Abstract
This report represents an unusually large parathyroid carcinoma (PC) mimicking thyroid nodule recurrence after hemithyroidectomy. PC is a rare endocrine malignancy accounting for less than 1% of hyperparathyroidism cases. This is the first case report where contrast-enhanced ultrasound (CEUS) was performed on a PC. A 63-year-old female presented with an enlarged mass on the left side of the neck. In 2012, left-side hemithyroidectomy was done due to a benign goiter. In 2020, laboratory analysis showed markedly elevated parathyroid hormone and calcium. Multiparametric neck ultrasonography was performed including B-mode, color Doppler, shear wave elastography, and CEUS. Computed tomography revealed an irregular mass in proximity to the trachea, esophagus, and dislocation of the common carotid artery. Perifocal fatty tissue appeared normal. Scintigraphy displayed a suspected parathyroid tumor or a suspected left lobe nodule of thyroid. Based on the biochemical diagnosis of primary hyperparathyroidism and radiological examinations, a suspected parathyroid tumor was considered. Intraoperative findings demonstrated an unusually large 9 × 6 cm tumor (84 g) adjacent to the common carotid artery anterolaterally and the recurrent laryngeal nerve medially. Pathohistological examination revealed a tumor solid in structure, with focal necrosis penetrating the capsule. Immunohistochemical analysis was positive for chromogranin, CD56, and Ki-67 (8–10%) and negative for CK20 and CK7. The morphological and immunohistochemical results correspond to PC. PC is a challenging diagnosis requiring a multidisciplinary approach, especially in the case of previous neck surgery. The only curative treatment for PC is radical surgery. Lifelong monitoring of PCs is mandatory due to the high recurrence rate.
Keywords: Parathyroid surgery, Parathyroid carcinoma, Hyperparathyroidism, Parathyroid hormone, Contrast-enhanced ultrasound
Introduction
Parathyroid carcinoma (PC) is a rare endocrine malignancy accounting for less than 1% of hyperparathyroidism cases with a reported incidence of 0.015 cases per 100,000 population and a prevalence of 0.005% in the USA [1]. PC affects both genders equally with peak incidence a decade earlier than parathyroid adenoma − 45–51 years. Nonfunctioning carcinomas have been reported; however, over 90% are functionally active and secrete parathyroid hormone (PTH) [2]. Due to the lack of specific clinical characteristics, diagnosis and patient management of PC patients may prove challenging and most PCs are diagnosed either incidentally or postoperatively.
We present a case of a large PC diagnosed 8 years after thyroid surgery mimicking thyroid nodule recurrence.
Case Report/Case Presentation
A 63-year-old female presented with an enlarged mass on the left side of the neck after left-side thyroid lobe resection due to a benign goiter in 2012. In 2013, a routine postoperative ultrasonography (US) examination was done, which displayed a homogeneous, hypoechoic mass 1.8 × 1.9 cm in size. In 2014, the same hypoechoic mass was found on US with no alteration in size. No routine follow-up or US examinations were performed for 5 years. The patient was under the supervision of a general practitioner.
In 2020, the laboratory analysis performed by the general practitioner showed elevated calcium 3.42 mmol/L (normal range 2.08–2.65 mmol/L) and PTH 1,242 pg/mL (normal range 15–68.3 pg/mL). The reason for performing analysis was a palpable neck mass described by the patient; however, there were no mass-associated symptoms. As a part of an ongoing research project, a multiparametric neck US was performed: B-mode examination revealed a hypoechoic, irregular, nonhomogeneous mass size 5.5 × 3.2 × 5.0 cm with multiple central calcifications and mixed vascularization pattern on color Doppler and superb microvascularization imaging (SMI) modalities. Mass appeared less elastic on 2D shear wave elastography compared to the normal thyroid tissue (28.8 kPa and 16.8 kPa, respectively). As for contrast-enhanced ultrasound (CEUS) − after injection of 2 mL of the contrast agent SonoVue, followed by saline bolus, 10 mL intravenously, heterogeneous, rapid enhancement of the left side neck mass with the presence of washout was observed. CEUS (SonoVue) showed diffuse, homogeneous hypervascularity in the early arterial phase − at 6 s, quickly reaching the peak contrast concentration − 12 s, following homogeneous early washout from 23 s (Fig. 1).
