Abstract
Background:
Carbohydrate antigen 19-9 (CA19-9) is the only biomarker for monitoring responses during treatments of pancreatic cancer, but its accuracy for disease outcome is controversial. Fluid biopsy is a new method for diagnosis and monitoring treatment response. In this study, we investigate the usefulness of cell-free DNA (cfDNA) in predicting disease progression during the treatment of pancreatic cancer.
Methods:
Biopsy-proved advanced pancreatic cancer patients who received systemic chemotherapy were enrolled after signed informed consent. CA19-9 and cfDNA in blood were measured before and after every two cycles of treatments, and the disease progression was monitored by computed tomography (CT) with 3-month interval.
Results:
In total, 74 patients and 148 blood samples were enrolled in this study. Patients whose average blood cfDNA concentration of >9.71 ng/mL before and after first two courses of chemotherapy would subsequently show new distant metastasis (NDM) on CT scans 3 months later. The accuracy was 94.37% (AUC 0.9705, p < 0.0001) and the progression-free survival (PFS) and overall survival (OS) of patients with cfDNA concentration of >9.71 ng/mL were worse than those patients with cfDNA concentration of <9.71 ng/mL (median PFS: 95 days versus 322 days, p < 0.0001; median OS: 150 days versus 431 days, p < 0.0001). The cfDNA concentration of >9.71 ng/mL is a predictor for PFS, OS, and distant metastasis-free survival by multivariate analysis. Comparison of KRAS G12 variants detected by next-generation sequencing from tumor tissue issue and remnant DNA of cfDNA showed that increased cfDNA was primarily derived from cancer cells.
Conclusion:
The cancer-cell-derived cfDNA levels could be served as a powerful biomarker for prediction of NDM in patients with advanced/metastatic pancreatic cancer.
Keywords: CA19-9, cfDNA, distant metastasis, KRAS G12 variant, pancreatic cancer
Clinical relevance
This study investigated the usefulness of cell-free DNA (cfDNA) in predicting the outcome during the treatment of pancreatic cancer. KRAS G12 variants were also tested from tumor specimen and remnant DNA of cfDNA by next-generation sequencing (NGS). We found that the value of cancer-cell-derived cfDNA is a better marker than carbohydrate antigen 19-9 (CA19-9). The elevated cfDNA value could predict the outcome and new distal metastasis earlier than abdominal computed tomography (CT) during the treatment of pancreatic cancer patients.
Background
Pancreatic cancer is the 12th and 11th most common cancer in men and women worldwide, respectively, with more than 460,000 new cases in 2018. It is estimated to become the second leading cause of death in the United States before 2030.1–4 Surgery remains an effective therapy for patients with pancreatic ductal adenocarcinoma (PDAC), whose main lesion sites are controlled or defined locally.5,6 Early and accurate prediction of patient outcomes with advanced or metastatic cancer after systemic treatments to determine resectability is critical; however, there is still no convincing biomarker.7–10 For example, current methods are unable to adequately define the accurate level of response to systemic treatments, because restaging after systemic treatments is mostly based on imaging findings via CT or magnetic resonance imaging, which are not obtained timeously for predictive prognosis of treatment and do not often accurately reflect the therapeutic effect after systemic therapy.10,11 Hence, novel biomarkers that can be acquired in real time during intervals between chemotherapy and surgery are required.
Currently, CA19-9 in serum is the only clinical indicator used to monitor responses to treatments in PDAC; however, its application in predicting disease outcomes and treatment responses in advanced and metastatic cancer is unclear, with low correlation to imaging data for re-evaluation of responses. 12 Furthermore, CA19-9 expression in pancreatic cancer patients is based on gene regulation of the Lewis blood group (Le). Generally, approximately 5–7% of patients with Le(a-b-) could not express CA19-9, and the ratio was up to 10% in Caucasian population. 13 To overcome this, several liquid biopsy biomarkers from blood, which are less invasive and can be repeatedly obtained, have been developed.11,14 cfDNA is a minimally invasive biomarker that originates from mechanisms of cell lysis, apoptosis, necrosis, and active release of DNA fragments into circulating cells during tumorigenesis 15 and may be used as a reliable method to detect tumor-specific mutations.16,17 It is believed to be primarily derived from apoptotic cells in healthy individuals of hematopoietic origin. In cancer patients, it is derived from both hematopoietic and tumor origins.11,18,19 Previous studies showed that cfDNA levels correlated with tumor burden and were elevated with tumor growth in colorectal, breast, lung, and ovarian cancer patients.18,20–23 Currently, the clinical use of cfDNA in lung cancer, breast cancer, and colon cancer is relatively established, and several commercial kits have been approved by the U.S. Food and Drug Administration that are used to predict treatment outcome, analyze genetic mutations, test drugs, and personalize medicine use.24,25 However, the application of cfDNA in pancreatic cancer remains elusive, 26 with only few reports.27,28
In this study, we aimed to determine the prognostic utility of cfDNA with longitudinal, serial sampling from patients with PDAC, who were initially diagnosed with advanced or metastatic cancer under systemic chemotherapies, to predict early treatment response. As CA19-9 was not elevated in every pancreatic cancer patient, its limitations were predicting disease outcomes, especially in those patients who did not express CA19-9 or those who had a CA19-9 level greater than the linear range of the machine.
