Abstract
Severe hemophilia A and moyamoya (SHAM) syndrome is a rare condition that combines hemophilia A and moyamoya disease (MMD) due to an Xq28 microdeletion encompassing the F8 and BRCC3 genes. Here, we report the case of a 19-year-old male patient with hemophilia A and hypogonadism that presented with right-sided hemiparesis and dysarthria. Brain magnetic resonance imaging and angiography revealed an ischemic lesion in the left lobe and stenosis of both middle cerebral arteries with a concomitant thick vascular network, compatible with moyamoya disease. Next-generation sequence revealed a large Xq28 deletion compatible with SHAM syndrome. The patient was treated with acetylsalicylic acid and neurosurgical intervention was scheduled. Our patient is one of the few cases reported in the literature with Xq28 microdeletion encompassing the F8, hemophilia A causative gene, and BRCC3, responsible for MMD, presenting with a compound phenotype that included neurological manifestations and hypogonadism. In conclusion, diagnosis of MMD should be considered in any male, young patient with symptoms of ischemic stroke with no obvious explanation, and especially in patients with known hemophilia, since a relationship between the two conditions has been documented.
Keywords: Hemophilia A, Moyamoya syndrome, Stroke, SHAM syndrome
Introduction
Hemophilia A is an X-linked inherited bleeding disorder, caused by deficiency or dysfunction of coagulation factor VIII. Patients with hemophilia A are at increased risk of bleeding, especially into muscles, weight-bearing joints, and soft tissues. Replacement therapy with intravenous administration of the deficient coagulation factor is required for life. The prevalence of hemophilia A is 17/100,000 male births, with that of severe hemophilia being 6/100,000 male births [1].
Moyamoya disease (MMD) is a chronic, occlusive cerebrovascular angiopathy [2]. It is characterized by progressive bilateral stenosis at the intracranial portion of the internal carotid artery with concurrent formation of an abnormal vascular network at the base of the brain [2]. Possible complications of MMD are transient ischemic attacks, strokes, or seizures due to brain ischemia as well as brain hemorrhage and headaches due to mechanisms that counterbalance ischemia (i.e., fragile collateral vessels and aneurysms). The prevalence of MMD varies worldwide; it is estimated at 10.5/100,000 individuals in Japan, but it is much lower in the rest of the world, around 0.09/100,000 individuals [3]. Setting the diagnosis of MMD can be challenging, with digital subtraction angiography remaining the “gold standard” for the diagnosis. However, since digital subtraction angiography is an invasive procedure, other radiographic methods, like single-photon emission computed tomography, dynamic perfusion computed tomography, magnetic resonance angiography (MRA), and arterial spin labeling are commonly used [4, 5]. Few cases of patients with an X-linked MMD and concomitant hemophilia A have been reported; in severe hemophilia A and MMD (SHAM) syndrome, as it is called, an Xq28 deletion seems to be responsible for the absence of both F8 gene (causing hemophilia) and BRCC3 gene (causing MMD) [6, 7, 8].
Case Report/Case Presentation
A 19-year-old male patient was admitted due to weakness of the right arm and leg, along with speech disorders in the past 12 h. His medical history was remarkable for severe hemophilia A; he had experienced multiple episodes of hemarthrosis and was treated with intravenous administration of factor VIII.
On admission, physical examination revealed right hemiparesis, dysarthria, and mild confusion. Delay of male sexual development was also noted; small testes, small phallus, and reduced body hair were observed. Hormonal measurements revealed hypergonadotropic hypogonadism. Coagulation tests were normal, apart from prolonged activated partial thromboplastin time (57 s). The level of factor VIII was 15%. The patient's laboratory results can be found in Table 1.
Table 1.