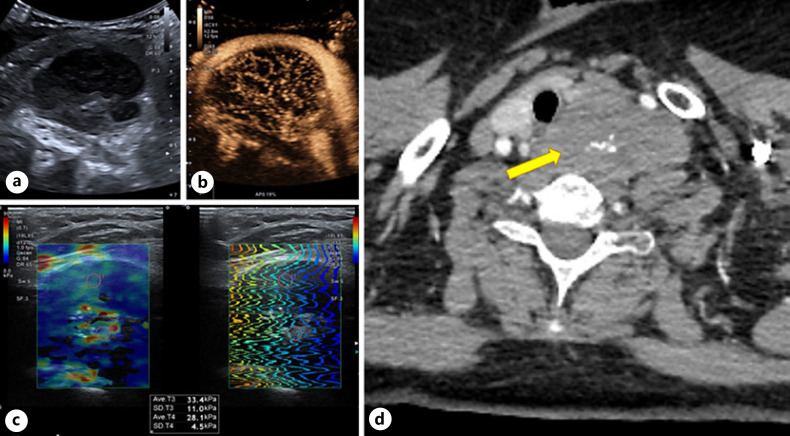
Fig. 1.
US: left side hypoechoic, irregular, inhomogeneous neck mass (5 × 53 × 25.0 cm) with multiple central calcifications (a), CEUS: homogeneous trabecular early hyperenhancement (b), moderate elasticity on SWE 28–33 kPa (c). CT: mass with anterolateral dislocation of the common carotid artery in the axial plane (d). SWE, shear wave elastography.
Computed tomography (CT) revealed an irregular mass in proximity to the trachea, esophagus, and marked ventral dislocation of the common carotid artery. Perifocal fatty tissue appeared normal. No cervical lymphadenopathy was observed (Fig. 1). Scintigraphy displayed a suspected parathyroid tumor or a suspected left lobe nodule of thyroid. Based on the biochemical diagnosis of primary hyperparathyroidism and radiological examinations, a suspected parathyroid tumor was considered; therefore, the patient was approved for elective surgery.
Standard cervicotomy was extended due to the size of the mass. For a better approach, both the left sternohyoid and sternothyroid muscles were divided. Intraoperative findings demonstrated a tumor adjacent to the common carotid artery displaced anterolaterally and the recurrent laryngeal nerve medially. The tumor was extirpated preserving the left common carotid artery and internal jugular vein. Left superior parathyroid gland was preserved. The size of the tumor removed from the neck was unusually large − 9 cm × 6 cm, with a mass of 84 g (Fig. 2). The PTH level before surgery was 1,545 pg/mL, PTH during operation 1,288 pg/mL, PTH 20 min after resection 121 pg/mL, PTH 6 h after operation 17 pg/mL, PTH next day − 13 pg/mL, and next day calcium − 2.77 mmol/L.
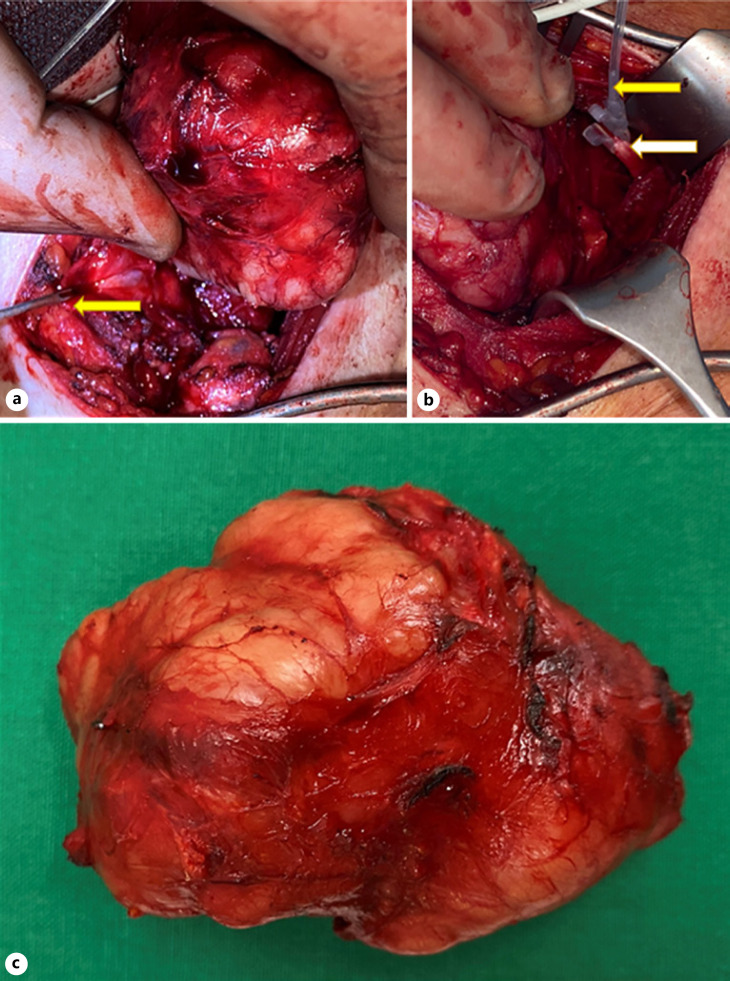
Fig. 2.