Methods
Patients’ information and grouping
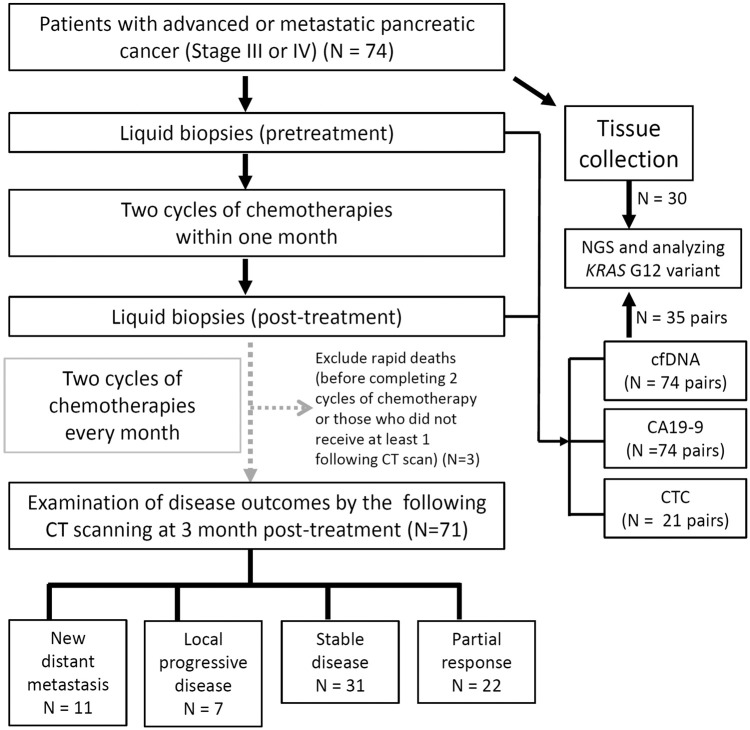
Patients were eligible for this study if they were aged >18 years old, had a cytologically and pathologically confirmed diagnosis of pancreatic adenocarcinoma, and staged via CT scanning. The eligibility criteria were as follows: pathologically proven PDAC with advanced stage (III) or metastatic cancer (IV) and Eastern Cooperative Oncology Group (ECOG) score ⩽1 before treatment. The exclusion criteria were those who could not be followed up by the study cutoff day. In all, 74 patients, who were initially staged III or IV, were enrolled between July 2020 and November 2021. The study was conducted ethically in accordance with the National Cheng Kung University Hospital’s Institutional Review Board (IRB) (B-ER-10594), and all blood samples were collected during daily practice at each outpatient appointment (with IRB approval and written informed consent from patients) or during admission. The workflow of this study is summarized and illustrated in Figure 1.
Figure 1.
Workflow of liquid biopsy analysis for pancreatic cancer patients in this study.
The clinical characteristics and demographic data of patients, including age, sex, tumor stage, tumor size, metastatic site, and outcome variables were collected. Disease progression and treatment response status, defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1), were confirmed via CT scan and followed up approximately every 8–12 weeks. According to CT imaging with RECIST criteria 1.1, we separated the progressive disease (PD) group into two different severities: new distant metastasis (NDM) and local progression disease (LPD). Patients with NDM were characterized with whole abdominal plus chest CT scan to identify any new target lesions occurring at new non-pancreatic organs and sites, such as the liver, lung, and peritoneum. In contrast, new lesions found at adjacent sites on the pancreas or the main pancreatic tumor enlargement were defined as local PD. For clear illustration of disease progression after chemotherapy, disease scores examined by CT were defined as follows: 2, NDM; 1, LPD; 0, initial diagnosis or stable disease (SD); −1, partial response (PR); −2, complete response.
Measurement of cfDNA and CA19-9
Approximately 6–8 mL of one patient’s whole blood specimen was collected in Streck Cell-Free DNA BCT® (Streck, La Vista, NE, USA. Cell-free plasma in the upper layer was separated using centrifugation at 1600g for 10 min at 25°C. The plasma was subsequently centrifuged at 12,000g for 10 min to remove any possible cells or debris (avoiding genomic DNA contamination), and cfDNA from the resulting supernatant was extracted using the iCatcher Circulating cfDNA 4000 Kit and iCatcher 12 instrument (Catchgene, Taipei, Taiwan) according to the manufacturer’s instructions. cfDNA quantities were measured using the Quant-iT™ dsDNA HS Assay Kit (Thermo Fisher Scientific, MA, USA). cfDNA integrity with target regions, 25–500 base pairs (bp), were measured using high-sensitivity large fragment 50 kb analysis kits (Thermo Fisher Scientific). cfDNA concentrations for each specimen (ng per mL of whole blood) shown in this study were calculated as follows: total extracted cfDNA (ng) × cfDNA integrity (%)/volume of whole blood (mL).
CA19-9 (U/mL) in blood of patients was routinely measured during every two cycles of chemotherapy. CA19-9 levels were measured via the electrochemiluminescence immunoassay using the Elecsys CA19-9 Immunoassay on a Cobas e601 platform (Roche, Basel, Sweden) according to the manufacturer’s instructions. The resulting CA19-9 values were in the range of 2–10,000 U/mL (normal range, ⩽34 U/mL).
DNA next-generation sequencing
The analyses of KRAS G12 variants in cfDNA were performed via NGS using the NextSeq 2000 System (Illumina, CA, USA). Approximately 40 ng cfDNA per sample was used for library preparation using the Trusight Oncology 500 Kit (Illumina) according to the manufacturer’s instructions. The sequence data were processed and analyzed using the TruSight Oncology 500 Local App version 1.3 (Illumina). The average sequencing depth was approximately 1500×. Variant allele frequencies (VAFs) were calculated as altered variant reads/total reads.
Genomic DNA was extracted from 6 × 10 μm tissue sections using the GeneRead DNA FFPE Kit (Qiagen, Venlo, The Netherlands). DNA concentrations were measured using a Qubit high-sensitivity kit (Thermo Fisher Scientific). Subsequently, 40 ng of DNA was used as input for library preparation. Input DNA was sheared on a Covaris M220 Focused-ultrasonicator (Covaris, MA, USA) using a microTUBE-50 AFA Fiber Screw-Cap (Covaris) following the manufacturer’s instructions. DNA libraries were prepared using the hybrid capture-based TruSight Oncology 500 Library Preparation Kit (Illumina) following Illumina’s assay protocol. Libraries were sequenced using NexSeq550 (Illumina).
