Abstract
Background
Allogeneic blood transfusions in oncologic surgery are associated with increased recurrence and mortality. Adverse effects on outcome could be reduced or avoided by using intraoperative autologous blood cell salvage (IOCS). However, there are concerns regarding the safety of the autologous IOCS blood. Previous meta-analyses from 2012 and 2020 did not identify increased risk of cancer recurrence after using autologous IOCS blood. The objective of this review was to reassess a greater number of IOCS-treated patients to present an updated and more robust analysis of the current literature.
Methods
This systematic review includes full-text articles listed in PubMed, Cochrane, Cochrane Reviews, and Web of Science. We analyzed publications that discussed cell salvage or autotransfusion combined with the following outcomes: cancer recurrence, mortality, survival, allogeneic transfusion rate and requirements, length of hospital stay (LOS). To rate the strength of evidence, a Grading of Recommendations Assessment, Development and Evaluation (GRADE) of the underlying evidence was applied.
Results
In the updated meta-analysis, 7 further observational studies were added to the original 27 observational studies included in the former 2020 analysis. Studies compared either unfiltered (n = 2,311) or filtered (n = 850) IOCS (total n = 3,161) versus non-IOCS use (n = 5,342). Control patients were either treated with autologous predonated blood (n = 484), with allogeneic transfusion (n = 4,113), or did not receive a blood transfusion (n = 745). However, the current literature still contains only observational studies on these topics, and the strength of evidence remains low. The risk of cancer recurrence was reduced in recipients of autologous salvaged blood with or without LDF (odds ratio [OR] 0.76, 95% confidence interval [CI]: 0.64–0.90) compared to nontransfused patients or patients with allogeneic transfusion. There was no difference in mortality (OR 0.95, 95% CI: 0.71–1.27) and LOS (mean difference −0.07 days, 95% CI: −0.63 to 0.48) between patients treated with IOCS blood or those in whom IOCS was not used. Due to high heterogeneity, transfusion rates or volumes could not be analyzed.
Conclusion
Randomized controlled trials comparing mortality and cancer recurrence rate of IOCS with or without LDF filtration versus allogeneic blood transfusion were not found. Outcome was similar or better in patients receiving IOCS during cancer surgery compared to patients with allogeneic blood transfusion or nontransfused patients.
Keywords: Cell salvage, Tumor surgery, Autologous transfusion, Cancer recurrence
Background
Perioperative allogeneic red blood cell (RBC) transfusion is associated with increased mortality and cancer recurrence in multiple retrospective studies [1, 2, 3, 4, 5, 6]. The underlying trials adjusted their empirical observations for confounding factors such as preoperative anemia, severity of illness, perioperative blood loss, variations in hemotherapy algorithms, and extent of surgical trauma [7]. Some results suggest that transfusion-related immunomodulation may affect transfusion-associated outcome results [8]. Although the controversy continues whether the observed outcome after allogeneic RBC transfusion is due to correlation or causation, blood-sparing techniques are important to reduce transfusion-associated adverse events [9, 10].
Blood-sparing techniques, especially in cancer surgery, should be investigated with regards to their intrinsic safety profile [10]. Intraoperative autologous blood cell salvage (IOCS) is one method to reduce the rate and amount of allogeneic RBC transfusion [11]. The principle of IOCS is to collect and process blood from the surgical site and reinfuse the autologous blood. IOCS has been shown to significantly reduce allogeneic transfusion in metastatic spine surgery as well as adult cardiac and orthopedic surgery [11, 12]. The modern IOCS nowadays uses autologous blood return following filtration with a leukocyte reduction filter (“LRF” or leukocyte depletion “LDF” [“depletion” is defined as a more stringent reduction of leucocytes to less than 106 per unit]) in order to reduce contamination from tumor cells. Despite this additional safety step, the safety of IOCS in cancer surgery is controversial, and we had yet to see prospective randomized controlled trials on this topic.
Two questions need to be answered regarding the causation for the prevented widespread use of IOCS in daily practice worldwide: Does IOCS in cancer surgery affect survival, metastasis, and cancer recurrence rates? Does a reduction of allogeneic transfusion that might be achieved by IOCS in cancer surgery lead to better oncological outcome primarily via a reduction of potential adverse effects of allogeneic transfusion? i.e., what is safer for the patient: allogeneic or IOCS blood?
To date, the outcomes of patients treated with or without IOCS are unknown. Multicenter randomized double-blinded controlled trials are needed. To assess the effect of IOCS in colorectal cancer surgery, assuming a 5-year survival rate of 40%, at least 1,000 cases would be required to detect and demonstrate a presumed 10% change in the survival rate [13, 14]. For a mixed tumor collective with greater survival rates such as in prostate cancer, a greater number would be required to improve statistical power. Our previous 2020 meta-analysis of 27 observational studies and 1,606 IOCS treatments across a number of surgical specialties suggested that cancer recurrence and survival after nonmodified IOCS are not inferior compared to intraoperative allogeneic RBC transfusion, no transfusion, or preoperative autologous donation (PAD) [15]. On the opposite, we found a slightly reduced recurrence rate in this small number of observational cohorts. This new analysis includes additional observational studies and new IOCS-treated patients published since the prior meta-analysis to confirm or correct the previous result.
Methods
The current literature since our last meta-analysis (May 2019 to March 2022) was systematically reviewed by two independent authors. All full-text publications in PubMed, Cochrane, Cochrane Reviews, and Web of Science with the primary keywords “autologous transfusion/autotransfusion/cell salvage” and the links “tumor, cancer, metastasis, outcome, oncology, recurrence, survival” were screened. Data extraction was done from full-text eligible publications in English, French, Spanish, Italian, and German in a Web-embedded database. The following study details and population demographic characteristics were extracted: study design, timeline, sample size, number in each intervention arm, kind of control group, median length of follow-up in the IOCS group, groupwise absolute numbers for cancer recurrence, mortality or survival, allogeneic-transfused patients' means and standard deviations for transfusion requirements (volume in mL), and length of hospital stay (LOS).
The quality of the evidence was rated as “high,” “moderate,” “low,” “very low” by both authors in the screening process according to a modified Grading of Recommendations Assessment, Development and Evaluation (GRADE) system. The risk of bias was not included in the assessment because the studies were exclusively observational studies, and therefore, we assumed a high risk of bias.
All studies with retransfusion of the salvaged blood with or without LDF filtration in tumor surgery were included in a systematic search, in which the study objective was survival/mortality, recurrence rate, transfusion requirements, and LOS. Studies were excluded if they were non-full-text articles, abstracts, poster presentations, and mixed surgeries for both benign and malign diseases. However, previously published meta-analyses, abstracts only, and case reports were included for qualitative analysis and were not included in the quantitative analysis. The effect estimates of the individual studies were summarized in a meta-analysis.
In some analyses, a distinction was made in the IOCS group between patients who were transfused with autologous blood and those who were not, and an “intention-to-treat” analysis was conducted, regardless of the actual “per-protocol treatment” provided (e.g., [16]). The same was applied in case of clinically irrelevant transfusion volumes and/or rates in the group of IOCS (e.g., [17], in whom only 5 out of 16 patients were retransfused with salvaged blood). If locoregional metastases and distant metastases were reported as outcome parameters, the rate of distant metastases was used. Overall mortality was calculated from the overall survival rate, if it was not stated separately. If general survival or mortality data and disease-specific data were reported, the general ones were used. For case-control studies, the matched results were used (e.g., [18]).
With regards to allogeneic transfusion requirements, some publications provided information on the transfusion volume and others on the rate of allogeneic transfusion recipients. Blood components such as RBC, platelet, and fresh frozen plasma were summed and estimated at 275 mL and whole blood at 450 mL, in order to establish comparability of the total transfusion volume. If a distinction was made between intraoperative and postoperative transfusion requirements, the intraoperative data were used, otherwise, we assumed the total transfusion requirement until discharge or the end of the observation period. If the transfusion rate was zero in a study, a continuity correction of 0.5 was conducted to calculate a risk estimate.
For binary endpoints (recurrences, mortality, patients requiring transfusion), the odds ratio (OR) with 95% confidence intervals (CI) was used. The mean difference (MD) with 95% CIs was used to calculate continuous data such as volume and LOS. The OR and Mantel-Haenszel method were also used to calculate blood volume and LOS. An estimation for MD was calculated using the inverse-variance method. All meta-analyses were based on a random-effects model. Statistical heterogeneity between studies was examined using the I2, with the following overlapping categorization: I2 of 0–30% characterizes little to no heterogeneity, 30–60% for moderate statistical heterogeneity, 50–90% for substantial statistical heterogeneity, and greater than 75% for significant statistical heterogeneity.