a Parathyroid tumor. Recurrent laryngeal nerve (yellow arrow). b Left vagus nerve X (white arrow) and continuous vagal nerve stimulation (yellow arrow). c Tumor after extirpation.
Pathohistological examination revealed that the tumor was solid in structure, with focal necrosis, penetrating the capsule. Immunohistochemical analysis was positive for chromogranin A, Ki-67 (8–10%) and negative for CD56, CK20, and CK7. The morphological analysis and proliferative index Ki-67 corresponded to PC. TNM staging: pT1N0M0 (confirmed capsular invasion) (Fig. 3).
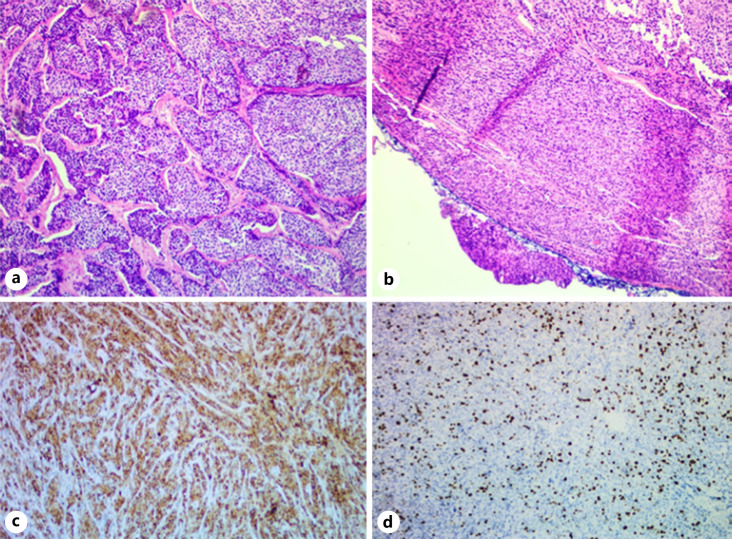
Fig. 3.
Hematoxilin and eosin staining of parathyroid tumor: tumor trabeculae (a) and capsular invasion (b). ICH: chromogranin positive (c) and Ki-67 index of 8–10% suggestive of carcinoma (d). ICH, immunohistochemistry.
Laboratory values 2 months after surgery showed calcium 2.25 mmol/L and PTH 3.7 pg/mL. No evidence of biochemical recurrence is present. Postoperative CT scan showed surgical clips in the tumor bed and no signs of recurrence, and scintigraphy did not show an uptake in that region, while US revealed an isoechoic suspicious residual tissue. Six months after surgery, routine US was done: the same isoechogenic mass on the left side of the neck was found, 14 mm long. Nine months after surgery, the calcium level was 2.36 mmol/L, PTH 43.9 pg/mL. Routine US was done: the same isoechogenic mass on the left side of the neck was found with no change in length. The follow-up for this patient is still ongoing.
Discussion/Conclusion
PC is usually strongly suspected based on clinical signs, for example, profound hypercalcemia and radiological appearance. In this case, the first suggestive sign of malignancy was the highly elevated PTH (1,242 pg/mL) and calcium (3.42 mmol/L). However, thyroid tumor recurrence was still considered a possibility.
Radiological examination is often useful for tumor localization and planning of surgery, which, however, cannot always accurately distinguish between malignant and benign disease. The most common modalities for locating parathyroid lesions include US, CT, magnetic resonance imaging, and Tc-99m sestamibi (MIBI) parathyroid scintigraphy [3]. Combining imaging modalities increases the diagnostic sensitivity and accuracy of the diagnosis and was also performed in this case.
A multiparametric US examination was performed, including CEUS, which has been reported to have sensitivity and specificity of over 97% for detecting abnormal parathyroid gland [4, 5]. For this carcinoma, CEUS was highly suggestive of malignancy with early arterial enhancement and a homogeneous early washout at 23 s. To the best of our knowledge, this is the first case report of PC where CEUS was performed, showing signs of malignancy and aiding the diagnosis. Signs of malignancy on B-mode US were hypoechoic inhomogeneous appearance, central calcifications, and mixed vascularization pattern on color Doppler and SMI.
