Abstract
Contact lens use is often associated with corneal infections. Pseudomonas aeruginosa is the most common cause of contact lens-associated infections. Its treatment is often challenging due to the ability of this opportunistic bacteria to be resistant to antibiotics that are, usually, prescribed empirically. Antiseptic could be an adjunctive therapy aiming to broaden the antimicrobial spectrum. Low concentration povidone iodine has rapid broad-spectrum activity against bacteria including P. aeruginosa, fungi, viruses, protozoa, and biofilms, lack of resistance and efficacy in wound healing process, along with an optimum safety and tolerability profile. The purpose of this case report was to show the effect of 0.66% povidone iodine added to the antimicrobial treatment of a corneal abscess caused by P. aeruginosa in a contact lens wearer. A 25-year-old female, with suspected microbial keratoconjunctivitis was empirically treated with topical antibiotics (gentamicin and moxifloxacin). After a worsening of the corneal abscess, subconjunctival injection of gentamicin was started and, with the aim of broadening the antimicrobial spectrum, 0.66% PVP-I (2 times a day) was added. Based on the antibiogram, registering abundant growth of P. aeruginosa, topical antibiotics were substituted with ciprofloxacin, while PVP-I was maintained until complete recovery. Combined treatment of antibiotics and PVP-I 0.66% was effective, safe, and well tolerated in treating ocular infection caused by P. aeruginosa. PVP-I could be a useful additional therapeutic tool for fighting P. aeruginosa infections, generally resistant to antibiotics, and to prevent clinical worsening pending the correct microbiological diagnosis.
Keywords: Contact lenses, Microbial keratitis, Pseudomonas aeruginosa, Povidone iodine
Introduction
Contact lenses (CLs) are widely used for vision correction. More than 140 million wearers are present worldwide [1]. Although CLs are generally well tolerated, they can often be the vector of corneal infection (microbial keratitis [MK]) with an annual incidence of around 2–4 per 10,000 wearers and 20 per 10,000 for overnight soft-lens wearers [2].
Based on several studies, the pathogenesis of MK associated with CL wearing would appear to be a complex and multifactorial process including different factors able to compromise the resistance to infection of the healthy cornea. Data report that blinking, tear fluid flow, the structure of corneal epithelium as well as the basal lamina of healthy eyes are all contributing factors along with regulatory elements to generate a barrier protecting the corneal stroma against penetration of microbial pathogens [1].
However, penetration of the corneal epithelium can occur in the presence of pathogenic microbial levels. CLs are considered a primary vector of this penetration for two reasons: they provide a substrate favoring the adhesion of microbes and their transfer onto the ocular surface, and at the same time, they provoke a disruption of the tear film as well as of the ocular surface barriers. They may therefore be the major predisposing factor to infection by allowing bacteria access to the deeper layer of the cornea [3].
Moreover, the ocular microbiota in CL wearers is characterized by contamination from bacterial species of the lid margins and Gram-negative bacteria which are generally present in domestic water supplies [4]. The ocular surface of CL wearers has lower levels of Gram-positive bacteria in comparison with non-lens wearers, while on the other hand, it has greater levels of Gram-negative species [5].
Among all the Gram-negative bacteria, Pseudomonas aeruginosa, although it is an opportunistic pathogen causing infection only rarely, is the most common cause of CL-associated ocular infections. It is one of the major organisms causing MK related to CL use due to favorable CL colonization [6], survival niche allowing microbial replication at the interface between the cornea and CL [7], and ability to produce a biofilm [8]. Particularly, the biofilm formation on CLs may prolong the retention time of the organisms in the site and increase their pathogenicity [9].
Moreover, the P. aeruginosa genome has several virulence factors, both cell-associated and extracellular, capable of starting and maintaining infection [10]. P. aeruginosa produces some enzymes which include protease which either invades or kills cornea cells and, through a complicated regulatory system, plays an important role in regulating virulence during infection. For example, it coordinates the production of virulence factors by means of the quorum-sensing system [11]. One of the effects of these virulence factors is the digestion of collagen which contributes to corneal melting and perforation along with an excessive activation of the host's immune response that favors tissue destruction [11].
Treatment of eye infections caused by P. aeruginosa is often challenging due to the ability of this opportunistic bacteria to be resistant to antibiotics with both intrinsic and acquired mechanisms [12]. Indeed, it is naturally immune to several antibiotics due to the presence of resistance genes in its genome. Moreover, it is also capable of acquiring mobile gene elements such as plasmids, integrons, and transposons that are capable of conferring rapid drug resistance [13, 14]. Mobile gene elements are crucial for the transmission of resistance to aminoglycosides and beta-lactams antibiotics. Furthermore, even some potential factors for the transmission of fluoroquinolones resistance have been recently identified [12].
