Abstract
We describe a new microtiter immunospore trapping device (MTIST device) that uses a suction system to directly trap air particulates by impaction in microtiter wells. This device can be used for rapid detection and immunoquantification of ascospores of Mycosphaerella brassicicola and conidia of Botrytis cinerea by an enzyme-linked immunosorbent assay (ELISA) under controlled environmental conditions. For ascospores of M. brassicicola correlation coefficients (r2) of 0.943 and 0.9514 were observed for the number of MTIST device-impacted ascospores per microtiter well and the absorbance values determined by ELISA, respectively. These values were not affected when a mixed fungal spore population was used. There was a relationship between the number of MTIST device-trapped ascospores of M. brassicicola per liter of air sampled and the amount of disease expressed on exposed plants of Brassica oleracea (Brussels sprouts). Similarly, when the MTIST device was used to trap conidia of B. cinerea, a correlation coefficient of 0.8797 was obtained for the absorbance values generated by the ELISA and the observed number of conidia per microtiter well. The relative collection efficiency of the MTIST device in controlled plant growth chambers with limited airflow was 1.7 times greater than the relative collection efficiency of a Burkard 7-day volumetric spore trap for collection of M. brassicicola ascospores. The MTIST device can be used to rapidly differentiate, determine, and accurately quantify target organisms in a microflora. The MTIST device is a portable, robust, inexpensive system that can be used to perform multiple tests in a single sampling period, and it should be useful for monitoring airborne particulates and microorganisms in a range of environments.
Airborne spores of fungal plant pathogens have commonly been detected and enumerated by microscopic examination of surfaces on which spores have been impacted (2, 13). Sampling procedures may involve passive collection of spores by gravitational deposition (14) and/or sampling specific volumes of air with “active” spore-trapping devices (1, 9, 18). Such techniques require considerable amounts of time and expertise if accurate counts are to be obtained. In addition, sample identification is often not a realistic option, especially when there is no selective medium and when there are morphologically similar spores (such as the spores produced by ascosporic fungi).
However, technological advances in fungal diagnostics in which either antibody or nucleic acid probes are used (4, 6, 11) offer the potential for developing rapid systems for detecting and quantifying airborne spores of fungal plant pathogens. An immunoassay system developed by Spore View (Chaparral Diagnostics, Burlington, Vt.) utilizes passive deposition of ascospores of Venturia inaequalis, the causal agent of apple scab, on a membrane surface. Similarly, studies to develop an antibody-based immunoassay for early detection of Sclerotinia sclerotiorum (15), a major fungal pathogen of oilseed rape (Brassica napus), have relied solely on passive deposition of ascospores on rapeseed petals and subsequent mycelial growth. It is unlikely that these systems could detect pathogens at concentrations below a critical threshold level since only small volumes of air or small sample sizes can be assayed with passive sampling. Consequently, sampling methods, location, and the efficiency of sampling are crucial factors when high cropping acreages are examined with these systems. As a result, rapid assay formats in which large volumes of air are sampled are a prerequisite if accurate immunomonitoring of air spora is to be achieved. With vegetable production systems detection of small amounts of inocula is important because the presence of disease organisms at low levels can result in a loss of quality.
During development of a portable remotely operated fiber optic biosensor system for detecting aerosolized bacteria (19), research carried out by the U.S. Naval Research Laboratory revealed the potential of an immunoassay system used to sample relatively large volumes of air. However, a limitation of this system was the low limit of detection, 3,000 CFU of Bacillus subtilis subsp. niger per ml of air sampled. At present there are few systems that can accurately detect small amounts of inocula. Nevertheless, other workers (8), using a Burkard 7-day volumetric suction trap (B 7-day trap) as a trapping device, quantified airborne pollen allergens on polyvinylidene difluoride membranes by using immunoblotting and chemiluminescence techniques. Similarly, workers have developed an immunofluorescence test to detect and quantify airborne ascospores of Mycosphaerella brassicicola, the ringspot pathogen of brassicas. Trapped ascospores are labeled directly on B 7-day trap Melinex tape with polyclonal antibodies (PAbs) and anti-rabbit fluorescein isothiocyanate-conjugated antibodies (17). However, when these two methods are used as routine quantification methods, both tests are laborious, require expensive laboratory equipment for analysis, and include processes which require manual operation. Nevertheless, for foliar diseases in which the inoculum is a fundamental aspect of disease spread, immunoassays have the potential to produce novel quantitative data on the epidemiology of airborne pathogens.
In this paper we describe a new microtiter immunospore trapping device (MTIST device) which uses a suction system to directly trap air spora by impaction in microtiter wells, and this device can be used for rapid detection and quantification of ascospores of M. brassicicola and conidia of Botrytis cinerea by an enzyme-linked immunosorbent assay (ELISA). M. brassicicola is a foliar plant pathogen that has significant economic importance and causes ringspot of brassicas, and B. cinerea is a general pathogen that affects a wide range of crops, including brassicas, in which it causes grey mold in the field and postharvest rot in stored white cabbage and other produce. Control of both diseases is problematic because of the nature of the airborne inoculum. A disease prediction model for M. brassicicola has been developed previously (16), but additional information concerning inoculum levels is required for effective disease control. Information derived from studies of environmental conditions and airborne B. cinerea inoculum levels should be important in disease prediction studies, rationalization of fungicide usage, scheduling harvest dates, and determining the storage conditions used for white cabbage and other brassica crops.
In this paper we describe using a MTIST device to immunomonitor airborne spores of two plant pathogens.
MATERIALS AND METHODS
MTIST device.