Isolation of circulating tumor cells
Circulating tumor cell (CTC) characterization was performed after collecting ~10 mL of peripheral blood before and after treatment. The blood was processed with Ficoll-Paque separation, red blood cell lysis and negative selection with depletion of CD45-positive leukocytes using magnetic microbeads. Thereafter, resulting cells were stained with fluorescence-conjugated anti-epithelial cell adhesion molecule (EpCAM), anti-cytokeratin 7 antibodies, and anti-CD45 antibodies. Stained cells were analyzed using a cytometry machine. Positive CTCs were defined as both EpCAM positive and cytokeratin 7 positive yet CD45 negative.
Statistical analysis
The cutoff values of cfDNA concentrations between the NDM group and the non-NDM group (including LPD, SD, and PR patients) were determined and calculated by receiver operating characteristic (ROC) curve analysis with the highest sensitivity and specificity. Survival analysis based on cutoff values was performed using the Kaplan–Meier method, providing p values, with the use of the log-rank (Mantel–Cox) test. Cox proportional hazards regression analysis was performed to analyze prognostic factors for progression-free survival (PFS), distant metastasis-free survival (DMFS), and overall survival (OS). Hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs) were estimated. Statistical analyses were performed using Microsoft Excel 2016; GraphPad Prism software, version 7.00; or SPSS software, version 17.0.
Results
Demographic of patients
In all, 74 patients were eligible for inclusion in the present study from July 2020 to November 2021. Basic demographics are shown in Table 1. The median age was 65.7 years (range, 36.2–84.1); the male–female ratio was 39:35. The liver was the most common metastatic site (54.1%), followed by the peritoneum (12.2%) and the lungs (5.4%) prior to any treatment. As all patients were initially diagnosed with locally advanced or metastatic cancer, they received systemic chemotherapy every 2 weeks or at least twice a month. cfDNA concentrations were monitored monthly until the patient died or underwent the operation. The median number of follow-up days was 232 (range, 68–456). In the 74 patients, 18 (24.3%) were stage III and 56 (75.7%) were stage IV. The median tumor size was 3.7 cm (range, 0.7–7.5 cm). Primary tumors of 37 patients (50%) were located in the pancreatic head and neck portion, 18 patients in the body (24.3%), and 18 patients in the tail (24.3%). The initial CA19-9 level before treatment was from <2 to >10,000 (the lower and upper limits of the linear range in the central laboratory). The normal value of CA19-9 was defined as ⩽34 U/mL in our study based on the laboratory results. The ECOG performance of all enrolled patients was 0 in 62 patients (83.8%), 1 in 10 patients (13.5%), and 2 in 2 patients (2.7%). Following several cycles of chemotherapy, 17 patients (23%) underwent surgery, which included eight patients at stage III and nine patients at stage IV.
Table 1.
Basic demographic characteristics of the patients and tumors (n = 74).
| Characteristics | Total (n = 74) | Percentage (%) |
|---|---|---|
| Age, years (median, range) | 65.7 (36.2–84.1) | |
| Sex | ||
| Male | 39 | 52.7 |
| Female | 35 | 47.3 |
| Stage | ||
| III | 18 | 24.3 |
| IV | 56 | 75.7 |
| ECOG status | ||
| 0 | 62 | 83.8 |
| 1 | 10 | 13.5 |
| 2 | 2 | 2.7 |
| Follow-up days (median, range) | 232 (68–456) | |
| Tumor site | ||
| Head and neck | 37 | 50.0 |
| Body | 18 | 24.3 |
| Tail | 18 | 24.3 |
| Liver | 1 | 1.4 |
| Primary tumor size (median, range) | 3.7 (0.7–7.5) | |
| N stage (n = 52) | ||
| N0 | 4 | 5.4 |
| N1 | 38 | 51.4 |
| N2 | 32 | 43.2 |
| Initial metastatic site (n = 56) | ||
| Oligometastatic organ | 40 | 71.4% |
| Multiple metastatic organ | 16 | 28.6% |
| New distant metastatic site (n = 11) | ||
| Oligometastatic organ | 9 | 81.8% |
| Multiple metastatic organ | 2 | 18.2% |
| Initial cfDNA (ng/mL) | ||
| Median (range) | 5.27 (1.21–48.5) | |
| Initial CA19-9 (U/mL) | ||
| <34 | 11 (<2–33.1) | 14.9% |
| 35–10,000 | 51 (38.8–8650) | 68.9% |
| >10,000 | 12 | 16.2% |
CA19-9, carbohydrate antigen 19-9; cfDNA, cell-free DNA; ECOG, Eastern Cooperative Oncology Group.
