Abstract
Study Design:
Case-control study.
Objectives:
To investigate the incidence of symptomatic spinal epidural hematoma (SSEH) and recognize its risk factors in a cohort of patients undergoing posterior thoracic surgery in isolation.
Methods:
From January 2010 to December 2019, patients who developed SSEH after posterior thoracic surgery and underwent hematoma evacuation were enrolled. For each SSEH patient, 2 or 3 controls who did not develop SSEH and underwent the same procedures with similar complexity at the same section of the thoracic spine in the same period were collected. The preoperative and intraoperative factors, blood pressure-related factors and radiographic parameters were collected to identify possible risk factors by comparing between the 2 groups.
Results:
A total of 24 of 1612 patients (1.49%) were identified as having SSEH after thoracic spinal surgery. Compared to the control group (53 patients), SSEH patients had significant differences in the APTT (p = 0.028), INR (p = 0.009), ratio of previous spinal surgery (p = 0.012), ratio of cerebrospinal fluid leakage (p = 0.004), thoracic kyphosis (p<0.05), local kyphosis angle (p<0.05), epidural fat ratio at T7 (p = 0.003), occupying ratio of the cross-sectional area (p<0.05) and spinal epidural venous plexus grade (p<0.05). Multiple logistic regression analysis revealed 3 risk factors for SSEH: cerebrospinal fluid leakage, the local kyphosis angle (>8.77°) and the occupying ratio of the cross-sectional area (>49.58%).
Conclusions:
The incidence of SSEH was 1.49% in posterior thoracic spinal surgeries. Large local kyphosis angle (>8.77°), high occupying ratio of cross-sectional area (>49.58%) and cerebrospinal fluid leakage were identified as risk factors for SSEH.
Keywords: symptomatic spinal epidural hematoma, posterior thoracic surgery, local kyphosis angle, occupying ratio of cross-sectional area, cerebrospinal fluid leakage
Introduction
Symptomatic spinal epidural hematoma (SSEH) is one of the most devastating complications after spinal surgery. It was first reported in 1869, and since then, many scholars have focused on this complication with its incidence, etiology and treatment. The reported incidence of asymptomatic SSEH confirmed by magnetic resonance imaging (MRI) ranges from 14.6%-58%, while symptomatic SSEH occurs less frequently (0.1%-0.4%).1,2 Although symptomatic SSEH is very rare, it often causes serious consequences, including severe pain, paralysis, lower extremity weakness and bowel dysfunction, by compressing the spinal cord. Once the diagnosis is established, emergency surgery for evacuation, hemostasis and possible additional decompression are performed as early as possible to prevent poor outcomes. In a clinical experiment, authors revealed that revision surgery within 8 hours could achieve full or partial recovery of neurological function. 3 A study on dogs also demonstrated that 6 hours was the time line for neurological recovery. 4 Thus, it is important for spine surgeons to identify the risk factors for SSEH in order to make an early diagnosis and prevent the occurrence of SSEH.
Numerous studies have identified many risk factors for SSEH, including an age >60, coagulopathy, a high body mass index, the use of nonsteroidal anti-inflammatory drugs, multilevel procedures, previous spine surgery, an Rh-positive blood type, blood loss >1 L, hemoglobin <10 g/dl, an international normalized ratio >2.0 within 48 hours after surgery, a high diastolic blood pressure before surgery, a large increase in blood pressure after extubation, the use of gelfoam and so on.5-10 However, some factors remain controversial. For instance, Bovonratwet et al found that following anterior cervical discectomy and fusion, patients who had a lower BMI easily developed hematoma, whereas another study identified that a high BMI was a risk factor in a whole spine level sample.5,8 Additionally, most studies have drawn conclusions based on a cohort of lumbar and cervical spine patients. Few studies have focused on the incidence of and risk factors for SSEH in thoracic patients.