Patient's laboratory findings on presentation
| Examined substance, units | Patient's values | Normal values | Examined substance, units | Patient's values | Normal values |
|---|---|---|---|---|---|
| Hb, mg/dL | 11.8 | 13.5–18.0 | Cortisole, µg/dL | 18.4 | 6.24–18.0 |
| Hct, % | 31.5 | 40.0–54.0 | E2, pg/mL | <5.0 | 11.3–43.2 |
| WBC, K/nL | 6.99 | 4.5–11.0 | T-Testo, ng/mL | 0.3 | 2.5–8.4 |
| PLTs, K/nL | 442.0 | 140.0–440.0 | DHEAS, ng/dL | 212.0 | 70.2–492.0 |
| ESR, mm/h | 20.0 | 0.0–20.0 | SHBG, nmol/L | 46.7 | 18.3–54.1 |
| CRP, mg/L | 28.7 | 0.0–5.0 | PT | 14.2 | 11.0–13.0 |
| Glucose, g/dL | 113.0 | 72.0–106.0 | INR | 1.1 | 0.9–1.2 |
| Cr, mg/dL | 0.5 | 0.7–1.2 | aPTT | 57.0 | 29.0–40.0 |
| Urea, mg/dL | 41.0 | 15.0–43.0 | Fibrinogen | 402.0 | 180.0–400.0 |
| Na, mmol/L | 140.0 | 136.0–143.0 | FVIII, % | 15.0 | 70.0–150.0 |
| K, mmol/L | 4.1 | 3.7–4.9 | Inhibitor FVIII | Negative | Negative |
| Mg, mg/dL | 2.1 | 1.6–2.4 | FII 20210 G-A | GG- negative | Negative |
| Ca, mg/dL | 8.9 | 8.6–10.2 | FV 1691 G-A | GG- negative | Negative |
| ALP, U/L | 118.0 | 56.0–167.0 | MTHFR 677 C-T | CC negative | Negative |
| g-GT, U/L | 15.0 | 8.0–61.0 | ATIII, % | 100.0 | 75.0–140.0 |
| ALT, U/L | 13.0 | <41.0 | PrC, % | 98.0 | 75.0–140 |
| AST, U/L | 18.0 | 15.0–40.0 | C3, mg/dL | 102.0 | 90.0–180.0 |
| tBil, mg/dL | 0.3 | 0.3–1.2 | C4, mg/dL | 25.5 | 10.0–40.0 |
| LDH, U/L | 225.0 | 135.0–225.0 | ANA | Negative | <1:80 |
| TC, mg/dL | 91.0 | 140.0–200.0 | pANCA | 0.0–20.0 | 3.2 |
| HDL, mg/dL | 40.1 | 40.0–60.0 | cANCA | 0.0–20.0 | 5.6 |
| LDL, mg/dL | 41.0 | <100.0 | RF, IU/mL | <8.9 | <20.0 |
| Tg, mg/dL | 49.0 | 50.0–150.0 | IgG, mg/dL | 1,080.0 | 700.0–1,600.0 |
| Trop, pg/mL | 3.0 | <14.0 | IgA, mg/dL | 83.6 | 70.0–400.0 |
| Fe, ng/dL | 16.0 | 59.0–158.0 | IgM, mg/dL | 68.8 | 40.0–230.0 |
| Ferritin, ng/mL | 185.0 | 30.0–400.0 | Anti- cardiolipin IgG, U/mL) | <9.0 | 0.0–15.0 |
| B12 vitamin, pg/mL | 448.0 | 223.0–925.0 | |||
| Follic acid, ng/mL | 11.6 | 4.0–26.8 | Anti- cardiolipin IgM, U/mL) | <9.0 | 0.0–15.0 |
| TSH, mlU/L | 1.1 | 0.3–4.2 | Anti-b2GPI IgG, U/mL | <6.25 | 0.0–9.0 |
| Free T4, pmol/L | 16.0 | 12.0–22.0 | |||
| 1GF-1, ng/mL | 40.0 | Anti-b2GPI IgM, U/mL | <6.25 | 0.0–9.0 | |
| HCG b, mIU/mL | <0.2 | <2.0 | |||
| Prolactin, mIU/mL | 475.0 | 87.0–392.0 | |||
| FSH, mIU/mL | 102.8 | 1.3–11.8 | |||
| LH, mIU/mL | 42.1 | 2.8–6.8 |
Hb, hemoglobin; Hct, hematocrit; WBC, white blood cells; PLTs, platelets; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; Cr, creatinine; Na, sodium; K, potassium; Mg, magnesium; Ca, calcium; ALP, alkaline phosphatase; g-GT, gamma-glutamyl transpeptidase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; tBil, total bilirubin; LDH, lactate dehydrogenase; TC, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein; Tg, triglycerides; Trop, troponin T; Fe, ferrous; TSH, thyroid-stimulating hormone; T4: thyroxine; IGF-1, insulin-like growth factor-1; HCG b, human chorionic gonadotropin beta; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; T-Testo, total testosterone; DHEAS, dehydroepiandrosterone sulfate; SHBG, sex hormone binding globulin; INR, international normalized ratio; aPTT, activated partial thromboplastin time; PT, prothrombin time; FVIII, factor VIII; FII, factor II; FV, factor V; MTHFR, methylene tetrahydrofolate reductase; ATIII, antithrombin (AT); PrC, protein C; C3, complement component C3; C4, complement component C4; ANA, anti-nuclear antibodies; pANCA, perinuclear anti-neutrophil cytoplasmic antibodies; cANCA, cytoplasmic anti-neutrophil cytoplasmic antibodies; RF, rheumatoid factor; IgG, immunoglobulin G; IgA, immunoglobulin A; IgM, immunoglobulin M; anti-b2GPI, anti-beta-2-glycoprotein I IgG (U/mL).