Antibiotics are routinely used for the treatment of MK as well as for many others ocular infections, although their overuse and misuse is increasing the risk of higher antibiotic resistance. This may favor a quali-quantitative selection of specific microbial strains that may worsen the clinical condition [15].
Sometimes antiseptics can be considered as an alternative to antibiotics or an adjuvant therapy so as to prevent the development of resistance [16]. Povidone iodine (PVP-I) is the gold standard antiseptic in ophthalmology. It is known for its rapid broad-spectrum activity against bacterial spores, bacteria, fungi, viruses, and protozoa [17]. Even when compared with other antiseptic agents such as chlorhexidine gluconate, polyhexanide, or octenidine, PVP-I showed a broader antimicrobial spectrum against Gram-negative bacteria, actinobacteria, bacterial spores, fungi, and viruses and a similar broad spectrum against Gram-positive bacteria [18]. It acts by releasing free iodine, which causes cell death by penetrating the microbial membranes and causing intracytoplasmic oxidation of proteins [17]. Instead, chlorhexidine is a much larger molecule that is not able to penetrate the cell wall and cannot be absorbed by some Gram-negative bacterial cell membranes. It is not active against several Enterobacteriaceae, P. aeruginosa, all Actinobacteria, and all spores [16]. On the contrary, PVP-I activity, especially at low concentration, against Gram-negative bacteria, including P. aeruginosa, has been clearly demonstrated in in vitro and ex vivo studies [19, 20, 21]. Low-dose PVP-I has been shown to eradicate also robust biofilms of multidrug-resistant S. aureus, K. pneumoniae, P. aeruginosa, and Candida albicans in vitro [22]. Furthermore, it can prevent the selection of resistant bacterial strain or cross-resistance caused by antibiotics [23]. In fact, notwithstanding the long and extensive use of PVP-I, to date, no reports