Therefore, the purpose of this study was to investigate the incidence of SSEH and recognize the risk factors based on patients who underwent posterior thoracic surgery.
Methods
Ethical Consideration
This study was approved by the ethics committee of our hospital (IRB00006761) and conducted according to the principles of the Declaration of Helsinki. For this type of study, formal consent is not required.
Study Design
Between January 2010 and December 2019, 1612 patients underwent thoracic decompression surgery due to thoracic spinal stenosis (TSS) caused by thoracic disc herniation and ossification of the posterior longitudinal ligament and ligamentum flavum with/without posterior instrumentation in our institution. The SSEH group was determined according to symptoms as well as MRI and intraoperative findings, and these patients underwent hematoma evacuation within 1 week of the initial surgery. The symptoms of the patients included (1) severe or increasing radiating pain in the lower limbs; (2) lower limb numbness; (3) a progressive decrease in muscle power; and (4) bladder or bowel dysfunction. The exclusion criteria were as follows: (1) TSS caused by trauma, tumors, infections, deformities or other diseases; (2) SSEH occurring at the cervicothoracic or thoracolumbar junctions; (3) initial surgeries performed at other hospitals; and (4) incomplete clinical data. In the control group, those who did not develop SSEH but underwent the same procedures with similar complexity at the same section of the thoracic spine in the same period (the same year or the following year) were randomly selected from the pool of patients. All patients underwent surgery with general anesthesia. The drainage tubes were removed when the volume was less than 50 ml per 24 hours.
Risk Factors for SSEH
The medical documents associated with the 2 groups were reviewed in detail. Possible risk factors were divided into 4 categories: preoperative factors, intraoperative factors, blood pressure-related factors and radiographic parameters.
Preoperative factors
Preoperative factors, including age, sex, body mass index, comorbidities, pre- and postoperative routine blood test results (blood cell number, hemoglobin level, hematocrit, platelet count), coagulation status (prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, international standardization rate), medications recorded (use of nonsteroidal anti-inflammatory drugs and anticoagulation drugs), smoking and drinking status, and history of spine surgery, were recorded.
Intraoperative factors
The intraoperative factors, including estimated blood loss, duration of surgery and anesthesia, volume of crystalloids and colloidal solution, blood transfusion (red cell, blood plasma, autotransfusion), cerebrospinal fluid leakage, number of drainage tubes and gelfoam dural coverage, were recorded.
Blood pressure-related factors
Blood pressure was measured at admission, intraoperatively, after extubation, in the anesthesia recovery room, and postoperatively. The intraoperative blood pressure was measured every 10 minutes when general anesthesia began and was recorded as an average level. Blood pressure variability (BPV) was recorded as a type of intraoperative blood variance.
Radiographic parameters
The following radiographic parameters were evaluated during full spine CT scans and thoracic MRI by Surgimap as previously described (version: 2.2, Nemaris Inc.): thoracic kyphosis (TK), local kyphosis angle, lumbar lordosis (LL), occupying ratio of the spinal canal anterior-posterior diameter, occupying ratio of the cross-sectional area, spinal epidural venous plexus (SEVP) grade, epidural fat at T7 and maximum level. The local kyphosis angle was determined by the measurement of the Cobb angle. All measurements were performed by 3 independent observers who were blinded to the patients. All patients were measured twice, and the average value was recorded. TK, LL and the local kyphosis angle were measured during full spine CT using traditional methods. It should be noted that in our hospital, TSS patients do not usually undergo full spine X-ray. Therefore, we obtained data from full spine CT in the supine position.
In the maximum compression segment, the osseous spinal canal diameters and maximum thicknesses of ossification were measured on sagittal and axial CT images by Surgimap. The occupying ratio of the spinal canal anterior-posterior diameter was calculated as follows 11 (Figures 1 and 2):
Figure 1.