Results
The updated meta-analysis included 34 observational studies, with 8,503 enrolled subjects, and 3,161 of those patients were IOCS-treated patients (see Table 1, search methodology Fig. 1). From all studies, 27 studies with n = 2,181 IOCS-treated subjects reported recurrence, 22 studies with n = 1,610 in the IOCS group reported mortality, 20 studies reported the number of transfused patients (n = 1,973 IOCS), 10 studies with n = 842 IOCS subjects reported transfusion volumes, and 10 studies (n = 401 IOCS) stated LOS.
Table 1.
Outcome studies for IOCS in cancer surgery [12, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55]
| Author/level of evidence (GRADE) | Study type, observation period (IOCS group) | IOCS group | Control group | Cancer | Allogeneic transfusion | Cancer recurrence, tumor-free survival, metastases, tumor progress | Mortality | LOS |
|---|---|---|---|---|---|---|---|---|
| Akbulut et al. [24] GRADE category: very low | Retrospective cohort, observation period 26+/–15 months | n = 24 | n = 59, non-IOCS | Hepatocellular carcinoma | No data | No difference for tumor-free survival (p = 0.9), cancer recurrence 29.2% versus. 25.4% (p = 0.7), same for local and distant metastases (p = 0.8) | Overall survival: no difference (p = 0.6) | − |
|
| ||||||||
| Akchurin et al. [25] GRADE category: very low | Retrospective observation, observation period 12 months | n = 8 | No control group | Intravascular tumor growth or lung metastases from kidney cancer or sarcoma | − | All 8 subjects survived the first year without further metastasis or progress | − | − |
|
| ||||||||
| Aning et al. [26] GRADE category: low | Prospective longitudinal study (2001–2010 vs. 2001–2003), observation period 24 months | n = 194 | n = 19, non-IOCS | Urothelial carcinoma of the bladder | IOCS reduced the mixed autologous with allogeneic transfusion rates by 70%, allogeneic only by 50–60% | − | − | IOCS reduced the LOS from 18.4 days auf 10.7 |
|
| ||||||||
| Araujo et al.[27] GRADE category: low | Retrospective observational study Observation period 5 years | n = 122 | n = 36 non-IOCS | HCC | − | No difference for recurrence (p = 0.953, 59.7% vs. 83.3%), IOCS is not independently predictive for recurrence (p = 0.512) | No difference for survival rate (p = 0.51) | |
|
| ||||||||
| Bower et al. [28] GRADE category: low | Prospective cohort, observation period 18 months | n = 32 | n = 60, no IOCS −g autologous transfusion at low collections | Gastrointestinal cancer | − | No difference for total cancer recurrence (28% vs. 43%; p = 0.9), recurrence for pancreatic carcinoma (33% vs. 24%; p = 0.07), local recurrence (22% vs. 17%; p = 0,58), less distance metastases (16% vs. 25%; p = 0.0427) | − | − |
|
| ||||||||
| Connor et al. [16] GRADE category: low | Prospective cohort, observation period 24 months, 12.4 years later from Engle et al. [29] | n = 31 | Control group 1: n = 40, no autologous transfusion of collected blood Control group 2: n = 231 matched historical subjects | Uterine cervix cancer | IOCS reduced the allogeneic transfusion rate during surgery (p < 0.001) and afterward (p = 0.02) | 3 local recurrences in the pelvis (one in the IOCS group), but no distant metastases | After 12.4 years, lower mortality with IOCS 12.9% versus non-IOCS 17.5%. | − |
|
| ||||||||
| Davis et al. [30] GRADE category: low | Retrospective cohort, n = 389, observation period 40.2 months | n = 87 | Control 1:PAD,n = 245 Control 2: NT, n = 57 | Prostate carcinoma | − | No difference for recurrence (biochemical prostate-specific antigen) IOCS versus PAD versus NT (15% vs. 16% vs. 19%; p = 0.784). Lowest risk of recurrence with the use of IOCS (OR 0.81; 95% CI: 0.33–2.00) versus NT (OR 0.66; 95% CI: 0.21–2.08) | − | − |
|
| ||||||||
| Elmalky et al. [12] GRADE category: low | Retrospective observation (single center), retrospective ca. 4 years | n = 63, + LDF | n = 113, allogeneic transfusion | Metastatic spine tumors | IOCS + LDF is cost effective and reduces allogeneic transfusion risk by 59% (OR = 0.407, p = 0.03) | − | Comparable survival and complication rates (χ2 =0.245, p = 0.62) | Shorter length of stay with IOCS by 3.76 days (p = 0.028) |
|
| ||||||||
| Foltys et al.[31] GRADE category: low | Prospective cohort, observation period 38 months | n = 40 | n = 96 non-IOCS | HCC | − | Recurrence rate similar IOCS 13% (5/40) versus non-IOCS 19% (18/96) (p = 0.29) | − | − |
|
| ||||||||
| Fujimoto et al. [18] GRADE category: low | Prospective cohort, observation period 12 months | n = 50 | n = 54, PAD | HCC | Allogeneic transfusion rate reduced (814±397 mL vs. PAD 3466±1811 mL;p< 0.05) | No difference in the recurrence rate (62.8% vs. 67.3%) and survival (61.9% vs. 52.8%) | − | − |
|
| ||||||||
| Gilbert et al. [32] GRADE category: low | Prospective cohort, cost efficiency | n = 86 together with PAD | n = 86 sole PAD without IOCS | Prostate cancer | Allogeneic transfusion rate for both groups similar (each 8%, 3.3 units PAD vs. 2.9 units PAD + IOCS; p = 0.76) | − | IOCS increased cost by 433 US $ | − |
|
| ||||||||
| Gorinetal.[33] GRADE category: low | Retrospective cohort, observation period 47 months | n = 395 | n = 1,467, non-IOCS | Prostate cancer | Allogeneic transfusion rate can be reduced with IOCS (0.6% vs. 3-20%) | With or without IOCS comparable recurrence-free survival periods (5 yr: 82.4% vs. 83.7%; 8 yr: 73.4 vs. 76.6%; p = 0.32) | − | − |
|
| ||||||||
| Gray et al. [34] GRADE category: low | Prospective cohort, historical case-control study, observation period 25 months | n = 62, + LDF | n = 101, non-IOCS | Prostate cancer | Allogeneic transfusion rate reduced by IOCS + LDF (3% vs. 14%, p = 0.04) | − | Progression-free survival similar (p = 0.41) with a trend to be longer in the IOCS + LDF group | − |
|
| ||||||||
| Han et al. [21] GRADE category: low | Retrospective cohort, observation period 1,2, und 5 years | n = 222, +LDF | n = 97, non-IOCS | HCC | − | Comparable total tumor recurrence (HR 0.85; 95% CI: 0.47-1.53; p = 0.579), hepatic recurrence (HR 0.75; 95% CI: 0.36-1.56, p = 0.44), identical risk of extrahepatic recurrence (HR 1.00; 95% CI: 0.49-2.04, p = 0.999) | − | − |
|
| ||||||||
| Hart et al. [35] GRADE category: very low or low | Prospective observation, not included in the analysis, 24 months observation period | n = 49 | No control group | Carcinoma of the bladder | − | No sign of tumor spread, recurrence in 21% | 2-yr survival 88% | − |
|
| ||||||||
| Hirano et al. [36] GRADE category: low | Prospective observation, observation period 8 years | n = 46 | n = 50, allogeneic packed red cells and plasma | HCC | − | Higher survival and tumor-free periods after 10 years in the IOCS population (20% vs. 8%, p < 0.05), especially following curative resection and stage I/II HCC (while not different for stage III/IV HCC) | Same subjects as in the article of Fujimoto et al. [18] now longer observation period | − |
|
| ||||||||
| Kang et al. [37] GRADE category: moderate to low | Retrospective cohort with 1:1 matching longest observation period 60 months | n = 76, Matched 24 | n = 34 non-IOCS, matched 24 | HCC | Comparable transfusion rate and used units IOCS versus non-IOCS 64.5% vs. 55.9%; p = 0.39 | Similar recurrence rate 0%,1.8%, 1.8% versus non-IOCSA 0%, 3.2%, 3.2%, respectively; p = 0.55; HR of recurrence 2.64, 95% CI: 0.28–25.30; p = 0.40 | Survival rate at 1, 3, and 5 years IOCS versus non-IOCS similar (6.0%, 88.4%, 83.0% vs. 97.1%, 91.1%, 87.8%, respectively; p = 0.79), hazard ratio of death for matched pairs (IOCS vs. non-IOCS [HR]1.26, 95% CI: 0.52-3.05, p = 0.61) | − |
|
| ||||||||
| Kangetal.[38] GRADE category: moderate to low | Retrospective cohort, observation period 59 months | n= 74, +LDF, solely IOCS (excluded were mixed allogeneic and IOCS) | n = 73, no transfusion | Colorectal cancer liver metastases | − | Median recurrence-free survival was 13.7 months in the IOCS group and 18.7 months in the NT group (p = 0.22) | Overall survival was similar between IOCS versus no transfusion (HR 0.58; 95% CI: 0.31 to 1.11) | − |
|
| ||||||||
| Kim et al. [39] GRADE category: low | Retrospective cohort, observation period 53 months | n = 121, + LDF | n = 109, non-IOCS | HCC | Allogeneic transfusion reduced with IOCS (3.7 units vs. 9.9; p < 0.01) | − | Comparable tumor-free survival (83.3% vs. 77.4%, p= 0.31) | − |
|
| ||||||||