concerning resistance or cross-resistance to PVP-I have been recorded even in ophthalmic use [23]. The favorable resistance profile of PVP-I is probably due to the multiple mechanisms of action of PVP-I [24, 25]. This effect is in contrast with other antiseptics [18]. Unlike PVP-I, chlorhexidine, for example, has been found to act on one specific bacterial target: the bacterial cell wall [26]. Therefore, the adaptation of this target may result in resistance to its activity. Despite recent reports suggesting that the use of chlorhexidine does not favor any resistance among pathogens [27, 28, 29], there is some evidence to the contrary in several studies [30, 31, 32, 33, 34]. This report describes the resolution of a corneal abscess case in a CL wearer with ocular infection caused by P. aeruginosa, treated with broad-spectrum empiric antibiotic treatment associated with antiseptic formulation containing 0.66% PVP-I.
Case Report/Case Presentation
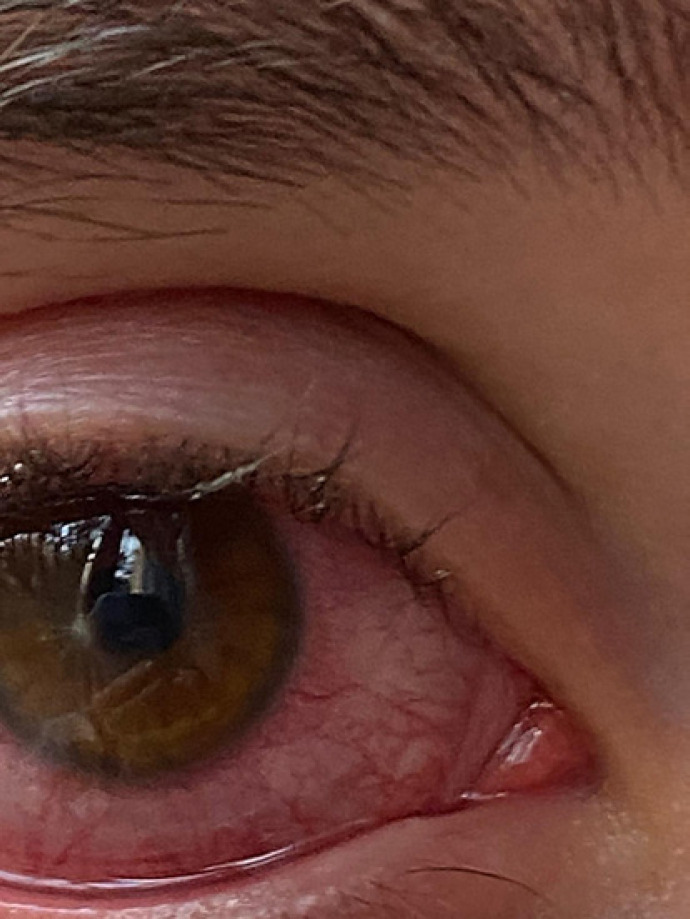
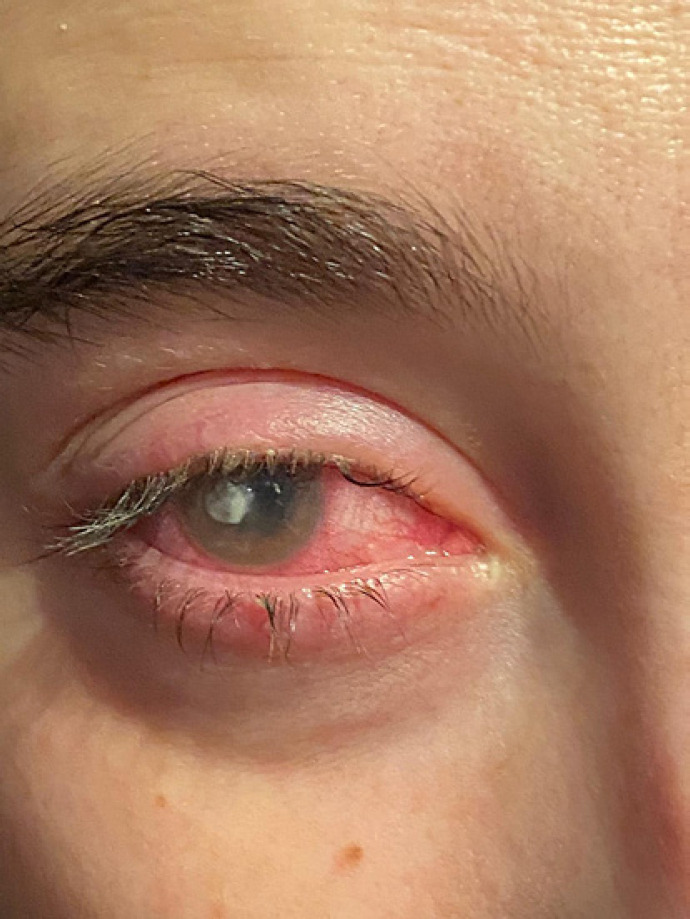
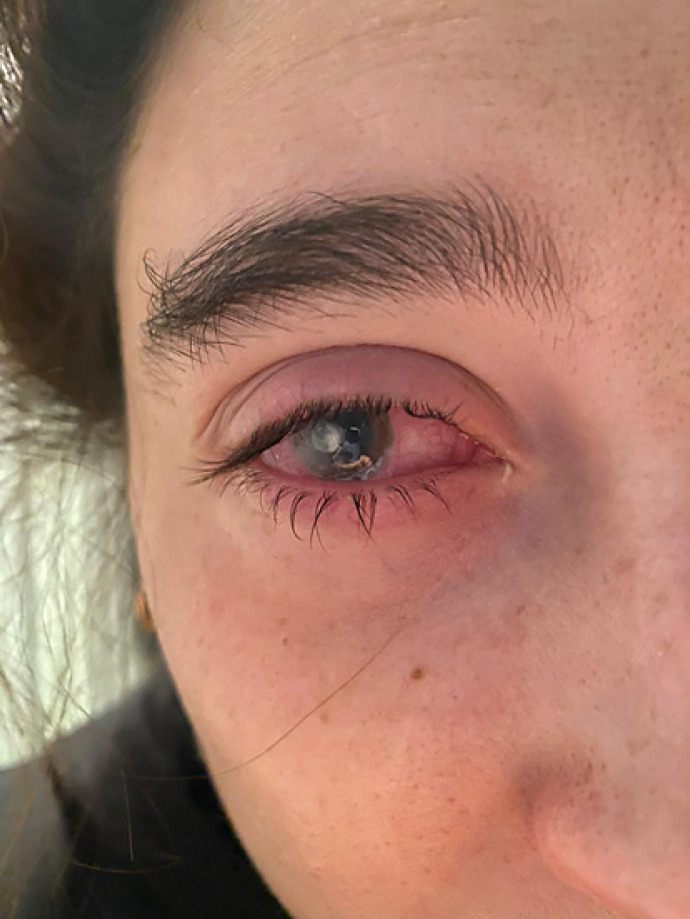
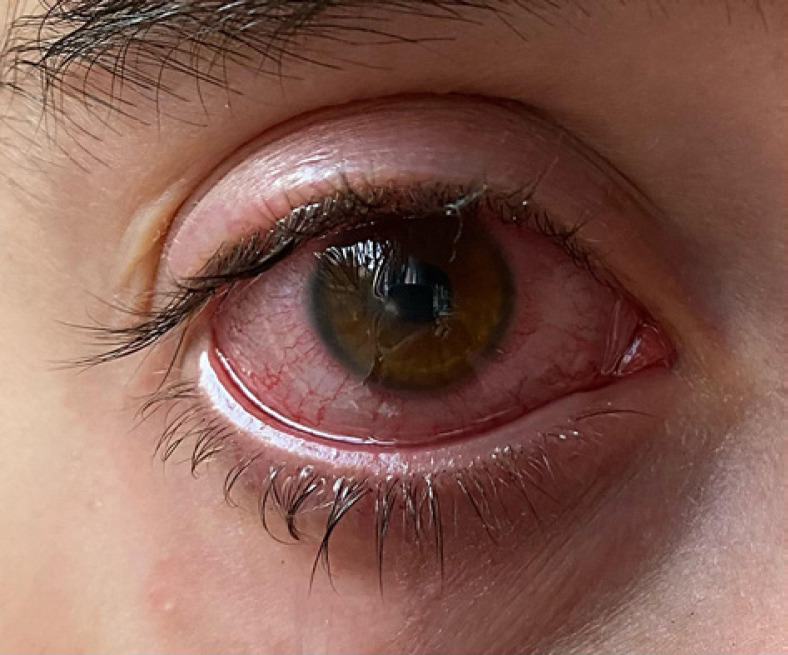
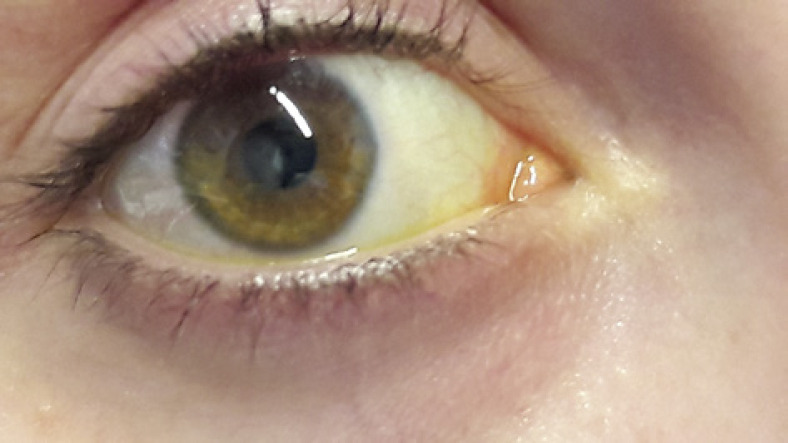
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A 25-year-old female, CL wearer, came to the surgery with clear signs and symptoms of keratoconjunctivitis (day 0, shown in Fig. 1). A conjunctival swab was promptly performed, and she was empirically treated with broad-spectrum antibiotics, moxifloxacin 0.5% (VIGAMOX eye drops; Sooft Italia) and gentamicin 0.3% (GENTICOL eye drops; Sifi) 4 times a day, in order to fight potential infection caused by both Gram-positive and Gram-negative germs, respectively. The following day, the patient worsened as the pain increased and was not adequately controllable even with systemic painkillers. The keratitis, indeed, evolved into corneal ulcer with hypopion involving the deeper corneal layers and the anterior chamber. Even the visual