Abstract
Malic enzyme is one of at least five enzymes, known to be present in Corynebacterium glutamicum, capable of carboxylation and decarboxylation reactions coupling glycolysis and the tricarboxylic acid cycle. To date, no information is available concerning the physiological role of the malic enzyme in this bacterium. The malE gene from C. glutamicum has been cloned and sequenced. The protein encoded by this gene has been purified to homogeneity, and the biochemical properties have been established. Biochemical characteristics indicate a decarboxylation role linked to NADPH generation. Strains of C. glutamicum in which the malE gene had been disrupted or overexpressed showed no detectable phenotype during growth on either acetate or glucose, but showed a significant modification of growth behavior during lactate metabolism. The wild type showed a characteristic brief period of exponential growth on lactate followed by a linear growth period. This growth pattern was further accentuated in a malE-disrupted strain (ΔmalE). However, the strain overexpressing malE maintained exponential growth until all lactate had been consumed. This strain accumulated significantly larger amounts of pyruvate in the medium than the other strains.
Corynebacterium glutamicum is widely used in the industrial production of amino acids, particularly l-glutamate and l-lysine. The modified carbon flux distribution during the shift from cell growth to amino acid overproduction involves a high flux through the anaplerotic reactions. In C. glutamicum, four carboxylating enzymes able to convert pyruvate or phosphoenolpyruvate (PEP) to four-carbon atom dicarboxylic acids oxaloacetate (OAA) or malate have been demonstrated, namely PEP carboxykinase (PPCk), PEP carboxylase (PPC), pyruvate carboxylase (PC), and malic enzyme. A fifth reaction, OAA decarboxylase, has also been demonstrated, but this reaction is generally considered to operate only in the gluconeogenic direction (22). To date, three of the carboxylating anaplerotic enzymes have been characterized, although little information exists concerning the properties of malic enzyme in C. glutamicum. The PPCk catalyzes the reversible carboxylation of PEP to OAA, although the strong inhibition of the OAA-forming reaction by ATP indicates that this enzyme functions predominantly as a gluconeogenic decarboxylase (21). The PPC catalyzes the irreversible carboxylation of PEP to OAA. The enzyme was purified and shown to be activated by acetylcoenzyme A (CoA) and fructose-1,6-bisphosphate and inhibited by both aspartate and α-ketoglutarate (32, 33). This enzyme was initially considered to be the major anaplerotic enzyme in C. glutamicum (51) and has been the target of genetic engineering strategies to increase flux toward OAA (35, 46). However, various groups (19, 37; L. Mathieu, C. Rollin, A. Guyonvarch, F. Wojeik, and N. D. Lindley, rDNA Biotechnol. 4:Metab. Eng. I, poster abstr. 53, 1996) have demonstrated that deletion of PPC has no significant effect on either growth or amino acid production, except under biotin limitation. The PC catalyzes the irreversible carboxylation of pyruvate to OAA. This activity was first described by Tosaka et al. (50) and was postulated to be the major anaplerotic enzyme during rapid growth on glucose (10), although the enzyme has been notoriously difficult to assay precisely. Recently, this biotin-dependent enzyme has been characterized (38) and was shown to be inhibited by acetyl-CoA, aspartate, and ADP. This enzyme was shown to be essential for rapid growth on lactate, but deletion mutants retained their capacity to grow on glucose (39). Double mutants lacking both PPC and PC activity were unable to grow on glucose (39). Nuclear magnetic resonance (NMR) analysis has, however, demonstrated that carboxylation of pyruvate accounts for approximately 90% of the anaplerotic flux (36). Strains lacking PPC activity grown under biotin limitation were unable to synthesize glutamate (Mathieu et al., rDNA Biotechnol. 4:Metab. Eng. I, poster abstr. 53, 1996), although some production of tricarboxylic acid (TCA) intermediates did occur. This indicates that at least one other anaplerotic reaction must be able to fuel the TCA cycle. Malic enzyme catalyzes the reversible carboxylation of pyruvate to malate coupled with NADPH oxidation. This activity has been measured in various strains and under different growth conditions (9, 10, 18, 51), although the role of this activity has never been clearly established. The fact that this enzyme can contribute to NADP+-NADPH equilibrium has led to the suggestion that this enzyme may play an important role in NADPH synthesis on substrates other than glucose (9, 13). However, the role of malic enzyme in the metabolism of C. glutamicum remains unclear, and in view of the importance of NADP+-NADPH cycling in this organism, it is necessary to establish the physiological role of this enzyme. In this paper, the biochemical characterization of the malic enzyme and the molecular analysis of the corresponding malE gene of C. glutamicum are described. The physiological role of malic enzyme has been investigated by studying genetically engineered strains of C. glutamicum in which the expression of the malE gene has been modified.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study, their relevant characteristics, and their sources or references are given in Table 1. Escherichia coli strain DH5α was grown aerobically at 37°C in Luria-Bertani medium, with the addition of ampicillin (100 μg/ml), kanamycin (25 μg/ml), and Bacto agar when appropriate. For molecular studies, C. glutamicum 2262 was grown aerobically at 30°C in brain heart infusion medium, with the addition of kanamycin (25 μg/ml) and Bacto agar when appropriate. The medium and conditions for growth of C. glutamicum 2262 in 3.5-liter fermentors (Chemap) have been described previously (8). The pH was maintained at 7.6 by automatic addition of KOH or HCl depending on the substrate. The cultures were inoculated with precultures grown overnight in shake flasks at 30°C on a rotary shaker (200 rpm) with the same medium and carbon source. The cell concentration was determined by spectrophotometric A650 measurements after dilution in fresh medium so as to remain within the optical linear response range. The concentrations of glucose and organic acids in the culture supernatant were determined as previously described (18) by high-performance liquid chromatography with a Bio-Rad HPX87H column at 48°C with H2SO4 (5 mM) as the eluant. Detection was performed with UV and refractometric detectors. The C. glutamicum DNA bank used in this study was described by Reyes et al. (42). Individual recombinant E. coli clones were cultivated in 96-well microtitration plates and replica plated onto hybridization membranes. Membranes were treated for colony hybridization as described by Ausubel et al. (1), and master microtitration plates were stored at −80°C until used.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| Strains | ||
| E. coli DH5a | F−endA1 hsdR17 supE44 thi-1 λ− recA1 gyrA96 relA1 Δ(lacZYA-argF)U169 F80d lacZ ΔM15 | 20 |
| C. glutamicum | ||
| Wild type | Wild-type strain 2262 | 18 |
| ΔmalE | 2262 malE::integron pMF14::malEint | This work |
| Plasmids | ||
| pMF11 | 2.9-kb fragment, obtained by partial Sau3A digestion of C. glutamicum DNA, cloned in BclI-digested pUN121 | 33; this work |
| pMF12 | 4.35-kb fragment, obtained by partial Sau3A digestion of C. glutamicum DNA, cloned in BclI-digested pUN121 | This work |
| pMF13 | 4.4-kb fragment, obtained by partial Sau3A digestion of C. glutamicum DNA, cloned in BclI-digested pUN121 | This work |
| pCGL243 | Integron-bearing cloning vector | 42 |
| pMF14 | 0.8-kb BamHI-StuI fragment from pMF12 cloned in BamHI-SmaI-digested pCGL243 | This work |
| pKK388-1 | Expression vector carrying the trc promoter | 5 |
| pMF 21 | RcaI-DraI fragment corresponding to the malE ORF, cloned in NcoI-SmaI-digested pKK388-1 | This work |
| pMF22 | SalI-SalI fragment from pMF21, cloned in SalI-digested pCGL243 | This work |
DNA preparation and transformation.