CfDNA is a potential predictive biomarker
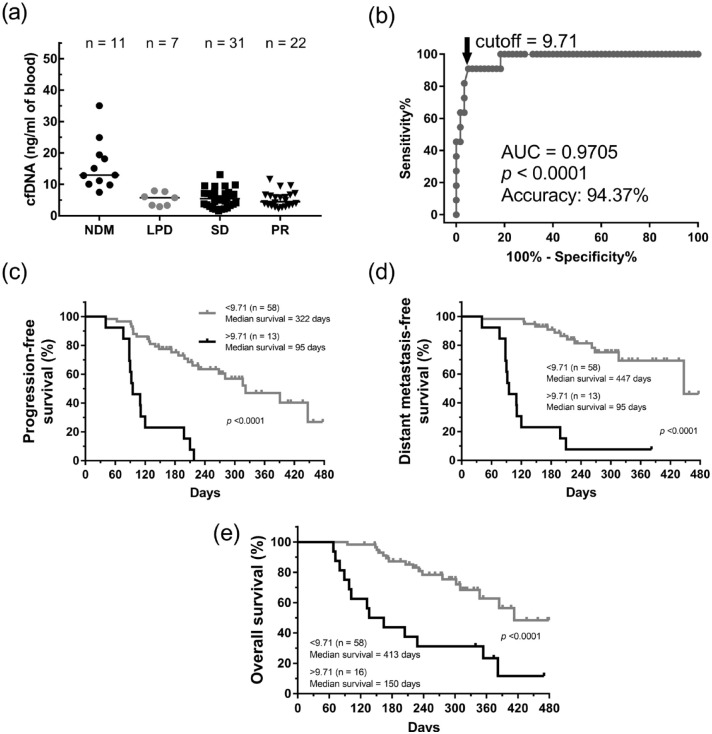
To prove this hypothesis, liquid biopsies of patients before and after two cycles of chemotherapy were collected, and the levels of cfDNA, CA19-9, and CTC were examined, as shown in Figure 1. According to RECIST criteria with minor modifications, disease progression and responses of these patients in the first CT scan after the 2- or 3-month treatment were classified as NDM, LPD, SD, and PR. Notably, cfDNA concentrations in both pre- and post-treatment in NDM patients showed high yet random levels (Figure 2(a)). To determine the optical threshold between the NDM group and non-NDM (LPD + SD + PR) groups, ROC analysis was performed. The average values of cfDNA with a cutoff concentration of 9.71 ng/mL before and after the two courses of treatment showed the best predictive AUC value (0.9705; p < 0.0001) and accuracy (94.37%) with 90.91% sensitivity (95% CI: 58.72–99.77%) and 95% specificity (95% CI: 86.08–98.96%) (Figure 2(b)). Those patients with an average cfDNA concentration of >9.71 ng/mL showed significantly worse PFS compared to other patients with cfDNA concentration of <9.71 ng/mL (median PFS: 95 days versus 322 days, p < 0.0001; Figure 2(c)). In addition, those patients also had shorter DMFS (median DMFS: 95 days versus 447 days, p < 0.0001) and OS (median OS: 150 days versus 413 days, p < 0.0001) (Figure 2(d) and 2(e)). These results indicated that monitoring the value of cfDNA in the first month could predict disease progression. When the cfDNA concentration of patients was over the cutoff value (9.71 ng/mL), they would potentially get worse within 3 months and succumb to death in the following half year.
Figure 2.
The average of cfDNA concentration before and after treatments provided a predictive biomarker for disease progression. (a) The dot plots of average of cfDNA concentrations from patients with pre-treatment and post-treatment in four groups of disease outcomes. (b) The ROC curve analyses of NDM and non-NDM (LPD + SD + PR). Kaplan–Meier analysis of PFS(c), new DMFS (d), and OS (e) in pancreatic cancer patients were showed according to the cutoff value by the ROC curve. We also showed the difference of median survival days of patients calculated by the value of cfDNA.
Bars in the dot plots = median.
cfDNA, cell-free DNA; DMFS, distant metastasis-free survival; LPD, local progressive disease; NDM, new distant metastasis; OS: overall survival; PFS, progression-free survival; PR, partial response; ROC, receiver operating characteristic; SD, stable disease.
For comparison, CA19-9 concentration measurements were also included in this study. Unlike cfDNA, the CA19-9 levels of the 74 patients were evidently fluctuated and diverse (Supplemental Figure 1(a)). In the four groups with different diseases status outcomes (NDM, LPD, SD, and PR), the CA19-9 concentrations before and after two cycles of chemotherapy were similar and scattered from <35 (the normal standard) to >10,000 U/mL (above the detection limit of the assay used). The ROC analysis results showed that the AUC values of pre- and post-treatment were only 0.7121, p = 0.0261 (Supplemental Figure 1(b)), indicating that the initial CA19-9 concentration was not a suitable early predictive biomarker for disease progression. CTC numbers were also evaluated, and the trends were also different among the four disease outcomes (Supplemental Figure 2).
To confirm that the average cfDNA concentration was a suitable indicator of prognosis, Cox regression analysis was used. The possible risk factors, such as age, disease stage, tumor size, T or N status, cfDNA, and CA19-9 levels, were also evaluated. In univariate Cox regression analyses, the average cfDNA concentration of >9.71 ng/mL was highly associated with worse PFS (HR = 6.85; p < 0.001) and OS (HR = 4.16; p < 0.001). Multivariable analyses of factors associated with PFS and OS showed that average cfDNA concentration of >9.71 ng/mL was the best variable for PFS (HR = 6.25, 3–13.02; p < 0.001) and OS (HR = 6.75, 2.85–16; p < 0.001) (Table 2). In contrast, other factors, such as CA19-9 concentration in blood (>34 U/mL), did not show any significant HR. Moreover, the association between average cfDNA and NDM was further confirmed using univariate and multivariate Cox regression analyses with statistically significant HR; 11.34, 4.81–26.71, p < 0.001 (Table 3). Collectively, these analyses demonstrated that early detection of cfDNA in blood could be a predictive prognostic biomarker for later disease progression.
Table 2.
Analysis of factors associated with PFS and OS.
| Factor | PFS | OS | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age >65 years | 1.09 (0.56–2.10) | 0.8 | 0.98 (0.47–2.04) | 0.964 | ||||
| Stage (III versus IV) | 1.57 (0.72–3.44) | 0.258 | 2.85 (0.99–8.19) | 0.052 | 3.21 (1.05–9.78) | 0.04 | ||
| Tumor size: >3.7 cm | 0.83 (0.43–1.59) | 0.572 | 2.15 (2.03–4.49) | 0.041 | 4.2 (1.73–10.31) | 0.002 | ||
| N stage | 1.88 (1.06–3.32) | 0.03 | 1.69 (0.94–3.02) | 0.079 | 3.07 (1.52–6.18) | 0.002 | 3.44 (1.59–7.42) | 0.002 |
| cfDNA average: >9.71 ng/mL | 6.85 (3.28–14.27) | <0.001 | 6.25 (3–13.02) | <0.001 | 4.16 (2.01–8.63) | <0.001 | 6.75 (2.85–16) | <0.001 |
| CA19-9: >34 U/mL | 1.70 (0.6–4.82) | 0.317 | 1.97 (0.60–6.51) | 0.267 | ||||
CA19-9, carbohydrate antigen 19-9; cfDNA, cell-free DNA; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Table 3.