| Kinnear et al.[40] GRADE category: low | Retrospective cohort, observation period 9 months | n = 16 (only 5 subjects of the 16 IOCS patients received the IOCS autologous blood) | n = 24, non-IOCS | Kidney cancer | Both groups had similar allogeneic transfusion rates (6% vs. 4%; p = 0.96) | Recurrence rates were not different (18% vs. 7%; p = 0.40) | − | Both groups had similar complication rates (19% vs. 29%; p = 0.46) and same LOS (7 days) |
|
| ||||||||
| Kinnear et al. [17] GRADE category: low | Retrospective cohort, observation period 33 months | n = 29, + LDF | n = 30, non-IOCS | Prostate cancer | Allogeneic transfusion rates were similar (9 vs. 6 patients; p = 0.41), fewer red blood products transfused in the IOCS group (12 vs. 40 units) | Similar recurrence rates (6 vs. 3 patients; p = 0.30) | Similar complications (10 vs. 5 patients; p = 0.16 | − |
|
| ||||||||
| Kwon et al. [20] GRADE category: moderate to low | Retrospective cohort, propensity score match, longest observation periods 60 months | n = 74, + LrF | n = 74 | HCC | − | Recurrence rate after 1, 3, 5 years IOCS versus non-IOCS 16.2%/23.1%/32.5% versus 24.6%/38.3%/39.7% overall recurrence ([HR] = 0.72 [0.43–1.21]), intrahepatic recurrence (HR=0.70 [0.35–1.40]), and extrahepatic recurrence (HR = 0.82 [0.46–1.47]) | No differences in overall death (HR = 0.57 [0.29–1.12]), HCC-related death (HR = 0.59 [0.29–1.20]), and HCC-unrelated death (HR = 0.48 [0.09–2.65]) | − |
|
| ||||||||
| Lyon et al. [41] GRADE category: low | Retrospective observation (single center, observation period 23 months) | n = 33 | n = 34, allogeneic transfusion | Renal cancer | In the IOCS group, a trend for higher allogeneic transfusion requirements (21% vs. 8%), but associated with a 3-fold higher blood loss and longer surgeries | No difference for recurrence, metastases, or cancer-related mortality | 1 death in the non-IOCS group 23 months after surgery (interquartile: 8–42 M) | No difference in complications (21% vs. 17%, p= 0.83), LOS (3 vs. 3 days, p = 0.09) |
|
| ||||||||
| MacIvor et al. [42] GRADE category: very low bis low | Retrospective observational study, observation periods up to 3 years | n=40, + LRF | n = 63, PAD | Prostate cancer | PAD avoidance of allogeneic transfusion more effective (0 vs. 6.3% in IOCS), influenced by a protocol-dependent selection of nonanemic subjects for PAD. After PAD, comparable or even higher anemia levels in this group | Similar recurrence rate | − | Similar LOS |
|
| ||||||||
| Martin et al. [23] GRADE category: very low | Prospective cohort | n = 20, + LDF | No control group | Colorectal carcinoma, pancreatic adenocarcinoma | Similar low allogeneic transfusion rate | − | No cancer cells in the autologous IOCS units after LDF | − |
|
| ||||||||
| Mirhashemi et al. [43] GRADE category: low | Retrospective cohort, observation period 22 months (0–89) | n = 50 | n = 106, non-IOCS | Cervical carcinoma | IOCS group was less exposed to allogeneic blood transfusion (12% vs. 30%; p = 0.02) | In comparison to a historical cohort, survival was similar (86% vs. 85.6%), only local recurrences in the IOCS group | Complications similar for fever, sepsis, ileus, pulmonary emboli, thrombosis, etc. | − |
|
| ||||||||
| Muscari et al. [22] GRADE category: low | Prospective cohort, observation period 48 months | n = 31 | n = 16, non-IOCS | HCC | Considerably increased allogeneic red cell transfusion in the IOCS group: 56% versus 10%; p < 0.0009; plasma 44% versus 13%,p = 0.02 | Tumor recurrence without difference (IOCS 6.4% vs. non-IOCS 6.3%) | More cirrhosis and assumed more complex surgery in the IOCS group | − |
|
| ||||||||
| Myrga et al. [44] GRADE category: low | Retrospective cohort, observation period 28 months | n = 87, + LDF | n = 70, non-IOCS | Bladder cancer | Similar allogeneic transfusion rate (IOCS vs. non-IOCS 15%vs.21%,p = 0.30), intra- as well as postoperative | Cancer recurrence was similar (23 % vs. 24%; p = 0.85) | Similar mortality (12% vs. 17%;p = 0.36) | No difference in LOS (IOCS vs. non-IOCS 6 vs. 5 days, p = 0.32), 90 day readmission rate, or infection rate |
|
| ||||||||
| Nieder et al.[45] GRADE category: low | Retrospective cohort, observation period 40 months | n = 265 | n = 773, non-IOCS | Prostate carcinoma | − | 5-yr recurrence rate (biochemical) not different (IOCS 15% vs. 18%; p = 0.76) | Tumor-free survival not different (IOCS 27.9±30.3 Mo vs. 32.1±29.5 Mo;p = 0.49) | − |
|
| ||||||||
| Nieder et al.[46] GRADE category: low | Retrospective cohort, observation period 19 months | n = 65 | n = 313, non-IOCS | Urothelial bladder carcinoma | lOCS-treated subjects had considerably higher blood loss (862 mL vs. 537 mL), were allogeneic transfused more frequently (37% vs. 16.3%), but similar transfusion volumes (652 vs. 639 mL) | Disease-specific survival similar (72.2% vs. 73.0%; p = 0.9) | Overall survival also (63.9% vs. 65.8%; p = 0.7) | − |
|
| ||||||||
| Nutu et al. [19] | Propensity score-matched pairs, retrospective cohort, observation period 60 months | n= 192 (matched = 127) | n = 378, matched, n = 127, non-IOCS | HCC | − | Similar HCC recurrence (cause-specific Cox model: HR 0.79,95% CI: 0.36–1.73; p = 0.549, Fine and Gray model: HR: 0.79,95% CI: 0.40–1.57; p= 0.50) | Similar disease-free survival (HR 1.07, 95% CI: 0.65–1.76, p = 0.800) | − |
|
| ||||||||
| Park et al. [47] GRADE category: very low | Prospective cohort, observation period 48 months | n = 6, IOCS alone | n = 4, IOCS in combination with PAD no control group without IOCS | Bladder carcinoma | IOCS + PAD were not transfused with allogeneic blood, allogeneic transfusion rate IOCS + PAD versus PAD 0% versus 66.6% | Survival IOCS + PAD versus IOCS 75% versus 33.3%, respectively | − | |
|
| ||||||||
| Pinto et al. [48] GRADE category: low | Retrospective cohort, observation period 84 month | n= 122 | n = 34, non-IOCS | HCC | − | For the 1-year, 5-year, and 7-year periods, the overall survival for IOCS versus non-IOCS groups was 84.2%, 67.7%, and 56.8% versus 85.3%, 67.5%, and 67.5% in the non-IOCS group (p = 0.77), disease-free survival 81.6%, 66.5%, and 55.4% versus 85.3%, 64.1 %, and 64.1 % (p = 0.74) | Median hospital stay was 13 versus 15 days (p = 0.4) In IOCS versus non- lOCS-treated subjects | − |
|
| ||||||||
| Pisters et al. [49] GRADE category: very low | Prospective observation | n = 20, IOCS unfiltered in combination with PAD | No control group | − | Great reductions in allogeneic transfusion, autologoustransfusion only 96% (1 case needed allogeneic blood) | − | − | − |
|
| ||||||||
| Raval et al.[50] GRADE category: low | Retrospective cohort (single center, single surgeon), observation period 57 months | n = 42 | n = 32, PAD | Prostate cancer | − | Rate for metastases in the PAD group higher (PAD 12.5% vs. IOCS 0%; p = 0.03). Tumor recurrence (biochemical) in the IOCS group reduced (PAD 34.4% vs. IOCS 9.5%; p = 0.02) | No difference in mortality (PAD 9.4% vs. 0%; p = 0.08) | − |
|
| ||||||||
| Stoffel et al. [51] GRADE category: low | Prospective cohort, observation period 46 months | n = 48 | n = 64, non-IOCS | Prostate cancer | − | Lower risk of cancer recurrence with IOCS (OR 0.766, p = 0.54) | − | − |
|
| ||||||||
| Ubee et al. [52] GRADE category: low | Prospective cohort, observation period 16 months | n = 25, + LRF | n = 25, non-IOCS | Prostate carcinoma | Allogeneic transfusion rate (proportion of subjects transfused) and transfusion needs (number of needed units) with IOCS reduced: (IOCS vs. non-IOCS 20% vs. 72%, n = 16 vs. n = 69) | Recurrence rate reduced (biochemical) with IOCS (4% vs. 16%), no indication of tumor spread | − | − |
|
| ||||||||
| Vagner et al. [53] GRADE category: low | Prospective cohort, observation period 60 months | n = 20 | n = 19, allogeneic transfusion | Renal carcinoma | − | Recurrence rate comparable between groups | Mortality comparable between groups (40.9 vs. 42.1%) | − |
|
| ||||||||
| Zulim et al. [54] GRADE category: low | Retrospective observation | n = 39 | No control group | HCC | − | No difference to historical controls for cancer recurrence of patients not treated with IOCS | No difference in comparison to historical and literature overall survival rate, tumor-free survival | − |
|
| ||||||||
| Xu et al. [55] GRADE category: low | Retrospective cohort | n=42, + LDF | n = 126, non-IOCS | − | The control and ICS groups had equivalent use of allogeneic transfusion (3 vs. 2 patients; p = 0.33) | Similar rates of biochemical recurrence (17% vs. 14%; p = 0.90) | − | Similar adjuvant therapy use (30% vs. 29%; p = 0.85) and complications (14% vs. 19% patients; p = 0.46) |
GRADE, Grading of Recommendations Assessment, Development and Evaluation.