acuity worsened (day 1, shown in Fig. 2). Therefore, a subconjunctival injection of gentamicin was immediately added to the empirical antibiotic treatment. Furthermore, while waiting for the conjunctival swab results, it was decided to administer an adjunctive treatment with a solution containing 0.66% PVP-I, hyaluronic acid, and medium-chain triglycerides, 2 times a day (IODIM®, Medivis) with the aim of broadening the antimicrobial spectrum. The antibiotic therapy (topical and subconjunctival) and the treatment with PVP-I were continued for 5 days when the abscess appeared to be cured (day 5, shown in Fig. 3). At day 5, the conjunctival swab evaluation and the antibiogram results highlighted an infection caused by P. aeruginosa highly sensitive to aminoglycosides and more sensitive to ciprofloxacin than to gentamicin. Consequently, a therapy with ciprofloxacin 0.3% (OFTACILOX eye drops; Alcon) was started (instead of the previous topical moxifloxacin and gentamicin) and associated to PVP-I treatment until day 10, while the subconjunctival injection with gentamicin was continued until day 7. At day 10 (day 10, shown in Fig. 4), the patient showed no more signs of the corneal ulcer. From day 11 and for the following 2 months, the patient was maintained with an anti-inflammatory treatment based on a corticosteroid formulated in hyaluronic acid vehicle (CORTIVIS®, Medivis; hydrocortisone sodium phosphate and hyaluronic acid), leading to the complete resolution of the inflammatory processes (month 2, shown in Fig. 5). After the 2-month period, the spectacle corrected visual acuity was 0.8 with −3.75 D of myopia and central leukoma, not causing visual disturbance or significant aberration. This combined treatment of antibiotic and antiseptic may suggest the usefulness of routinely adding antiseptic agents for the treatment of this kind of infection, although this procedure should be verified in other large clinical studies.