Analysis of factors associated with distant metastasis PFS.
| Factor | Univariate of DMFS | Multivariate of DMFS | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age: >65 years | 1.16 (0.51–2.6) | 0.728 | ||
| Disease stage (III or IV) | 0.65 (0.29–1.48) | 0.306 | ||
| Tumor size: >3.7 cm | 1.11 (0.5–2.45) | 0.803 | ||
| N status | 1.61 (0.81–3.19) | 0.170 | 1.23 (0.60–2.52) | 0.576 |
| cfDNA average: >9.71 ng/mL | 12.40 (5.28–27.83) | <0.001 | 11.34 (4.81–26.71) | <0.001 |
| CA19-9: >34 U/mL | 2.61 (0.51–9.23) | 0.298 | ||
CA19-9, carbohydrate antigen 19-9; cfDNA, cell-free DNA; CI, confidence interval; DMFS, distant metastasis-free survival; HR, hazard ratio; PFS, progression-free survival.
Elevated cfDNA was primarily derived from tumor cells
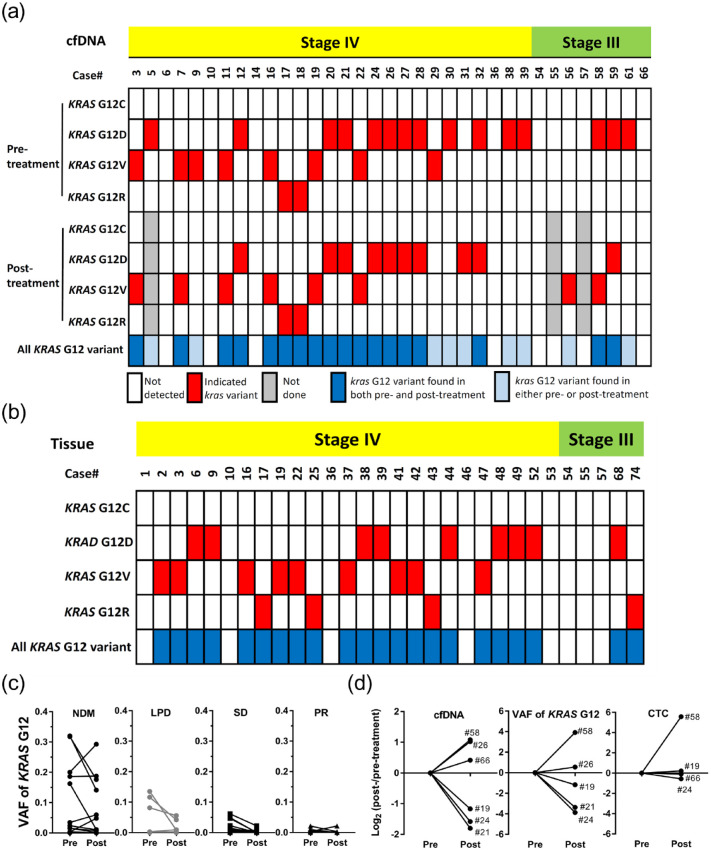
The early elevated cfDNA in blood revealed the occurrence of NDM, implying that tumor cells would release unexplored messages, such as circulating tumor DNA (ctDNA) into the blood before the formation of new lesions. To elucidate the increase in cfDNA from ctDNA, cfDNA samples were subjected to NGS to detect KRAS G12 variants, which were thought to be high in patients with pancreatic cancer. In total, 35 pairs of cfDNA from patients with pre- or post-treatment and 30 tissue DNA samples from patients were sent to detect the presence of KRAS G12C, G12D, G12V, or G12R (Figure 3(a) and 3(b)). Notably, there were no G12C variants detected in these patients, and the others were randomly present in cfDNA or tissue DNA of patients. In addition, there were no evident differences in G12 variant ratios in both cfDNA and tissue DNA from the four disease outcomes (Supplemental Table 1), indicating that KRAS G12 variants were not the main factor of disease progression in our study. VAFs of KRAS G12 from pre- and post-treated cfDNA were also summarized for comparison between the four disease outcomes (Figure 3(c)), and the VAFs in the NDM group appeared drastically increased or decreased, which was almost consistent with the cfDNA concentrations (Figure 3(c)). Therefore, we compared the cfDNA quantities, VAF, and CTC numbers in pre- and post-treated liquid biopsies of NDM patients (Figure 3(d)). Consistent with our hypothesis, cfDNA quantities were mostly synchronized with VAFs and extremely close to CTC numbers (Supplemental Figure S2). This suggested that elevated cfDNA observed in the NDM group, over the baseline level or indicated cutoff value in our study, may primarily originate from cancer cells, including CTCs.
Figure 3.
The KRAS G12 variant-containing cfDNA derived from tissue cancer cells and/or CTCs could be served as a predictive indicator for the occurrence of NDM. (a, b) Integrated analyses of KRAS G12 variants of patients’ cfDNA and tissue DNA. (c) The VAF of KRAS G12 in cfDNA from patients with different disease outcomes. (d) Integrated comparisons of cfDNA, VAF of KRAS G12 G12, and CTCs among indicated patients.
cfDNA, cell-free DNA; CTC, circulating tumor cell; NDM, new distant metastasis; VAF, variant allele frequency.