Measurement of the anterior-posterior diameter on a sagittal view. (a) Maximum compression segment on a sagittal view; (b) The anterior-posterior diameter of the osseous spinal canal is defined by the line AB; (c) Maximum thickness of ossification is defined by the line CD. The occupying ratio of the spinal canal anterior-posterior diameter on a sagittal view was calculated as follows: occupying ratio = (CD/AB)×100%.
Figure 2.
Measurement of the anterior-posterior diameter on an axial view. (a) Maximum compression segment on an axial view; (b, c) The anterior-posterior diameter of the osseous spinal canal is defined by line C, and the maximum thickness of ossification is defined by line B. The occupying ratio of the spinal canal anterior-posterior diameter on an axial view was calculated as follows: occupying ratio = (B/C)×100%.
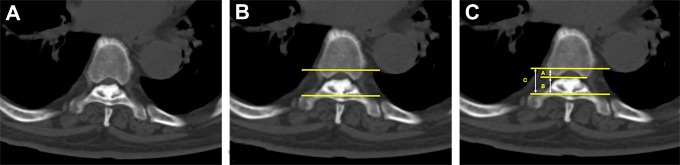
In the maximum compression segment, the normal cross-sectional area (CSA) was measured in the pedicle region (without ossification). If maximal compression occurred at the level of the intervertebral disc, the normal cross-sectional area was calculated as CSAn = (CSAn-1+CSAn+1)/2. The cross-sectional area of the narrowest level was measured in the same segment. There is usually no pedicle in this region. Therefore, we used the greatest distance between the bilateral pedicles to define the boundary of the spinal canal. Detailed measurements were described previously. 12 The occupying ratio was calculated as follows (Figure 3):
Figure 3.
Measurement of the cross-sectional area. (a, b) In the pedicle region, the normal cross-sectional area was measured by selecting several points around the boundary. (c) Line AB was used to define the lateral boundary of the area. (d, e) In the narrowest region, there is usually no pedicle; thus, line AB can define the lateral boundary of the area. (f) Within line AB, the narrowest cross-sectional area was measured as described previously.
The spinal epidural venous plexus (SEVP) is a tubular structure on axial T2-weighted MRI in the epidural space because of the flowing void effect. We measured the thickest diameter of the SEVP on axial T2-weighted MRI in the lesion segment. The degree of SEVP was classified into 4 grades as described previously: grade 0, no epidural veins were seen; grade 1, 1 or more epidural veins were seen and the thicknesses of all veins were < 1 mm (mild dilatation); grade 2, the thickest of the epidural veins were ≥ 1 mm and < 3 mm (moderate dilatation); and grade 3, the thickest of the epidural veins were ≥ 3 mm (severe dilatation). 13 The epidural space included the anterior and posterior space (Figure 4).
Figure 4.
Dilatation of the SEVP. (a, b) Axial T2-weighted MRI showed that the posterior epidural veins (white arrow) were dilatated. (grade 2).
Previous studies have shown that epidural lipomatosis can lead to SEVP dilation. 13 Therefore, we selected 2 positions to define the epidural fat in patients. T7 was chosen in most of the studies on EF in the literature. The maximum EF at the thoracic spine was also measured. Because T1-weighted MRI could provide a good way to distinguish epidural fat (EF) from the dural sac (DS), the EF and DS diameters were measured on sagittal T1-weighted MRI as described previously. 14 The EFR was calculated as follows (Figure 5):
Figure 5.
Measurement of epidural fat at T7 and the maximum level. (a) T1-weighted MRI of the thoracic spine. (b) Measurement at the T7 level. The measurements of the dural sac are shown in white (line AB), and epidural fat is shown in black (line BC). (c) Measurement at the maximum epidural fat level. The measurements of the dural sac are shown in white (line DE), and epidural fat is shown in black (line EF). The EFRT7 and EFRmax were calculated as follows: ; .