Plasmids from E. coli were isolated by the method of Birnboim (2). Plasmids and chromosomal DNA from C. glutamicum were obtained as described elsewhere (42). E. coli was transformed by electroporation (14) or by the CaCl2 method (44). C. glutamicum was transformed by electroporation (4). Oligonucleotide synthesis was performed as described by Caruthers et al. (6) with the Gene Assembler Plus from Pharmacia (Freiburg, Germany).
DNA manipulations.
Restriction enzymes, T4 DNA ligase, Klenow polymerase, proteinase K, DNase I, and RNase A, were obtained from Boehringer (Mannheim, Germany) or from Promega (Madison, Wis.) and used as instructed by the manufacturers. DNA labelling and Southern and colony hybridization experiments were performed as previously described (1). PCR experiments were performed in a Crocodile II microprocessor controlled incubation system (Appligene Oncor, Illkirch, France) with Ampli Taq Gold polymerase and its buffer from Perkin-Elmer (Foster City, Calif.). PCR consisted of a 10-min incubation at 94°C, followed by 35 amplification cycles (1 min at 94°C, 1 min at 50°C, 1 min at 72°C for each cycle). Plasmid pMF12 was sequenced by the dideoxy chain termination method (45) on a model 373 DNA sequencing system from Applied Biosystems. Sequencing was performed by a DNA walking approach, with pMF12 as the matrix and designed oligonucleotides as primers. Sequence data were compiled and analyzed by the Gene Jockey II, sequence processor program (Biosoft, Cambridge, United Kingdom).
Inactivation of the chromosomal malE gene.
The chromosomal malE gene of C. glutamicum was disrupted as described by Reyes et al. (42). A 0.8-kb BamHI-StuI DNA fragment was cloned into the pCGL243 shuttle vector to give pMF14. From pMF14, an XbaI-XbaI integron was obtained as described by Reyes et al. (42) and used to disrupt the chromosomal malE gene from C. glutamicum. The resulting ΔmalE strain was then tested for malic enzyme activity and displayed no detectable activity. Gene disruption was confirmed by Southern analysis.
Overexpression of the malE gene.
Overexpression of the malE gene in C. glutamicum was achieved by cloning the malE open reading frame (ORF) under the control of the Ptrc promoter, which promotes a high level of inducible gene expression in C. glutamicum (12). The resulting in-frame fusion was transferred into the E. coli-C. glutamicum shuttle vector pCGL243 (42). The malE ORF was obtained by PCR with C. glutamicum chromosomal DNA as the template and oligonucleotides 5′ GGCTAAATGTCATGACCATCGACC 3′ (corresponding to nucleotides 714 to 737, which allows the creation of an RcaI restriction site overlapping the ATG codon) and 5′GTTTGATTTAAAGGTCTGGTCTCG 3′ (reverse complement of nucleotides 2025 to 2048, located downstream of the putative terminator and containing a DraI restriction site) as primers. After PCR, the amplified DNA fragment was submitted to restriction with RcaI and DraI, and the product was cloned into NcoI- and SmaI-restricted plasmid pKK388-1 to give plasmid pMF21. The in-phase fusion was controlled by DNA sequencing. From pMF21, a SalI-SalI DNA fragment, encompassing the Ptrc promoter, the malE ORF, and the malE terminator, was isolated and cloned into pCGL243 at the SalI site to give pMF22. This plasmid was then introduced into C. glutamicum strains by electroporation. The overexpression was confirmed by direct measurement of enzyme activity.
Enzyme activity assay.
Malic enzyme activity was determined spectrophotometrically by measuring the change in the absorbance of NADPH at 340 nm (ɛ = 6,223 M−1 · cm−1) at 30°C. The reaction mixture for the oxidative decarboxylation of malate contained potassium phosphate buffer (100 mM, pH 7.8), MgCl2 (5 mM), NADP+ (0.6 mM), and sodium l-malate (40 mM). The reaction mixture for the reductive carboxylation of pyruvate contained Tris-HCl buffer (100 mM, pH 7.0), MgCl2 (5 mM), NADPH (0.3 mM), NH4Cl (8 mM), NaHCO3 (50 mM), and sodium pyruvate (30 mM). Specific activity was expressed relative to the protein content of the cell extract as determined by the Lowry method.
To study the effect of monovalent cation and divalent metal cation requirements, the potassium phosphate buffer used to measure the oxidative decarboxylation malate activity was replaced by Tris-HCl buffer (100 mM, pH 7.8).
Purification of malic enzyme.