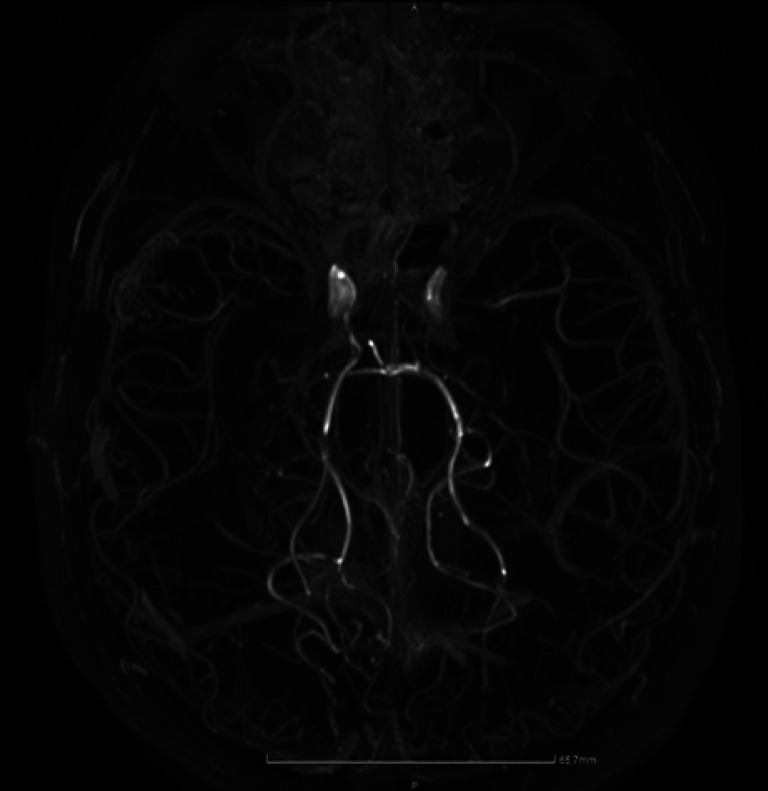
Due to the patient's medical history, intracranial hemorrhage was suspected. Brain computer tomography revealed an old ischemic lesion in the right frontal lobe, indicating a former silent ischemic stroke; the left brain hemisphere, however, seemed normal and no sign of hemorrhage was found. An hour after his admission, the patient experienced generalized tonic-clonic seizures that were treated with diazepam and levetiracetam. After stabilization, he underwent brain magnetic resonance imaging, which revealed a large ischemic lesion in the area supplied by the left middle cerebral artery, with low sign intensity on diffusion-weighted imaging and on apparent diffusion coefficient images, along with high sign intensity on T2 and fluid-attenuated inversion recovery weighted images, not enhanced after administration of intravenous contrast medium. A second lesion with characteristics of chronic ischemic injury was observed in the right frontal lobe, consistent with computer tomography findings. MRA revealed significant stenosis of both middle cerebral arteries with a concomitant thick vascular network in the circumference of both brain hemispheres, compatible with MMD (shown in Fig. 1). Since MMD was diagnosed on the ground of MRA, single-photon emission computed tomography was not performed at that time. Due to the history of hemophilia and advanced risk of bleeding, classic brain angiography was avoided.
Fig. 1.
Brain MRA revealing significant stenosis of both middle cerebral arteries with a concomitant thick vascular network in the circumference of both brain hemispheres.