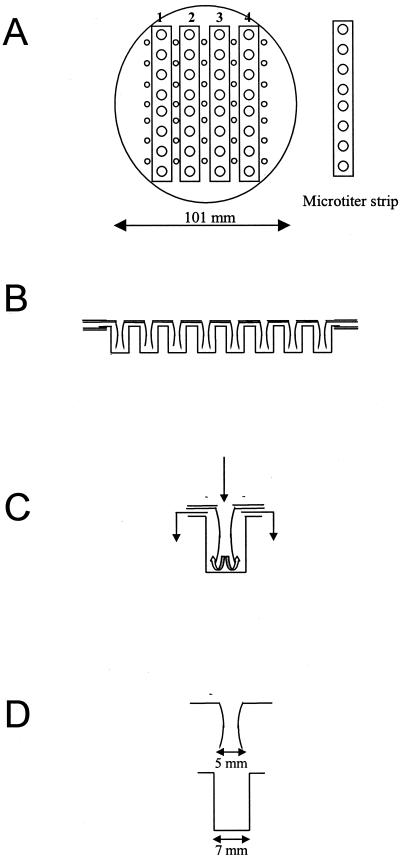
The MTIST device which we used (Fig. 1 and 2) is a modified personal volumetric air sampler produced by Burkard Manufacturing Co. Ltd. (Rickmansworth, Hertsfordshire, United Kingdom). This sampler is operated by a standard Burkard turbine suction unit, and air is drawn through the system at a constant rate of 20 liters per min (Fig. 1C). The volume of air sampled can be increased or decreased depending on the requirements of the test. Particulates in the airstream are channeled through delivery trumpet nozzles and directed across the base of each collection well of microtiter well strips [4 by 8 wells; catalog no. 9502 027; ThermoQuest (UK) Ltd., Basingstoke, Hampshire, United Kingdom]. The MTIST device is operated by rechargeable 6-V batteries which can provide 3 to 4 h of continuous operation. For longer periods or intermittent running conditions an electric mains model is available in 110- and 240-V versions. A sliding air control is located under the sampling chamber in order to allow rapid closure following sampling.
FIG. 1.
Microtiter immunospore suction trap. (A) Overhead view of the MTIST device. (B) Cross-sectional view of the MTIST device. (C) Air movement during operation of the MTIST device. (D) Cross section of the MTIST device delivery trumpet and microtiter well.
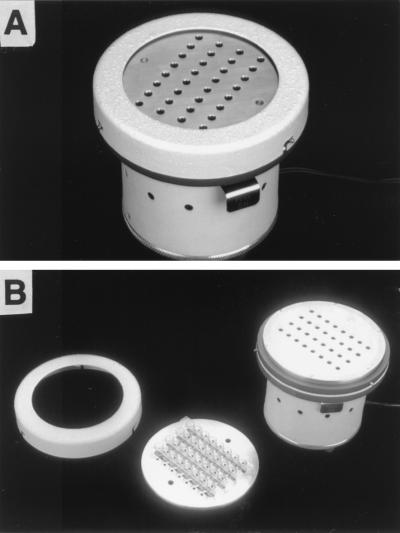
FIG. 2.
(A) MTIST device. (B) Components of the MTIST device.
Immunoquantification of M. brassicicola. (i) Production of PAbs to M. brassicicola.
A 100-ml ascospore suspension containing 2.5 × 104 ascospores that were collected in sterile distilled water (SDW) from cultures that were producing M. brassicicola pseudothecia (single-spore isolates CH195001, CH19500B, and C19500C) on senescent sprout leaf decoction agar (SLD agar) (17) was concentrated by freeze-drying (Modulyo 4K; Edwards, Crawley, United Kingdom). The sample was rehydrated in 15 ml of SDW and sonicated with a Soniprep apparatus (MSE, Crawley, United Kingdom) at a micron amplitude of 20 for a total of 15 min. The sonicated ascospore sample was freeze-dried as described above except that it was rehydrated in 5 ml of phosphate-buffered saline (PBS) (pH 7.2). The immunization protocol and serum collection method used have been described by Kennedy et al. (17). Using a DEAE Affi-Gel blue gel support (Bio-Rad, Hemel Hempstead, Herts., United Kingdom), we purified immune serum and collected the immunoglobulin G (IgG) fraction. The purified serum was designated polyclonal antiserum (PAb) 98/4/P, and the IgG concentration was 0.8 mg ml−1. As a preservative, 0.05% tincture of merthiolate (Tinc) (1 mg of thimerosal per ml and 1 mg of pararosanoline per ml in ethanol) was added to the purified IgG serum before storage at −20°C in 100-μl aliquots.
A second PAb, designated PAb 96/10/4, which was previously raised by using whole ascospores of M. brassicicola, was also used in this study (17).
(ii) Determination of the optimal working dilutions of PAb 98/4/P and PAb 96/10/4 in a PTA-ELISA.
Using a plate-trapped antigen ELISA (PTA-ELISA), we determined the optimal working dilution of each PAb for a whole-ascospore suspension of M. brassicicola, as described by Kennedy et al. (17).
(iii) Specificity of PAbs for fungal spores collected in SDW.
PAbs 98/4/P and 96/10/4 were screened for cross-reactivity with a range of airborne fungal spores (Table 1). Fungal cultures were either grown on synthetic media or collected from infected plant material. Fungal spores were collected in SDW, and the concentration was adjusted to ∼1,000 spores ml−1. The spore suspensions were stored at −20°C until they were processed by the PTA-ELISA as described below.
TABLE 1.
Fungal species used in cross-reactivity tests to determine PAb specificity
| Fungal species | Growth medium or planta |
|---|---|
| Aspergillus ruber | MSA |
| Paecilomyces variotii | MEA |
| Penicillium aurantiogriseum | MEA |
| Botrytis cinerea | PDA |
| Botrytis allii | PDA |
| Sclerotinia sclerotiorum | Triticumb |
| Alternaria brassicicola | PLY |
| Albugo candida | B. oleracea |
| Peronospora parasitica | B. oleracea |
| Erysiphe cruciferarum | B. oleracea |
| Puccinia allii | Allium porrum |
| Mycosphaerella brassicicola | SLD agar |
MEA, malt extract agar; PDA, potato dextrose agar; MSA, malt salt agar; PLY, prune lactose yeast extract agar.