Case demonstration
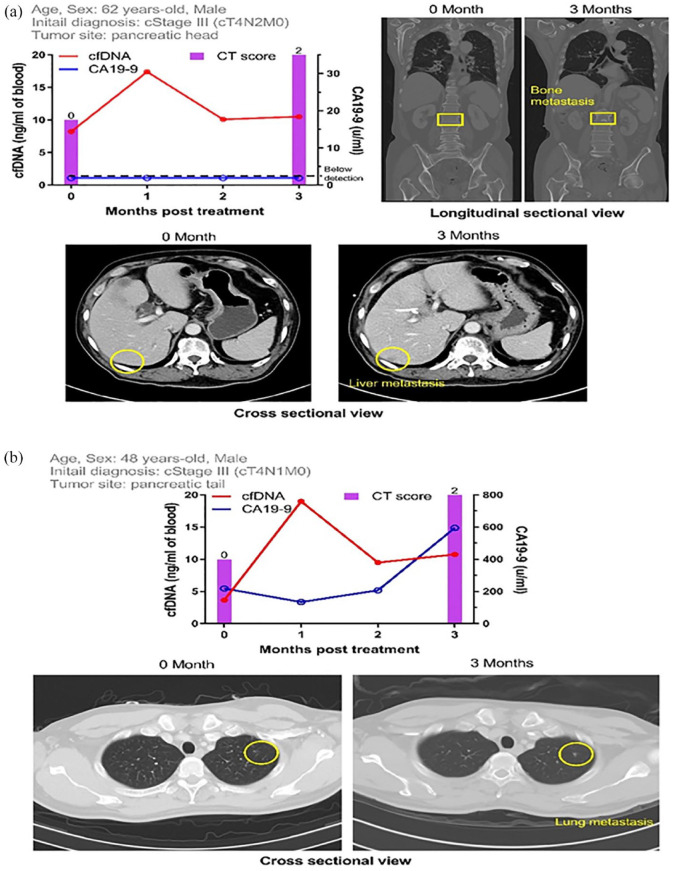
Two typical cases showed that the appearance of elevated cfDNA was prior to the observation of new distant lesions on CT scan. A 62-year-old male patient (Case #58) was initially diagnosed with clinical stage III cancer (T4N2M0) (Figure 4(a)). The cfDNA concentration before treatment was 8.22 ng/mL, drastically elevated to 17.40 ng/mL after two cycles of chemotherapy, and subsequently declined to 10.09 ng/mL in the second month post-treatment. New distant lesions at the S6 liver and L2 spine were found on the following CT scan in the third month post-treatment. In contrast, CA19-9 levels were all under detectable limitation (2 U/mL) throughout the monitoring period, indicating that CA19-9 could not serve as a marker in this case. The other case was a 48-year-old male patient (Case #61) initially diagnosed with clinical stage III cancer (T4N1M0) (Figure 4(b)). Similarly, the cfDNA concentration evidently increased 1-month post-treatment. A new distant lesion in the lung was revealed in the following CT scan in the third month post-treatment. CA19-9 levels were also monitored; notably, CA19-9 concentration increased in the third month post-treatment, which was later than the cfDNA concentration. Thus, these two cases raised the role of cfDNA in predicting the new distant lesions prior to CT scanning.
Figure 4.
Case demonstration of patients with new distal lesions found after monitoring a fluctuation of cfDNA, not CA19-9, concentrations. (a) This patient was diagnosed as locally advanced disease initially, with clinical stage III (T4N2M0). Initial cfDNA level was 8.22 ng/mL. He was treated with systemic chemotherapy, and the cfDNA increased rapidly to 17.40 ng/mL after two cycles of chemotherapy. The first following CT scan which was done at 3 months after treatment revealed new liver metastasis at S6 and bone metastasis at L2. However, the CA19-9 level was below the detection level (<2 U/mL) during the whole treatment course. (b) This patient was diagnosed as locally advanced disease initially, with clinical stage III (T4N1M0). Initial cfDNA level was 3.66 ng/mL. He was treated with systemic chemotherapy, and the cfDNA increased rapidly to 18.99 ng/mL after two cycles of chemotherapy. The first following CT scan after 3 months of chemotherapy showed one new lung metastasis. The CA19-9 level increased slower than cfDNA, with a late elevation at the same time when CT scan was done.
CA19-9, carbohydrate antigen 19-9; cfDNA, cell-free DNA; CT, computed tomography.
Discussion
This is the first study to demonstrate that average cfDNA concentrations within 1 month could predict disease outcomes in advanced/metastatic PDAC, especially in patients who suffered from rapidly NDM and succumbed to poor survival. The cfDNA can provide an earlier indication of disease progression than regular CT scan imaging, whereas CA19-9 cannot. The leading message of average cfDNA concentration could provide a first-step indicator to clinicians for filtering tumor irresectability due to the high risk of NDM in the high-level group, which is always decided by a regular image scan. However, this has limitations in detecting occult distant metastasis.29,30
Our study shows whole serial, longitudinal information of cfDNA quantity and disease progression, which started with pre-systemic chemotherapy and continued monitoring until surgery or quitting. Complete yet simple evidence is convenient in that it allows quick decision making regarding irresectability without complicated analyses and predicts the high possibility of extremely poor prognosis. Previous studies used ctDNA, rather than cfDNA, to serve as a prognostic biomarker in pancreatic cancer.24,27,28,31 One of the ctDNA criteria is the detection of genetic mutations in tumor-related gene panels or differential mutation allele frequency of target genes, for example, KRAS and tp53. Similar observations in those studies have also shown a positive correlation between the presence of ctDNA and disease progression.27,31,32 Our study used the quantity of cfDNA as a clear cutoff value and could alert approximately 13.5% (10 out of 74) of stage III or IV patients to the possibility of NDM lesions within 2–3 months after treatment. In cancer patients, the cfDNA is thought to be primarily derived from dead resident or CTCs. The cfDNA levels positively correlated with disease progression in several cancers.18,20 For example, the cfDNA concentrations in patients with colorectal cancer could serve as the first threshold to categorize ‘disease-positive’ and ‘disease-negative’ recurrent groups. 33 In the present study, we used the average cfDNA value between the initial and after two cycles of chemotherapy as the threshold to determine whether NDM occurred. There were no evident cfDNA levels in patients without NDMs, implying that there was a baseline cfDNA level in the blood. Our evidence showed that the KRAS G12 variant-containing cfDNA enforced the correlation between ctDNA and tumor cells. Therefore, an elevated cfDNA over the cutoff value would be derived from cancer cells, which may be functionally progressive before new lesions are established.