Bacterial cells were washed twice in KCl (0.2% [wt/vol]) and resuspended in Tris-tricarballylate buffer (270 mM, pH 7.8) containing MgCl2 (4.5 mM) and glycerol (22% [vol/vol]). Cells were disrupted by sonication (8 cycles of 30 s with 90-s cooling intervals) and were maintained on ice during sonication. The suspension was centrifuged at 10,000 × g for 15 min to remove cell debris. The supernatant was used to determine the specific activity of malic enzyme and for the protein purification process described below.
In step I, polyethyleneimine (PEI) was added to the crude extract with constant stirring to a final concentration of 0.04%. The mixture was stirred for 30 min at 4°C, and the precipitate was removed by centrifugation at 5,000 × g for 15 min.
In step II, the enzyme solution was applied to a Sepharose Q anion-exchange column (1.6 by 20 cm) (Pharmacia) previously equilibrated with Tris-HCl buffer (40 mM, pH 8.5) containing 5 mM MgCl2, 10 mM KCl, and 1 mM EDTA (buffer A). Malic enzyme was eluted with this buffer solution containing 0.34 M NaCl at a flow rate of 1 ml/min. The fractions containing the malic enzyme activity were pooled and desalted with a PD-10 column (Amersham Pharmacia Biotech).
In step III, the enzyme fraction was passed through a Blue-Sepharose CL-6B (Amersham Pharmacia Biotech) column (20 ml) which had been equilibrated with buffer A. The malic enzyme was eluted at 0.7 ml/min in buffer A for 30 min, followed by 30 min in buffer A containing 200 mM NaCl, and finally 30 min in buffer A containing 300 mM NaCl. (Malic enzyme was recovered in this last elution.) Fractions containing the enzyme activity were pooled and desalted.
In step IV, the protein extract from step III was run on a Red-Sepharose CL-6B (Amersham Pharmacia Biotech) column (10 ml) equilibrated with buffer A. The malic enzyme was eluted at 0.5 ml/min with buffer A for 30 min followed by the same buffer containing 200 mM NaCl for 30 min (malic enzyme was eluted during this period). The fractions exhibiting malic enzyme activity were combined, desalted, and stored at 4°C prior to enzymatic characterization.
Electrophoretic analysis of proteins.
Electrophoresis was carried out with the PHAST system (Amersham Pharmacia Biotech). Under denaturing conditions, the sample was diluted with denaturing buffer Tris-HCl (10 mM) containing EDTA (1 mM), sodium dodecyl sulfate (SDS; 2.5% [wt/vol]), β-mercaptoethanol (5% [wt/vol]), and bromophenol blue (0.01% [wt/vol]) and heated to 90°C for 1 min before being loaded on a polyacrylamide PhastGel gradient 8-25 (Amersham Pharmacia Biotech). The buffer system in PhastGel SDS buffer was tricine (0.2 M trailing ion)-Tris (0.2 M)-SDS (0.55%) at pH 8.1.
Under native conditions, the frontline marker was added to the sample and placed on a polyacrylamide PhastGel gradient 8-25. The buffer system in PhastGel native buffer was l-alanine (0.88 M)-Tris (0.25 M) at pH 8.8. For both types of electrophoresis, the proteins were revealed by AgNO3 (0.5% [wt/vol]) staining.
Nucleotide sequence accession number.
The nucleotide sequence reported in this work has been assigned GenBank accession no. AF234535.
RESULTS
Isolation of the malE gene from C. glutamicum.
Cloning of the malE gene from C. glutamicum was initiated by DNA-DNA hybridization with a designed DNA probe. Comparison of deduced amino acid sequences of NADP-dependent malic enzymes from the gram-positive bacteria Bacillus stearothermophilus (24) and Streptococcus bovis (23) revealed the presence of two highly conserved regions (AAMPVMEGKA and HDDQHGTAIV), which are located at the N and C termini, respectively, of these malic enzymes. From these peptide sequences, two degenerated oligonucleotides were designed, taking into account the codon bias index for moderately expressed genes of C. glutamicum (28) and the Wobble rule. These two oligonucleotides (5′ GCGATGCCWGTCATGGAAGGRAARGTK 3′ and 5′ GTRCTACTRGTYGTRCCRTGYCG 3′) were used for PCR amplification of C. glutamicum chromosomal DNA. The resulting 220-bp DNA fragment was cloned in the pGEMT cloning vector and sequenced. Comparison of the deduced polypeptide with known malic enzyme sequences highlighted a strong similarity between the cloned fragment and malE genes from various organisms. The amplified DNA fragment was isolated, radioactively labeled, and used as a probe against C. glutamicum-issued DNA fragments within the DNA bank. Three plasmids were isolated, namely pMF11, pMF12, and pMF13. NADP+-dependent malic enzyme activity was then tested in E. coli cells harboring either pMF11, pMF12, or pMF13. Plasmid pMF12 was chosen for further experiments, since it encodes a functional malE gene as estimated from enzyme measurements (malic enzyme specific activity of 0.127 μmol/mg of protein−1 · min−1 as compared to activities of <0.01 μmol/mg of protein−1 · min−1 in the other strains).
Southern hybridization was performed to confirm that the cloned fragment originated from C. glutamicum. Chromosomal DNAs from C. glutamicum and E. coli (plasmid pMF12) were digested with PstI or EcoRV, size fractionated, and transferred onto a membrane. Radioactively labeled pMF12 was used as a probe. The probe hybridized specifically to chromosomal DNA from C. glutamicum and pMF12 (data not shown), confirming that the cloned fragment originated from C. glutamicum and that it corresponds to a fragment within the genome with no detectable structural alterations.
Nucleotide sequence of the malE gene.
The nucleotide sequence of a 2,226-bp fragment from pMF12 was determined from both strands by the dideoxy chain termination method. The sequence has been deposited in GenBank under accession no. AF234535. Computer analysis revealed a main ORF extending from bp 725 to 1900. This ORF exhibited a codon usage (P/T = 0.725) indicative of a highly expressed gene of C. glutamicum (28). Database searches with the deduced polypeptides of this ORF revealed similarities to sequences stored in the GenBank and SwissProt databases with significant identity to known malic enzymes. These results indicate that this ORF represents the malE gene from C. glutamicum. From comparison data, the predicted translational initiation site at nucleotide 212 is an ATG codon. The malE gene is not preceded by a typical ribosome binding site (AAGGAG), but is followed by a structure resembling a rho-independent transcription terminator (43). According to the rules of Tinoco et al. (49), a ΔG value of −23.7 kcal/mol at 37°C can be predicted for the predicted mRNA hairpin loop sequence. The predicted malE gene product consists of 392 amino acids with a molecular weight of 40,950, which is in agreement with the subunit molecular weight of 42,000 determined by SDS-polyacrylamide gel electrophoresis (PAGE) for the purified enzyme from C. glutamicum (see below).
