BACKGROUND
Anaphylaxis is an acute, potentially life-threatening systemic allergic reaction with an increasing burden in the United States and abroad.1 During the past decade there have been significant advances in the understanding of the epidemiology, pathogenesis, acute management, and long-term prevention of anaphylaxis among high-risk patients.2 These advances have resulted in improved care for patients with and at risk of anaphylaxis; however, pressing data gaps and research needs remain that should be addressed to optimize patient care and clinical outcomes and to reduce the societal burden of this disease.2
To this end, a 25-member multidisciplinary panel of anaphylaxis experts was convened in 2020 to systematically describe and appraise anaphylaxis knowledge gaps and research priorities.2 This study group previously used Delphi methodology to develop consensus anaphylaxis outcome definitions, including persistent, refractory, and biphasic anaphylaxis (Box 1) as well as persistent and biphasic non-anaphylactic reactions (Box 2).3 The panel used similar methodology to develop a consensus severity grading system for acute allergic reactions to standardize their severity and harmonize language used in clinical care and research (Fig. 1).4 The severity grading system for acute allergic reactions (SGS-AR) is novel in that it can be used to assess the severity of allergic reactions on a continuum from mild local reactions to anaphylactic shock. It can also be used to evaluate reaction severity of initial and biphasic reactions. These studies, including the perspectives from the multidisciplinary panel, underscore that significant anaphylaxis research and knowledge gaps exist that may hinder clinical care and can result in suboptimal patient outcomes. As such, these same experts sought to systematically outline and appraise anaphylaxis knowledge gaps and research priorities by asking panelists to generate knowledge gaps/research priority statements. Panelists were then asked to review and revise all statements after which they rated the potential impact and feasibility of addressing statements on a 0 to 100 scale. The panel generated 98 statements across 4 anaphylaxis themes: Population Science, Basic and Translational Sciences, Emergency Department Care/Acute Management, and Long-term Management Strategies and Prevention. This study provides the framework for collaborative scientific pursuits to address these and other anaphylaxis knowledge and research gaps to improve the care and outcomes of patients with anaphylaxis.2
Box 1. Clinical criteria for diagnosing persistent, refractory, and biphasic anaphylaxis.
Persistent anaphylaxis is highly likely when the following criterion is fulfilled: a
Presence of symptoms/examination findings that fulfill the 2006 NIAID/FAAN anaphylaxis criteria that persist for at least 4 hours.
Refractory anaphylaxis is highly likely when both of the following 2 criteria are fulfilled: b
Presence of anaphylaxis following appropriate epinephrine dosing and symptom-directed medical management (eg, intravenous [IV] fluid bolus for hypotension).
The initial reaction must be treated with 3 or more appropriate doses of epinephrine (or initiation of an IV epinephrine infusion).c
Biphasic anaphylaxis is highly likely when all of the following 4 criteria are fulfilled: d
New/recurrent symptoms/examination findings must fulfill the 2006 NIAID/FAAN anaphylaxis criteria.
Initial symptoms/examination findings must completely resolve before the onset of new/recurrent symptoms/examination findings.
There cannot be allergen reexposure before the onset of new/recurrent symptoms/examination findings.
New/recurrent symptoms/examination findings must occur within 1 to 48 hours from complete resolution of initial symptoms/examination findings.
aThe diagnosis of persistent anaphylaxis is independent of the management of the initial reaction. For reactions that do not fulfill persistent anaphylaxis criteria, please refer to Box 2 (clinical criteria for diagnosing persistent nonanaphylactic reactions).
bRefractory anaphylaxis is not dependent on the duration of symptoms/examination findings.
cAppropriate epinephrine dosing: 0.01 mg/kg intramuscular epinephrine, maximum single dose 0.5 mg. Also includes manufacturer recommended dosing for epinephrine auto-injectors.
dThe diagnosis of biphasic anaphylaxis is independent of the management of the initial reaction. For reactions that do not fulfill biphasic anaphylaxis criteria, please refer to Box 2 (clinical criteria for diagnosing biphasic nonanaphylactic reactions).
From Dribin TE, Sampson HA, fCamargo CA Jr, Brousseau DC, Spergel JM, Neuman MI, Shaker M, Campbell RL, Michelson KA, Rudders SA, Assa’ad AH, Risma KA, Castells M, Schneider LC, Wang J, Lee J, Mistry RD, Vyles D, Vaughn LM, Schumacher DJ, Witry JK, Viswanathan S, Page EM, Schnadower D. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J Allergy Clin Immunol. 2020 Nov;146(5):1089-1096; with permission.
Box 2. Clinical criteria for diagnosing persistent and biphasic nonanaphylactic reactions.
Persistent allergic reactions are highly likely when the following criterion is fulfilled: a
Presence of symptoms/examination findings that do not fulfill the 2006 NIAID/FAAN anaphylaxis criteria that persist for at least 4 hours.
Biphasic allergic reactions are highly likely when all of the following 4 criteria are fulfilled: b
New/recurrent symptoms/examination findings do not fulfill the 2006 NIAID/FAAN anaphylaxis criteria.
Initial symptoms/examination findings must completely resolve before the onset of new/recurrent symptoms/examination findings.
There cannot be allergen reexposure before the onset of new/recurrent symptoms/examination findings.
New/recurrent symptoms/examination findings must occur within 1 to 48 hours from complete resolution of initial symptoms/examination findings.
aThe diagnosis of persistent allergic reaction is independent of the management of the initial reaction.
bThe diagnosis of biphasic allergic reaction is independent of the management of the initial reaction.