S. sclerotiorum-inoculated autoclaved wheat grain was placed 1 cm deep in John Innes no. 1 compost (23).
For each fungal isolate 100 μl of a spore suspension was pipetted into each well of an 8-well microstrip. The microstrips were incubated overnight at 4°C, after which the unbound material was removed and the wells were washed twice (1 min each) with 200 μl of PBS-Tinc per well. The microtiter wells were blocked with 200 μl of 3% casein buffer (3% [wt/vol] casein in PBS) and incubated in a Wellwarm shaker incubator (Denley Instruments Ltd., Sussex, United Kingdom) at 37°C for 45 min. The residual blocking buffer was removed, and the wells were washed four times (1 min each) with 200 μl of PBS–Tinc–0.05% Tween 20 (PBS-Tinc-Tw). Two paired wells of each microstrip received 100 μl of PAb 98/4/P in PBS-Tinc-Tw (1:15) per well and 100 μl of PAb 96/10/4 in PBS-Tinc-Tw (1:50) per well. The remaining four wells of each microstrip received 100 μl of PBS-Tinc-Tw per well. Following incubation as described above, the wells were washed four times (1 min each) with 200 μl of PBS-Tinc-Tw. An anti-rabbit IgG SEEKit kit (catalog no. SSB-2003; Harlan Sera-Lab Ltd., Belton, Loughbourough, United Kingdom) was used to amplify the signal generated by the bound antibodies of PAbs 98/4/P and 96/10/4. As a negative control, a paired well of each microstrip, which had received no PAb during the previous incubation stage, was probed with the anti-rabbit IgG SEEKit kit. The optimal working dilution in PBS-Tinc-Tw for both the biotinylated antibody and the horseradish peroxidase-streptavidin complex was determined to be 1:10. The instructions of the manufacturer were followed. The incubation temperature and the time for each stage were the temperature and time described above; four 1-min washes with 200 μl of PBS-Tinc-Tw were performed after each of the two stages. To each of the eight wells of each microstrip 100 μl of 3,3′,5,5′-tetramethylbenzidene substrate (catalog no. T-3405 and P-4922; Sigma) was then added. The reaction was stopped by adding 25 μl of 2 M H2SO4. Absorbance at 450 nm was determined with a model HT11 ELISA plate reader (Anthos Labtech Instruments, Salzburg, Austria).
(iv) Specificity of PAbs for fungal spores trapped with the MTIST device.
In an enclosed chamber, airborne spores of a number of fungal isolates (Table 1) were actively trapped in microtiter wells of microstrips (4 by 8 wells) by using the MTIST device. The number of spores trapped in each of the microtiter wells was determined by using a Nikon model TMS inverted binocular microscope. The microtiter strips were stored at −20°C until they were processed by PTA-ELISA. To determine the potential cross-reactivity of the two PAbs, only microstrips which contained approximately 1,000 spores per well were used in the PTA-ELISA. The PTA-ELISA was performed as described above, but no preincubation stage prior to blocking was included.
(v) Immunoquantification of trapped ascospores of M. brassicicola in controlled-environment cabinets with the MTIST device.
Twelve sporulating culture plates containing M. brassicicola (CH195001, CH19500C, C19500D, C195001, C19500B, and DS1) were placed in a controlled-environment cabinet (catalog no. SGC970/C/RO-HFL; Sanyo Gallenkamp, Loughborough, Leicestershire, United Kingdom) operating at 94% relative humidity with continuous light and intermittent wetting for 0.3 min every 60 min. Discharged ascospores were collected by impaction in the microtiter wells of microstrips (4 by 8 wells) of the MTIST device, which was operated with a continuous airflow rate of 20 liters per min. Over a 96-h sampling period, the microstrips were changed at 30-min and 1-, 2-, 3-, 4-, 6-, 12-, and 36-h intervals. For each sampling period the total number of ascospores in each of the wells of each microstrip was determined with a Nikon model TMS inverted binocular microscope. Eight negative control microstrips, which had been removed from the MTIST device following operation for 2 h in a controlled environment with no sporulating cultures, and ascospore-containing microstrip wells were stored in 96-well multiframe holders [catalog no. 9503 060; ThermoQuest (UK)] at −20°C. The mean percent distribution of impacted ascospores for each sample in microtiter wells was determined by performing a single-factor analysis of variance. The ascospores in wells were immunoquantified by PTA-ELISA as described previously, but no preincubation stage prior to blocking was included. For each strip four wells received PAb 98/4/P, and the remaining four received PAb 96/10/4.
To determine the sampling efficiency of the MTIST device, a B 7-day trap was placed adjacent to the MTIST device and used as a reference trap. B 7-day traps have been used routinely to monitor fungal air spora (12, 13, 24, 26) and in a wide variety of air-sampling studies (3, 10). Ascospores on the Melinex spore tape were detected and quantified by immunofluorescence, as described by Kennedy et al. (17).
(vi) Immunoquantification of ascospores of M. brassicicola in a mixed fungal airborne population by using the MTIST device.
Eight Brassica oleracea var. gemmifera plants (Brussels sprouts) which had been inoculated with Erysiphe cruciferarum (powdery mildew) and exhibited severe disease symptoms were placed in a controlled-environment cabinet operating as described above together with 12 sporulating culture plates containing M. brassicicola. Using the MTIST device as described above, the air spora in the cabinet was sampled over a 24-h period (20 liters of air min−1) by using sampling periods of 30 min and 1, 2, 4, and 12 h. For two of the 4-h sampling periods and one 12-h sampling period, 40 additional but disease-free Brussels sprouts seedlings (B. oleracea var. gemmifera cv. Golfer) with three true leaves were placed in the controlled-environment cabinet at the following four positions: top left, top right, bottom left, and bottom right. Following each of these sampling periods the 40 B. oleracea plants were removed and placed into an environment with 100% humidity for 24 h. The plants then were removed, placed in a glasshouse, and grown at 15°C for 21 days. The plants were visually examined for expression of ringspot lesions. To confirm that ringspot (M. brassicicola) symptoms were present, infected leaf tissue was removed and surface sterilized for 1 min in aqueous sodium hypochlorite (4% [wt/vol] available chlorine), and organisms were isolated by using SLD agar (17).