Analysis of the deduced amino acid sequence from C. glutamicum.
GenBank and SwissProt database searches with the deduced amino acid sequence of the C. glutamicum malic enzyme revealed close matches to the complete amino acid sequences of malic enzymes from a number of gram-positive and gram-negative bacteria. In an alignment, the C. glutamicum enzyme shows 69, 68, 63, 64, and 63% identity to the enzymes from B. stearothermophilus (24), S. bovis (23), Bacillus subtilis (26), E. coli (3), and Haemophilus influenzae (16), respectively. The alignment showed that identical residues are scattered throughout the sequence. A secondary structure prediction was made for the deduced amino acid sequence of the C. glutamicum malic enzyme with the ProDom domain search program (11). The calculated secondary structure of C. glutamicum malic enzyme indicates a compact, globular structure for the subunits. From these data, regions of functional significance could be highlighted. Region Y44 to C52 could be assigned to the binding site for malate (25). The sequence from K193 to D223 matches perfectly the consensus sequence for ADP-binding sites, with a predicted βαβ secondary structure (52), and can be assigned to the binding site of the ADP ring of NADP+.
Biochemical characteristics of malic enzyme. (i) Purification of malic enzyme.
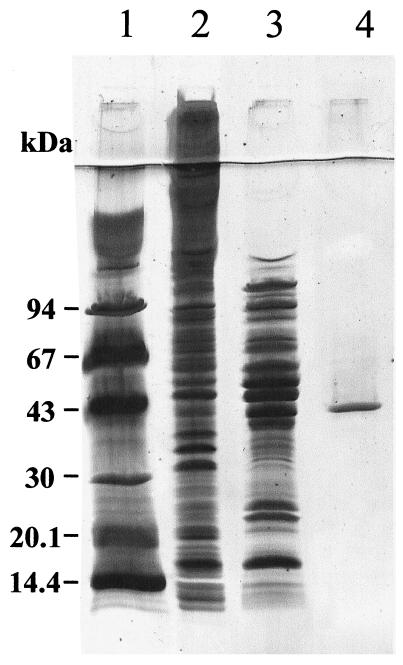
The results of the purification procedure are summarized in Table 2. The four-step procedure led to an 88-fold purification with a 17% recovery of initial activity. A single band was obtained on SDS-PAGE, demonstrating the homogeneity of the purified enzyme (Fig. 1). The molecular weight of the subunit was estimated to be 42,000, in close agreement with the molecular weight estimated from the gene sequence. From the purification data, the malic enzyme protein represents about 1% of total cytoplasmic proteins. This value is in full accordance with the calculated P/T value (28), estimated from the codon usage in the malE sequence, indicating a high abundance for the malic enzyme in C. glutamicum. Under native conditions, the estimated molecular weight was 105,000. This result suggested that the malic enzyme of C. glutamicum was a dimer.
TABLE 2.
Purification factor and activity recovery of malic enzyme from C. glutamicum during the different steps of protein purification
| Purification step | Total activity (μmol/min) | Yield (%) | Sp act (μmol · min−1 · mg of protein−1) | Purification factor |
|---|---|---|---|---|
| Crude extract | 47.4 | 100 | 0.124 | 1 |
| PEI | 46.5 | 98 | 0.16 | 1.3 |
| Sepharose Q | 30.3 | 64 | 2.6 | 21 |
| Blue trisacryl | 17 | 36 | 6.55 | 53 |
| Red Sepharose | 8 | 17 | 10.9 | 88 |
FIG. 1.
SDS-PAGE of the malic enzyme from C. glutamicum at various steps of purification. Lanes: 1, molecular mass markers (kilodaltons); 2, preparation after PEI precipitation; 3, preparation after elution from Sepharose Q; 4, preparation after elution from red Sepharose.
(ii) Effect of pH and temperature.
The optimum pH for the oxidative decarboxylation activity with regard to l-malate was 7.8 when using potassium phosphate or Tris-HCl buffer. This is similar to the optimum pH of other malic enzymes. In the reverse direction, the optimum pH was 7.5. The same value was found for the malic enzyme of S. bovis (23), although an optimal pH of 6.0 has been reported for the enzyme of B. stearothermophilus (24). The highest specific activity of the purified enzyme was obtained at 50°C, and no measurable activity could be detected at 60°C. Malic enzyme of C. glutamicum was stable for at least 120 min at 4°C, whereas incubation at temperatures between 33 and 40°C led to progressive inactivation of the enzyme. After a 120-min preincubation at 33 and 40°C, only 76 and 30%, respectively, of the initial activity was detected.
(iii) Kinetic parameters.
The C. glutamicum malic enzyme was strictly NADP+ dependent; no activity could be measured with NAD+ as a cofactor. Moreover, no measurable activity was detected with d-malate. The kinetics constants (Km and Vmax) of the purified enzyme were calculated by the double reciprocal plot for both directions. Kms of 3.8 ± 0.6 and 13.8 ± 3.5 mM were determined for malate and pyruvate, respectively. The Km for pyruvate is similar to values determined for the malic enzymes of E. coli (Km = 16 mM) (48) and S. bovis (Km = 11.4 mM) (23), but somewhat lower than that reported for Ascaris suum (Km = 45 mM) (27). The Kms for NADP+ and NADPH were estimated to be 0.083 ± 0.017 and 0.06 ± 0.015 mM, respectively. The Vmax for the carboxylating activity (2.25 μmol/min−1 · mg of protein−1) was fivefold lower than the Vmax for decarboxylating activity (10.9 μmol/min−1 · mg of protein−1).
(iv) Effect of NH4+ and K+.