Fig. 1.
Day 0, keratoconjunctivitis with corneal infiltrate.
Fig. 2.
Day 1, corneal abscess.
Fig. 3.
Day 5, clean corneal abscess.
Fig. 4.
Day 10, corneal abscess resolution.
Fig. 5.
Month 2, corneal leukoma in outcomes of corneal abscess.
Discussion/Conclusion
MK is a clinical condition potentially leading to blindness that requires prompt treatment in order to preserve vision. It is historically considered a nonviral infection caused by bacteria, fungi, and/or protozoa, although suitable diagnosis of causative organisms is still critical. The main diagnostic tool remains that of germ cultures, but often, they are inadequate, especially as they take a long time to provide a diagnostic result. Faster methods are still needed to minimize treatment failure and to limit antimicrobial resistance [35, 36].
Based on the advice from the Royal College of Ophthalmologists, MK should initially be treated with a broad-spectrum antibiotic able to fight both Gram-negative and Gram-positive bacteria [37]. Dual therapy targeting both Gram-negative and Gram-positive germs is usually based on topical aminoglycosides and fourth generation fluoroquinolones [38]. However, it is becoming clear that a more targeted treatment to improve the clinical outcome is going to be needed [10]. Since microbiological evaluation of conjunctival swabs often takes a long time to be performed and is not always completely reliable due to the lack of material recovered, this often leads to the choice of incorrect and ineffective therapies responsible for worsening of the ocular pathology.
P. aeruginosa, which is the major causative microbe isolated in MK associated with CL use, is an important pathogen in worldwide healthcare which has been included − by the Infectious Diseases Society of America − in the group of antibiotic-resistant bacteria Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter spp., acronymically dubbed “the ESKAPE pathogens” since they are capable of “escaping” the microbicidal action of antibiotics [39]. Moreover, P. aeruginosa is a bacterium that regularly forms biofilms [40], and biofilm formation during infection is a highly critical factor which confers a drastic increase in microbial resistance to antibiotics, even when the strains are susceptible during planktonic growth. Antibiotics that are clinically useful, such as aminoglycosides and fluoroquinolones, are shown to be effective in killing P. aeruginosa in planktonic growth but can induce severe biofilm production when the microbes are exposed at subinhibitory concentrations. This may be one of the reasons why P. aeruginosa infection is hard to treat. Microbes living in a biofilm community are, in fact, exposed to lower concentrations of antibiotics when medications are administered, a condition which may contribute to the exacerbation of chronic infections by strengthening the existing biofilms [41]. Moreover, although fluoroquinolones are currently used in clinical practice as an empirical therapy, resistance to this class of antibiotics is likely to increase according to various reports concerning the acquired fluoroquinolones resistance genes in P. aeruginosa isolates [42]. Clinicians should bear in mind that clinical resistance may occur even with the newer fluoroquinolones [43].
Another aspect to be considered is toxicity related to the prolonged use of antibiotics. For example, it is known that prolonged use of aminoglycosides such as gentamicin may result in host cell toxicity and potential damage to corneal epithelium, resulting in prolonged healing time [37]. For the aforementioned reasons, sometime the first-line treatment may fail or may be inappropriate due to a resistance identified by the later analysis of conjunctival swab. Therefore, combining from the outset an antibiotic therapy together with a broad-spectrum antiseptic agent that is also capable of fighting biofilms and is immune to resistance could be the appropriate choice to reduce the worsening of the clinical condition.
This report describes the successful treatment of MK with an association of antibiotics and antiseptic. In the first instance, the treatment with the association of aminoglycoside and fluoroquinolone, prescribed empirically, did not produce any improvement and the MK turned into a corneal abscess. Only 5 days after starting the empirical treatment did the ineffectiveness of the moxifloxacin become clear (due to the identification of the Gram-negative germ, P. aeruginosa that was maintaining the infection), and the appropriate antibiotic was prescribed (ciprofloxacin). In this scenario, the additional antiseptic treatment based on PVP-I may have contributed to the improvement of the clinical condition that was resolved just a few days later. Indeed, although intense conjunctival irritation was still present, it might be related to the toxic side effect of topical antibiotics. In contrast, a reduction of the microbial load resulting in the improvement of the abscess could be hypothesized as being linked to the PVP-I activity. Based on this hypothesis, the improvement of the clinical condition of the patient may be attributable to the antiseptic therapy added to the antibiotic ones.