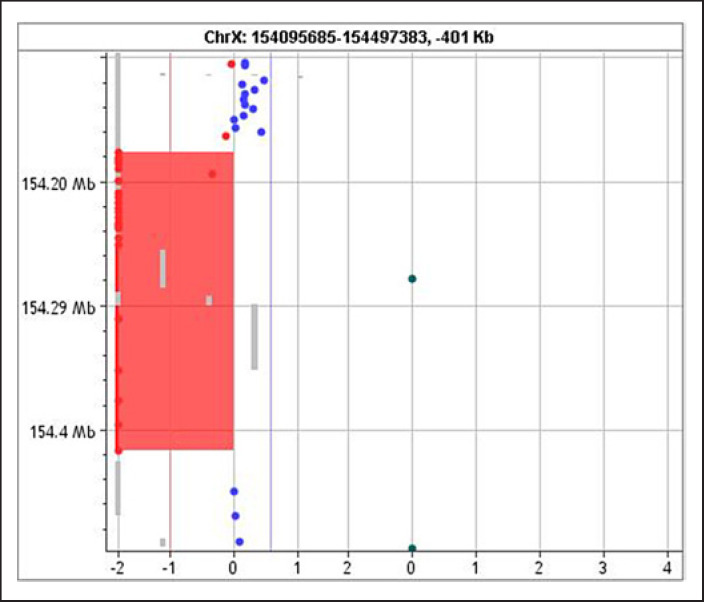
Genomic deoxyribonucleic acid (DNA) was manually extracted from peripheral blood samples of the patient using the QiAamp DNA Mini Kit (Qiagen, Hilden, Germany). The quality and quantity of the DNA were determined using the NanoDrop 2000c UV-VIS spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Chromosomal microarray analysis was performed using the 4 × 180K G3 comparative genomic hybridization (CGH) and single nucleotide polymorphism microarray platform (G5890A, design ID 029830, Agilent Technologies, Santa Clara, CA, USA). The platform features a total of 110,712 oligonucleotide CGH probes covering the whole genome annotated against NCBI Build 38 (UCSC GRCh38, Dec. 2013), with a median CGH probe spacing of 25.3 kb, as well as 59,647 single nucleotide polymorphism probes for the detection of copy-neutral loss of heterozygosity. The laboratory protocol was carried out according to the manufacturer's instructions (agilent oligonucleotide array-based CGH for genomic DNA analysis) [9]. Chromosomal microarray analysis identified a 141 Kb microdeletion in the Xq28 chromosomal region (arr[GRCh37] Xq28[154176025_154417044] × 1) that included the F8, FUNDC2, CMC4, MTCP1, and BRCC3 genes (shown in Fig. 2). The two genes included in the microdeletion interval that were responsible for the patient's full phenotype are F8 (causing hemophilia) and BRCC3 (MMD).
Fig. 2.
Chromosomal microarray analysis of patient's DNA. 141 Kb microdeletion in the Xq28 chromosomal region (arr[GRCh37] Xq28[154176025_154417044] × 1) that included the F8, FUNDC2, CMC4, MTCP1, and BRCC3 genes.
The patient was treated with acetylsalicylic acid 100 mg/day. He attended intensive sessions of physiotherapy and was discharged and referred for possible neurosurgical intervention.
Discussion/Conclusion
We present the case of a 19-year-old male patient with hemophilia A and an ischemic stroke, which was attributed to SHAM syndrome. Molecular genotyping revealed an Xq28 microdeletion encompassing both genes (F8 and BRCC3) responsible for the final phenotypic features, including developmental delay and hypergonadotropic hypogonadism.
Only a few cases of SHAM syndrome have been reported in the literature. A case similar to ours, a 10-year-old boy with hemophilia A and an Xq28 microdeletion has been previously presented [6]. Two additional cases have been described with contiguous manifestations; no genotypic testing was however performed [7, 10]. Another relevant case presented is a 37-year-old patient with hemophilia A detected to carry an Xq28 deletion, including F8 and BRCC3 genes; he was noted to have short stature, premature grey hair, and hypergonadotropic hypogonadism; brain MRA, however, was negative for MMD [11]. Myskinite et al. [8],on the other hand, studied three unrelated families with MMD; they observed that common findings among affected patients were short stature, facial dysmorphism, hypergonadotropic hypogonadism, hypertension, dilated cardiomyopathy, early bilateral acquired cataract, premature coronary heart disease, and grey hair. The syndrome was attributed to Xq28 microdeletion and more specifically to BRCC3 deletion [8]. The patients in the aforementioned study − although not tested − probably presented with a microdeletion that did not extend to the F8 gene and therefore did not have hemophilia A; the study supports the association between MMD and BRCC3 deletion [8]. In our case, hypergonadotropic hypogonadism and mild hypertension but no other characteristics of the syndrome were documented.
The treatment of a patient with SHAM syndrome is challenging as MMD predisposes to ischemia, while hemophilia increases the risk of hemorrhage. Acetylsalicylic acid has been used in some patients in order to prevent emboli distracted from microthrombi from sites of arterial narrowing, however, the cornerstone of MMD treatment is surgical restoration of brain circulation [2].