For each MTIST device sampling period each well of each microstrip was examined with a Nikon model TMS inverted binocular microscope to determine the total number of impacted M. brassicicola and E. cruciferarum spores. For each of four sampling periods two wells were examined to determine the spatial distribution of the impacted spores. Microstrips were stored at −20°C until they were examined by using the PTA-ELISA as described above.
Immunoquantification of B. cinerea. (i) Production of a Botrytis-specific MAb.
A hybridoma cell line secreting a Botrytis-specific monoclonal antibody (MAb), designated BC12.CA4, was raised by using splenocytes from a mouse which had been coimmunized with surface washings from a plate culture of B. cinerea (designated P-6 g) and supernatant from a hybridoma cell line that secreted the near genus-specific MAb BC-KH4 (5) at a 1:1 (vol/vol) ratio (22). Genus-specific MAb BC12.CA4 is an IgG1 antibody that has been shown to recognize a heat-stable epitope on an antigen expressed along the extracellular matrix of hyphae and on the surfaces of conidia of B. cinerea (22).
(ii) Immunoquantification of conidia of B. cinerea trapped with the MTIST device.
A 3-mg conidial sample collected with a cyclone surface sampler (Burkard) in a sterile chamber by using six sporulating cultures of B. cinerea BC 404 grown on prune lactose yeast extract erythromycin agar (21) was added to a spore settling tower. With the MTIST device at the base of the spore settling tower and operating as described above, conidia of B. cinerea were actively trapped for 5, 10, and 15 s. Following each sampling period the microstrips (4 by 8 wells) were removed, and 100 μl of PDBG buffer (0.1% [wt/vol] potato dextrose broth [Difco], 1% [wt/vol] glucose in PBS) was added to each well. As a negative control, PDBG buffer was added to a microstrip which had not been exposed to B. cinerea conidia. The individual microstrips were incubated at room temperature for 16 h, after which the PDBG buffer was removed and the total number of germinated conidia in each well of each microstrip was determined by using a microscope. The microtiter wells then were washed with PBS containing 0.05% (vol/vol) Tween 20 (PBS-Tw) and blocked with 200 μl of 0.3% (wt/vol) casein in PBS containing 0.02% (wt/vol) NaN3. Following incubation at room temperature for 10 min, the residual blocking buffer was removed, and the wells were sequentially incubated for 1 h at room temperature with 100-μl volumes of BC12.CA4 hybridoma supernatant and goat anti-mouse polyvalent (IgG, IgA, and IgM) peroxidase conjugate (catalog no. A-0412; Sigma) diluted 1:500 in PBS-Tw. After this the substrate 3,3′,5,5′-tetramethylbenzidene (catalog no. T-3405; Sigma) in acetate buffer (5) was added to each well, and the preparations were incubated for 10 min. The reaction was stopped by adding 50 μl of 3 M H2SO4. Absorbance at 450 nm was determined with a model MRX ELISA plate reader (Dynatech Laboratories Inc., Chantilly, Va.). The wells were washed between incubation steps four times with PBS-Tw.
RESULTS
Immunoquantification of M. brassicicola. (i) Specificity of PAbs 96/10/4 and 98/4/P in the PTA-ELISA.
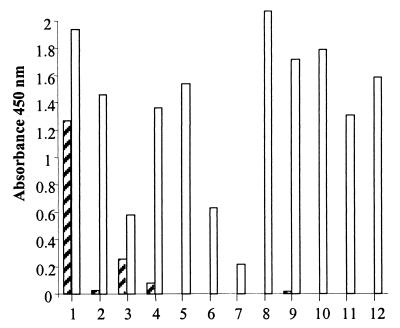
When fungal spores were collected in water and incubated in microtiter wells overnight, both antisera cross-reacted with each of the fungal spore suspensions tested. However, when fungal spores were actively trapped in microtiter wells by the MTIST device and were examined by PTA-ELISA without preincubation in water, a different pattern of recognition was observed. In the later tests the cross-reactivity with both antisera was limited to the ascospore stage of S. sclerotiorum (Fig. 3) (PAb 96/10/4).
FIG. 3.
Reactivity of PAb 96/10/4 with a range of airborne fungal spores, as shown by PTA-ELISA. 1, Mycosphaerella brassicicola; 2, Peronospora parasitica; 3, Sclerotinia sclerotiorum; 4, Albugo candida; 5, Aspergillus ruber; 6, Botrytis allii; 7, Puccinia allii; 8, Botrytis cinerea; 9, Erysiphe cruciferarum; 10, Penicillium aurantiogriseum; 11, Paecilomyces variotti; 12, Alternaria brassicicola. Cross-hatched bars, spores collected with the MTIST device; open bars, spores collected in water.
(ii) Immunoquantification of MTIST device-trapped ascospores of M. brassicicola.