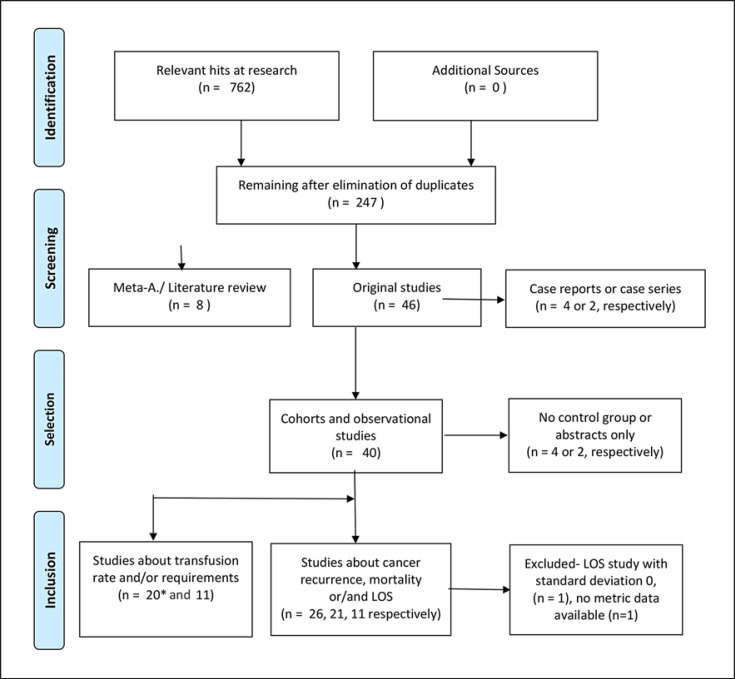
Fig. 1.
Systematic research history − Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow. The numbers of studies does not sum up due to the report of more than one outcome per study. n, numbers of publications; LOS, length of hospital stay.
In comparison to the last meta-analysis published in 2020 [15], 9 full-text articles (observational studies) with additional n = 653 IOCS-treated subjects were found. Of those, 7 articles were published after the last analysis, while two studies were included due to a wider inclusion criterion. A most recent study with 127 matched pairs could not be included for the recurrence and mortality analysis since the study estimated “time-to-event” data from a model [19].
We included two study pairs from the same institution (Kwon et al. [20] and Han et al. [21], Fujimoto et al. [18] and Hirano et al. [56]) despite a potentially overlapping patient population (overlap by 74 vs. 222, 46 vs. 50 IOCS-treated and analyzed subjects). Since enrollment periods and numbers of enrolled subjects varied, we assumed two different populations. All studies included observational data of varying cancer surgery for a wide range of cancer types. We also included studies about procedures associated with higher blood loss such as liver transplantation or spine surgery. The median observation period after surgery was almost 3 years: 35.6 months (1., 3. quartiles 23–48 months).
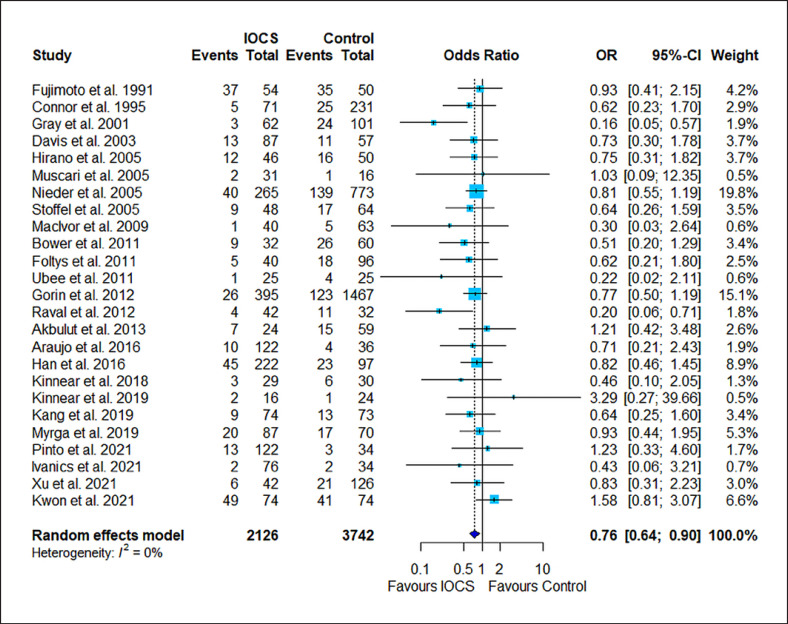
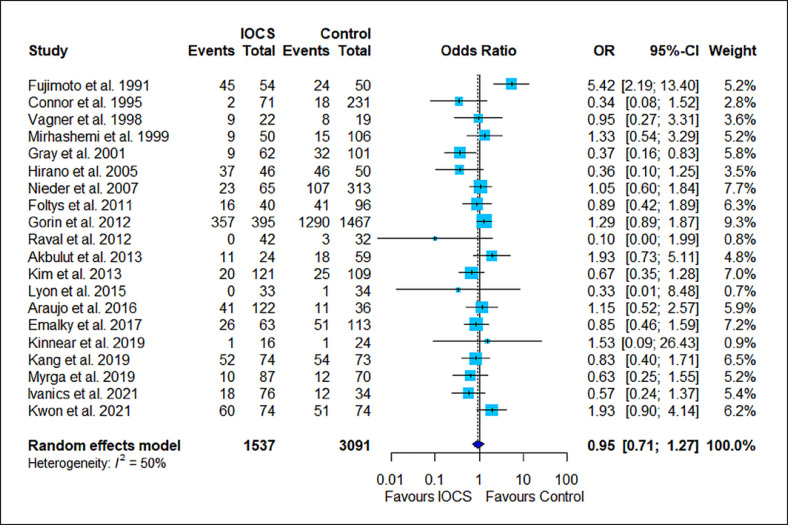
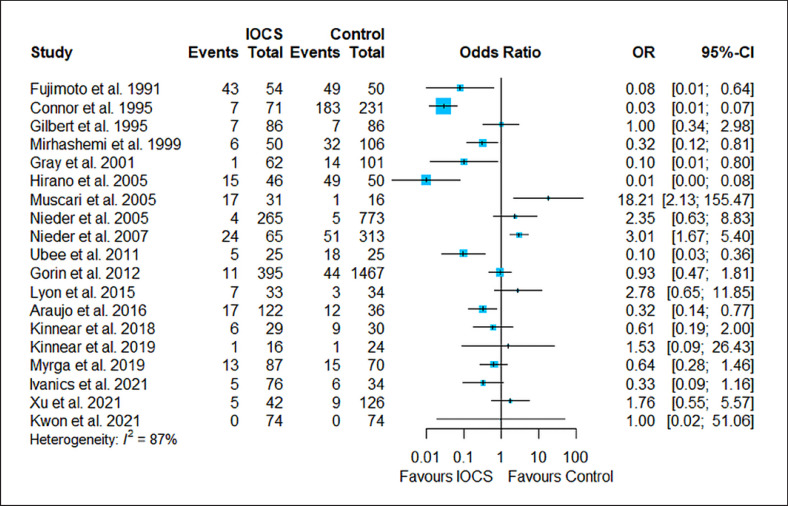
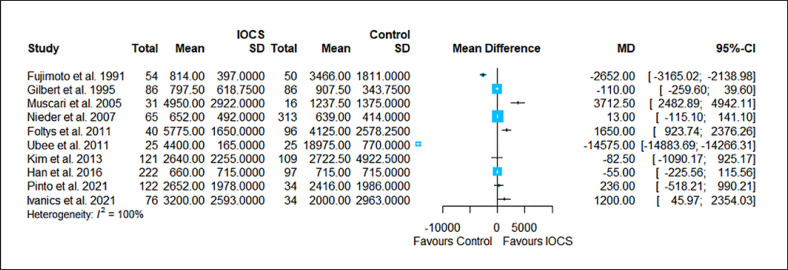
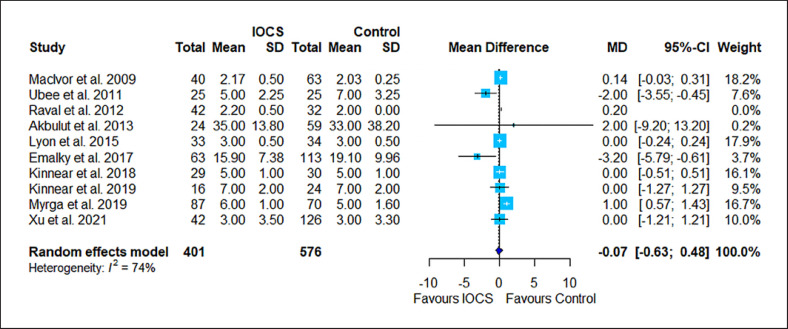
The results of the risk estimation are presented in Figures 2, 3, 4, 5, 6, a series of forest plots generated from included cancer surgeries for various reported outcomes comparing IOCS use and retransfusion versus control (no transfusion, allogeneic transfusion only), presented as ORs and 95% CI. The cancer recurrence rate decreased in the IOCS groups (OR 0.76 [95% [CI]: 0.64–0.94], heterogeneity I2 = 0%). There was no observed difference between the groups regarding mortality (OR 0.95, 95% CI: 0.71–1.27) and LOS (MD −0.07, 95% CI: −0.63 to 0.48). Due to the high amount of heterogeneity between the studies, we did not calculate a pooled effect for transfusion rate (I2 = 87%) and transfused volume (I2 = 100%).
Fig. 2.
Forest plot for all studies that reported cancer recurrence. Twenty-five studies compared n = 2,126 subjects treated with IOCS versus n = 3,742 controls. The weight of a single study in the meta-analysis is reflected by the size of the blue square (and dependent from number of subjects and width of CIs). The horizontal lines are confidential intervals. The length of follow-up is not displayed (for this see Table 1). Control groups were mentioned as “no IOCS use” without further details (n = 3,283) or specified as allogeneic transfusion (n = 358), PAD with or without allogeneic blood (n = 394), or no transfusion necessary (n = 125).
Fig. 3.
Forest plot for all studies that reported mortality. Twenty studies compared n = 1,537 subjects treated with IOCS versus n = 3,091 controls. The weight of a single study in the meta-analysis is reflected by the size of the blue square (and dependent from number of subjects and width of CIs). The horizontal lines are confidential intervals. The length of follow-up is not displayed (for this see Table 1). Control groups were mentioned as “no IOCS use” without further details (n = 2,729) or specified as allogeneic transfusion (n = 64), PAD with or without allogeneic blood (n = 105), or no transfusion necessary (n = 91).
Fig. 4.
Forest plot for all studies that reported the transfusion rate (numbers of subjects transfused per group). Nineteen studies compared n = 1,629 subjects treated with IOCS versus n = 3,646 controls. The weight of a single study in the meta-analysis is reflected by the size of the blue square (and dependent from the number of subjects and width of CIs). The length of follow-up is not displayed (for this see Table 1). Heterogeneity I2 = 87% prohibits reliable calculation of OR. Control groups were mentioned as “no IOCS use” without further details (n = 3,287) or specified as allogeneic transfusion (n = 80), PAD with or without allogeneic blood (n = 140), or no transfusion necessary (n = 57).
Fig. 5.