From Dribin TE, Sampson HA, Camargo CA Jr, Brousseau DC, Spergel JM, Neuman MI, Shaker M, Campbell RL, Michelson KA, Rudders SA, Assa’ad AH, Risma KA, Castells M, Schneider LC, Wang J, Lee J, Mistry RD, Vyles D, Vaughn LM, Schumacher DJ, Witry JK, Viswanathan S, Page EM, Schnadower D. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J Allergy Clin Immunol. 2020 Nov;146(5):1089-1096; with permission.
Fig. 1.
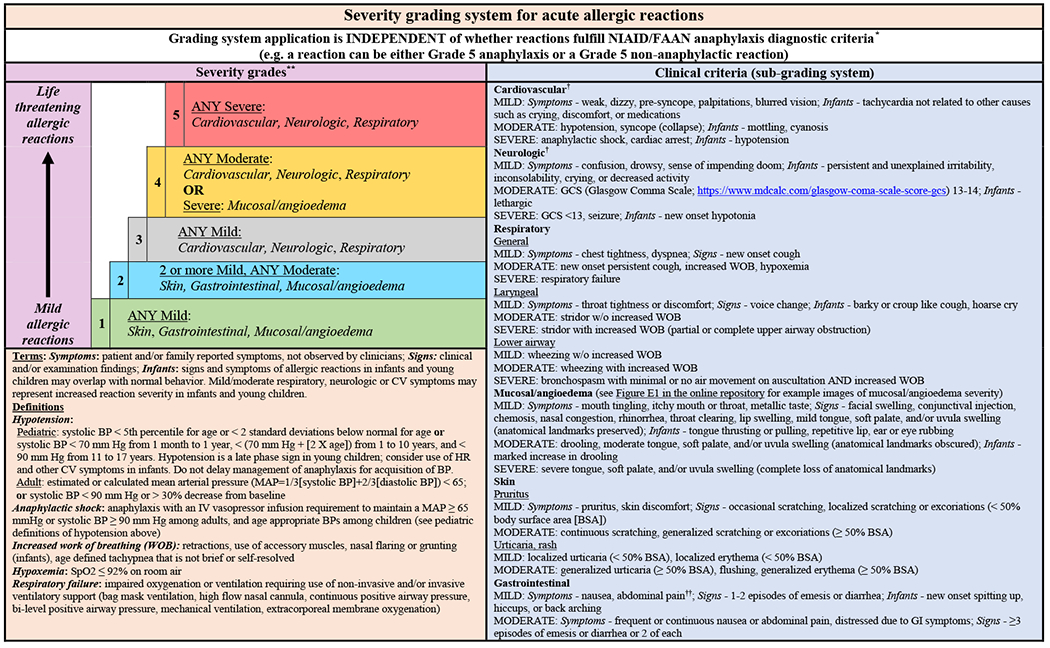
Severity grading system for acute allergic reactions. a The severity grading system is designed for use across the spectrum of acute allergic reactions as depicted by the vertical arrow (mild to life-threatening reactions), whether they fulfill NIAID/FAAN criteria for anaphylaxis or not. b For patients with multiple symptoms, reaction severity is based on the most severe symptom; symptoms that constitute more severe grades always supersede symptoms from less severe grades. The grading system can be used to assign reaction severity at any time during the course of reactions; reactions may progress rapidly (within minutes) from one severity grade to another. The grading system does not dictate management decisions; reactions of any severity grade may require treatment with epinephrine. c Patients with severe cardiovascular and/or neurologic involvement may have urinary or stool incontinence. However, the significance of incontinence as an isolated symptom is unclear, and it is therefore not included as a symptom in the subgrading system. d Abdominal pain may also result from uterine cramping. (From Dribin TE, Schnadower D, Spergel JM, Campbell RL, Shaker M, Neuman MI, Michelson KA, Capucilli PS, Camargo CA Jr, Brousseau DC, Rudders SA, Assa’ad AH, Risma KA, Castells M, Schneider LC, Wang J, Lee J, Mistry RD, Vyles D, Pistiner M, Witry JK, Zhang Y, Sampson HA. Severity grading system for acute allergic reactions: A multidisciplinary Delphi study. J Allergy Clin Immunol. 2021 Jul;148(1):173-181; with permission.)
The objective of this review is to summarize anaphylaxis data gaps and research needs consistent with the aforementioned study by Dribin and colleagues, given it is the most comprehensive, systematic appraisal of anaphylaxis research and knowledge gaps to date.2 Addressing these gaps will result in improved care of patients with or at risk of anaphylaxis with the ultimate goal of optimizing patient outcomes and lessening the burden of anaphylaxis on patients, families, communities, and the health care system. Of note, some anaphylaxis data gaps and research needs do not directly reference anaphylaxis but instead conditions that predispose to anaphylaxis, such as food, venom, and medication allergy; this is intentional, given that reducing the risk and burden of anaphylaxis is predicated on preventing and treating allergic conditions.2,5,6
DISCUSSION
The aforementioned themes align with principal anaphylaxis research and clinical domains. It will only be possible to address these gaps and improve patient care and clinical outcomes through integrated research strategies that align expertise in basic science, translational, and clinical research as well as epidemiology, public health, implementation science,7 drug development, and bioengineering.