Malic enzyme activity from various microorganisms has been shown to be activated by NH4+ and K+ (15, 17, 23, 24). This was shown to also be the case for the malic enzyme of C. glutamicum. Addition of NH4+ or K+ increased the measured activity in both the oxidative decarboxylation and the reductive carboxylation directions. The Kds determined were 13 mM for K+ and 1.25 mM for NH4+.
(v) Divalent cation requirements.
Malic enzyme activity was demonstrated to be dependent on the presence of divalent metal cations. Maximum activity was observed in the presence of 5 mM Mn2+ (Table 3), although other divalent cations (Mg2+, Co2+, and Ni2+, but not Ca2+) also had a positive effect on enzyme activity. In the absence of such divalent cations, a residual activity of 12% was measured, due probably to the presence of trace amounts of MgCl2 in the enzyme preparation. This positive response of malic enzyme to the presence of divalent cations appears to be a conserved feature of this enzyme.
TABLE 3.
Effect of divalent metal ions, OAA, and glutamate on in vitro malic enzyme activity from C. glutamicuma
| Divalent cation | Concn (mM) | Activity (%) | Compound | Concn (mM) | Activity (%) |
|---|---|---|---|---|---|
| None | 12 | None | 100 | ||
| Mn2+ | 5 | 100 | OAA | 2.5 | 87 |
| 1 | 96 | 5 | 79 | ||
| Mg2+ | 5 | 94 | 10 | 67 | |
| 1 | 84 | ||||
| Co2+ | 5 | 56 | Glutamate | 25 | 92 |
| 1 | 73 | 100 | 88 | ||
| Ni2+ | 5 | 31 | 200 | 75 | |
| 1 | 37 | ||||
| Ca2+ | 5 | 5 | |||
| 1 | 0 |
All specific activities were measured at least three times per assay condition, and values are averaged values in which standard errors were less than ±5%.
(vi) Effect of various compounds on malic enzyme activity.
The possible effect of phosphorylated glycolytic intermediates on malic enzyme activity was examined by using concentrations corresponding to the in vivo concentrations seen during both the exponential growth phase and the amino acid overproduction phase associated with glutamate accumulation (18). None of these metabolites exerted a significant effect on malic enzyme activity at the following concentrations: 6-phosphogluconate (2.5 and 5 mM), glucose-6-phosphate (10, 20, and 40 mM), fructose-6-phosphate (2, 4, and 8 mM), fructose-1,6-bisphosphate (10 and 20 mM), glyceraldehyde-3-phosphate (2 and 4 mM), PEP (1.5 and 10 mM), acetyl-CoA (0.02, 0.2, and 0.4 mM), ATP (0.5 and 1 mM), and ADP (0.5 and 1 mM). However, addition of OAA or glutamate provoked some, although limited, inhibition (Table 3). With respect to OAA, the malic enzyme of C. glutamicum was less sensitive than others (7, 17, 23), while sensitivity to inhibition by glutamate has not been examined previously.
Effect of modified expression of the malE gene in C. glutamicum.
To study whether C. glutamicum requires the malE gene for growth on various carbon sources, and to what extent the malE gene is of metabolic significance, the malE-overexpressing wild-type strain carrying the pMF22 plasmid [WT(pMF22)] and the malE-deficient (ΔmalE) strain were constructed (see Materials and Methods). Malic enzyme measurements in transformed cells shows that such strains showed the appropriate levels of enzyme activity (Table 4). The ΔmalE strain had no detectable malic enzyme activity, while the WT(pMF22) strain had high and constitutive malic enzyme activity during growth on various carbon substrates.
TABLE 4.
Malic enzyme specific activities in C. glutamicum strains grown on different carbon substrates
| Strain | Sp act (μmol · min−1 · mg of protein−1) on C sourcea:
|
||
|---|---|---|---|
| Glucose | Lactate | Acetate | |
| Wild type | 0.14 ± 0.01 | 0.18 ± 0.01 | 0.034 ± 0.005 |
| ΔmalE | <0.01 | <0.01 | <0.01 |
| WT(pMF22) | 1.0 ± 0.04 | 1.4 ± 0.06 | 1.6 ± 0.06 |
The results are average values obtained with protein extracts taken from at least two independent experiments and analyzed in triplicate, and the standard errors are indicated.
(i) Malic enzyme activity.
For the wild-type strain, the highest activity was found in cells grown on lactate as the carbon source (Table 4). On glucose, the activity was slightly lower, whereas on acetate, only low measurable activity was detected. These results suggest that the C. glutamicum malic enzyme is regulated by the carbon source and might therefore be expected to have a physiological role during growth on those substrates for which high activity was measured, i.e., glucose and lactate. In light of the low level of malE expression during growth on acetate, any significant physiological role during acetate metabolism would seem unlikely.
(ii) Growth behavior.
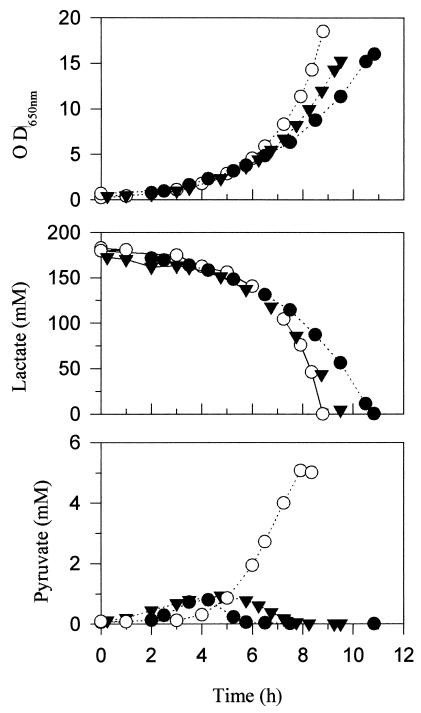
The growth behaviors of the C. glutamicum wild-type, ΔmalE, and WT(pMF22) strains were determined on minimal media containing glucose, lactate, or acetate as the sole carbon source. The ΔmalE strain is able to grow on all three substrates, indicating that malic enzyme is not an essential enzyme during growth on any of these substrates. Indeed, on both glucose and acetate, the genetically engineered strains showed identical specific growth rates to the wild-type strain (results not shown). However, significant differences were observed during growth on lactate (Fig. 2), for which the extent of the exponential growth period was dependent upon the level of malic enzyme activity. The ΔmalE strain showed a significantly diminished rate of growth after 8 h of fermentation compared to the wild type, while the WT(pMF22) strain maintained exponential growth throughout the entire fermentation. During the initial hours of the culture, all strains showed an exponential growth rate of 0.5 h−1, but after 8 h, the growth rates were 0.3, 0.4, and 0.5 h−1 for the ΔmalE, wild-type, and WT(pMF22) strains, respectively. The maintained exponential growth phase was seen to be correlated to the more rapid rate of lactate consumption during this period of the fermentation. Diminished growth rates of C. glutamicum on lactate have previously been attributed to pyruvate accumulation (9). When this was examined in the three strains used in this study, the WT(pMF22) strain was shown to have a profile of pyruvate accumulation different from those of the other strains. Pyruvate accumulation was somewhat delayed, but then increased to significantly higher levels than those seen for the other strains (Fig. 2).