Forest plot for all studies that reported transfusion requirements (number of transfused volumes/units per group). Ten studies compared n = 842 subjects treated with IOCS versus n = 860 controls. The weight of a single study in the meta-analysis is reflected by the size of the blue square (and dependent from the number of subjects and width of CIs). Horizontal lines are confidential intervals. The length of follow-up is not displayed (for this see Table 1). Heterogeneity I2 = 100% prohibits reliable calculation of OR. Control groups were mentioned as “no IOCS use” without further details (n = 1,116) or specified as allogeneic transfusion (n = 16), PAD with or without allogeneic blood (n = 140) or no transfusion necessary (n = 125).
Fig. 6.
Forest plot for all studies that reported LOS. Studies with nonpositive values for standard deviations were not included in the meta-analysis. Ten studies compared n = 401 subjects treated with IOCS versus n = 576 controls. The weight of a single study in the meta-analysis is reflected by the size of the blue square (and dependent from the number of subjects and width of CIs). The horizontal lines are confidential intervals. The length of follow-up is not displayed (for this see Table 1). Control groups were mentioned as “no IOCS use” without further details (n = 483) or specified as allogeneic transfusion (n = 87), PAD with or without allogeneic blood (n = 95), or no transfusion necessary (n = 0).
Discussion
This updated meta-analysis on IOCS use for cancer surgery identified 7 new studies and 520 more IOCS-treated cancer patients (to a total sum of n = 3,161). Combining the outcomes from 34 studies, the result of the previous meta-analyses could be confirmed: IOCS in cancer surgery is associated with a reduced risk ratio for cancer recurrence and metastasis [15, 57]. Mortality and cancer-free survival episodes are not influenced by the use or avoidance of IOCS. The homogeneity of results from available trials suggests that tumor cells in IOCS blood may not impact survival and cancer recurrence. The most recent study by Nutu et al. [19] on IOCS use for hepatocellular cancer is consistent with our results [19]. However, these findings are based on a low level of evidence since quality of data is derived from observational studies. The only study protocol of RCT for IOCS in ovarian cancer (entitled “TICTOC,” n = 30 vs. n = 30 controls) that can be found is a feasibility trial not yet published [58]. Although it is important to note that there could be confounding results since in almost a third of the observational studies, nontransfused patients were compared to IOCS-treated subjects. Nevertheless, our meta-analysis is based on the best evidence available as randomized trials have yet to be done.
IOCS was used in many institutions exclusively on high blood loss situations (e.g., [22]) and, in most instances, accompanied or augmented with allogeneic blood transfusion. In a subgroup with comparable blood loss in the control and IOCS patients, the usage of IOCS tends to reduce allogeneic transfusion rate. This aspect was already shown for well-standardized surgical procedures such as liver transplantation for malignancy. However, in mixed data such as in this analysis, trials dealing with transfusion rates and blood requirement were too heterogeneous to draw conclusions. Surprisingly, this meta-analysis did not identify allogeneic transfusion as a risk factor for an increased recurrence rate, which stands in contrast to most studies on transfusion effects in oncologic surgery [1, 2, 3, 4, 5, 6]. Since a large number of studies of this meta-analysis compared patients treated with IOCS supplemented with allogeneic transfusion to groups of patients not transfused, allogeneic transfused only, or transfused with predonated autologous blood, this design is not appropriate to assess allogeneic transfusion risks. As in many meta-analysis, the study protocols have to be considered heterogeneous: Included are control groups without any transfusion or the selection of IOCS patients by blood loss extend, coming down to an unfavorable selection bias according to the major surgical trauma and/or more advanced malignancy state in the IOCS group. The majority of retrospective studies identified the use or nonuse of IOCS and presented the institution's standards as the reason for group allocation. It has to be considered that a majority of “controls” are patients bled sparingly intraoperatively; thus, IOCS was not used. However, there was a reduced recurrence rate of patients receiving autologous IOCS blood. The immune modulation by allogeneic blood would be the most plausible explanation for the group difference to the subgroups of controls of transfused subjects. The methodological description of observational studies rarely quantified the extent of allogeneic augmentation following autologous predonation. Patients without transfusion were too few (for recurrence 3.8%) to impact the overall result.