The size of cfDNA is approximately 160 bp because cfDNA fragments are associated with nucleosomes.33,34 A study using 344 plasma samples from 200 cancer patients via paired-end sequencing showed that cfDNA fragments were dominant at 167 bp, suggesting that they were released from apoptotic caspase-dependent cleavage. 35 In pancreatic cancer, the cfDNA integrity, known as the percentage of fragment length, was investigated and showed no evident correlation with disease severity. 36 However, a recent report indicated that small mutant cfDNA fragments are prevalent in patients with pancreatic cancer. 37 In addition, tumor-derived mutations are carried by short cfDNA fragments. Targeting short sequencing amplicons increases the sensitivity of cfDNA assays and should be considered when evaluating their clinical performance. 38 Therefore, our study focused on the 25–500 bp cfDNA fragments, evaluated the cfDNA integrity, and calculated the cfDNA concentrations by combining both cfDNA quantity and integrity to achieve real cfDNA conditions in patients with pancreatic cancer. Detecting ctDNA is relatively expensive and difficult to obtain when compared with detecting cfDNA. In this study, most of the cfDNA fragments are released from tumor cells, which is equal to that of ctDNA. Monitoring cancer-cell-derived cfDNA in liquid biopsies is quicker and simpler than other costly practices. These findings suggest that we could longitudinally and continuously measure cfDNA in our daily practice to predict treatment responses and disease outcomes in the future.
Although our study provides the first step in predicting possible distal metastasis in patients with pancreatic cancer, there exist several limitations. First, these naïve patients receiving two cycles of chemotherapy only, without evaluation via a CT scan after treatment, we cannot stop chemotherapy or change regimen. So, patients may be lost rapidly with possible new distal lesions that could not be defined. Second, unresectability owing to new distal lesions was predicted in stage III patients; because we cannot define unresectability only by elevated cfDNA levels observed in this study. Third, there was no validation cohort. Such biomarker study needs a large number of pancreatic cancer patients, and it is also a challenge to enroll patients to complete within several years. For example, our previous genome-wide study in pancreatic cancer patients recruited subjects for the past 5 years. 39 However, we are confident in providing more evidence in the near future to confirm that changes in cfDNA concentration is a predictive biomarker. Our findings indicate that the average value of cfDNA levels within 1 month was a leading marker in pancreatic cancer, which is alarming for patients with the occurrence of new lesions on distal tissues/organs. A quick and simple analysis of cfDNA quantity allows clinicians to re-evaluate patients or to change the systemic treatment strategy because of the increased risk of rapid NDM. In addition, this is the first study to recruit pancreatic cancer patients at stage III and stage IV, who have low resectability, to strive for any possible cure. We are planning a clinical trial to validate our findings in a prospectively randomized study. Furthermore, clinical pilot study applying cfDNA quantification after neoadjuvant therapies in resectable or borderline resectable cancer patients to determine the most suitable patients to receive curative surgical resection is ongoing.
Conclusion
The cfDNA could be a leading marker for predicting NDM of advanced pancreatic cancer during treatment. The utility of cfDNA in predicting early recurrence in resectable disease and the possible resectability in borderline resectable/advanced disease are highly expected.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank Prof. Sheng-Hiang Lin for providing statistic analyzing consults and methods.
Footnotes
Ethics approval and consent to participate: Informed consent was provided by all patients, and the National Cheng Kung University Hospital’s Institutional Review Board (B-ER-10594) approved all aspects of this study.
Consent for publication: All authors agree to publish this paper by the correspondent author.
Author contributions: Chien-Jui Huang: Data curation; Formal analysis; Investigation; Writing – original draft.
Wen-Yen Huang: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Chien-Yu Chen: Data curation; Formal analysis.
Ying-Jui Chao: Data curation; Investigation.
Nai-Jung Chiang: Data curation; Formal analysis.
Yan-Shen Shan: Conceptualization; Data curation; Funding acquisition; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Ministry of Science and Technology of Taiwan (MOST) (MOST 109-2321-B-006-011, MOST 110-2321-B-006-005, MOST 110-2745-B-006-001, and MOST 110-2813-C-006-035-B) and Ministry of Health and Welfare (MOHW) (MOHW 110-TDU-B-212-144026 and MOHW110-TDU-B-211-124003).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chien-Jui Huang, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan; Division of Gastroenterology and Hepatology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Wen-Yen Huang, Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Chien-Yu Chen, School of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Ying-Jui Chao, Division of General Surgery, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Nai-Jung Chiang, Department of Oncology, Taipei Veterans General Hospital, Taipei; School of Medicine, National Yang Ming Chiao Tung University, Taipei; National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan.
Yan-Shen Shan, Division of General Surgery, Department of Surgery, National Cheng Kung University Hospital, Tainan; Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, No. 138 Sheng-Li Road, Tainan 704, Taiwan.
References
- 1. World Cancer Research Fund International. Pancreatic cancer statistics, https://www.wcrf.org/dietandcancer/pancreatic-cancer-statistics/ (2020, accessed 7 December 2021).
- 2. Neoptolemos JP, Kleeff J, Michl P, et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018; 15: 333–348. [DOI] [PubMed] [Google Scholar]
- 3. Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020; 395: 2008–2020. [DOI] [PubMed] [Google Scholar]
- 4. American Cancer Society. Cancer facts and figures 2021, https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html (2022, accessed 7 December 2021).