CT features of PC are high short-to-long axis ratio, irregular shape, presence of peritumoral infiltration, low-contrast enhancement, and occasionally − calcification [5]. In this case, an irregular shape and central calcification were visualized as well as dislocation of the common carotid artery. No affected lymph nodes were seen on CT, and this was later confirmed histopathologically with TNM staging − pT1N0M0.
Sestamibi scan can help locate abnormal or ectopic parathyroid tissue, which, however cannot differentiate between benign and malignant lesions [3]. Different degrees of MIBI uptake in PC have been reported; moreover, it may be related to the size of the tumor as well as serum calcium levels and oxyphilic cell count [6, 7, 8]. On a Tc-99m MIBI scintigraphy, malignancy-related signs are delayed uptake and washout [3]. Scintigraphy after surgery did not display a suspected parathyroid tissue or a suspected left thyroid lobe nodule.
Histopathological examination remains the golden standard for the definitive diagnosis of PC. Carcinoma is usually larger than the adenoma, and the average size at presentation is 3 cm and shows adherence to surrounding structures. Macroscopically, the color ranges from gray to white [9]. In this case, the tumor was 9 × 6 cm in size and was visible and palpable on the neck. Several histological malignancy criteria for PC have been described, and Schanctz and Castleman propose the following: fibrous trabeculae, mitotic figures, capsular invasion, and blood vessel invasion [10]. Not all malignancy criteria were met in this case; for example, no intravascular invasion was seen; however, penetration of the capsule as well as fibrous bands was both present. In addition, this tumor showed focal necrosis. It has been reported that areas of coagulative necrosis can be seen in up to 1/3 of the cases [11].
Immunohistochemistry plays a crucial role in diagnosing PC and differentiating from other tissue tumors, especially follicular thyroid carcinoma and parathyroid adenoma [12]. PC usually stains positively for PTH and negatively for thyroglobulin helping the diagnostic process. Ki-67 and protein parafibromin (HPRT-2 gene) are immunohistochemistry markers that can help differentiate between parathyroid adenoma and carcinoma. Complete loss of parafibromin expression as well as the Ki-67 index of more than 5% has a high correlation with carcinomas [13]. As for the present carcinoma, immunohistochemical analysis was positive for chromogranin and Ki-67 (8–10%) and negative for CD56, CK20, and CK7; therefore, a diagnosis of PC was made.
The prognosis of patients with PC depends on multiple factors and is therefore variable. Best prognosis can be achieved in cases of early recognition and complete “en bloc” excision when the 5-year survival rate can reach 90% and 67% at 10 years [14, 15]. Two months after surgery, our patient received a routine US showing an isoechogenic mass on the left side of the neck sized 2 cm. A scintigraphy was further performed and showed no evidence of parathyroid pathology. Further US follow-up after 6 months was performed with no alteration in the locoregional status; therefore, observation and routine US scans are indicated in the following years due to the high recurrence rate of >50% [15].
In conclusion, PC is a challenging diagnosis requiring a multidisciplinary approach, especially in the case of previous neck surgery. CEUS proved to be a valuable additional tool in differentiating PC from other tissue. The only curative treatment for PC is radical surgery. Lifelong monitoring of PCs is mandatory due to the high recurrence rate.