Statistical Analysis
Data was recorded and analyzed using SPSS software version 24.0 (IBM, Armonk, New York). Univariate analysis was carried out to identify the potential risk factors at first. Continuous data is presented as the mean ± standard deviation and were assessed by independent sample t test. Categorical variables were compared using the X 2 test and Fisher’s exact test. Multiple logistic regression analysis was applied to identify the independent risk factors for SSEH. Adjusted odds ratios and 95% confidence intervals were calculated. The difference was considered significant if the p value was < 0.05.
Results
Incidence
Twenty-four patients (1.49%) were identified as having SSEH after surgery and underwent hematoma evacuation. Moreover, 2 patients who had incomplete clinical data were excluded. The average time of symptom occurrence was 36.45 ±68.56 hours after surgery. Another 53 patients who underwent the same procedure at the same level were enrolled as the control group. The detailed information is shown in Table 1.
Table 1.
Preoperative Factors.
| SSEH group (n = 22) | Control group (n = 53) | p Value* | |
|---|---|---|---|
| Age (years) | 53.27 ± 10.96 | 52.98 ± 9.29 | 0.91 |
| Sex (Male/Female) | 16/6 | 27/26 | 0.08 |
| % Male | 72.73% | 50.94% | |
| BMI(kg/m2) | 27.20 ± 4.54 | 27.89 ± 4.22 | 0.54 |
| Anticoagulation (%) | 1 (4.55%) | 0 (0) | 0.29 |
| Nonsteroidal drug (%) | 20 (90.91%) | 41 (77.36%) | 0.17 |
| Hypertension (%) | 10 (45.45%) | 17 (32.08%) | 0.27 |
| Diabetes (%) | 3 (13.64%) | 8 (15.09%) | 1.00 |
| Smoking or drinking (%) | 1 (4.55%) | 8 (15.09%) | 0.27 |
| History of spinal surgery (%) | 7 (31.81%) | 4 (7.54%) | 0.012 |
| Red Blood cell (×109/L) | 4.81 ± 0.49 | 4.85 ± 0.49 | 0.71 |
| Hb (g/L) | 147.36 ± 16.51 | 142.37 ± 19.89 | 0.30 |
| HCT | 0.44 ± 0.04 | 0.43 ± 0.05 | 0.82 |
| PLT (×109/L) | 233.41 ± 65.78 | 237.26 ± 68.98 | 0.82 |
| PT (s) | 10.83 ± 0.85 | 10.65 ± 0.61 | 0.31 |
| APTT (s) | 36.41 ± 10.70 | 32.63 ± 4.02 | 0.028 |
| TT (s) | 13.92 ± 1.48 | 13.75 ± 1.06 | 0.58 |
| Fib (g/L) | 2.79 ± 0.54 | 3.03 ± 0.54 | 0.086 |
| INR | 1.03 ± 0.07 | 0.98 ± 0.05 | 0.009 |
| After surgery | |||
| Red Blood cell (×109/L) | 4.00 ± 0.49 | 4.17 ± 0.58 | 0.39 |
| Hb (g/L) | 121.00 ± 13.52 | 125.55 ± 17.90 | 0.42 |
| HCT | 0.36 ± 0.04 | 0.37 ± 0.05 | 0.41 |
| PLT (×109/L) | 202.33 ± 42.50 | 212.20 ± 61.08 | 0.58 |
Values in Bold indicates p value < 0.05, which is considered as significance difference.
BMI body mass index; Hb hemoglobin; HCT Hematocrit; PLT platelet count; PT prothrombin time; APTT activated partial thromboplastin time; TT thrombin time; Fib fibrinogen; INR international normalized ratio.
* By independent sample t test or Chi-square test.
Univariate Analysis
The preoperative factors of the subjects are listed in Table 1. The results showed that although the APTT (SSEH: 36.41 ± 10.70 vs control: 32.63 ± 4.02, p = 0.028) and INR (SSEH: 1.03 ± 0.07 vs control: 0.98 ± 0.05, p = 0.009) were significantly higher in the SSEH group, they were all in the normal range. The ratio of patients who previously received spine surgery was significantly higher in the SSEH group (31.81%) than in the control group (SSEH: 31.81% vs control: 7.54%, p = 0.012).