- 5. Okusaka T, Nakamura M, Yoshida M, et al. Clinical practice guidelines for pancreatic cancer 2019 from the Japan Pancreas Society: A synopsis. Pancreas 2020; 49: 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Satoi S, Unno M, Motoi F, et al. The effect of neoadjuvant chemotherapy with gemcitabine and S-1 for resectable pancreatic cancer (randomized phase II/III trial; Prep-02/JSAP-05). J Clin Oncol 2019; 37: 4126–4126. [DOI] [PubMed] [Google Scholar]
- 7. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 8. Yang Z, LaRiviere MJ, Ko J, et al. A Multianalyte panel consisting of extracellular vesicle miRNAs and mRNAs, cfDNA, and CA19-9 shows utility for diagnosis and staging of pancreatic ductal adenocarcinoma. Clin Cancer Res 2020; 26: 3248–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019, 16: 11–26. [DOI] [PubMed] [Google Scholar]
- 10. Oba A, Ho F, Bao QR, et al. Neoadjuvant treatment in pancreatic cancer. Front Oncol 2020; 10: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitahata Y, Kawai M, Hirono S, et al. Circulating tumor DNA as a potential prognostic marker in patients with borderline-resectable pancreatic cancer undergoing neoadjuvant chemotherapy followed by pancreatectomy. Ann Surg Oncol 2021; 29: 1596–1605. [DOI] [PubMed] [Google Scholar]
- 12. Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg 2016; 223: 52–65. [DOI] [PubMed] [Google Scholar]
- 13. Su YY, Chiang NJ, Tsai HJ, et al. The impact of liposomal irinotecan on the treatment of advanced pancreatic adenocarcinoma: real-world experience in a Taiwanese cohort. Sci Rep 2020; 10: 7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crowley E, Di Nicolantonio F, Loupakis F, et al. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 2013; 10: 472–484. [DOI] [PubMed] [Google Scholar]
- 15. Chen E, Cario CL, Leong L, et al. Cell-free DNA concentration and fragment size as a biomarker for prostate cancer. Sci Rep 2021; 11: 5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stroun M, Maurice P, Vasioukhin V, et al. The origin and mechanism of circulating DNA. Ann N Y Acad Sci 2000; 906: 161–168. [DOI] [PubMed] [Google Scholar]
- 17. Slostad JA, Liu MC, Allred JB, et al. BRAF V600 mutation detection in plasma cell-free DNA: NCCTG N0879 (alliance). Mayo Clin Proc Innov Qual Outcomes 2021; 5: 1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thierry AR, Mouliere F, Gongora C, et al. Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res 2010; 38: 6159–6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang L, Liang Y, Li S, et al. The interplay of circulating tumor DNA and chromatin modification, therapeutic resistance, and metastasis. Mol Cancer 2019; 18: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 2017; 9: eaan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molina-Vila MA, de-Las-Casas CM, Bertran-Alamillo J, et al. cfDNA analysis from blood in melanoma. Ann Transl Med 2015; 3: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008; 14: 985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multicenter randomized trial of the Dartmouth regimen versus dacarbazine in patients with metastatic melanoma. J Clin Oncol 1999; 17: 2745–2751. [DOI] [PubMed] [Google Scholar]
- 24. Hou J, Li X, Xie KP. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol Cancer 2021; 20: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rolfo C, Cardona AF, Cristofanilli M, et al. Challenges and opportunities of cfDNA analysis implementation in clinical practice: perspective of the International Society of Liquid Biopsy (ISLB). Crit Rev Oncol Hematol 2020; 151: 102978. [DOI] [PubMed] [Google Scholar]
- 26. Wang SE, Shyr BU, Shyr BS, et al. Circulating cell-free DNA in pancreatic head adenocarcinoma undergoing pancreaticoduodenectomy. Pancreas 2021; 50: 214–218. [DOI] [PubMed] [Google Scholar]
- 27. Bernard V, Kim DU, San Lucas FA, et al. Circulating nucleic acids are associated with outcomes of patients with pancreatic cancer. Gastroenterology 2019; 156: 108–118.e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Botrus G, Kosirorek H, Sonbol MB, et al. Circulating tumor DNA-based testing and actionable findings in patients with advanced and metastatic pancreatic adenocarcinoma. Oncologist 2021; 26: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc 2002; 55: 232–237. [DOI] [PubMed] [Google Scholar]
- 30. Heinrich S, Lang H. Neoadjuvant therapy of pancreatic cancer: definitions and benefits. Int J Mol Sci 2017; 18: 1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee B, Lipton L, Cohen J, et al. Circulating tumor DNA as a potential marker of adjuvant chemotherapy benefit following surgery for localized pancreatic cancer. Ann Oncol 2019; 30: 1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sugimori M, Sugimori K, Tsuchiya H, et al. Quantitative monitoring of circulating tumor DNA in patients with advanced pancreatic cancer undergoing chemotherapy. Cancer Sci 2020; 111: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeh YM, Lin PC, Lee CT, et al. Treatment monitoring of colorectal cancer by integrated analysis of plasma concentration and sequencing of circulating tumor DNA. Mol Cancer 2020; 19: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017; 17: 223–238. [DOI] [PubMed] [Google Scholar]
- 35. Mouliere F, Chandrananda D, Piskorz AM, et al. Enhanced detection of circulating tumor DNA by fragment size analysis. Sci Transl Med 2018; 10: eaat4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Utomo WK, Janmaat VT, Verhaar AP, et al. DNA integrity as biomarker in pancreatic cyst fluid. Am J Cancer Res 2016; 6: 1837–1841. [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X, Liu L, Ji Y, et al. Enrichment of short mutant cell-free DNA fragments enhanced detection of pancreatic cancer. EBioMedicine 2019; 41: 345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zvereva M, Roberti G, Durand G, et al. Circulating tumour-derived KRAS mutations in pancreatic cancer cases are predominantly carried by very short fragments of cell-free DNA. EBioMedicine 2020; 55: 102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shan YS, Chen LT, Wu JS, et al. Validation of genome-wide association study-identified single nucleotide polymorphisms in a case-control study of pancreatic cancer from Taiwan. J Biomed Sci 2020; 27: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-2-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-3-tam-10.1177_17588359221106558 for Cancer-cell-derived cell-free DNA can predict distant metastasis earlier in pancreatic cancer: a prospective cohort study by Chien-Jui Huang, Wen-Yen Huang, Chien-Yu Chen, Ying-Jui Chao, Nai-Jung Chiang and Yan-Shen Shan in Therapeutic Advances in Medical Oncology