The concern of tumor dissemination or plantation of distant metastases by reinfusion of IOCS blood is based on physiological assumptions and is not well investigated. The warnings against the use of IOCS in cancer surgery are based on a single published case report that was cited when warnings against IOCS use are issued. On closer inspection, there is a misinterpretation of the results in the case report by Yaw et al. from 1975 [59]. The case report describes the tragic death of a 52-year-old patient with lung cancer following a bilobectomy. Malignant cells were identified in a microscopic evaluation of the transfused blood. However, the collected blood was not retransfused to the patient and was discarded due to the diagnosis of the frozen section. Hence, the IOCS did not cause the fatal outcome.
In light of the favorable results in more than 2,500 cancer patients and the fact that no single case report of cancer spread by IOCS use exists, theoretical concerns about the spread of autologous malign cells by IOCS lose weight. Since randomized controlled trials are difficult to conduct in these settings, this analysis of observational trials is the best available approach despite low numbers and high heterogeneity in the included observations.
Due to the limitation in data quality, we conclude that the safest option is to use IOCS in combination with LDF filtration. The filtration efficacy in terms of cancer cell reduction using a newer generation of LDFs is 99.6% [60] to 99.9% [61]. This was demonstrated for a large variety of tissues such as prostate and renal cancer [62]; osteosarcoma [63]; breast [64]; colorectal [65]; pancreatic [23]; hepatocellular [66]; endometrium, cervical, and ovarian [67]; spinal metastases [68]; and lung cancer [69].
In conclusion, the use of IOCS in cancer surgery in many observational studies was shown to be safe and positive in terms of a favorable outcome. Nevertheless, data quality is low, and the theoretical risk to cause hematogenous spread of malignant cells by reinfusion of the IOCS product is to be considered. Hence, multicenter randomized controlled trials are urgently needed to confirm the results of this meta-analysis. Meanwhile, the recommendation of some societies and groups of experts is to use IOCS in cancer surgery with some restrictions [70, 71, 72, 73, 74, 75].
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature.
Conflict of Interest Statement
T.F.: honoraria and reimbursements for travel expenses, lectures, investigator meetings, and presentations from Janssen-Cilag, AstraZeneca, Vifor Pharma, Pharmacosmos, the German Red Cross, Aspect Medical, Organon, Alliance Pharmaceuticals, and Baxter Healthcare Corp; expert consulting contracts with Janssen-Cilag, Vifor Pharma, Pharmacosmos; research grants from the Else-Groenert-Foundation and the University Medicine Mannheim, University of Heidelberg. A.U.S.: research grant from Pharmacosmos, Denmark, to perform a single-center, prospective trial on preoperative anemia treatment. A.U.S. is supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) grant STE 1895/9-1 and STE 1895/10-1 as part of the DFG research consortium FerrOS-FOR5146. A.H.: no conflicts of interest to declare. M.M.: no conflicts of interest to declare. G.D.: no conflicts of interest to declare. M.A.W.: no conflicts of interest to declare. J.H.W.: no conflicts of interest to declare. D.F.: no conflicts of interest to declare.
Funding Sources
The German Interdisciplinary Task Force for Clinical Hemotherapy IAKH supports the topic by covering for cost during research, analysis, writing, and publication such as publication fees, salaries, article prints, and presentation expenses.
Author Contributions
T.F.: study director and manager, project design, communication with the IAKH, selection of reviewers and authors, research and data extraction, manuscript drafting, and final check. A.U.S.: manuscript and scientific content control. M.M.: statistics. A.H.: manuscript native language check. G.D.: project design, scientific content check, and manuscript. M.A.W.: scientific content check and manuscript refinement. J.H.W.: project design and manuscript comments check. D.F.: analysis and data check, research and data extraction, manuscript drafting and refinement, and author coordination.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the German Interdisciplinary Task Force for Clinical Hemotherapy IAKH for funding and support.
References
- 1.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235–44. doi: 10.1097/SLA.0b013e31825b35d5. [DOI] [PubMed] [Google Scholar]
- 2.Schneider M, Schafer N, Potthoff AL, Weinhold L, Eichhorn L, Weller J, et al. Perioperative red blood cell transfusion is associated with poor functional outcome and overall survival in patients with newly diagnosed glioblastoma. Neurosurg Rev. 2022;45:1327–33. doi: 10.1007/s10143-021-01633-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HL, Tai YH, Lin SP, Chan MY, Chen HH, Chang KY. The impact of blood transfusion on recurrence and mortality following colorectal cancer resection: a propensity score analysis of 4,030 patients. Sci Rep. 2018;8((1)):13345. doi: 10.1038/s41598-018-31662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Wang Z, Jiang S, Shao B, Liu J, Zhang S, et al. Perioperative allogenenic blood transfusion is associated with worse clinical outcomes for hepatocellular carcinoma: a meta-analysis. PLoS One. 2013;8:e64261. doi: 10.1371/journal.pone.0064261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102–10. doi: 10.1016/j.ijsu.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 6.Hanna DN, Gamboa AC, Balch GC, Regenbogen SE, Holder-Murray J, Abdel-Misih SRZ, et al. Perioperative blood transfusions are associated with worse overall survival but not disease-free survival after curative rectal cancer resection: a propensity score-matched analysis. Dis Colon Rectum. 2021;64((8)):946–54. doi: 10.1097/DCR.0000000000002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isbister JP, Shander A, Spahn DR, Erhard J, Farmer SL, Hofmann A. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev. 2011;25:89–101. doi: 10.1016/j.tmrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Goubran H, Sheridan D, Radosevic J, Burnouf T, Seghatchian J. Transfusion-related immunomodulation and cancer. Transfus Apher Sci. 2017;56:336–40. doi: 10.1016/j.transci.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 9.Dickson EA, Acheson AG. Allogeneic blood and postoperative cancer outcomes: Correlation or causation? Anaesthesia. 2020;75:438–41. doi: 10.1111/anae.14965. [DOI] [PubMed] [Google Scholar]
- 10.Fischer D, Neb H, Choorapoikayil S, Zacharowski K, Meybohm P. Red blood cell transfusion and its alternatives in oncologic surgery-a critical evaluation. Crit Rev Oncol Hematol. 2019;134:1–9. doi: 10.1016/j.critrevonc.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;17:Cd001888. doi: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Elmalky M, Yasin N, Rodrigues-Pinto R, Stephenson J, Carroll C, Smurthwaite G, et al. The safety, efficacy, and cost-effectiveness of intraoperative cell salvage in metastatic spine tumor surgery. Spine J. 2017;17:977–82. doi: 10.1016/j.spinee.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Harrison S, Steele RJ, Johnston AK, Jones JA, Morris DL, Hardcastle JD. Predeposit autologous blood transfusion in patients with colorectal cancer: a feasibility study. Br J Surg. 1992;79:355–7. doi: 10.1002/bjs.1800790426. [DOI] [PubMed] [Google Scholar]
- 14.Valbonesi M, Bruni R, Lercari G, Florio G, Carlier P, Morelli F. Autoapheresis and intraoperative blood salvage in oncologic surgery. Transfus Sci. 1999;21:129–39. doi: 10.1016/s0955-3886(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 15.Frietsch T, Steinbicker AU, Hackbusch M, Nguyen XD, Dietrich G. [safety of cell salvage in tumor surgery: systematic review with meta-analysis] Anaesthesist. 2020;69:331–51. doi: 10.1007/s00101-020-00751-4. [DOI] [PubMed] [Google Scholar]