Population Science
Background
The incidence of anaphylaxis is increasing globally, although there does not seem to be an increase in deaths. It is difficult to assess the true rate of anaphylaxis-induced deaths for a variety of reasons, including difference in diagnostic codes globally.8 Increasing cases of anaphylaxis are attributed to nonsteroidal antiinflammatory drugs, monoclonal antibodies, and chemotherapeutic agents.9–11 Food-induced anaphylaxis has also increased, particularly in children and adolescents. Between 1.6% and 5.1% of the US population have had anaphylaxis, and 1% of hospitalizations and 0.1% of emergency department (ED) encounters result in fatalities.12 ED visits for anaphylaxis doubled among all ages and tripled in children during the past decade in the United States.13 There is need for an accurate population database to support disease surveillance, to assess trends in anaphylaxis across diverse, broad populations and geographies, and to develop targeted interventions to mitigate disease burden and evaluate the effectiveness of these interventions longitudinally.
Data gaps and research needs
A review of central data gaps and research needs for Population Science is described in (Box 3). The 2006 National Institute of Allergy and Infectious Disease and Food Allergy and Anaphylaxis Network (NIAID/FAAN) anaphylaxis diagnostic criteria are widely used in clinical practice and research and are retrospectively and prospectively validated. However, there is a lack of universal consensus whether or not the NIAID/FAAN criteria should be modified based on new data and proposed recommendations from the World Allergy Organization.1,14–19 This includes how to account for isolated respiratory involvement after a known/suspected allergen exposure in sensitive patients, mild symptoms (eg, “throat tightness and nausea”) reported in food allergic patients, and how to define persistent gastrointestinal symptoms associated with the route of exposure (eg, food ingestion). Refining and achieving an international consensus as to the definition of anaphylaxis will promote improved disease surveillance globally.2
Box 3. Population Science data gaps and research needs.
Population Science
There is a need to
Establish international consensus about what constitutes anaphylaxis to support population research, including disease surveillance.
Evaluate the barriers (eg, geographic, socioeconomic) of patient access to allergists/immunologists for the long-term management (eg, venom immunotherapy, oral immunotherapy) of anaphylaxis and related conditions (eg, food, medication, venom allergies).
Better evaluate for socioeconomic disparities in the risk of, care, and outcomes of patients with anaphylaxis. There is a need to develop novel personal and community interventions to target these sectors of the population and to address these disparities to improve health outcomes for all patients with anaphylaxis.
Clarify geographic practice variation in anaphylaxis management (eg, emergency medical service, protocols, access to epinephrine auto-injectors, prescription patterns for epinephrine auto-injectors).
Evaluate the epidemiology of anaphylaxis severity, fatal anaphylaxis, as well as persistent, refractory, and biphasic anaphylaxis; this includes evaluating the association between specific allergens and these outcomes, as well as individual patient characteristics.
Understand the influence of prior anaphylaxis on quality of life and allergen avoidance behavior.
Better understand primary care physician’s understanding of anaphylaxis and specific allergy management.
Identify risk factors for future anaphylaxis severity, including tools that can identify patients/individuals who are at low risk of future anaphylaxis.
Evaluate the role of epinephrine auto-injectors in public spaces. Assuming there is a role for epinephrine auto-injectors in public spaces, what is the best way to implement such programs accounting for location (restaurants, schools, planes, stadiums) and specific costs (eg, pricing models, cost-effectiveness)?
Evaluate the long-term follow-up care of patients with anaphylaxis (eg, proportion of patients who follow-up with allergists/immunologists, have up-to-date epinephrine auto-injectors, and undergo testing to identify eliciting allergens), including barriers, facilitators, and strategies for improvement. Such evaluation would need to be sensitive to the differing resources available in different contexts (eg, developed vs developing countries, urban vs rural areas)
Modified from Dribin TE, Schnadower D, Wang J, Camargo CA Jr, Michelson KA, Shaker M, Rudders SA, Vyles D, Golden DBK, Spergel JM, Campbell RL, Neuman MI, Capucilli PS, Pistiner M, Castells M, Lee J, Brousseau DC, Schneider LC, Assa’ad AH, Risma KA, Mistry RD, Campbell DE, Worm M, Turner PJ, Witry JK, Zhang Y, Sobolewski B, Sampson HA. Anaphylaxis knowledge gaps and future research priorities: A consensus report. J Allergy Clin Immunol. 2021 Aug 12:S0091-6749(21)01209-4; with permission.
