Abstract
Syringocystadenocarcinoma papilliferum (SCACP) is a rare cutaneous adnexal neoplasm. To the best of our knowledge, fewer than 50 cases have been described in the literature. We describe the first reported case of an SCACP in an immunocompromised patient. We report the development of an SCACP over 3 months in a 77-year-old organ transplant recipient undergoing regular dermatological follow-up. The lesion was excised with clear margins with Mohs micrographic surgery. This lesion's rapid development and small size contrasts with the predominantly slow-growing, larger lesions described in immunocompetent patients. Lastly, this case further highlights the importance of close dermatological follow-up of immunosuppressed patients.
Keywords: Cancer, Malignancy, Skin cancer, Adnexal neoplasms, Immunosuppression
Introduction
Syringocystadenocarcinoma papilliferum (SCACP) is an exceedingly rare malignancy [1, 2]. There are fewer than 50 reported cases in the literature [2]. SCACP usually arises from pre-existing syringocystadenoma papilliferum (SCAP) and/or nevus sebaceous of Jadassohn [3]. The lesions show adnexal differentiation; however, there is no specific immunohistochemical stain for SCACP to guide diagnosis. The disease is more frequent in the elderly but can occur from the third decade onward, with a slight male predilection [2]. SCACP may occur anywhere on the body but particularly on the head and neck followed by the trunk [2]. The disease may behave aggressively, with cases of locoregional and distant metastatic spread described [2, 4]. Treatment consists of surgical removal.
Case Report
A 77-year-old man presented for regular 3-monthly dermatology review. He had received a liver transplant 15 years previously for polycystic liver disease. His current medications comprised tacrolimus 1 mg twice daily, acitretin 20 mg daily, calcium/vitamin D, perindopril, gliclazide, and linagliptin. He had a history of multiple keratinocyte cancers, with 11 squamous and basal cell carcinomas excised within the past 2 years and a 0.25-mm Breslow thickness lentigo maligna melanoma excised 2.5 years previously.
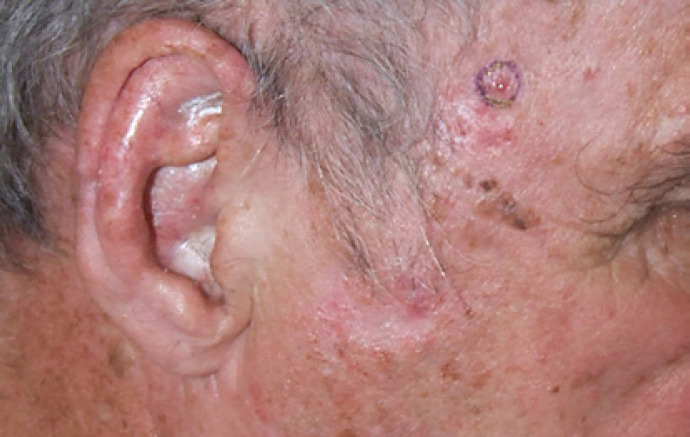
Physical examination revealed a 3-mm diameter pearly papule on the right temple without ulceration (shown in Fig. 1). There was no regional lymphadenopathy. The lesion had grown in less than 3 months and the patient had been unaware of its development.
Fig. 1.
SCACP on the right temple of a 77-year-old liver transplant recipient. A 3-mm-diameter pearly papule without ulceration is seen.