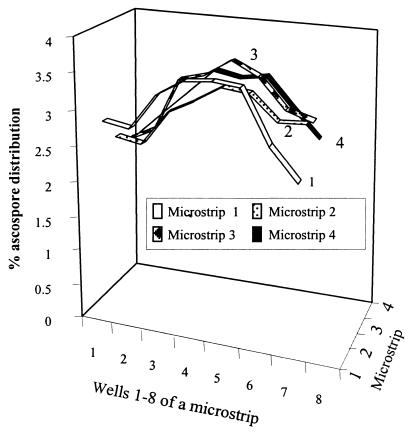
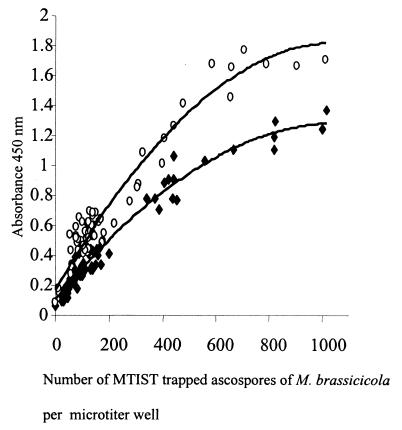
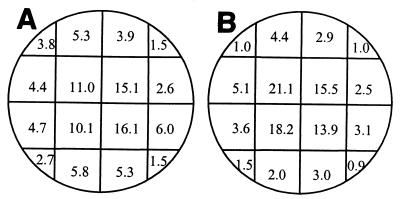
Using a Nikon model TMS inverted binocular microscope at a magnification of ×200 with bright-field illumination, we confirmed that MTIST device-trapped ascospores of M. brassicicola were present in the base of each collection microtiter well, and the total number in each well was determined. We found that ascospores were distributed throughout the base of each well, and, as the ascospore concentration increased, the spores remained spatially separated. Employing a single-factor analysis of variance, we determined that significant variation in the mean percent ascospore distribution occurred in the microtiter well strips (Table 2); greater numbers of ascospores were collected in the inner four wells of each microstrip (Fig. 4). Except for wells 1 and 8, there was no significant difference between the strips (Table 2). Following PTA-ELISA, correlation coefficients (r2) of 0.943 and 0.9514 (polynomial) were obtained with PAbs 96/10/4 and 98/4/P, respectively, when we compared the number of ascospores per microtiter well and the absorbance values obtained from PTA-ELISA analyses (Fig. 5). The relative collection efficiency of the MTIST device in a controlled plant growth chamber with limited airflow was 1.7 times greater than the collection efficiency of the B 7-day trap.
TABLE 2.
Mean distribution of MTIST device-trapped ascospores of M. brassicicola in microstrips (4 by 8 wells), expressed as percentages for the 96-h sampling period
| Well no. | % of ascospores in:
|
||||
|---|---|---|---|---|---|
| Microstrip 1 | Microstrip 2 | Microstrip 3 | Microstrip 4 | SEDa | |
| 1 | 2.94 | 2.56 | 2.37 | 2.33 | 0.23 |
| 2 | 2.87 | 2.48 | 2.57 | 2.73 | 0.26 |
| 3 | 3.44 | 3.04 | 3.43 | 3.11 | 0.24 |
| 4 | 3.69 | 3.26 | 3.56 | 3.53 | 0.26 |
| 5 | 3.74 | 3.53 | 3.83 | 3.44 | 0.24 |
| 6 | 3.69 | 3.48 | 3.62 | 3.50 | 0.21 |
| 7 | 2.88 | 3.09 | 3.13 | 3.03 | 0.22 |
| 8 | 2.38 | 3.11 | 3.01 | 2.59 | 0.21 |
| SEDb | 0.25 | 0.26 | 0.199 | 0.229 | |
SED, standard error of difference. The degrees of freedom was 36.
The degrees of freedom was 72.
FIG. 4.
Mean distribution of impacted ascospores of M. brassicicola for each of the microstrips (4 by 8 wells), expressed as percentages, for the 96-h sampling period.
FIG. 5.
Immunoquantification of MTIST device-trapped ascospores of M. brassicicola in a controlled-environment chamber, as determined with PAb 98/4/P (⧫) and PAb 96/10/4 (○) by performing a PTA-ELISA.
(iii) Immunoquantification of ascospores of M. brassicicola in a mixed fungal airborne population by using the MTIST device.
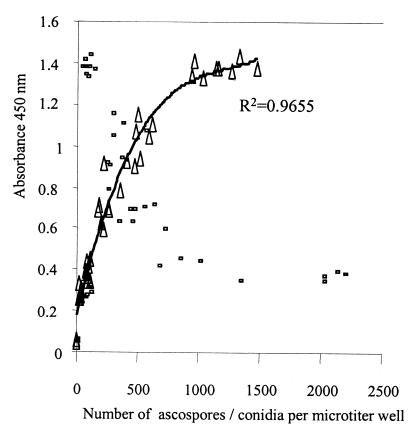
MTIST device-trapped ascospores of M. brassicicola and conidia of E. cruciferarum were identified in the bases of the collection microtiter wells (Fig. 6). The total number of ascospores of M. brassicicola and the total number of conidia of E. cruciferarum in each well were determined. Ascospores of M. brassicicola and conidia of E. cruciferarum were distributed throughout the base of each well, but the greatest numbers occurred in the central region of each microtiter well (Fig. 7). Low numbers of conidia of E. cruciferarum were observed on the sides of the microtiter collection vessels. As the conidial concentration increased (>100 conidia per microtiter well), aggregation of conidia was observed, and at levels of >1,000 conidia per microtiter well the ascospores of M. brassicicola were obscured. Following PTA-ELISA correlation coefficients of 0.8963 and 0.9655 (polynomial) were obtained with PAbs 96/10/4 and 98/4/P, respectively, when we compared the number of ascospores per microtiter well and the absorbance values (Fig. 8). There was not a correlation between the number of conidia of E. cruciferarum per microtiter well and the absorbance value when either PAb was used (Fig. 8).
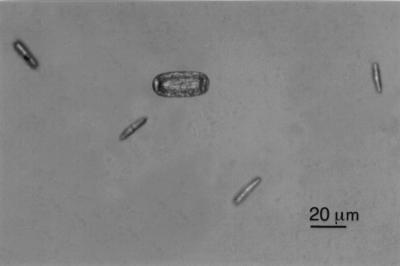
FIG. 6.
MTIST device-trapped ascospores of M. brassicicola and a conidium of E. cruciferarum in the base of a microtiter well.
FIG. 7.
Total numbers of MTIST device-trapped spores collected in eight wells, expressed as percentages in a single microtiter well. (A) M. brassicicola. (B) E. cruciferarum.
FIG. 8.