There also is a need to evaluate global anaphylactic practice variations, including Emergency Medical Services protocols, access to and use of epinephrine auto-injectors (EAIs), and EAI prescription patterns. There is a need to evaluate barriers to patient access to allergy/immunology care, particularly in research-deficient settings/geographies, and the long-term management of anaphylaxis and predisposing conditions such as that occurs with food, medication, and venom allergy. Investigations pursuing this line of research should account for differences in resource allocation/availability, particularly in resource-limited communities and countries. This work should support novel, efficacious, cost-effective, and sustainable community and public health interventions to mitigate the burden of anaphylaxis by accounting for socioeconomic, infrastructure, and environmental differences.2
Basic and Translational Sciences
Background
There have been exciting advances in the understanding of anaphylaxis and its pathogenesis during the past decade20; however, these have not been translated into reliable or improved bedside care of patients. Promising breakthroughs include identifying the role of anaphylaxis effector cells (mast cells, basophils, neutrophils, monocytes/macrophages) and their mediators (histamine, tryptase, platelet-activating factor, prostaglandins, interleukins, complement) as well as immunoglobulin E (IgE) and non-IgE pathways.21–24 In addition, there is now an improved understanding of risk factors, including hereditary alpha tryptasemia (the only presently known genetic risk factor) caused by the duplication of alpha tryptase genes at the TPSAB1 gene locus on chromosome 16. The prevalence in Western populations is believed to be between 4% and 6%.25,26 Mast cell activation disorders, including mastocytosis, are associated with an increased frequency of anaphylaxis. Anaphylaxis can be the presenting manifestation that leads to the diagnosis of clonal and nonclonal mast cell activation disorders.27–29 Research as to the role of the intestinal microbiome in protecting infants from developing food allergies, the most common cause of anaphylaxis in children, has also evolved during the past decade.30,31
Data gaps and research needs
A review of central data gaps and research needs for Basic and Translational Sciences is described in Box 4.2 Despite the identification of promising anaphylaxis biomarkers, there is a need to determine how biomarkers, for example, histamine and tryptase, can be incorporated into routine clinical care and to identify novel biomarkers to improve the diagnosis and treatment of anaphylaxis. Although anaphylaxis is a clinical diagnosis, identifying accurate, easy to obtain biomarkers would improve and help standardize care, particularly when there is diagnostic uncertainty, for example, “Is this allergen induced asthma or anaphylaxis?” Biomarkers would also help to standardize observation periods to monitor for persistent and biphasic reactions by allowing clinicians to trend biomarker levels and optimize treatment strategies as to if and when persistent and biphasic symptoms warrant additional treatment with epinephrine. Developing and translating the use of biomarkers into routine care would be the first step in transitioning the current model of care to a precision medicine care model that also incorporates host characteristics, for example, phenotypes, comorbidities, causative agents, and endotypes to predict and prognosticate management strategies both for short- and long-term care.32 In addition, identifying novel biological markers/mediators is an important first step to develop targeted, mechanistic-based therapies to treat refractory anaphylaxis, prevent anaphylaxis among high-risk individuals, and possibly cure common predisposing allergic conditions such as food, medication, and venom allergy.2
Box 4. Basic and Translational Sciences data gaps and research needs.
Basic and Translational Sciences
There is a need to
Develop strategies (eg, therapies, early food exposures) to prevent the development of food allergies in infancy.
Determine whether the basophil activation test can be configured (with standardized technique, reporting of results, and clinical threshold) to predict risk for anaphylaxis occurrence, severity, and course.
Evaluate the clinical usefulness of current biomarkers (tryptase, basophil activation test, urinary histamine, or leukotrienes) in confirming the diagnosis of anaphylaxis and in predicting future reaction severity, clinical courses, and informing optimal management strategies (eg, when to administer epinephrine, observation periods).
Clarify the compensatory mechanisms responsible for anaphylaxis recovery, the impact of anaphylaxis risk factors and triggers on these mechanisms, and how timing of epinephrine administration, intravenous fluid, and oxygen affect these mechanisms before and after the onset of multiorgan involvement.
Determine what other mediators are important in anaphylaxis that may serve as more reliable biomarkers for identification of anaphylaxis (during the episode) and risk of anaphylaxis (before the episode).
Develop biomarkers to indicate who is at risk for severe anaphylactic reactions.
There is a need to explore the role of cytokines, histamine, leukotrienes, metabolomics, and other factors in the severity and response to allergic triggers and therapies used to treat allergic reactions.
Determine why some foods are more likely to induce severe/fatal anaphylaxis (eg, peanut, cashew, seafood) than others (eg, egg, soybean).
Define clinically meaningful and reliable thresholds to screen/detect specific allergens in food for patients with life-threatening allergies.
Evaluate how the use of tryptase as a biomarker can be improved (eg, optimal timing).
Modified from Dribin TE, Schnadower D, Wang J, Camargo CA Jr, Michelson KA, Shaker M, Rudders SA, Vyles D, Golden DBK, Spergel JM, Campbell RL, Neuman MI, Capucilli PS, Pistiner M, Castells M, Lee J, Brousseau DC, Schneider LC, Assa’ad AH, Risma KA, Mistry RD, Campbell DE, Worm M, Turner PJ, Witry JK, Zhang Y, Sobolewski B, Sampson HA. Anaphylaxis knowledge gaps and future research priorities: A consensus report. J Allergy Clin Immunol. 2021 Aug 12:S0091-6749(21)01209-4; with permission.