Our patient is one of the few cases reported in the literature with Xq28 microdeletion encompassing the F8, hemophilia A causative gene, and BRCC3, responsible for MMD, presenting with a compound phenotype that included neurological manifestations and hypogonadism. In conclusion, diagnosis of MMD should be considered in any male, young patient with symptoms of ischemic stroke with no obvious explanation, and especially in patients with known hemophilia, since a relationship between the two conditions has been documented.
Statement of Ethics
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. The procedures followed were in accordance with the ethical guidelines of the Declaration of Helsinki. Ethical approval from a hospital/university committee is not required for this case report in accordance with “Laiko” General Hospital of Athens regulations.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was acquired for this manuscript.
Author Contributions
Evangelia Tzeravini attended the patient, acquired patient's informed consent, drafted, reviewed, and approved the final version of the manuscript; Stamatia Samara attended the patient, drafted, reviewed, and approved the final version of the manuscript; Anna Kourampa was the consultant hematologist, drafted, reviewed, and approved the final version of the manuscript. Georgios Vakrinos was the radiologist who diagnosed moyamoya syndrome based on magnetic resonance angiography findings, provided the radiographic images, drafted, reviewed, and approved the final version of the manuscript; Athina Efthimiou was the consultant neurologist, drafted, reviewed, and approved the final version of the manuscript; Maria Tzetis conducted the genetic analysis, provided the microarray analysis found in Figure 2, drafted, reviewed, and approved the final version of the manuscript; Theodoros Androutsakos attended the patient, acquired patient's informed consent, drafted, reviewed, and approved the final version of the manuscript.
Data Availability Statement
All data that support the findings of this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgements
The authors would like to thank the “Hemophilia Society of Greece” for the financial support in order to perform genetic testing of the patient.
References
- 1.Iorio A, Stonebraker JS, Chambost H, Makris M, Coffin D, Herr C, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019 Oct 15;171((8)):540–6. doi: 10.7326/M19-1208. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS. Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke. 2016 Jan;18((1)):2–11. doi: 10.5853/jos.2015.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Zheng L, Feng L. Epidemiology, diagnosis and treatment of moyamoya disease. Exp Ther Med. 2019 Mar;17((3)):1977–84. doi: 10.3892/etm.2019.7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Jin M, Sun X, Li J, Liu Y, Xi Y, et al. Imaging of moyamoya disease and moyamoya syndrome: current status. J Comput Assist Tomogr. 2019 Mar/Apr;43((2)):257–63. doi: 10.1097/RCT.0000000000000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liming Z, Weiliang S, Jia J, Hao L, Yang L, Ludtka C, et al. Impact of blood pressure changes in cerebral blood perfusion of patients with ischemic Moyamoya disease evaluated by SPECT. J Cereb Blood Flow Metab. 2021 Jun;41((6)):1472–80. doi: 10.1177/0271678X20967458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janczar S, Fogtman A, Koblowska M, Baranska D, Pastorczak A, Wegner O, et al. Novel severe hemophilia A and moyamoya (SHAM) syndrome caused by Xq28 deletions encompassing F8 and BRCC3 genes. Blood. 2014 Jun 19;123((25)):4002–4. doi: 10.1182/blood-2014-02-553685. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Enomoto T, Yanaka K, Nose T. Moyamoya disease associated with hemophilia A. Case report. Pediatr Neurosurg. 2002 Mar;36((3)):157–60. doi: 10.1159/000048372. [DOI] [PubMed] [Google Scholar]
- 8.Miskinyte S, Butler MG, Herve D, Sarret C, Nicolino M, Petralia JD, et al. Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. Am J Hum Genet. 2011 Jun 10;88((6)):718–28. doi: 10.1016/j.ajhg.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzetis M, Kitsiou-Tzeli S, Frysira H, Xaidara A, Kanavakis E. The clinical utility of molecular karyotyping using high-resolution array-comparative genomic hybridization. Expert Rev Mol Diagn. 2012 Jun;12((5)):449–57. doi: 10.1586/erm.12.40. [DOI] [PubMed] [Google Scholar]
- 10.Saini AG, Goswami JN, Suthar R, Sankhyan N, Vyas S, Singhi P. Probable moyamoya syndrome in association with hemophilia A in an infant. Indian J Pediatr. 2017 Feb;84((2)):164–5. doi: 10.1007/s12098-016-2229-5. [DOI] [PubMed] [Google Scholar]
- 11.Lavin M, Jenkins PV, Keenan C, White B, Betts DR, O'Donnell JS, et al. X-linked moyamoya syndrome associated with severe haemophilia A. Haemophilia. 2016 Jan;22((1)):e51–4. doi: 10.1111/hae.12806. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included in this article. Further inquiries can be directed to the corresponding author.