FIG. 2.
Comparative growth behavior, lactate consumption, and pyruvate accumulation with the C. glutamicum wild-type (▾), ΔmalE (●), and malE-overexpressing WT(pMF22) (○) strains on lactate. OD650 nm, optical density at 650 nm.
DISCUSSION
The malE gene, encoding malic enzyme from C. glutamicum, has been cloned and studied at the molecular level. The cloned malE gene exhibits classical features for a highly expressed gene from C. glutamicum, and the corresponding protein is clearly homologous to other NADP+-dependent malic enzymes. Malic enzyme was purified from C. glutamicum, and its biochemical characteristics were shown to be similar to those of other microbial malic enzymes. The enzyme exhibits similar requirements for divalent cations, is activated by NH4+ and K+, and has typical pH and temperature optima. When the two anaplerotic enzymes involving pyruvate (malic enzyme and pyruvate carboxylase) are compared, it can be seen that affinity for pyruvate was approximately 10-fold lower for the malic enzyme (Km = 13.4 mM) than for the pyruvate carboxylase activity (Km = 1.3 mM) (38). In order for malic enzyme to contribute to TCA cycle replenishment, pyruvate would need to accumulate to high intracellular concentrations. This was observed for Δppc strains grown under biotin limitation (Mathieu et al., rDNA Biotechnol. 4: Metab. Eng. I, poster abstr. 53, 1996), indicating that malic enzyme can fulfill such a function, but such a role in wild-type cells growing on sugars seems highly improbable. Recent NMR studies have indicated some degree of back flux from the TCA cycle to pyruvate during both growth and lysine overproduction (29, 47), and in view of the kinetic characteristics of the malic enzyme, the implication of this enzyme in such a back flux would seem feasible. This exchange reaction can occur as a deviation of the TCA cycle flux, bypassing cytoplasmic malate dehydrogenase activity or via the recently described membrane-bound flavoprotein which can reduce OAA to malate (31). The presence of this ubiquinone-dependent enzyme makes possible a cycle involving pyruvate carboxylation to OAA followed by reduction to malate and then decarboxylation back to pyruvate. The operation of such a cycle would generate additional NADPH without carbon loss via CO2 production and has been proposed previously for growth on substrates known to have a relatively low flux through the pentose pathway (9, 13). Such a role may explain the high level of expression on glucose and also the difficulty in obtaining evidence from simple analysis of the growth response. As shown recently, C. glutamicum is capable of modulating NADPH production in response to demand in various ways (30). These authors significantly diminished the NADPH requirement during lysine production by replacing the NADPH-dependent glutamate dehydrogenase with the NADH-dependent enzyme from Peptostreptococcus asaccharolyticus, but did not improve lysine production. Instead, the flux through the NADPH-generating pentose pathway was significantly diminished. The absence of a phenotype on glucose for the strains used here might therefore indicate that other pathways generating NADPH were modified in the strains used, indicating a certain degree of redundancy in the metabolic pathway network.
The absence of a phenotype during growth on acetate is not surprising, since malic enzyme is present at only low intracellular concentrations indicative of low expression of malE on this substrate. This is indicative of some carbon source-related regulation of malE expression, and a more detailed molecular characterization of the promoter sequence may facilitate the identification of the underlying mechanism. On acetate, the glyoxylate cycle was found to be the essential anaplerotic pathway (40, 41).
The modified growth behavior of the ΔmalE strain and the WT(pMF22) strain on lactate compared to that of the wild-type strain indicates a more important role for malic enzyme during growth on lactate. A C. glutamicum strain lacking both pyruvate and PPCs was unable to grow on lactate (39), so again, an anaplerotic function can be excluded, despite the high pyruvate pool associated with lactate metabolism (8). A more probable role here is the NADPH-generating reaction, discussed above and postulated to be a potential manner of NADPH generation during lactate metabolism by Cocaign-Bousquet and Lindley (9), who used stoichiometric flux analysis to formulate their hypothesis. The higher activities of both malic enzyme and pyruvate carboxylase (38) on lactate add supportive evidence to this model. This bacterium appears to have evolved an extremely complex network of reactions linking glycolysis to the TCA cycle. Malic enzyme offers one such possibility and is probably physiologically important as a means of supplying NADPH availability on gluconeogenic substrates unable to orientate a high carbon flux through alternative NADPH-generating pathways, such as the pentose pathway. Presumably, the high pyruvate concentration in the WT(pMF22) strain during growth on lactate is observed because of the maintenance of a high rate of lactate consumption, although the reasons for pyruvate accumulation during the prolonged exponential growth phase will require a more detailed analysis of the central pathways. It will be interesting to see whether modulation of this activity will be a useful genetic engineering strategy to increase NADPH availability so as to improve the production yields of compounds requiring significant amounts of NADPH for their biosynthetic pathways.
ACKNOWLEDGMENTS
We thank Pierre Escalier for valuable technical assistance.