However, conducting basic and clinical research during human anaphylaxis is difficult because of the challenge of timely enrolling patients in EDs, collecting serial biospecimens, and the need to simultaneously collect short- and long-term phenotypic and outcome data.2 An additional challenge is that mature mast cells, the key effector cells of anaphylaxis, are tissue based and not in the circulation and therefore difficult to obtain during reactions. Therefore, it is important that scientists with expertise in animal-based research collaborate with clinical/translational researchers to study human anaphylaxis. Likewise, there is a need for large prospective studies to facilitate the collection and banking of clinical and biological data to accelerate scientific discoveries and support grant applications.2
Acute Management
Background
Anaphylaxis continues to be underrecognized, misdiagnosed, and mismanaged,6 and this includes the underuse of epinephrine, the first line of therapy to treat primary and biphasic reactions, and the overuse of “second-line therapies” (antihistamines and glucocorticosteroids) for which there are insufficient data to support their use.6,33 Likewise, there are no validated data for the use of epinephrine, specifically for persistent and biphasic reactions. In addition, practice variations exist regarding the lengths of observation following initial reaction onset and/or treatment with epinephrine to monitor for a biphasic reaction, nor are there validated clinical criteria to standardize hospitalization criteria and care. These gaps may contribute to unnecessary hospitalizations, increasing health care costs, and undo personal and financial strain on patients and families.2,13,34,35
A limitation of conducting rigorous research specific to acute anaphylaxis management, including the prevalence of and risk factors for biphasic reactions, is the lack of consensus definitions for adverse anaphylaxis outcomes. To address this gap, a 19-member panel developed consensus definitions of persistent, refractory, and biphasic anaphylaxis (see Box 1) as well as persistent and biphasic nonanaphylactic reactions (see Box 2).3 Dissemination and application of these definitions in research and clinical care will serve as a foundation to optimize and standardize clinical management and patient outcomes for future investigations. This project also highlighted the need to develop a consensus severity grading system for acute allergic reactions, including anaphylaxis and nonanaphylactic reactions. The same researchers, using Delphi methodology, developed the SGS-AR (see Fig. 1).4 Validation, dissemination, and application of the grading system will help standardize the language and outcomes used in clinical care and research and will serve as a tool to standardize adverse reaction reporting for clinical trials.2,4
Data gaps and research needs
A review of central data gaps and research needs for Acute Management are described in Box 5.2 An important first step is to validate the SGS-AR in different clinical settings, for example, EDs and allergy/immunology centers, to ensure it can be used universally and is reliable. Successful validation of the SGS-AR will help harmonize clinical care language and standardize outcomes in observational and interventional trials. It may also lead to the development of SGS-AR–embedded patient technologies and result in standardized reporting of acute allergic reactions in non–health care settings. Validation of the SGS-AR may also have the positive impact of improving real-time management decisions in non–health care settings for patients and their families, including when to administer epinephrine or seek emergent medical care.4
Box 5. Acute Management data gaps and research needs.
Acute Management
There is a need to
Validate the severity grading system for acute allergic reactions.
Improve the evidence-based practice of the emergency treatment of anaphylaxis.
Conduct a randomized controlled trial to evaluate the efficacy of adjunctive systemic glucocorticoids in treating anaphylaxis, including reducing initial reaction severity, and preventing biphasic reactions.
Develop tools and strategies to improve anaphylaxis recognition by caregivers and health care professionals.
Develop clinical prediction models to determine if hospitalization is indicated after initial reaction management and to inform patient-centric periods of observation.
Identify shortcomings of current anaphylaxis action plans used in the ED, inpatient and outpatient settings, and develop optimal, patient-centered anaphylaxis action plans for these settings; this includes determining the minimum number of elements to be included in the action plan to achieve efficacy.
- Identify signs and symptoms of anaphylaxis in infants/young children accounting for:
- The challenge of recognizing signs and symptoms of anaphylaxis in nonverbal children (challenges: lack of subjective symptoms; assessing mental status, eg, inconsolability, lethargy).
- Differences in cardiovascular involvement in infants/young children compared with adults (eg, hypotension in children is a late finding of decompensated shock, and tachycardia may be the only sign of compensated shock in children).
- Signs and symptoms of anaphylaxis in infants/young children can overlap with normal behavior.
- Develop an anaphylaxis management guideline specific to treatment with epinephrine that takes into account all patient ages and care settings to address the following questions:
- After treatment of anaphylaxis with epinephrine, which recurrent/new signs and/or symptoms should be treated with epinephrine versus those that can be monitored without treatment with epinephrine?
- Can mild anaphylactic reactions be safely managed without epinephrine?
- What constitutes delayed epinephrine administration and the degree to which this increases the risk for adverse outcomes including refractory and/or biphasic reactions?
- What is the optimal timing for repeat epinephrine administration?
Develop a model (including information such as past medical history, reaction severity, response to treatment with epinephrine) to identify patients with anaphylaxis who can be safely managed at home instead necessitating emergency care evaluation.
Evaluate the role of alternative epinephrine delivery mechanisms (beyond currently available epinephrine auto-injectors) to treat anaphylaxis.
Modified from Dribin TE, Schnadower D, Wang J, Camargo CA Jr, Michelson KA, Shaker M, Rudders SA, Vyles D, Golden DBK, Spergel JM, Campbell RL, Neuman MI, Capucilli PS, Pistiner M, Castells M, Lee J, Brousseau DC, Schneider LC, Assa’ad AH, Risma KA, Mistry RD, Campbell DE, Worm M, Turner PJ, Witry JK, Zhang Y, Sobolewski B, Sampson HA. Anaphylaxis knowledge gaps and future research priorities: A consensus report. J Allergy Clin Immunol. 2021 Aug 12:S0091-6749(21)01209-4; with permission.