Relationship of MTIST device-trapped ascospores of M. brassicicola (▴) and conidia of E. cruciferarum (□) in a PTA-ELISA analysis, as determined with PAb 98/4/P.
Ringspot latent infection.
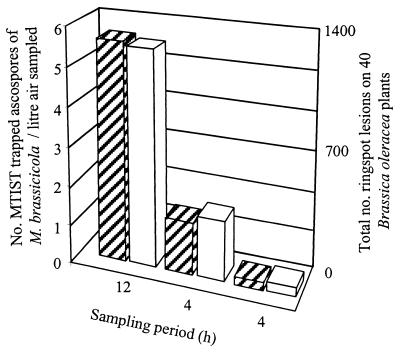
All B. oleracea var. gemmifera cv. Golfer seedlings (Brussels sprouts) that had been exposed to the mixed fungal spore population had ringspot lesions (Fig. 9). Organisms isolated from the infected lesions on SLD agar were confirmed to be M. brassicicola (7). The position within the cabinet had little effect on the total number of ringspot lesions which developed on brassica seedlings exposed to spores. We observed that there was an association between the number of MTIST device-trapped ascospores of M. brassicicola per liter of air sampled and the total number of ringspot lesions that developed (Fig. 10). Leaf immaturity inhibited the development of E. cruciferarum lesions.
FIG. 9.
Leaves of B. oleracea cv. Golfer exhibiting ringspot lesions.
FIG. 10.
Number of MTIST device-trapped ascospores of M. brassicicola per liter of air sampled and total number of ringspot lesions per sampling period on 40 exposed B. oleracea (Brussels sprouts) plants. Cross-hatched bars, ascospores trapped with MTIST device; open bars, ringspot lesions.
Immunoquantification of MTIST device-trapped B. cinerea conidia.
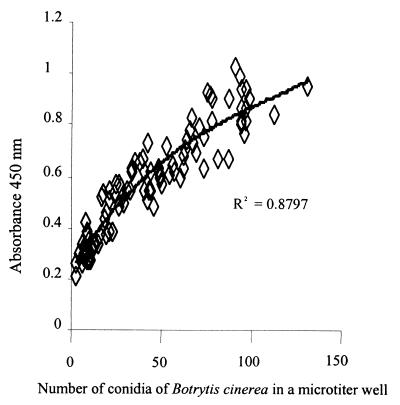
As determined with the MTIST device for each sampling period (5, 10, and 15 s), B. cinerea conidia were present in the bases of the microtiter wells. To optimize the immunoassay, B. cinerea conidia were germinated by incubating them for 16 h in PBDG buffer prior to the PTA-ELISA. Recognition of ungerminated conidia by Botrytis-specific MAb BC12.CA4 is poor (22); this antibody predominantly binds to epitopes present in the extracellular mucilage of the conidial germ tube and mycelial wall. Using the absorbance values generated and the observed numbers of germinated conidia per microtiter well, we fitted a polynomial curve to the data to give a correlation coefficient of 0.8797 (Fig. 11).
FIG. 11.
Relationship between MTIST device-trapped germinated conidia of B. cinerea and corresponding PTA-ELISA absorbance values.
DISCUSSION
In the past it has been impossible to quantify organisms in an airborne microflora accurately and quickly. A wide variety of methods have been used, which can be broadly divided on the basis of active or passive sampling techniques (1). With most of these techniques researchers depend on microscopic examination of impaction surfaces. Nonmicroscopic techniques have usually involved using selective agar media or trap plants. However, these methods have limitations since they rely on passive sampling and are not universally applicable. For example, problems can arise in detection of some slowly growing fungal species on agar, especially if the target organism occurs at low levels in the presence of large populations of other microfloral organisms. Additional problems are the incubation conditions used to express the target organism after it is trapped on agar and determining what might constitute a CFU. Using plants to express levels of target organisms in a microflora is equally problematic. For some diseases, symptoms are not expressed until weeks after infection has occurred. An additional problem is that not all organisms in an airborne microflora are pathogenic on plants. Therefore, it has been difficult in the past to measure the degree of airborne dispersal of fungal propagules, particularly when low levels have been present.
In the present study we developed new techniques and equipment which can be used to actively sample airborne microfloras. The equipment can be used to rapidly differentiate and accurately quantify target organisms in a microflora without microscopic examination. Additionally, target microflora can be quantified without long incubation periods after sampling. The values are also independent of the type and quantity of airborne spores in the air at the time of sampling. However, the specificity of the test depends on the antibodies used. The range of organisms used in cross-reactivity tests must reflect the range of organisms likely to be encountered in the field. In the cross-reactivity tests performed in the present study we used many pathogens and fungi likely to be found in horticultural brassica crops. Cross-reactivity with ascospores of other fungal species, including Sclerotinia species, might be problematic if the system described here was used to detect M. brassicicola in arable brassica crops. It is possible that Sclerotinia infection of arable brassica crops might interfere with counts in locations adjacent to horticultural brassica crops. However, this problem can be overcome by using more than one antibody in the system to accurately quantify the degree of cross-reactivity which may occur at any location. Additional information concerning the long-distance dispersal of the fungal pathogens would also be required.
