in medication costs, $468,000 in public facility costs, and $144,000 in nonmedication out-of-pocket costs.
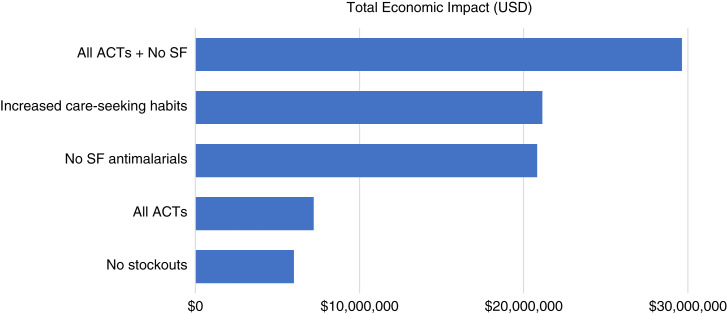
Figure 2 presents the results of five potential scenarios compared with the current malaria landscape in Benin. Results of the model simulation demonstrated that increased care-seeking habits by 20% across all health facilities had the second greatest health and economic impact after the scenario of increasing the availability of quality-assured ACTs. Approximately 27.8% of children under five with malaria have been reported to not seek treatment.19 Increasing care-seeking habits could prevent around 900 fewer deaths among children under five annually and result in $21.1 million in total cost savings. Another scenario we investigated was replacing the current stock of antimalarials with ACTs. Ensuring access to first-line antimalarials would prevent around 280 deaths annually and would result in $7.2 million in total annual cost savings. The impact of having access to first-line antimalarials was compounded when the ACTs were also quality assured.
Figure 2.
Estimated cost savings of simulated scenarios compared with baseline malaria burden in Benin. ACTs = artemisinin-based combination therapies; SF = substandard or falsified; USD = U.S. dollars. This figure appears in color at www.ajtmh.org.
Although the probability of encountering a stockout was high for those who sought care at a private facility (52.5%) or a drug store (61.5%), only 8.4% of pediatric malaria patients sought care at private facilities, and 11.1% at drug stores.19 Therefore, having no stockouts had a lower impact compared with other scenarios, by preventing 180 fewer deaths annually, and amounted to $6 million in total annual cost savings.
DISCUSSION
This is a novel study evaluating the health and economic impact of substandard and falsified antimalarials on the pediatric population in Benin. We simulated that improving the quality of antimalarials can reduce 11% of malaria deaths and save a total of $20.8 million annually, including nearly $430,000 in facility costs and $465,000 in medication costs. The results of this study are important in demonstrating the potential reduction of unnecessary costs to the health care system and the population by ensuring that antimalarials are safe, efficacious, and trustworthy.
We demonstrated that access to ACTs—especially quality-assured ACTs—contributes significantly to reducing the malaria burden. Replacing all antimalarials with ACTs can result in $7.2 million in annual cost savings, and an additional $22.4 million in cost savings could be achieved if all ACTs were quality-assured. This was primarily a result of the lack of availability of ACTs, which constituted 26% to 52% of antimalarials at various health facilities.19 Efforts in providing quality-assured ACTs could benefit Benin significantly, especially in the private sector, where the probability of experiencing a stockout is as high as 61.5% at these health care facilities.15
Among the potential scenarios we simulated, increasing care seeking behavior by 20% demonstrated the second largest health and economic impact. If more children are able to seek care for malaria, the likelihood of obtaining effective treatment increases, which could prevent at least 900 deaths and save $21.1 million a year. In Benin, 27.8% of children with malaria reported not seeking medical treatment.19 This could prolong treatment duration, increase the risk of disease progression, and lead to preventable deaths and disabilities. Although we acknowledge that conducting interventions to increase care seeking by 20% may be a challenge, our scenarios demonstrate the comparative impact of improving different aspects of access to malaria care for children in Benin.
It is important to acknowledge how far Benin has come in reducing the burden of malaria. In 1982, Benin created the National Malaria Control Program (NMCP), which has been responsible for monitoring and evaluating all activities to control the disease.46 The NMCP aimed to reduce the burden of malaria in Benin with help provided by key stakeholders such as the U.S. Agency for International Development (USAID) and the U.S. President’s Malaria Initiative (PMI).46 The NMCP has provided free access to ACTs and testing for children under five years of age at the community level and in health facilities.47 In addition, it has contributed to the destruction of 118 tons of counterfeit medicines (including ACTs) in 2019 alone.47 Despite these efforts, malaria remains endemic in Benin, and only 28% of children under five in 2016 received first-line treatment for uncomplicated malaria according to ACTwatch.13
Although ACTs are largely available through public facilities, more than 70% of antimalarials are distributed through the private sector in Benin.13 In addition, the private sector is rarely present in NMCP activities, and the country’s surveillance system does not cover drug stores.46 Efforts in providing access to ACTs could be directed toward the private sector through regulation and increased surveillance of private facilities, including drug stores.22 In 2019, the NMCP, in collaboration with PMI, partnered with the Private Sector Health Platform to manage and monitor the performance of the antimalarial supply chain through the presence of young professional logisticians in private-sector facilities.47 Continued engagement with the private sector is critical to reduce substandard and falsified medicines, and to increase access to first-line antimalarial medications.