There is also a need to improve anaphylaxis diagnostic criteria as discussed in the Population Science section of this review, which includes modifying diagnostic criteria to account for signs/symptoms of infant anaphylaxis, which may overlap with normal infant behavior, for example, crying, irritability, spitting up, and back arching.36 Likewise, cardiovascular or neurologic signs/symptoms in infants and young children may represent a more severe/advanced disease state than in adults.4,36,37
There is also a need to derive and validate clinical prediction models to standardize ED observation periods and hospitalization criteria.2 Such models would positively affect the length of observation periods and potentially prevent unnecessary, costly hospitalizations. Furthermore, given the importance of timely epinephrine administration, there is a need to better understand the pharmacodynamics, pharmacokinetics, and the clinical outcomes of epinephrine administered by different devices and routes. This includes evaluating the efficacy of noninjectable epinephrine delivery systems, which would may preferable to patients and families compared with EAIs. Furthermore, although delayed epinephrine use is a potential risk factor for biphasic anaphylaxis, there is a need to determine what constitutes “delay” and the degree to which it increases the risk of adverse anaphylaxis outcomes, including fatal, refractory, persistent, and biphasic reactions.2,38,39
Although epinephrine is the first-line anaphylaxis therapy it is often underused and replaced by second-line therapies, such as H1 and H2 antagonists and systemic glucocorticosteroids, for which there are insufficient data to support their use.6 Therefore, there is a need for randomized controlled trials to evaluate the efficacy of these therapies at reducing reaction severity and preventing biphasic reactions. Such a line of investigation is difficult to conduct, given the obstacles associated with ED enrollment, randomization, and because of the perceived lack of equipoise, given the routine use of these medications in clinical care.2,33 There is also a need to evaluate how to best standardize and implement anaphylaxis action plans for patients and families and to identify best practices for EAI prescription programs to ensure access to EAIs.2
In summary, there is a need for large, prospective observational and interventional trials to address research gaps specific for the management of acute anaphylaxis. Such research will allow investigators to collect accurate longitudinal data using novel techniques and patient-friendly technologies. In addition, it would be ideal to consent patients to biobank specimens at the time of study enrollment to accelerate basic and translational research discoveries. Finally, it is essential to incorporate the perspectives of patients and families when designing prospective ED-based research to ensure study findings translate into equitable and patient-centric outcomes.2
Long-Term Management
Background
The long-term management and prevention of anaphylaxis is contingent on allergen avoidance, drug desensitization, allergen immunotherapy, and appropriate observation periods for patients at high risk of acute allergic reactions or anaphylaxis.5,6 Providing a diagnosis of mast cell activation disorders is critical. Despite the benefit of allergen immunotherapy in mitigating the risk of anaphylaxis, particularly for food allergy, patients are at risk of immunotherapy-induced anaphylaxis. A central tenet of the long-term management and prevention is the need for clinicians to appropriately discuss the potential benefits and risks of different therapies and treatment strategies with patients and families.2
Data gaps and research needs
A review of central data gaps and research needs for Long-Term Management is described in Box 6.2 There is a need to determine immunotherapy best practices, including oral immunotherapy for food allergies. Immunotherapy protocols must be patient-centered and modified to meet the needs of patients and families from diverse communities and backgrounds. For example, there is a potential for maintenance immunotherapy to be available in nonmedical facilities. There is also a need to evaluate practice differences that contribute to patients being labeled or delabeled with drug allergy and the underutilization of drug desensitization protocols.2
Box 6. Long-Term Management data gaps and research needs.
Long-Term Management
There is a need to
Delabel individuals who are unnecessarily labeled as medication allergic/at risk of anaphylaxis, particularly to antibiotics, due to vague reactions or reactions that occurred a long time ago. Use of expensive, broader spectrum antibiotics, due to incorrect or outdated diagnosis, is very costly and may promote more antimicrobial resistance.
Determine how risk perceptions influence quality of life for patients at risk for anaphylaxis and determine what anaphylaxis outcomes matter most to patients. These patient-oriented outcomes are key to evaluating the effectiveness of current and novel anaphylaxis therapies and management strategies.
Evaluate the impact of device cost and pragmatic device limitations as a barrier to effective anaphylaxis treatment in the community.
There is a need to understand barriers to self-injectable epinephrine carriage and use and how these barriers can be addressed.
Determine best practices for oral immunotherapy in patients with food allergy.
Understand how the health literacy of patients from diverse racial, cultural, and socioeconomic backgrounds affects their understanding of and application of written anaphylaxis action plans to provide anaphylaxis self-management.
Develop validated decision aids (eg, use of therapies/strategies to prevent anaphylaxis) to address the needs of patients/families from diverse racial, cultural, and socioeconomic backgrounds.
Identify and address barriers to early allergen introduction to prevent food allergies that lead to risk of recurrent anaphylaxis.
Understand the psychological impact of anaphylaxis and anaphylaxis therapies on patients and caregivers and develop novel interventions and/or strategies to address them.
Understand the unwarranted geographic practice variation in the underutilization of drug desensitization, particularly in high-risk populations (eg, patients with cystic fibrosis treated with β-lactam antibiotics, patients treated with carboplatin).
Modified from Dribin TE, Schnadower D, Wang J, Camargo CA Jr, Michelson KA, Shaker M, Rudders SA, Vyles D, Golden DBK, Spergel JM, Campbell RL, Neuman MI, Capucilli PS, Pistiner M, Castells M, Lee J, Brousseau DC, Schneider LC, Assa’ad AH, Risma KA, Mistry RD, Campbell DE, Worm M, Turner PJ, Witry JK, Zhang Y, Sobolewski B, Sampson HA. Anaphylaxis knowledge gaps and future research priorities: A consensus report. J Allergy Clin Immunol. 2021 Aug 12:S0091-6749(21)01209-4; with permission.
Identifying and addressing barriers to early allergen introduction to prevent food allergies, the most common cause of anaphylaxis in children, is also necessary.40 There are practice guideline variations for such recommendations, which are confusing to families and clinicians and lead to suboptimal care and patient outcomes. When designing investigations specific to the long-term management of patients at risk for anaphylaxis, it is imperative to include patients and families from diverse backgrounds to ensure that study procedures, outcomes, and interventions result in optimal health outcomes.2
SUMMARY
This article outlines central anaphylaxis data gaps and research needs related to the following anaphylaxis themes: Population Science, Basic and Translational Sciences, Acute Management, and Long-Term Management. There is need for multidisciplinary collaboration among basic, translational, clinical, and population scientists with input from patients/families, policymakers, and other stakeholders to address these gaps with the ultimate goal of reducing the societal burden of anaphylaxis.2
KEY POINTS.