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of University of Latvia (protocol code P-1.2/01, June 5, 2019) and Ethics Committee of Riga Stradins University (protocol code 6-1/09/16, September 10, 2020). Written informed consent was obtained from the patient for the publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Funding Sources
This case report was funded by the Fundamental and Useful Research Project (FLPP) within the project “Multiparametric ultrasound correlation with morphology in patients with primary hyperparathyroidism [Nr. lzp-2020/2-0297].” The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
Conceptualization: Maija Radzina and Zenons Narbuts; methodology: Maija Radzina; validation: Sergejs Pavlovics; formal analysis: Janis Gardovskis; investigation: Maija Radzina, Sergejs Pavlovics, Mara Liepa, and Peteris Prieditis; resources: Maija Radzina, Sergejs Pavlovics, and Madara Ratniece; data curation: Arturs Ozolins and Elina Tauvena; writing − original draft preparation: Madara Ratniece, Elina Tauvena, and Sergejs Pavlovics; writing − review and editing: Maija Radzina, Arturs Ozolins, Zenons Narbuts, and Rita Niciporuka; visualization: Sergejs Pavlovics and Maija Radzina; supervision: Maija Radzina and Zenons Narbuts; project administration: Janis Gardovskis, Maija Radzina, and Zenons Narbuts.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available yet due to ongoing research.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty‐six cases of parathyroid carcinoma treated in the U.S. between 1985–1995. Cancer. 1999;86((3)):538–44. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Wilkins BJ, Lewis JS. Non-functional parathyroid carcinoma: a review of the literature and report of a case requiring extensive surgery. Head Neck Pathol. 2009 Jun;3((2)):140–9. doi: 10.1007/s12105-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheon M, Choi JY, Chung JH, Lee JY, Cho SK, Yoo J, et al. Differential findings of Tc-99m sestamibi dual-phase parathyroid scintigraphy between benign and malignant parathyroid lesions in patients with primary hyperparathyroidism. Nucl Med Mol Imaging. 2011;45((4)):276–84. doi: 10.1007/s13139-011-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha A, Hornung M, Stroszczynski C, Schlitt HJ, Jung EM. Highly efficient localization of pathological glands in primary hyperparathyroidism using contrast-enhanced ultrasonography (CEUS) in comparison with conventional ultrasonography. J Clin Endocrinol Metab. 2013;98((5)):2019–25. doi: 10.1210/jc.2013-1007. [DOI] [PubMed] [Google Scholar]
- 5.Takumi K, Fukukura Y, Hakamada H, Nagano H, Kumagae Y, Arima H, et al. CT features of parathyroid carcinomas: comparison with benign parathyroid lesions. Jpn J Radiol. 2019;37((5)):380–9. doi: 10.1007/s11604-019-00825-3. [DOI] [PubMed] [Google Scholar]
- 6.Meng Z, Tan J, Zhang M, Dong F, Jia Q, Zhang F. Tc-99m pertechnetate/sestamibi imaging in a case of recurrent parathyroid carcinoma with metabolic bone disorder. Clin Nucl Med. 2009;34((7)):479–82. doi: 10.1097/RLU.0b013e3181a7d136. [DOI] [PubMed] [Google Scholar]
- 7.Clark P, Wooldridge T, Kleinpeter K, Perrier N, Lovato J, Morton K. Providing optimal preoperative localization for recurrent parathyroid carcinoma: a combined parathyroid scintigraphy and computed tomography approach. Clin Nucl Med. 2004;29((11)):681–4. doi: 10.1097/00003072-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier A, Jeannotte S, Verreault J, Lefebvre B, Bisson G, Mongeau CJ, et al. Preoperative localization of parathyroid lesions in hyperparathyroidism: relationship between technetium-99m-MIBI uptake and oxyphil cell content. J Nucl Med. 1998;39((8)):1441–4. [PubMed] [Google Scholar]
- 9.DeLellis RA. Parathyroid carcinoma: an overview. Adv Anat Pathol. 2005;12((2)):53–61. doi: 10.1097/01.pap.0000151319.42376.d4. [DOI] [PubMed] [Google Scholar]
- 10.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31((3)):600–5. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Bondeson L, Sandelin K, Grimelius L. Histopathological variables and DNA cytometry in parathyroid carcinoma. Am J Surg Pathol. 1993;17((8)):820–9. doi: 10.1097/00000478-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Noguchi S, Murakami N, Toda M, Adachi M, Daa T. Immunohistological study of nonfunctional parathyroid carcinoma. Report of a case. Acta Pathol Jpn. 1992;42((4)):279–85. doi: 10.1111/j.1440-1827.1992.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 13.Ryhänen EM, Leijon H, Metso S, Eloranta E, Korsoff P, Ahtiainen P, et al. A nationwide study on parathyroid carcinoma. Acta Oncol. 2017;56((7)):1–13. doi: 10.1080/0284186X.2017.1306103. [DOI] [PubMed] [Google Scholar]
- 14.Cetani F, Frustaci G, Torregrossa L, Magno S, Basolo F, Campomori A, et al. A nonfunctioning parathyroid carcinoma misdiagnosed as a follicular thyroid nodule. World J Surg Oncol. 2015;13((1)):270. doi: 10.1186/s12957-015-0672-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleinpeter KP, Lovato JF, Clark PB, Wooldridge T, Norman ES, Bergman S, et al. Is parathyroid carcinoma indeed a lethal disease? Ann Surg Oncol. 2005;12((3)):260–6. doi: 10.1245/ASO.2005.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available yet due to ongoing research.