The antiseptic agent used in the present case was a 0.66% PVP-I solution also containing hyaluronic acid, glycerol, and medium-chain triglycerides. Among the different antimicrobial agents, formulations containing PVP-I have been used for antisepsis for a long time. Particularly, 0.66% PVP-I has been described in cases of corneal ulcers [44, 45] as well as in adenoviral keratoconjunctivitis [46] treated successfully. It also has several advantages when compared to other antiseptics: it has the broadest spectrum of activity, it is effective in killing ESKAPE pathogens and biofilms, and there has been no resistance or cross-resistance described even if it has been widely used for decades [18]. PVP-I can also promote wound healing through different mechanisms such as increased expression of transforming growth factor beta, neovascularization, and reepithelialization, so it could be considered the ideal agent for the management of antisepsis in wound care [24, 47, 48].
Noteworthy is also its optimum tolerability profile [24]. The safety and tolerability of PVP-I has been clinically evaluated in an observational, prospective study involving patients with mild-moderate dry-eye disease. The study demonstrated that PVP-I, 2 drops/BID for a 4-week period, was well tolerated and efficacious in improving symptoms and reducing the severity of the injury of the ocular surface as showed by the increase in TBUT and the reduction of epithelial distress highlighted with corneal-conjunctival staining [49]. On the other hand, the presence in the formulation of hyaluronic acid, glycerol, and medium-chain triglycerides may have contributed to the improvement of the ocular surface health. The undisputed effectiveness of PVP-I along with the safety profile of the specific formulation may have contributed to the resolution of the case by early expansion of the antimicrobial spectrum and by favoring the healing process toward the final recovery. PVP-I 0.66%, because of its broad antimicrobial spectrum, its immunity to resistance/cross-resistance, its efficacy against ESKAPE microorganisms, and biofilms and because of its favorable safety profile, even in long-term use and, also in the wound healing process, could be an ideal treatment to be added promptly to the antibiotic treatment that is usually prescribed in an empirical way while waiting for the exact microbiological diagnosis.
Statement of Ethics
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. The guidelines of the institute Ethic Committee Brianza do not require ethical approval for single case reports.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
Funding Sources
This manuscript did not receive any funding.
Author Contribution
The author who contributed entirely to this work is Stefano Castelnuovo. An acknowledgment section has not been created because no other person has contributed to the present study.
Data Availability Statement
All data analyzed during this case report are included in this article. Further inquiries can be directed to the corresponding author.
References
- 1.Fleiszig SMJ, Kroken AR, Nieto V, Grosser MR, Wan SJ, Metruccio MME, et al. Contact lens-related corneal infection: intrinsic resistance and its compromise. Prog Retin Eye Res. 2020 May;76:100804. doi: 10.1016/j.preteyeres.2019.100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carnt N, Samarawickrama C, White A, Stapleton F. The diagnosis and management of contact lens-related microbial keratitis. Clin Exp Optom. 2017 Sep;100((5)):482–93. doi: 10.1111/cxo.12581. [DOI] [PubMed] [Google Scholar]
- 3.Willcox MD. Which is more important to the initiation of contact lens related microbial keratitis, trauma to the ocular surface or bacterial pathogenic factors? Clin Exp Optom. 2006 Sep;89((5)):277–9. doi: 10.1111/j.1444-0938.2006.00054.x. [DOI] [PubMed] [Google Scholar]
- 4.Willcox MD, Power KN, Stapleton F, Leitch C, Harmis N, Sweeney DF. Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci. 1997 Dec;74((12)):1030–8. doi: 10.1097/00006324-199712000-00025. [DOI] [PubMed] [Google Scholar]
- 5.Høvding G. The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol. 1981 Jun;59((3)):387–401. doi: 10.1111/j.1755-3768.1981.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 6.Stapleton F, Dart JK, Seal DV, Matheson M. Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 1995 Jun;114((3)):395–402. doi: 10.1017/s0950268800052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stapleton F, Carnt N. Contact lens-related microbial keratitis: how have epidemiology and genetics helped us with pathogenesis and prophylaxis. Eye. 2012 Feb;26((2)):185–93. doi: 10.1038/eye.2011.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YT, Zhu H, Willcox M, Stapleton F. Removal of biofilm from contact lens storage cases. Invest Ophthalmol Vis Sci. 2010 Dec;51((12)):6329–33. doi: 10.1167/iovs.10-5796. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin-Borlace L, Stapleton F, Matheson M, Dart JK. Bacterial biofilm on contact lenses and lens storage cases in wearers with microbial keratitis. J Appl Microbiol. 1998 May;84((5)):827–38. doi: 10.1046/j.1365-2672.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilliam Y, Kaye S, Winstanley C. Pseudomonas aeruginosa and microbial keratitis. J Med Microbiol. 2020 Jan;69((1)):3–13. doi: 10.1099/jmm.0.001110. [DOI] [PubMed] [Google Scholar]