There are significant anaphylaxis data and knowledge gaps across key clinical care and research domains—Population Science, Basic and Translational Sciences, Acute Management, and Long-Term Management—that contribute to suboptimal patient outcomes.
Population science: refine anaphylaxis diagnostic criteria and develop reliable ways to record and measure it using multinational datasets.
Basic and translational sciences: identify reliable diagnostic, predictive, and prognostic anaphylaxis biomarkers to standardize and optimize short and long-term management strategies.
Acute management: develop clinical prediction models to standardize post-anaphylaxis observation periods and hospitalization criteria.
Long-term management: determine immunotherapy best practices including oral immunotherapy for patients with food allergy.
CLINICS CARE POINTS.
There have been promising advances in the care of patients with or at risk of anaphylaxis, including the long-term management and prevention of common predisposing allergic conditions. Despite these advances, significant data gaps and research needs remain. These needs include the need to refine anaphylaxis diagnostic criteria; identify accurate and reliable diagnostic, predictive, and prognostic biomarkers; standardize postanaphylaxis care, for example, observation periods, hospitalization criteria; and determine allergen/immunotherapy best practices. Addressing these and other gaps through multidisciplinary research collaborations will result in improved clinical care and optimal outcomes for patients with or at risk of anaphylaxis.
Funding:
The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2UL1TR001425 - 05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health, under Award Number 2KL2TR001426 -05A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURE
T.E. Dribin receives funding from the NIH. M. Castells is the BWH PI for the PIONEER BluePrint Clinical trial for Indolent Systemic Mastocytosis.
REFERENCES
- 1.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: Summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol 2006;117(2):391–7. [DOI] [PubMed] [Google Scholar]
- 2.Dribin TE, Schnadower D, Wang J, et al. Anaphylaxis knowledge gaps and future research priorities: a consensus report. J Allergy Clin Immunol 2021. 10.1016/j.jaci.2021.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dribin TE, Sampson HA, Camargo CA Jr, et al. Persistent, refractory, and biphasic anaphylaxis: a multidisciplinary Delphi study. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dribin TE, Schnadower D, Spergel JM, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol 2021. ;148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ J 2020;13(10):100472. Available at: https://www.sciencedirect.com/science/article/pii/S1939455120303756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaker MS, Wallace DV, Golden DBK, et al. Anaphylaxis–a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 2020; 145(4)1082–123. [DOI] [PubMed] [Google Scholar]
- 7.Bauer MS, Damschroder L, Hagedorn H, et al. An introduction to implementation science for the non-specialist. BMC Psychol 2015;3(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner PJ, Campbell DE, Motosue MS, et al. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J Allergy Clin Immunol Pract 2020;8(4):1169–76. Available at: http://www.sciencedirect.com/science/article/pii/S2213219819309675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloane D, Govindarajulu U, Harrow-Mortelliti J, et al. Safety, Costs, and Efficacy of Rapid Drug Desensitizations to Chemotherapy and Monoclonal Antibodies. J Allergy Clin Immunol Pract 2016;4(3):497–504. Available at: https://www.sciencedirect.com/science/article/pii/S2213219816000118. [DOI] [PubMed] [Google Scholar]
- 10.Isabwe GAC, Garcia Neuer M, de las Vecillas Sanchez L, et al. Hypersensitivity reactions to therapeutic monoclonal antibodies: phenotypes and endotypes. J Allergy Clin Immunol 2018;142(1):159–70.e2. Available at: https://www.sciencedirect.com/science/article/pii/S0091674918303063. [DOI] [PubMed] [Google Scholar]
- 11.Aun MV, Blanca M, Garro LS, et al. Nonsteroidal Anti-Inflammatory Drugs are Major Causes of Drug-Induced Anaphylaxis. J Allergy Clin Immunol Pract 2014;2(4): 414–20. Available at:https://www.sciencedirect.com/science/article/pii/S2213219814001354. [DOI] [PubMed] [Google Scholar]
- 12.Turner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: An analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol 2015;135(4):956–63.e1. Available at: http://www.sciencedirect.com/science/article/pii/S0091674914015164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michelson KA, Dribin TE, Vyles D, et al. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract 2020;8(2):767–8.e2. [DOI] [PubMed] [Google Scholar]
- 14.Turner PJ, Worm M, Ansotegui IJ, et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ J 2019;12(10):100066. Available at: https://pubmed.ncbi.nlm.nih.gov/31719946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motosue MS, Bellolio MF, Van Houten HK, et al. Outcomes of Emergency Department Anaphylaxis Visits from 2005 to 2014. J Allergy Clin Immunol Pract 2017;6(3):1002–9.e2. [DOI] [PubMed] [Google Scholar]
- 16.Brauer CEL, Motosue MS, Li JT, et al. Prospective Validation of the NIAID/FAAN Criteria for Emergency Department Diagnosis of Anaphylaxis. J Allergy Clin Immunol Pract 2016;4:1220–6. [DOI] [PubMed] [Google Scholar]