It has been shown that long-distance dispersal of microorganisms does occur (25, 27). It has been demonstrated (20) that Erysiphe graminis spores can be transported 110 km year−1 in the main wind direction. The authors of these studies concluded that the probability that viable spores of plant-pathogenic species could cause disease was low but not zero. However, the importance of low numbers of propagules may be outweighed if, for example, they are of a different biotype than the resident population. Recent work has revealed the importance of transmission of a biotype of Mycosphaerella graminicola when sexual recombination with existing populations occurred at a significant level (26). In previous studies it was difficult to determine whether the levels of inocula were related to increases or decreases in disease occurrence. In previous studies of the dispersal and diurnal periodicity of airborne microflora the researchers used standard trapping techniques, such as B 7-day traps or rotorods. However, in controlled environmental tests in which the trapping efficiency of a B 7-day trap was compared to the trapping efficiency of the MTIST device, the latter trap was 1.7 times more efficient based on the number of spores trapped per cubic meter of air. However, this was not surprising given the characteristics of the B 7-day trap. The initial comparisons between the MTIST device and the B 7-day trap were carried out under controlled environmental conditions under which the airflow was low. For field operation some alteration in the design of the MTIST device will be required since at present collection occurs 90° to the direction of the airflow. A field-based wind directional MTIST device (Fig. 12) is currently under evaluation. Further studies will determine and optimize the trapping efficiency of the field-based MTIST device for different wind speeds and with variable airflow. Tests will be carried out to compare the trapping efficiency of the MTIST device and the trapping efficiencies of the B 7-day and rotorod traps and other samplers, notably the Anderson type of samplers, under variable-airflow conditions found in the field.
FIG. 12.
Wind directional field-based MTIST device, operated by a data logger, for immunoquantification of field inoculum of M. brassicicola. This device is currently being evaluated.
In the present study we found that with the MTIST device spores impact in the bases of microtiter wells, and observations in this study confirmed that ascospores of M. brassicicola, conidia of E. cruciferarum, and germinated conidia of B. cinerea adhere permanently to the microtiter wells. However, if adhesion to the plate does not occur (due to spore aggregation), there may be some loss of spores during the ELISA process (B. cinerea and E. cruciferarum). This may be overcome in the future by precoating microtiter wells with poly-l-lysine. Further investigations of such effects in which a range of propagule types from different airborne microfloras are used are needed in order to establish the general usefulness of this device. Aggregation, even at very high spore concentrations, was not observed for M. brassicicola.
Our results indicate that there is likely to be little interaction between large and small propagules provided that the antibodies used for one target pathogen do not cross-react with the other pathogen. However, when cross-reactivity occurs, it can be overcome by using appropriate pathogen-specific MAbs or even specific PCR probes. Also, since many propagules have a distinct diurnal periodicity and require specific conditions for release into the air, cross-reactivity might not be a significant problem in some applications. Interestingly, in this study the cross-reactivity observed with the PAbs was considerably reduced when airborne spores were collected with the MTIST device. This suggests that the nonsoluble ascospore wall antigens of M. brassicicola are specific.
The results obtained with the device under controlled environmental conditions indicated that there was a close correlation between the amount of ringspot disease, which appeared on trap plants placed inside the cabinet, and the number of MTIST device-trapped ascospores of M. brassicicola from source plates. The device could, therefore, prove to be useful in determining the potential risk of infection at specific locations. However, information on the numbers of target propagules trapped at any location could be interpreted easily only if additional information or models describing the effects of environmental variables on the biological responses are available. This approach would be particularly useful for plant-pathogenic fungi, with which the technology could be used to predict rapidly, with relatively little effort, the degree of disease transmission and the potential levels of disease.
For successful usage of the MTIST device further information is needed concerning the placement of traps in relation to sources of a target inoculum because of the vertical variation in the airborne concentration of propagules. The vertical variation in the aerial concentration of V. inaequalis ascospores in apple orchards has been determined by Aylor (2) by using rotorods. As determined by the Venturia studies the aerial concentration of ascospores 3 m above the ground was 94% less than the concentration 0.15 m above the ground. The vertical variation in the concentration of airborne spores is one factor which determines the likelihood of transport of microflora spatially.
Potentially, the MTIST device system could be used to assess the presence or absence and quantity of several target air spores at the same time either by immunoassay or by PCR in which specific probes are used. This is often important in studies in which organisms occur as complexes in particular ecological niches. Advances in environmental data capture systems should allow operation of the MTIST device only when conditions are favorable for target spore release and/or infection. This should result in greater accuracy in epidemiological disease studies. If the device is to be used to detect several pathogens simultaneously, a complete microstrip should be used for each target airborne inoculum type. This conclusion is based on the observation that the number of ascospores of M. brassicicola trapped in microtiter wells varied significantly within a microtiter strip but not between microtiter strips when the same sample was examined.
New techniques which can be used to rapidly and reliably differentiate airborne microflora should be useful in research programs. The information obtained could be used to predict spatial variation in plant-pathogenic propagules and the interaction between agricultural and natural ecosystems. However, the present system can operate only in a nonautomated format, and there is some delay between sampling and quantification of the microflora in a sample. Reducing this time interval will require further major advances in technology if sample processing is to be automated. However, by using immunochromatographic techniques it is already possible to process samples on site. The MTIST device is a portable, robust, and inexpensive system that can be used for multiple tests during a single sampling period, and it should be useful for monitoring airborne particulates and microorganisms in a range of environments.
ACKNOWLEDGMENTS
The studies related to the use of the MTIST device for immunoquantification of M. brassicicola were supported by the Ministry of Agriculture Fisheries and Food at Horticulture Research International.
Molly Dewey thanks the Leverhulme Trust for support. Ulla Meyer thanks R. Spotts (Oregon State University), in whose laboratory she trapped and immunoquantified conidia of B. cinerea. Molly Dewey thanks Geoff and Stewart Wiley at Burkard for acting on her suggestion to develop a spore trap that allows spores to be trapped directly in microtiter wells (MTIST device). We thank R. B. Maude, P. R. Mills, and G. Keane for critically reviewing the manuscript.