- 16.Connor JP, Morris PC, Alagoz T, Anderson B, Bottles K, Buller RE. Intraoperative autologous blood collection and autotransfusion in the surgical management of early cancers of the uterine cervix. Obstet Gynecol. 1995;86:373–8. doi: 10.1016/0029-7844(95)00183-R. [DOI] [PubMed] [Google Scholar]
- 17.Kinnear N, Heijkoop B, Hua L, Hennessey DB, Spernat D. The impact of intra-operative cell salvage during open radical prostatectomy. Transl Androl Urol. 2018;7:S179–187. doi: 10.21037/tau.2018.04.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto J, Okamoto E, Yamanaka N, Oriyama T, Furukawa K, Kawamura E, et al. Efficacy of autotransfusion in hepatectomy for hepatocellular carcinoma. Arch Surg. 1993;128:1065–9. doi: 10.1001/archsurg.1993.01420210129021. [DOI] [PubMed] [Google Scholar]
- 19.Nutu OA, Sneiders D, Mirza D, Isaac J, Perera MTPR, Hartog H. Safety of intra-operative blood salvage during liver transplantation in patients with hepatocellular carcinoma, a propensity score-matched survival analysis. Transpl Int. 2021;34((12)):2887–94. doi: 10.1111/tri.14150. [DOI] [PubMed] [Google Scholar]
- 20.Kwon JH, Han S, Kim D, Kuk JH, Cho H, Kim S, et al. Blood salvage and autotransfusion does not increase the risk of tumor recurrence after liver transplantation for advanced hepatocellular carcinoma. Ann Surg. 2021 doi: 10.1097/SLA.0000000000004866. [DOI] [PubMed] [Google Scholar]
- 21.Han S, Kim G, Ko JS, Sinn DH, Yang JD, Joh JW, et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann Surg. 2016;264:339–43. doi: 10.1097/SLA.0000000000001486. [DOI] [PubMed] [Google Scholar]
- 22.Muscari F, Suc B, Vigouroux D, Duffas JP, Migueres I, Mathieu A, et al. Blood salvage autotransfusion during transplantation for hepatocarcinoma: does it increase the risk of neoplastic recurrence? Transpl Int. 2005;18:1236–9. doi: 10.1111/j.1432-2277.2005.00207.x. [DOI] [PubMed] [Google Scholar]
- 23.Martin RC, Wellhausen SR, Moehle DA, Martin AW, McMasters KM. Evaluation of intraoperative autotransfusion filtration for hepatectomy and pancreatectomy. Ann Surg Oncol. 2005;12:1017–24. doi: 10.1245/ASO.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Akbulut S, Kayaalp C, Yilmaz M, Ince V, Ozgor D, Karabulut K, et al. Effect of autotransfusion system on tumor recurrence and survival in hepatocellular carcinoma patients. World J Gastroenterol. 2013;19:1625–31. doi: 10.3748/wjg.v19.i10.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akchurin RS, Davidov MI, Partigulov SA, Brand JB, Shiriaev AA, Lepilin MG, et al. Cardiopulmonary bypass and cell-saver technique in combined oncologic and cardiovascular surgery. Artif Organs. 1997;21:763–5. doi: 10.1111/j.1525-1594.1997.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 26.Aning J, Dunn J, Daugherty M, Mason R, Pocock R, Ridler B, et al. Towards bloodless cystectomy: a 10-year experience of intra-operative cell salvage during radical cystectomy. BJU Int. 2012;110:E608–13. doi: 10.1111/j.1464-410X.2012.11338.x. [DOI] [PubMed] [Google Scholar]
- 27.Araujo RL, Pantanali CA, Haddad L, Rocha Filho JA, D'Albuquerque LA, Andraus W. Does autologous blood transfusion during liver transplantation for hepatocellular carcinoma increase risk of recurrence? World J Gastrointest Surg. 2016;8:161–8. doi: 10.4240/wjgs.v8.i2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower MR, Ellis SF, Scoggins CR, McMasters KM, Martin RC. Phase ii comparison study of intraoperative autotransfusion for major oncologic procedures. Ann Surg Oncol. 2011;18:166–73. doi: 10.1245/s10434-010-1228-4. [DOI] [PubMed] [Google Scholar]
- 29.Engle DB, Connor JP, Morris PC, Bender DP, De Geest K, Ahmed A, et al. Intraoperative autologous blood transfusion use during radical hysterectomy for cervical cancer: long-term follow-up of a prospective trial. Arch Gynecol Obstet. 2012;286:717–21. doi: 10.1007/s00404-012-2351-1. [DOI] [PubMed] [Google Scholar]
- 30.Davis M, Sofer M, Gomez-Marin O, Bruck D, Soloway MS. The use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? BJU Int. 2003;91:474–6. doi: 10.1046/j.1464-410x.2003.04129.x. [DOI] [PubMed] [Google Scholar]
- 31.Foltys D, Zimmermann T, Heise M, Kaths M, Lautem A, Wisser G, et al. Liver transplantation for hepatocellular carcinoma − is there a risk of recurrence caused by intraoperative blood salvage autotransfusion? Eur Surg Res. 2011;47:182–7. doi: 10.1159/000330746. [DOI] [PubMed] [Google Scholar]
- 32.Gilbert JB, Malkowicz SB, Wein AJ. Cell saver and radical retropubic prostatectomy: analysis of cost-effectiveness. Urology. 1995;46:542–4. doi: 10.1016/S0090-4295(99)80269-6. [DOI] [PubMed] [Google Scholar]
- 33.Gorin MA, Eldefrawy A, Manoharan M, Soloway MS. Oncologic outcomes following radical prostatectomy with intraoperative cell salvage. World J Urol. 2012;30:379–83. doi: 10.1007/s00345-011-0746-4. [DOI] [PubMed] [Google Scholar]
- 34.Gray CL, Amling CL, Polston GR, Powell CR, Kane CJ. Intraoperative cell salvage in radical retropubic prostatectomy. Urology. 2001;58:740–5. doi: 10.1016/s0090-4295(01)01365-6. [DOI] [PubMed] [Google Scholar]
- 35.Hart OJ, 3rd, Klimberg IW, Wajsman Z, Baker J. Intraoperative autotransfusion in radical cystectomy for carcinoma of the bladder. Surg Gynecol Obstet. 1989;168:302–6. [PubMed] [Google Scholar]
- 36.Hirano T, Yamanaka J, Iimuro Y, Fujimoto J. Long-term safety of autotransfusion during hepatectomy for hepatocellular carcinoma. Surg Today. 2005;35:1042–6. doi: 10.1007/s00595-005-3082-8. [DOI] [PubMed] [Google Scholar]
- 37.Ivanics T, Shubert CR, Muaddi H, Claasen MPAW, Yoon P, Hansen BE, et al. Blood cell salvage and autotransfusion does not worsen oncologic outcomes following liver transplantation with incidental hepatocellular carcinoma: a propensity score-matched analysis. Ann Surg Oncol. 2021;28((11)):6816–25. doi: 10.1245/s10434-021-09863-6. [DOI] [PubMed] [Google Scholar]
- 38.Kang R, Seath BE, Huang V, Barth RJ., Jr Impact of autologous blood transfusion on survival and recurrence among patients undergoing partial hepatectomy for colorectal cancer liver metastases. J Am Coll Surg. 2019;228:902–8. doi: 10.1016/j.jamcollsurg.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Kim JM, Kim GS, Joh JW, Suh KS, Park JB, Ko JS, et al. Long-term results for living donor liver transplant recipients with hepatocellular carcinoma using intraoperative blood salvage with leukocyte depletion filter. Transpl Int. 2013;26:84–9. doi: 10.1111/tri.12001. [DOI] [PubMed] [Google Scholar]
- 40.Kinnear N, Hua L, Heijkoop B, Hennessey D, Spernat D. The impact of intra-operative cell salvage during open nephrectomy. Asian J Urol. 2019;6:346–52. doi: 10.1016/j.ajur.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon TD, Ferroni MC, Turner RM, 2nd, Jones C, Jacobs BL, Davies BJ. Short-term outcomes of intraoperative cell saver transfusion during open partial nephrectomy. Urology. 2015;86:1153–8. doi: 10.1016/j.urology.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 42.MacIvor D, Nelson J, Triulzi D. Impact of intraoperative red blood cell salvage on transfusion requirements and outcomes in radical prostatectomy. Transfusion. 2009;49:1431–4. doi: 10.1111/j.1537-2995.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 43.Mirhashemi R, Averette HE, Deepika K, Estape R, Angioli R, Martin J, et al. The impact of intraoperative autologous blood transfusion during type iii radical hysterectomy for early-stage cervical cancer. Am J Obstet Gynecol. 1999;181:1310–6. doi: 10.1016/s0002-9378(99)70369-8. discussion 1315–6. [DOI] [PubMed] [Google Scholar]