Currently, there is minimal publicly available information on the drug regulatory landscape in Benin. Yet the systems in place appear inadequate to ensure that all medicines sold within the country are quality-assured, with reports of importation of illegal and unregistered medicines.48 The Systems for Improved Access to Pharmaceuticals and Services Program was funded by USAID “to improve the availability of quality pharmaceutical products and effective pharmaceutical services by strengthening pharmaceutical systems.”48 In 2016, the program conducted an assessment of the medicine registration system in Benin and provided recommendations that would regulate medicines more effectively, including the implementation of an overall quality management system as well as the development and implementation of guidelines and standard operating procedures to strengthen the registration of pharmaceutical products.48 The results from our study demonstrate the need for the Benin National Medicines Regulatory Authority and other key stakeholders to put such pharmaceutical regulatory policy changes into action.
Our results are in line with the existing literature. Model estimates of malaria cases and deaths are comparable to current epidemiological data projecting 1.7 million child malaria cases and approximately 6,400 deaths annually in Benin.22,49 Our Benin SAFARI model results were similar to the Democratic Republic of the Congo SAFARI model, in which the increasing care-seeking scenario had a large impact on reducing the malaria burden as a result of current low levels of care-seeking rates.10 Although quality of antimalarials and access to ACTs were ranked second and third after stockouts in the Nigeria SAFARI model,11 the combination of improving antimalarial quality and access to ACTs resulted in the most impactful scenario in Benin. This can be attributed to the overall lower administration of ACTs in Benin compared with Nigeria, and higher estimated prevalence of substandard and falsified antimalarials in Benin.11,15 Yet, we found the Benin and Nigeria models to be comparable in scale; in both models, we found there was a 11% reduction in total economic impact without substandard and falsified antimalarials.
There are several limitations to note. When compiling data inputs for the model, there was a noticeable lack of data, especially on the quality and efficacy of antimalarials in Benin, as well as country-specific cost data. This made it difficult to capture the true heterogeneity within the country. Although we conducted an extensive literature search to use the best and most recent data available, the model included some estimates from neighboring countries and previous SAFARI models. We have conducted rigorous probabilistic sensitivity analyses to capture the uncertainty around our main estimates. We believe the gaps in data demonstrate an opportunity for further research to generate more accurate information to combat the malaria burden in Benin. Last, this study focused on the benefits of various scenarios rather than the costs of interventions to implement these scenarios. Future studies to estimate the costs alongside benefits could be helpful to decision makers in estimating the returns on their investments. Despite these limitations, we believe our analysis is important in demonstrating the impact and raising awareness of substandard and falsified antimalarials in Benin.
CONCLUSION
To our knowledge, this study is the first to assess the impact of substandard and falsified antimalarials in the pediatric population of Benin. Access to quality-assured, first-line ACTs is still a predominate challenge in Benin. Results of this study can be used to inform policy makers and key stakeholders of the impact of poor-quality antimalarials and advocate for increased access to quality-assured ACTs. For example, this study could be used to advocate for better medication access to achieve Benin’s national strategic plan to reduce the number of annual malaria cases and the national mortality rate, and strengthen the management and coordination of the malaria program.22 Implementation of policies and programs that aim to improve medicinal quality and access to ACTs can reduce the burden of malaria and may mitigate the development of antimalarial resistance in Benin and beyond. These efforts may set the precedent for other African nations where malaria remains endemic.
ACKNOWLEDGMENTS
We thank the Research and Scholarship in Pharmacy (RASP) program at the UNC Eshelman School of Pharmacy at the University of North Carolina at Chapel Hill for facilitating this research. We also thank Antonio Bush and Kathryn Morbitzer for their valuable guidance.
REFERENCES
- 1. Ozawa S et al. 2018. Prevalence and estimated economic burden of substandard and falsified medicines in low- and middle-income countries: a systematic review and meta-analysis. JAMA Netw Open 1: e181662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, 2017. Seventieth World Health Assembly Update, 29 May 2017. Geneva, Switzerland: WHO.