REFERENCES
- 1.Aylor A E. Relative collection efficiency of Rotorod and Burkard spore samplers for airborne Venturia inaequalis ascospores. Phytopathology. 1993;83:1116–1119. [Google Scholar]
- 2.Aylor A E. The aerobiology of apple scab. Plant Dis. 1998;82:838–849. doi: 10.1094/PDIS.1998.82.8.838. [DOI] [PubMed] [Google Scholar]
- 3.Battarbee J L, Rose N L, Long X. A continuous, high resolution record of urban airborne particulates suitable for retrospective microscopical analysis. Atmos Environ. 1997;31:171–181. [Google Scholar]
- 4.Beck J, Ligon J J, Ettienne L, Binder A. Diagnostics in crop production. BCPC Symposium Proceedings no. 65. Nottingham, United Kingdom: Major Print Ltd.; 1996. Detection of crop fungal pathogens by polymerase chain reaction technology; pp. 111–118. [Google Scholar]
- 5.Bossi R, Dewey F M. Development of a monoclonal antibody-based immunodetection assay for Botrytis cinerea. Plant Pathol. 1992;41:472–482. [Google Scholar]
- 6.Dewey F M, Thornton C. Fungal immunodiagnostics in plant agriculture. In: Skerritt J H, Appels R, editors. New diagnostics in crop science. Biotechnology in Agriculture no. 13. Wallingford, United Kingdom: CAB International; 1995. pp. 151–170. [Google Scholar]
- 7.Dring D M. Studies on Mycosphaerella brassicicola (Duby) Linday. Trans Br Mycol Soc. 1961;44:253–264. [Google Scholar]
- 8.Ekebom A, Vesterberg O, Hjelmroos M. Detection and quantification of airborne birch pollen allergens on PVDF membranes by immunoblotting and chemiluminescence. Grana. 1996;35:113–118. [Google Scholar]
- 9.Evenhuis A, Verdam B, Zadoks J C. Splash dispersal of conidia of Mycocentrospora acerina in the field. Plant Pathol. 1997;46:459–469. [Google Scholar]
- 10.Frei T. Pollen distribution at high elevation in Switzerland: evidence for medium range transport. Grana. 1997;36:34–38. [Google Scholar]
- 11.Gray D I, Strachan N J C. Diagnostics in crop production. BCPC Symposium Proceedings no. 65. Nottingham, United Kingdom: Major Print Ltd.; 1996. Towards “on-line PCR”; pp. 375–382. [Google Scholar]
- 12.Hirst J M. Changes in atmospheric spore content: diurnal periodicity and the effects of weather. Trans Br Mycol Soc. 1953;36:375–393. [Google Scholar]
- 13.Hunter T, Coker R R, Royle D L. The teleomorph stage, Mycosphaerella graminicola, in epidemics of Septoria tritici blotch on winter wheat in UK. Plant Pathol. 1999;48:51–57. [Google Scholar]
- 14.Jamaux I, Gelie B, Lamarque C. Early stages of infection of rapeseed petals and leaves by Sclerotinia sclerotiorum revealed by scanning electron-microscopy. Plant Pathol. 1995;44:22–30. [Google Scholar]
- 15.Jamaux I, Spire D. Development of a polyclonal antibody-based immunoassay for the early detection of Sclerotinia sclerotiorum in rapeseed petals. Plant Pathol. 1994;43:847–862. [Google Scholar]
- 16.Kennedy R, Graham A M. 10th Biennial Australasian Plant Society Conference. Christchurch, N.Z.: Lincoln University; 1995. Forecasting Alternaria brassicae and Mycosphaerella brassicicola in vegetable brassicas; p. 66. [Google Scholar]
- 17.Kennedy R, Wakeham A J, Cullington J. Production and immunodetection of ascospores of Mycosphaerella brassicicola: ringspot of vegetable crucifers. Plant Pathol. 1999;48:297–307. [Google Scholar]
- 18.Kohl R, Blanco J, Kollar A. Detection of infection and evaluation of the parameters for the epidemiology of the apple scab disease. J Plant Dis Prot. 1994;101:378–385. [Google Scholar]
- 19.Ligler F S, Anderson G P, Davidson P T, Foch R J, Ives J T, King K D, Page G, Stenger D A, Whelan J P. Remote sensing using an airborne biosensor. Environ Sci Technol. 1998;32:2461–2466. [Google Scholar]
- 20.Limpert E. Barley mildew in Europe: evidence of wind-dispersal of the pathogen and its implications for improved use of host resistance and of fungicides for mildew control. In: Wolfe M S, Limpert E, editors. Intergrated control of cereal mildews: monitoring the pathogen. Dortrecht, The Netherlands: Martinus Nijhof Publishers; 1987. pp. 31–33. [Google Scholar]
- 21.Maude R B. Testing the viability of Septoria on celery seed. Plant Pathol. 1963;12:15–17. [Google Scholar]
- 22.Meyer, U. M., and F. M. Dewey. Efficacy of different immunogens for raising monoclonal antibodies to fungi to Botrytis cinerea. Mycol. Res., in press.
- 23.Mylchreest S J, Wheeler B E J. A method for inducting apothecia from sclerotia of Sclerotinia sclerotiorum. Plant Pathol. 1987;36:16–20. [Google Scholar]
- 24.Paulitz T C. Diurnal release of ascospores by Gibberella zeae in inoculated wheat plots. Plant Dis. 1996;80:674–678. [Google Scholar]
- 25.Roelfs A P. Wheat and rye stem rust. In: Roelfs A P, Bushnell W R, editors. The cereal rusts. Vol. 2. 1985. pp. 1–37. Diseases, distribution, epidemiology, and control. Academic Press, Orlando, Fla. [Google Scholar]
- 26.Stensvand A, Amundsen T, Semb L, Gadoury D M, Seem R C. Discharge and dissemination of ascospores by Venturia inaequalis during dew. Plant Dis. 1998;82:761–764. doi: 10.1094/PDIS.1998.82.7.761. [DOI] [PubMed] [Google Scholar]
- 27.Watson I A, De Sousa C N A. Long distance transfer of spores of Puccinia graminis tritici in the southern hemisphere. Proc Linn Soc NSW. 1982;106:311–321. [Google Scholar]
- 28.Zhan J, Mundt C C. Measuring immigration and sexual reproduction in field populations of Mycosphaerella graminicola. Phytopathology. 1998;88:1330–1337. doi: 10.1094/PHYTO.1998.88.12.1330. [DOI] [PubMed] [Google Scholar]