- 17.Campbell RL, Hagan JB, Manivannan V, et al. Evaluation of National Institute of Allergy and Infectious Diseases/Food Allergy and Anaphylaxis Network criteria for the diagnosis of anaphylaxis in emergency department patients. J Allergy Clin Immunol 2012;129:748–52. [DOI] [PubMed] [Google Scholar]
- 18.Cox L, Larenas-Linnemann D, Lockey RF, et al. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol 2010;125(3):569–74.e7. [DOI] [PubMed] [Google Scholar]
- 19.Cox LS, Sanchez-Borges M, Lockey RF. World Allergy Organization Systemic Allergic Reaction Grading System: Is a Modification Needed? J Allergy Clin Immunol Pract [Internet] 2017;5(1):58–62.e5. Available at: http://www.sciencedirect.com/science/article/pii/S2213219816305669. [DOI] [PubMed] [Google Scholar]
- 20.Muraro A, Lemanske RF, Castells M, et al. Precision medicine in allergic disease — food allergy , drug allergy , and anaphylaxis — PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy , Asthma and Immunology. Allergy 2017;72:1006–21. [DOI] [PubMed] [Google Scholar]
- 21.Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol 2017;140(2):335–48. Available at: https://www.sciencedirect.com/science/article/pii/S0091674917310205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SGA, Stone SF, Fatovich DM, et al. Anaphylaxis: Clinical patterns, mediator release, and severity. J Allergy Clin Immunol 2013;132(5):1141–9.e5. Available at: http://www.sciencedirect.com/science/article/pii/S0091674913009834. [DOI] [PubMed] [Google Scholar]
- 23.Cianferoni A Non–IgE-mediated anaphylaxis. J Allergy Clin Immunol 2021; 147(4):1123–31. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Khodoun MV, Strait R. Human IgE-independent systemic anaphylaxis. J Allergy Clin Immunol 2016;137(6):1674–80. Available at: https://www.sciencedirect.com/science/article/pii/S0091674916003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol 2020;147(2):622–32. Available at: http://www.sciencedirect.com/science/article/pii/S0091674920310290. [DOI] [PubMed] [Google Scholar]
- 26.Luskin KT, White AA, Lyons JJ. The Genetic Basis and Clinical Impact of Hereditary Alpha-Tryptasemia. J Allergy Clin Immunol Pract 2021. ;9(6):2235–42. Available at: https://www.sciencedirect.com/science/article/pii/S2213219821003068. [DOI] [PubMed] [Google Scholar]
- 27.Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation-related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol 2021;126(6):655–60. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs D, Kilbertus A, Kofler K, et al. Scoring the Risk of Having Systemic Mastocytosis in Adult Patients with Mastocytosis in the Skin. J Allergy Clin Immunol Pract 2021. ;9(4):1705–12.e4. Available at: https://www.sciencedirect.com/science/article/pii/S2213219820313544. [DOI] [PubMed] [Google Scholar]
- 29.Valent P, Akin C, Bonadonna P, et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. J Allergy Clin Immunol Pract 2019;7(4):1125–33.e1. Available at: https://www.sciencedirect.com/science/article/pii/S221321981930056X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feehley T, Plunkett CH, Bao R, et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 2019;25(3):448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang YJ, Marsland BJ, Bunyavanich S, et al. The microbiome in allergic disease: Current understanding and future opportunities–2017 PRACTALL document of the American Academy of Allergy, Asthma & Immunology and the European Academy of Allergy and Clinical Immunology. J Allergy Clin Immunol 2017;139(4):1099–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castells M Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol 2017;140:321–33. [DOI] [PubMed] [Google Scholar]
- 33.Michelson KA, Monuteaux MC, Neuman MI. Variation and Trends in Anaphylaxis Care in United States Children’s Hospitals. Acad Emerg Med 2016;23(5):623–7. [DOI] [PubMed] [Google Scholar]
- 34.Rudders SA, Banerji A, Vassallo MF, et al. Trends in pediatric emergency department visits for food-induced anaphylaxis. J Allergy Clin Immunol 2010;126(2): 385–8. [DOI] [PubMed] [Google Scholar]
- 35.Robinson LB, Arroyo AC, Faridi MK, et al. Trends in US hospitalizations for anaphylaxis among infants and toddlers: 2006 to 2015. Ann Allergy Asthma Immunol 2021;126(2):168–74.e3. Available at: https://www.sciencedirect.com/science/article/pii/S1081120620310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenhawt M, Gupta RS, Meadows JA, et al. Guiding Principles for the Recognition , Diagnosis , and Management of Infants with Anaphylaxis : An Expert Panel Consensus. J Allergy Clin Immunol Pract 2019;7(4):1148–56.e5. [DOI] [PubMed] [Google Scholar]
- 37.Pistiner M, Mendez-Reyes JE, Eftekhari S, et al. Caregiver Reported Presentation of Severe Food-induced Allergic Reactions in Infants and Toddlers. J Allergy Clin Immunol Pract 2020. 10.1016/j.jaip.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Lee JM, Greenes DS. Biphasic Anaphylactic Reactions in Pediatrics. Pediatrics 2000;106(4):762–6. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Peterson A, Lohse CM, et al. Further Evaluation of Factors That May Predict Biphasic Reactions in Emergency Department Anaphylaxis Patients. J Allergy Clin Immunol Pract 2017;5(5): 1295–301. [DOI] [PubMed] [Google Scholar]
- 40.Schroer B, Groetch M, Mack DP, et al. Practical Challenges and Considerations for Early Introduction of Potential Food Allergens for Prevention of Food Allergy. J Allergy Clin Immunol Pract 2021. ;9(1):44–56.e1. [DOI] [PubMed] [Google Scholar]