- 44.Myrga JM, Ayyash OM, Bandari J, Fam MM, Macleod LC, Jacobs BL, et al. The safety and short-term outcomes of leukocyte depleted autologous transfusions during radical cystectomy. Urology. 2020;135:106–10. doi: 10.1016/j.urology.2019.08.056. [DOI] [PubMed] [Google Scholar]
- 45.Nieder AM, Carmack AJ, Sved PD, Kim SS, Manoharan M, Soloway MS. Intraoperative cell salvage during radical prostatectomy is not associated with greater biochemical recurrence rate. Urology. 2005;65:730–4. doi: 10.1016/j.urology.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 46.Nieder AM, Manoharan M, Yang Y, Soloway MS. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology. 2007;69:881–4. doi: 10.1016/j.urology.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 47.Park KI, Kojima O, Tomoyoshi T. Intra-operative autotransfusion in radical cystectomy. Br J Urol. 1997;79:717–21. doi: 10.1046/j.1464-410x.1997.00122.x. [DOI] [PubMed] [Google Scholar]
- 48.Pinto MA, Grezzana-Filho TJM, Chedid AD, Leipnitz I, Prediger JE, Alvares-da-Silva MR, et al. Impact of intraoperative blood salvage and autologous transfusion during liver transplantation for hepatocellular carcinoma. Langenbecks Arch Surg. 2021;406((1)):67–74. doi: 10.1007/s00423-020-01997-7. [DOI] [PubMed] [Google Scholar]
- 49.Pisters LL, Wajsman Z. Use of predeposit autologous blood and intraoperative autotransfusion in urologic cancer surgery. Urology. 1992;40:211–5. doi: 10.1016/0090-4295(92)90476-d. [DOI] [PubMed] [Google Scholar]
- 50.Raval JS, Nelson JB, Woldemichael E, Triulzi DJ. Intraoperative cell salvage in radical prostatectomy does not appear to increase long-term biochemical recurrence, metastases, or mortality. Transfusion. 2012;52:2590–3. doi: 10.1111/j.1537-2995.2012.03682.x. [DOI] [PubMed] [Google Scholar]
- 51.Stoffel JT, Topjian L, Libertino JA. Analysis of peripheral blood for prostate cells after autologous transfusion given during radical prostatectomy. BJU Int. 2005;96:313–5. doi: 10.1111/j.1464-410X.2005.05621.x. [DOI] [PubMed] [Google Scholar]
- 52.Ubee S, Kumar M, Athmanathan N, Singh G, Vesey S. Intraoperative red blood cell salvage and autologous transfusion during open radical retropubic prostatectomy: a cost-benefit analysis. Ann R Coll Surg Engl. 2011;93:157–61. doi: 10.1308/003588411X561044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vagner EA, Davidov MI. [blood reinfusion during nephrectomy in patients with kidney neoplasm'] Khirurgiia. 1998:23–7. [PubMed] [Google Scholar]
- 54.Zulim RA, Rocco M, Goodnight JE, Smith GJ, Krag DN, Schneider PD. Intraoperative autotransfusion in hepatic resection for malignancy. Is it safe? Arch Surg. 1993;128:206–11. doi: 10.1001/archsurg.1993.01420140083013. [DOI] [PubMed] [Google Scholar]
- 55.Xu J, Kinnear N, Johns Putra L. Safety, efficacy and cost of intra-operative cell salvage during open radical prostatectomy. Transl Androl Urol. 2021;10((3)):1241–9. doi: 10.21037/tau-20-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirano Y, Miyoshi Y, Kondo Y, Okamoto K, Tanaka H. Liberal versus restrictive red blood cell transfusion strategy in sepsis or septic shock: a systematic review and meta-analysis of randomized trials. Crit Care. 2019;23:262. doi: 10.1186/s13054-019-2543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waters JH, Yazer M, Chen YF, Kloke J. Blood salvage and cancer surgery: a meta-analysis of available studies. Transfusion. 2012;52:2167–73. doi: 10.1111/j.1537-2995.2011.03555.x. [DOI] [PubMed] [Google Scholar]
- 58.Galaal K, Lopes A, Pritchard C, Barton A, Wingham J, Marques EMR, et al. Trial of intraoperative cell salvage versus transfusion in ovarian cancer (tic toc): protocol for a randomised controlled feasibility study. BMJ Open. 2018;8:e024108. doi: 10.1136/bmjopen-2018-024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaw PB, Sentany M, Link WJ, Wahle WM, GGlover JL. Tumor cells carried through autotransfusion. Contraindication to intraoperative blood recovery? JAMA. 1975;231:490–1. [PubMed] [Google Scholar]
- 60.Fruhauf NR, Dumpich O, Kaudel CP, Kasimir-Bauer S, Oldhafer KJ. Filtration of malignant cells: tumour cell depletion in an ex vivo model using a leukocyte adhesion filter. Perfusion. 2001;16((Suppl l)):51–5. doi: 10.1177/026765910101600i107. [DOI] [PubMed] [Google Scholar]
- 61.Marraccini C, Merolle L, Berni P, Boito K, Tamagnini I, Kuhn E, et al. Safety of leucodepleted salvaged blood in oncological surgery: an in vitro model. Vox Sang. 2017;112:803–5. doi: 10.1111/vox.12565. [DOI] [PubMed] [Google Scholar]
- 62.Edelman MJ, Potter P, Mahaffey KG, Frink R, Leidich RB. The potential for reintroduction of tumor cells during intraoperative blood salvage: reduction of risk with use of the rc-400 leukocyte depletion filter. Urology. 1996;47:179–81. doi: 10.1016/S0090-4295(99)80411-7. [DOI] [PubMed] [Google Scholar]
- 63.Müller M, Kuhn DF, Hinrichs B, Schindler E, Dreyer T, Hirsch C, et al. Ist die elimination von osteosarkomzellen durch „maschinelle autotransfusion“ und leukozytendepletionsfilter möglich? Anaesthesist. 1996;45:834–8. doi: 10.1007/s001010050318. [DOI] [PubMed] [Google Scholar]
- 64.Kongsgaard UE, Wang MY, Kvalheim G. Leucocyte depletion filter removes cancer cells in human blood. Acta Anaesthesiol Scand. 1996;40:118–20. doi: 10.1111/j.1399-6576.1996.tb04397.x. [DOI] [PubMed] [Google Scholar]
- 65.Futamura N, Nakanishi H, Hirose H, Nakamura S, Tatematsu M. The effect of storage on the survival of cancer cells in blood and efficient elimination of contaminating cancer cells by a leukocyte depletion filter. Am Surg. 2005;71:585–90. [PubMed] [Google Scholar]
- 66.Liang TB, Li DL, Liang L, Li JJ, Bai XL, Yu W, et al. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–9. doi: 10.1097/TP.0b013e3181671f2e. [DOI] [PubMed] [Google Scholar]
- 67.Catling S, Williams S, Freites O, Rees M, Davies C, Hopkins L. Use of a leucocyte filter to remove tumour cells from intra-operative cell salvage blood. Anaesthesia. 2008;63:1332–8. doi: 10.1111/j.1365-2044.2008.05637.x. [DOI] [PubMed] [Google Scholar]
- 68.Kumar N, Ahmed Q, Lee VK, Zaw AS, Goy R, Wong HK. Are we ready for the use of intraoperative salvaged blood in metastatic spine tumour surgery? Eur Spine J. 2016;25:3997–4007. doi: 10.1007/s00586-015-4112-x. [DOI] [PubMed] [Google Scholar]
- 69.Perseghin P, Viganò M, Rocco G, Della Pona C, Buscemi A, Rizzi A. Effectiveness of leukocyte filters in reducing tumor cell contamination after intraoperative blood salvage in lung cancer patients. Vox Sang. 1997;72:221–4. doi: 10.1046/j.1423-0410.1997.7240221.x. [DOI] [PubMed] [Google Scholar]
- 70.IAKH: recommendation of the interdisciplinary working group for clinical hemotherapy. https://www.iakh.de/der-einsatz-der-maschinellen-autotransfusion-in-der-onkochirurgie.html (last access 1.5.2022)
- 71.Klein AA, Bailey CR, Charlton AJ, Evans E, Guckian-Fisher M, McCrossan R, et al. Association of anaesthetists guidelines: cell salvage for peri-operative blood conservation 2018. Anaesthesia. 2018;73:1141–50. doi: 10.1111/anae.14331. [DOI] [PubMed] [Google Scholar]
- 72.Kozek-Langenecker SA, Ahmed AB, Afshari A, Albaladejo P, Aldecoa C, Barauskas G, et al. Management of severe perioperative bleeding: guidelines from the european society of anaesthesiology: First update 2016. Eur J Anaesthesiol. 2017;34:332–95. doi: 10.1097/EJA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 73.Leal-Noval SR, Munoz M, Asuero M, Contreras E, Garcia-Erce JA, Llau JV, et al. Spanish expert panel on alternatives to allogeneic blood T: Spanish consensus statement on alternatives to allogeneic blood transfusion − the 2013 update of the “seville document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Ferraris VA, Brown JR, Despotis GJ, Hammon JW, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 75.Waters J, Dyga RM, Yazer MH. AABB guidelines for blood recovery and reinfusion in surgery and trauma. AABB; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.