- 11.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84((4)):273–8. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 12.Subedi D, Vijay AK, Willcox M. Overview of mechanisms of antibiotic resistance in Pseudomonas aeruginosa: an ocular perspective. Clin Exp Optom. 2018 Mar;101((2)):162–71. doi: 10.1111/cxo.12621. [DOI] [PubMed] [Google Scholar]
- 13.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002 Mar 1;34((5)):634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 14.Kung VL, Ozer EA, Hauser AR. The accessory genome of Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2010 Dec;74((4)):621–41. doi: 10.1128/MMBR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013 Jan 24;368((4)):299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grzybowski A, Turczynowska M. More antisepsis, less antibiotics whenever possible. Asia Pac J Ophthalmol. 2018 Mar-Apr;7((2)):72–5. doi: 10.22608/APO.2017343. [DOI] [PubMed] [Google Scholar]
- 17.Lachapelle JM, Castel O, Casado AF, Leroy B, Micali G, Tennstedt D, et al. Antiseptics in the area of bacterial resistance: a focus on povidone iodine. Clin Pract. 2013;10((5)):579–92. [Google Scholar]
- 18.Barreto R, Barrois B, Lambert J, Malhotra-Kumar S, Santos-Fernandes V, Monstrey S. Addressing the challenges in antisepsis: focus on povidone iodine. Int J Antimicrob Agents. 2020 Sep;56((3)):106064. doi: 10.1016/j.ijantimicag.2020.106064. [DOI] [PubMed] [Google Scholar]
- 19.Musumeci R, Bandello F, Martinelli M, Calaresu E, Cocuzza CE. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur J Ophthalmol. 2019;29((6)):673–7. doi: 10.1177/1120672118802541. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Khun D, Kumarasinghe GL, De Zoysa GH, Sarojini V, Vellara HR, et al. Ex vivo evaluation of the stability, safety and antibacterial efficacy of an extemporaneous povidone-iodine preparation for ophthalmic applications. Clin Exp Optom. 2019 Nov;102((6)):583–9. doi: 10.1111/cxo.12899. [DOI] [PubMed] [Google Scholar]
- 21.Pinna A, Donadu MG, Usai D, D'amico-Ricci G, Boscia F, Zanetti S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®) Acta Ophthalmol. 2020;98((2)):e178–e180. doi: 10.1111/aos.14243. [DOI] [PubMed] [Google Scholar]
- 22.Capriotti K, Pelletier J, Barone S, Capriotti J. Efficacy of dilute povidone-iodine against multidrug resistant bacterial biofilms, fungal biofilms and fungal spores. Clin Res Dermatol Open Access. 2018;5((1)):1–5. [Google Scholar]
- 23.Grzybowski A, Kanclerz P, Myers WG. The use of povidone-iodine in ophthalmology. Curr Opin Ophthalmol. 2017;29((1)):19–32. doi: 10.1097/ICU.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 24.Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg. 2017;44:260–8. doi: 10.1016/j.ijsu.2017.06.073. [DOI] [PubMed] [Google Scholar]
- 25.Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017 Jul;30((3)):827–60. doi: 10.1128/CMR.00112-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung HY, Wong MM, Cheung SH, Liang LY, Lam YW, Chiu SK. Differential actions of chlorhexidine on the cell wall of Bacillus subtilis and Escherichia coli. PLoS One. 2012;7((5)):e36659. doi: 10.1371/journal.pone.0036659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marolf CT, Alter R, Lyden E, Fey PD, Rupp ME. Susceptibility of nosocomial Staphylococcus aureus to chlorhexidine after implementation of a hospital-wide antiseptic bathing regimen. Infect Control Hosp Epidemiol. 2017 Jul;38((7)):873–5. doi: 10.1017/ice.2017.80. [DOI] [PubMed] [Google Scholar]
- 28.Musuuza JS, Sethi AK, Roberts TJ, Safdar N. Analysis of multidrug-resistant organism susceptibility to chlorhexidine under usual clinical care. Infect Control Hosp Epidemiol. 2017 Jun;38((6)):729–31. doi: 10.1017/ice.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derde LPG, Cooper BS, Goossens H, Malhotra-Kumar S, Willems RJL, Gniadkowski M, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an Interrupted Time Series Study and cluster randomised trial. Lancet Infect Dis. 2014 Jan;14((1)):31–9. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vali L, Dashti AA, Mathew F, Udo EE. Characterization of heterogeneous MRSA and MSSA with reduced susceptibility to chlorhexidine in Kuwaiti hospitals. Front Microbiol. 2017 Jul 20;8:1359. doi: 10.3389/fmicb.2017.01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Htun HL, Hon PY, Holden MTG, Ang B, Chow A. Chlorhexidine and octenidine use, carriage of qac genes, and reduced antiseptic susceptibility in methicillin-resistant Staphylococcus aureus isolates from a healthcare network. Clin Microbiol Infect. 2019 Sep;25((9)):1154–e7. doi: 10.1016/j.cmi.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 32.Prag G, Falk-Brynhildsen K, Jacobsson S, Hellmark B, Unemo M, Söderquist B. Decreased susceptibility to chlorhexidine and prevalence of disinfectant resistance genes among clinical isolates of Staphylococcus epidermidis. APMIS. 2014 Oct;122((10)):961–7. doi: 10.1111/apm.12239. [DOI] [PubMed] [Google Scholar]