- 3. World Health Organization , 2017. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. Geneva, Switzerland: WHO. [Google Scholar]
- 4. World Health Organization , 2017. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. Geneva, Switzerland: WHO. [Google Scholar]
- 5. Ozawa S Evans DR Higgins CR Laing SK Awor P , 2019. Development of an agent-based model to assess the impact of substandard and falsified anti-malarials: Uganda case study. Malar J 18: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nayyar GM Breman JG Newton PN Herrington J , 2012. Poor-quality antimalarial drugs in Southeast Asia and sub-Saharan Africa. Lancet Infect Dis 12: 488–496. [DOI] [PubMed] [Google Scholar]
- 7. Talisuna AO et al. 2012. Mitigating the threat of artemisinin resistance in Africa: improvement of drug-resistance surveillance and response systems. Lancet Infect Dis 12: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, 2021. WHO Guidelines for Malaria. Geneva, Switzerland: World Health Organization, 1--214.
- 9. World Health Organization , 2018. World Malaria Report 2018. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 10.Evans D, Higgins C, Laing S, Awor P, Ozawa S, 2019. Poor-quality antimalarials further health inequities in Uganda. Health Policy Plan 34 (Suppl 3): iii36–iii47. [DOI] [PMC free article] [PubMed]
- 11. Ozawa S et al. 2019. Modeling the economic impact of substandard and falsified antimalarials in the Democratic Republic of the Congo. Am J Trop Med Hyg. 100: 1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beargie SM Higgins CR Evans DR Laing SK Erim D Ozawa S , 2019. The economic impact of substandard and falsified antimalarial medications in Nigeria. PLoS One 14: e0217910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jackson KD et al. 2020. Impact of substandard and falsified antimalarials in Zambia: application of the SAFARI model. BMC Public Health 20: 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zinsou C Cherifath AB , 2017. The malaria testing and treatment landscape in Benin. Malar J 16: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Conte R Paolucci M , 2014. On agent-based modeling and computational social science. Front Psychol 5: 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ACTwatch Group and Association Beninoise Pourle Marketing Social (ABMS), 2016. ACTwatch Study Reference Document: Benin Outlet Survey 2014. Washington, DC: Population Services International.
- 17. Abdulla S et al. 2008. Efficacy and safety of artemether– lumefantrine dispersible tablets compared with crushed commercial tablets in African infants and children with uncomplicated malaria: a randomised, single-blind, multicentre trial. Lancet 372: 1819–1827. [DOI] [PubMed] [Google Scholar]
- 18. Faucher JF et al. 2009. Comparison of sulfadoxine– pyrimethamine, unsupervised artemether–lumefantrine, and unsupervised artesunate–amodiaquine fixed-dose formulation for uncomplicated Plasmodium falciparum malaria in Benin: a randomized effectiveness noninferiority trial. J Infect Dis 200: 57–65. [DOI] [PubMed] [Google Scholar]
- 19.Institut National de la Statistique et de l’Analyse Économique (INSAE) and ICF, 2019. Benin Demographic and Health Survey 2017–18 [Dataset]. Rockville, MD: INSAE and ICF [Producers]. ICF [Distributor], 2019.
- 20. United Nations Conference on Trade and Development , n.d. Currency Exchange Rates, Annual, 2018. Available at: https://unctadstat.unctad.org/wds/TableViewer/tableView.aspx?ReportId=117. Accessed September 29, 2021.
- 21.The World Bank, 2018. GDP per capita (current US$) - Benin 2018. Available at: https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=BJ. Accessed September 10, 2021.
- 22.United States President’s Malaria Initiative, 2018. Benin: Malaria Operational Plan FY 2018. Available at: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2018-benin-malaria-operational-plan.pdf. Accessed October 1, 2021.
- 23. Lubell Y et al. 2011. Likely health outcomes for untreated acute febrile illness in the tropics in decision and economic models: a Delphi survey. PLoS One 6: e17439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baba-Moussa F et al. 2015. Quality control of selected antimalarials sold in the illicit market: an investigation conducted in Porto-Novo City (Republic of Benin). Adv Biosci Biotechnol 6: 637–644. [Google Scholar]
- 25. Kaur H et al. 2015. Quality of artemisinin-based combination formulations for malaria treatment: prevalence and risk factors for poor quality medicines in public facilities and private sector drug outlets in Enugu, Nigeria. PLoS One 10: e0125577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaur H et al. 2016. Fake anti-malarials: start with the facts. Malar J 15: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ioset JR Kaur H , 2009. Simple field assays to check quality of current artemisinin-based antimalarial combination formulations. PLoS One 4: e7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ochekpe NAAA Attah SE , 2010. Correlation of price and quality of medicines: assessment of some artemisinin antimalarials in Nigeria based on GPHF minilab. Int J Drug Dev Res. 2: 211–218. [Google Scholar]
- 29. Onwujekwe O et al. 2009. Quality of anti-malarial drugs provided by public and private healthcare providers in south-east Nigeria. Malar J 8: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarbartova JT, 2011. Survey of the Quality of Selected Antimalarial Medicines Circulating in Six Countries of Sub-Saharan Africa. Geneva, Switzerland: WHO, 1–118.