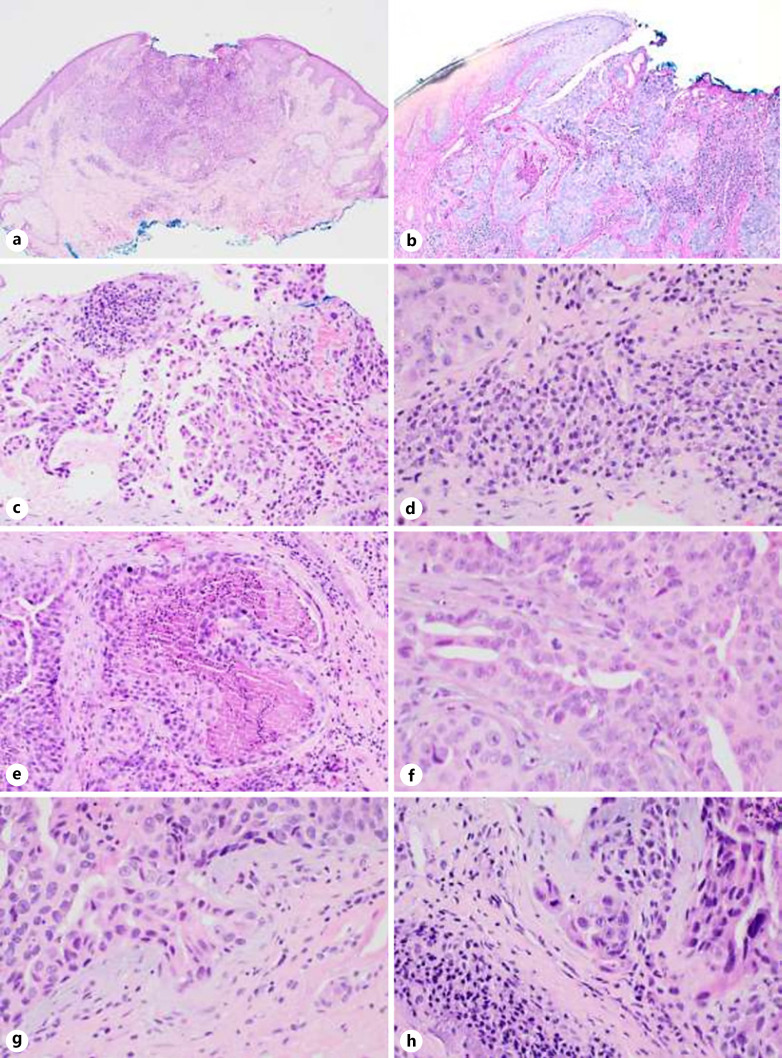
Histopathologic evaluation revealed an endophytic lesion pushing into the dermis to a depth of 1.4 mm (shown in Fig. 2). The lesion was composed of atypical cells forming gland-like structures and cystic spaces with focal papillary/micropapillary projections. The glands were lined by multilayered epithelium composed of atypical cells with large, anisomorphic nuclei and abundant eosinophilic cytoplasm. Mitoses were easily identified, and there was focal luminal necrosis. The lesion was generally well demarcated, but single cells and small cell clusters were focally present at the edge of the lesion associated with a desmoplastic stroma, indicative of early invasion. The surrounding stroma contained a dense plasma cell infiltrate. No lymphovascular or perineural invasion was seen. Immunohistochemically, the lesion was strongly and diffusely positive for CK7 and EMA. CEA was positive at the luminal borders. GCDFP15 and S100 were negative in the lesional cells. Ki67 was mildly elevated at 5–10%. The lesion was excised utilizing Mohs micrographic surgery requiring two layers to achieve clearance. A further rim of tissue was excised after Mohs clearance with no histological evidence of residual malignancy.
Fig. 2.
SCACP. a Endophytic profile of the tumor, with central surface ulceration and a surrounding inflammatory infiltrate (hematoxylin and eosin. ×40). b Connection of lesion to epidermis (periodic acid-Schiff. ×100). c Large, atypical nuclei and eosinophilic cytoplasm, with focal papillary/micropapillary-like structures (hematoxylin and eosin. ×100). d Dense plasma cell infiltrate at periphery of lesion with focal Russell bodies (hematoxylin and eosin. ×400). e Necrosis within a glandular lumen (hematoxylin and eosin. ×100). f Multilayered epithelium with mitoses (hematoxylin and eosin. ×400). g, h Atypical tumor buds and single cells infiltrating at periphery of lesion, with desmoplastic stroma (hematoxylin and eosin. ×400).
Discussion
We describe the first reported case of SCACP in an immunocompromised patient. SCACP may arise rapidly and behave aggressively. Of the reported cases, 22% have demonstrated locoregional lymphatic metastases [2] and 6% had distant metastatic disease which was fatal in all cases [2]. Late recurrences of SCACP have been described, and therefore long-term follow-up is warranted [5]. The mean duration of lesions prior to SCACP diagnosis is usually many years [2, 3, 6]. The mean maximum diameter of reported cases is close to 4 cm (range 0.4 cm–16 cm) [2]. Lesions often have a connection to the overlying epidermis by a residuum of a benign SCAP, supporting a stepwise progression from SCAP to SCACP [4]. The dense stromal infiltrate of plasma cells is a characteristic feature of SCAP and SCACP [2, 5]. Features of malignant transformation include multilayering of the glandular epithelium, increased nuclear atypia, and mitotic activity. They may be in situ or invasive. The invasive component is usually an adenocarcinoma; however, it sometimes can show squamous differentiation [4]. There is no specific immunohistochemical stain for the diagnosis of SCACP [2]. The lesions show adnexal differentiation, with positivity for broad spectrum cytokeratin as well as CK7, and are negative for CK20. EMA is frequently positive [2, 7], while CEA and GCDFP15 reactivity are variable [2, 4, 7]. CK5/6, SMA, S100, and p63 may highlight associated myoepithelial cells. Metastatic adenocarcinoma should be excluded clinically.