- 33.Hijazi K, Mukhopadhya I, Abbott F, Milne K, Al-Jabri ZJ, Oggioni MR, et al. Susceptibility to chlorhexidine amongst multidrug-resistant clinical isolates of Staphylococcus epidermidis from bloodstream infections. Int J Antimicrob Agents. 2016 Jul;48((1)):86–90. doi: 10.1016/j.ijantimicag.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Cheng A, Sun HY, Tsai YT, Wu UI, Chuang YC, Wang JT, et al. In vitro evaluation of povidone-iodine and chlorhexidine against outbreak and nonoutbreak strains of mycobacterium abscessus using standard quantitative suspension and carrier testing. Antimicrob Agents Chemother. 2017 Dec 21;62((1)):e01364–17. doi: 10.1128/AAC.01364-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017 Nov;124((11)):1678–89. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019 May-Jun;64((3)):255–71. doi: 10.1016/j.survophthal.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuft S, Burton M. Microbial Keratitis. London: The Royal College of Ophthalmology; 2013. [Google Scholar]
- 38.Cury ESJ, Chang MR, Cury Pontes ERJ. Non-viral microbial keratitis in adults: clinical and laboratory aspects. Braz J Microbiol. 2018 Nov;49((Suppl 1)):205–12. doi: 10.1016/j.bjm.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pendleton JN, Gorman SP, Gilmore BF. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther. 2013 Mar;11((3)):297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 40.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001 Jul 14;358((9276)):135–8. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 41.Morita Y, Tomida J, Kawamura Y. Responses of Pseudomonas aeruginosa to antimicrobials. Front Microbiol. 2014 Jan 8;4:422. doi: 10.3389/fmicb.2013.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan M, Summers S, Rice SA, Stapleton F, Willcox MDP, Subedi D. Acquired fluoroquinolone resistance genes in corneal isolates of Pseudomonas aeruginosa. Infect Genet Evol. 2020 Nov;85:104574. doi: 10.1016/j.meegid.2020.104574. [DOI] [PubMed] [Google Scholar]
- 43.Epstein SP, Bottone EJ, Asbell PA. Susceptibility testing of clinical isolates of Pseudomonas aeruginosa to levofloxacin, moxifloxacin, and gatifloxacin as a guide to treating pseudomonas ocular infections. Eye Contact Lens. 2006 Sep;32((5)):240–4. doi: 10.1097/01.icl.0000215432.96891.1f. [DOI] [PubMed] [Google Scholar]
- 44.Bordin P. Corneal ulcer treated with 0.66% nanoemulsion povidone-iodine: a case report. Am J Case Rep. 2020;21:21e919822. doi: 10.12659/AJCR.919822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bordin P. Low-concentration (0.66%) povidone iodine treatment of a corneal ulcer in a rheumatoid arthritis patient. Am J Case Rep. 2021 Mar 22;22:e928748. doi: 10.12659/AJCR.928748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricciardelli G, Giannaccare G, Di Zazzo A, Coassin M, Scorcia V, Romano MR, et al. Efficacy and tolerability of polyvinylpyrrolidone-iodine 0.6% treatment in adenoviral keratoconjunctivitis: a Prospective Randomized Controlled Study. Eye. 2021 Mar 2;36((1)):160–6. doi: 10.1038/s41433-020-01344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripa S, Bruno R, Reder R. Handbook of topical antimicrobials industrial applications, industrial applications in consumer products and pharmaceuticals. 1st ed. Boca Raton, FL: CRC Press; 2002. Clinical applications of povidone-iodine as a topical antimicrobial. p. ISBN 978–0–429–22172–9. [Google Scholar]
- 48.Wang L, Qin W, Zhou Y, Chen B, Zhao X, Zhao H, et al. Transforming growth factor beta plays an important role in enhancing wound healing by topical application of povidone-iodine. Sci Rep. 2017 Apr 20;7((1)):991. doi: 10.1038/s41598-017-01116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliverio GW, Spinella R, Postorino EI, Inferrera L, Aragona E, Aragona P. Safety and tolerability of an eye drop based on 0.6% povidone-iodine nanoemulsion in dry eye patients. J Ocul Pharmacol Ther. 2021 Mar;37((2)):90–6. doi: 10.1089/jop.2020.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this case report are included in this article. Further inquiries can be directed to the corresponding author.