This work received financial support from ORSAN-Amylum, the CNRS, and the European Union Cell Factory program (BIO4-CT96-0145).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1987. [Google Scholar]
- 2.Birnboim H C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- 3.Blattner F R, Plunkett G, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner F D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Man B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 4.Bonamy C, Guyonvarch A, Reyes O, David F, Leblon G. Interspecies electro-transformation in Corynebacteria. FEMS Microbiol Lett. 1990;66:263–270. doi: 10.1016/0378-1097(90)90294-z. [DOI] [PubMed] [Google Scholar]
- 5.Brosius J. A survey of molecular cloning vectors and their uses. In: Rodriguez R L, Denhardt D T, editors. Vectors. Boston, Mass: Butterworth; 1988. pp. 205–225. [Google Scholar]
- 6.Caruthers M H, Barone A D, Beaucage S L, Dodds D R, Fischer E F, McBride L J, Matteuci M, Staninsky Z, Tang J Y. Chemical synthesis of deoxynucleotides by the phosphoramidite method. Science. 1985;230:281. [Google Scholar]
- 7.Chen F, Okabe Y, Osano K, Tajima S. Purification and characterization of the NADP-malic enzyme from Bradyrhizobium japonicum A1017. Biosci Biotechnol Biochem. 1997;61:384–386. doi: 10.1271/bbb.61.384. [DOI] [PubMed] [Google Scholar]
- 8.Cocaign-Bousquet M, Monnet C, Lindley N D. Batch kinetics of Corynebacterium glutamicum during growth on various substrates: use of substrate mixtures to localise metabolic bottlenecks. Appl Microbiol Biotechnol. 1993;40:526–530. [Google Scholar]
- 9.Cocaign-Bousquet M, Lindley N D. Pyruvate overflow and carbon flux within the central metabolic pathways of Corynebacterium glutamicum during growth on lactate. Enzyme Microb Technol. 1995;17:260–267. [Google Scholar]
- 10.Cocaign-Bousquet M, Guyonvarch A, Lindley N D. Growth rate-dependent modulation of carbon flux through central metabolism and the kinetic consequences for glucose-limited chemostat cultures of Corynebacterium glutamicum. Appl Environ Microbiol. 1996;62:429–436. doi: 10.1128/aem.62.2.429-436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpet F, Gouzy J, Kahn D. The ProDom database of protein domain families. Nucleic Acids Res. 1998;26:323–326. doi: 10.1093/nar/26.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaunay S, Uy D, Baucher M F, Engasser J M, Guyonvarch A, Goergen J L. Importance of phosphoenolpyruvate carboxylase of Corynebacterium glutamicum during the temperature triggered glutamic acid fermentation. Metab Eng. 1999;1:334–343. doi: 10.1006/mben.1999.0131. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez H, Rollin C, Guyonvarch A, Guerquin-Kern J-L, Lindley N D. Carbon flux distribution in the central metabolic pathways of Corynebacterium glutamicum during growth on fructose. Eur J Biochem. 1998;254:96–102. doi: 10.1046/j.1432-1327.1998.2540096.x. [DOI] [PubMed] [Google Scholar]
- 14.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988;1:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driscoll B T, Finan T M. Properties of NAD+- and NADP+-dependent malic enzymes of Rhizobium (Sinorhizobium) meliloti and differential expression of their genes in nitrogen-fixing bacteroids. Microbiology. 1997;143:489–498. doi: 10.1099/00221287-143-2-489. [DOI] [PubMed] [Google Scholar]
- 16.Fleishmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, Fitzhugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritschman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 17.Garrido-Pertierra A, Martinez Marcos C, Martin Fernandez M, Ruiz-Amil M. Properties and function of malate enzyme from Pseudomonas putida. Biochimie. 1983;65:629–635. doi: 10.1016/s0300-9084(84)80026-7. [DOI] [PubMed] [Google Scholar]
- 18.Gourdon P, Lindley N D. Metabolic analysis of glutamate production by Corynebacterium glutamicum. Metab Eng. 1999;1:224–231. doi: 10.1006/mben.1999.0122. [DOI] [PubMed] [Google Scholar]
- 19.Gubler M E, Jetten M S M, Lee S H, Sinskey A J. Effects of phosphoenol pyruvate carboxylase deficiency on metabolism and lysine production in Corynebacterium glutamicum. Appl Microbiol Biotechnol. 1994;40:857–863. [Google Scholar]
- 20.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Jetten M S M, Sinskey A J. Characterization of phosphoenolpyruvate carboxykinase from Corynebacterium glutamicum. FEMS Microbiol Lett. 1993;111:183–188. [Google Scholar]
- 22.Jetten M S M, Sinskey A J. Purification and properties of oxaloacetate decarboxylase from Corynebacterium glutamicum. Antonie Leeuwenhoek. 1995;67:221–227. doi: 10.1007/BF00871217. [DOI] [PubMed] [Google Scholar]
- 23.Kawai S, Suzuki H, Yamamoto K, Inui M, Yakawa H, Kumagai H. Purification and characterization of a malic enzyme from the ruminal bacterium Streptococcus bovis ATCC 15352 and cloning and sequencing of its gene. Appl Environ Microbiol. 1996;62:2692–2700. doi: 10.1128/aem.62.8.2692-2700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi K, Doi S, Negoro S, Urabe I, Okada H. Structure and properties of malic enzyme from Bacillus stearothermophilus. J Biol Chem. 1989;6:3200–3205. [PubMed] [Google Scholar]
- 25.Kulkarni G, Cook P F, Harris B G. Cloning and nucleotide sequence of a full-length cDNA encoding Ascaris suum malic enzyme. Arch Biochem Biophys. 1993;300:231–237. doi: 10.1006/abbi.1993.1032. [DOI] [PubMed] [Google Scholar]
- 26.Lapidus A, Galleron N, Sorokin A, Ehrlich S D. Sequencing and functional annotation of the Bacillus subtilis genes in the 200 kb rrnB-dnaB region. Microbiology. 1997;143:3431–3441. doi: 10.1099/00221287-143-11-3431. [DOI] [PubMed] [Google Scholar]