In this case, the patient was undergoing regular dermatological review due to his immunocompromised status and history of multiple skin cancers. Clinically, the lesion had a “glistening” appearance, possibly due to the partly cystic nature of the lesion. Histologically, the overall architecture, with the characteristic stromal plasmacytic infiltrate, was suggestive of a possible precursor SCAP; however, no longstanding precursor lesion had been noted at the site previously. The lesion had arisen rapidly (within 3 months) and showed unequivocal features of malignancy, but was detected and removed at an early stage, accounting for the small clinical size of the lesion compared to previously reported cases.
While there are no reported cases of SCACP in immunocompromised patients in the literature, it is worthwhile to note that this patient group does have increased rates of adnexal neoplasms [8, 9]. In renal transplant recipients, adnexal tumors are more common and more frequently malignant than in immunocompetent patients [10].
In conclusion, we present the first documented case of an SCACP in an immunocompromised patient. This case highlights the importance of regular dermatologic follow-up of immunocompromised patients, to facilitate the early detection and treatment of aggressive cutaneous lesions in this population.
Statement of Ethics
This study conforms to the guidelines established by the Declaration of Helsinki. This retrospective review of patient data did not require ethical approval in accordance with Australian guidelines. This manuscript was created using existing collections of nonidentifiable data only. Written informed consent was obtained from the patient described for publication of case details and accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to declare.
Author Contributions
Diona Damian contributed to the conception of this article. She is the treating dermatologist of the patient described. Elizabeth Paver is a pathologist who was involved in the histopathological diagnosis of the lesion. Catherine Zilberg completed the literature search and drafted the article. Elizabeth Paver and Diona Damian edited the manuscript.
Data Availability Statement
All available data used in the generation of this case report are included in the article.
Acknowledgment
Elizabeth Paver would like to acknowledge the BB & A Miller Foundation, supporting the Jani Haenke Melanoma Pathology Fellowship.
References
- 1.Paradiso B, Bianchini E, Cifelli P, Cavazzini L, Lanza G. A new case of syringocystadenocarcinoma papilliferum: a rare pathology for a wide-ranging comprehension. Case Rep Med. 2014;2014:453874. doi: 10.1155/2014/453874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KG, Choi W, Lim JS, Hahn HJ, Myung KB, Cheong SH. Syringocystadenocarcinoma papilliferum: a case report and review of the literature. Ann Dermatol. 2019;31((5)):559–62. doi: 10.5021/ad.2019.31.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Requena L, Kiryu H, Ackerman A. Ackerman's histologic diagnosis of neoplastic skin disease: a method by pattern analysis. Neoplasms with apocrine differentiation. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- 4.Zhang Y, Kong YY, Cai X, Shen XX, Kong JC. Syringocystadenocarcinoma papilliferum: clinicopathologic analysis of 10 cases. J Cutan Pathol. 2017;44((6)):538–43. doi: 10.1111/cup.12934. [DOI] [PubMed] [Google Scholar]
- 5.Castillo L, Moreno A, Tardío JC. Syringocystadenocarcinoma papilliferum in situ: report of a case with late recurrence. Am J Dermatopathol. 2014;36((4)):348–52. doi: 10.1097/DAD.0b013e3182a38bb9. [DOI] [PubMed] [Google Scholar]
- 6.Park SH, Shin YM, Shin DH, Choi JS, Kim KH. Syringocystadenocarcinoma papilliferum: a case report. J Korean Med Sci. 2007;22((4)):762–5. doi: 10.3346/jkms.2007.22.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishida-Yamamoto A, Sato K, Wada T, Takahashi H, Iizuka H. Syringocystadenocarcinoma papilliferum: case report and immunohistochemical comparison with its benign counterpart. J Am Acad Dermatol. 2001;45((5)):755–9. doi: 10.1067/mjd.2001.117723. [DOI] [PubMed] [Google Scholar]
- 8.Kempf W, Mertz KD, Hofbauer GF, Tinguely M. Skin cancer in ogan transplant recipients. Pathobiology. 2013;80((6)):302–9. doi: 10.1159/000350757. [DOI] [PubMed] [Google Scholar]
- 9.D'Arcy ME, Castenson D, Lynch CF, Kahn AR, Morton LM, Shiels MS, et al. Risk of rare cancers among solid organ transplant recipients. J Natl Cancer Inst. 2020;113((2)):199–207. doi: 10.1093/jnci/djaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harwood CA, McGregor JM, Swale VJ, Proby CM, Leigh IM, Newton R, et al. High frequency and diversity of cutaneous appendageal tumors in organ transplant recipients. J Am Acad Dermatol. 2003;48((3)):401–8. doi: 10.1067/mjd.2003.97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All available data used in the generation of this case report are included in the article.