- 31. Affum AO Lowor S Osae SD Dickson A Gyan BA Tulasi D , 2013. A pilot study on quality of artesunate and amodiaquine tablets used in the fishing community of Tema, Ghana. Malar J 12: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyarko SH Cobblah A , 2014. Sociodemographic determinants of malaria among under-five children in Ghana. Malar Res Treat 2014: 304361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Camponovo F Bever CA Galactionova K Smith T Penny MA , 2017. Incidence and admission rates for severe malaria and their impact on mortality in Africa. Malar J 16: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bruxvoort K et al. 2015. Are Tanzanian patients attending public facilities or private retailers more likely to adhere to artemisinin-based combination therapy? Malar J 14: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: WHO. [Google Scholar]
- 36. Dondorp AM et al. 2010. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376: 1647–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rashed S et al. 2000. Economic impact of febrile morbidity and use of permethrin-impregnated bed-nets in a malarious area I: study of demographics, morbidity, and household expenditures associated with febrile morbidity in the Republic of Benin. Am J Trop Med Hyg 62: 173–180. [DOI] [PubMed] [Google Scholar]
- 38. Hansen KS Clarke SE Lal S Magnussen P Mbonye AK , 2017. Cost-effectiveness analysis of introducing malaria diagnostic testing in drug shops: a cluster-randomised trial in Uganda. PLoS One 12: e0189758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Batwala V Magnussen P Hansen KS Nuwaha F , 2011. Cost-effectiveness of malaria microscopy and rapid diagnostic tests versus presumptive diagnosis: implications for malaria control in Uganda. Malar J 10: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jimoh A Sofola O Petu A Okorosobo T , 2007. Quantifying the economic burden of malaria in Nigeria using the willingness to pay approach. Cost Eff Resour Alloc 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salawu AT Fawole OI Dairo MD , 2016. Patronage and cost of malaria treatment in private hospitals in Ibadan North L.G.A. southwestern, Nigeria. Ann Ib Postgrad Med 14: 81–84. [PMC free article] [PubMed] [Google Scholar]
- 42. Uzochukwu BS Obikeze EN Onwujekwe OE Onoka CA Griffiths UK , 2009. Cost-effectiveness analysis of rapid diagnostic test, microscopy and syndromic approach in the diagnosis of malaria in Nigeria: implications for scaling-up deployment of ACT. Malar J 8: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Onwujekwe O Uguru N Etiaba E Chikezie I Uzochukwu B Adjagba A , 2013. The economic burden of malaria on households and the health system in Enugu State southeast Nigeria. PLoS One 8: e78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Management Sciences for Health , n.d. International Medical Products Price Guide. Available at: https://www.msh.org/resources/international-medical-products-price-guide?DMFId=690&searchYear=2015. Accessed September 28, 2021.
- 45. World Health Organization , 2010. Country-Specific Inpatient and Outpatient Estimates in 2010 Currency. Geneva, Switzerland: WHO. [Google Scholar]
- 46. Ganfon H Ekanmian G Amoussou L Daniel-Garcia E Allabi AC , 2017. Evaluation of the knowledge and attitude of pharmacists about the national malaria control policy in southern Benin. Malar J 16: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United States President's Malaria Initiative, 2020. Benin: Malaria Operational Plan FY 2020. Available at: https://d1u4sg1s9ptc4z.cloudfront.net/uploads/2021/03/fy-2020-benin-malaria-operational-plan.pdf. Accessed October 1, 2021.
- 48. Kikule K, Nfor E, Saleeb S, 2018. Optimizing the Marketing Authorization Process in Benin: Institutionalization and Development of Medicines Registration Standard Operating Procedures. Arlington, VA: Management Sciences for Health. [Google Scholar]
- 49. World Health Organization , 2018. Number of Deaths in Children Aged <5, by Cause. Geneva, Switzerland: WHO. Available at: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/number-of-deaths. 2018. Accessed September 28, 2021. [Google Scholar]