- 27.Mallick S, Harris B G, Cook P F. Kinetic mechanism of NAD:malic enzyme from Ascaris suum in the direction of reductive carboxylation. J Biol Chem. 1991;266:2732–2738. [PubMed] [Google Scholar]
- 28.Malumbres M, Gil J A, Martin J F. Codon preference in Corynebacteria. Gene. 1993;134:15–24. doi: 10.1016/0378-1119(93)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Marx A, Striegel K, de Graaf A A, Sahm H, Eggeling L. Response of the central metabolism of Corynebacterium glutamicum to different flux burdens. Biotechnol Bioeng. 1997;56:168–180. doi: 10.1002/(SICI)1097-0290(19971020)56:2<168::AID-BIT6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Marx A, Eikmanns B J, Sahm H, de Graaf A A, Eggeling L. Response of the central metabolism in Corynebacterium glutamicum to the use of an NADH-dependent glutamate dehydrogenase. Metab Eng. 1999;1:35–48. doi: 10.1006/mben.1998.0106. [DOI] [PubMed] [Google Scholar]
- 31.Molenaar D, van der Reste M E, Petrovic S. Biochemical and genetic characterization of the membrane associated malate dehydrogenase (acceptor) from Corynebacterium glutamicum. Eur J Biochem. 1998;254:395–403. doi: 10.1046/j.1432-1327.1998.2540395.x. [DOI] [PubMed] [Google Scholar]
- 32.Mori M, Shiio I. Purification and some properties of phosphoenolpyruvate carboxylase from Brevibacterium flavum and its aspartate-overproducing mutant. J Biochem. 1985;97:1119–1128. doi: 10.1093/oxfordjournals.jbchem.a135156. [DOI] [PubMed] [Google Scholar]
- 33.Mori M, Shiio I. Synergistic inhibition of phosphoenolpyruvate carboxylase by aspartate and 2-oxoglutarate in Brevibacterium flavum. J Biochem. 1985;98:1621–1630. doi: 10.1093/oxfordjournals.jbchem.a135432. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson B, Uhlen M, Josephson S, Gatenbeck S, Philipson L. An improved positive selection vector constructed by oligonucleotide mediated mutagenesis. Nucleic Acids Res. 1983;11:8019–8030. doi: 10.1093/nar/11.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Regan M, Thierbach G, Bachmann B, Villeval D, Lepage P, Viret J F, Lemoine Y. Cloning and nucleotide sequence of phosphoenolpyruvate carboxylase-coding gene of Corynebacterium glutamicum ATCC13032. Gene. 1989;77:237–251. doi: 10.1016/0378-1119(89)90072-3. [DOI] [PubMed] [Google Scholar]
- 36.Park S M, Shaw-Reid C, Sinskey A J, Stephanopoulos G. Elucidation of anaplerotic pathways in Corynebacterium glutamicum via 13C-NMR spectroscopy and GC-MS. Appl Microbiol Biotechnol. 1997;47:430–440. [Google Scholar]
- 37.Peters-Wendisch P G, Eikmanns B J, Thierbach G, Bachmann B, Sahm H. Phosphoenolpyruvate carboxylase in Corynebacterium glutamicum is dispensable for growth and lysine production. FEMS Microbiol Lett. 1993;112:269–274. [Google Scholar]
- 38.Peters-Wendisch P G, Wendisch W F, Paul S, Eikmanns B J, Sahm H. Pyruvate carboxylase as an anaplerotic enzyme in Corynebacterium glutamicum. Microbiology. 1997;143:1095–1103. doi: 10.1099/00221287-143-4-1095. [DOI] [PubMed] [Google Scholar]
- 39.Peters-Wendisch P G, Kreutzer C, Kalinowski J, Patek M, Sahm H, Eikmanns B J. Pyruvate carboxylase from Corynebacterium glutamicum: characterisation, expression and inactivation of the pyc gene. Microbiology. 1998;144:915–927. doi: 10.1099/00221287-144-4-915. [DOI] [PubMed] [Google Scholar]
- 40.Reinscheid D J, Eikmanns B J, Sahm H. Characterization of the isocitrate lyase gene from Corynebacterium glutamicum and biochemical analysis of the enzyme. J Bacteriol. 1994;176:3474–3483. doi: 10.1128/jb.176.12.3474-3483.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reinscheid D J, Eikmanns B J, Sahm H. Malate synthase from Corynebacterium glutamicum: sequence analysis of the gene and biochemical characterization of the enzyme. Microbiology. 1994;140:3099–3108. doi: 10.1099/13500872-140-11-3099. [DOI] [PubMed] [Google Scholar]
- 42.Reyes O, Guyonvarch A, Bonamy C, Salti V, David F, Leblon G. Integron-bearing vectors: a method suitable for stable chromosomal integration in highly restrictive Corynebacteria. Gene. 1991;107:61–68. doi: 10.1016/0378-1119(91)90297-o. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg M, Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sano K, Ito K, Miwa K, Nakamori S. Amplification of the phosphoenolpyruvate carboxylase gene of Brevibacterium glutamicum to improve amino acid production. Agric Biol Chem. 1987;51:597–599. [Google Scholar]
- 47.Sonntag K, Schwinde J, de Graaf A A, Marx A, Eikmanns B J, Sahm H. 13C NMR studies of the fluxes in the central metabolism of Corynebacterium glutamicum during growth and overproduction of amino acids in batch cultures. Appl Microbiol Biotechnol. 1995;44:489–495. [Google Scholar]
- 48.Stols L, Donnelly M I. Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol. 1997;63:2695–2701. doi: 10.1128/aem.63.7.2695-2701.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tinoco I, Borer P N, Dengler B, Levine M D, Uhlenbeck O C, Crothers D M, Gralla J. Improved estimation of secondary structure in ribonucleic acid. Nat New Biol. 1973;246:40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- 50.Tosaka O, Morioka H, Takinami K. The role of biotin-dependent pyruvate carboxylase in L-lysine production. Agric Biol Chem. 1979;43:1513–1519. [Google Scholar]
- 51.Vallino J J, Stephanopoulos G. Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Biotechnol Bioeng. 1993;41:633–646. doi: 10.1002/bit.260410606. [DOI] [PubMed] [Google Scholar]
- 52.Wierenga R K, Terpstra P, Hol W G J. Prediction of the occurrence of the ADP-binding βαβ fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]