The univariate analysis results for intraoperative factors between the 2 groups are shown in Table 2. The results showed that the proportion of cerebrospinal fluid leakage was significantly higher in the SSEH group (SSEH: 45.45% vs control: 11.32%, p = 0.004). Other intraoperative factors showed no significant difference between the 2 groups.
Table 2.
Intraoperative Factors.
| SSEH group (n = 22) | Control group (n = 53) | p value* | |
|---|---|---|---|
| Estimated Blood loss (ml) | 728.5 ± 762.74 | 605.00 ± 950.57 | 0.61 |
| Duration of surgery (min) | 168.70 ± 94.81 | 134.50 ± 72.08 | 0.11 |
| Duration of Anesthesia (min) | 230.70 ± 115.14 | 197.07 ± 79.72 | 0.16 |
| Crystalloids (ml) | 1625.00 ± 579.59 | 1599.04 ± 708.31 | 0.88 |
| Colloidal solution (ml) | 642.11 ± 325.43 | 538.46 ± 576.04 | 0.46 |
| Packed Red Blood Cell (units) | 1.00 ± 1.48 | 0.64 ± 2.03 | 0.46 |
| Plasma (ml) | 105.26 ± 224.78 | 42.30 ± 155.10 | 0.19 |
| Autotransfusion (ml) | 249.78 ± 241.46 | 244.32 ± 447.31 | 0.96 |
| Cerebrospinal fluid leakage (%) | 10 (45.45%) | 6 (11.32%) | 0.004 |
| Number of Drainage tube (number) | 1.05 ± 0.21 | 1.07 ± 0.26 | 0.64 |
| Gelfoam dural coverage (%) | 7 (31.82%) | 9 (16.98%) | 0.215 |
Values in Bold indicates p value < 0.05, which is considered as significance difference.
* By independent sample t test or Chi-square test.
Table 3 provides an overview of the blood pressure-related factors. The results showed that blood pressures at different time points were not significantly different between the 2 groups. Additionally, the increases in the SBP and DBP after extubation as well as the intraoperative SBPV and DBPV were also similar in the 2 groups.
Table 3.
Blood Pressure Related Factors.
| SSEH group (n = 22) | Control group (n = 53) | p Value* | |
|---|---|---|---|
| SBP at admission (mmhg) | 130.45 ± 14.73 | 129.37 ± 14.81 | 0.78 |
| DBP at admission (mmhg) | 79.54 ± 7.00 | 79.47 ± 9.13 | 0.97 |
| Intraoperative SBP (mmhg) | 123.57 ± 12.11 | 121.73 ± 11.20 | 0.54 |
| Intraoperative DBP (mmhg) | 76.76 ± 9.63 | 78.81 ± 8.34 | 0.37 |
| SBP after extubation (mmhg) | 145.81 ± 19.64 | 139.25 ± 13.95 | 0.11 |
| DBP after extubation (mmhg) | 89.71 ± 11.15 | 90.15 ± 8.02 | 0.85 |
| SBP at anesthesia recovery room (mmhg) | 139.95 ± 21.03 | 134.16 ± 14.55 | 0.17 |
| DBP at anesthesia recovery room (mmhg) | 84.37 ± 11.63 | 80.92 ± 8.87 | 0.21 |
| Postoperative SBP (mmhg) | 134.30 ± 15.08 | 128.11 ± 10.56 | 0.055 |
| Postoperative DBP (mmhg) | 79.20 ± 15.78 | 74.54 ± 7.38 | 0.09 |
| Increase of SBP after extubation (mmhg) | 23.33 ± 12.68 | 19.34 ± 6.75 | 0.08 |
| Increase of DBP after extubation (mmhg) | 13.86 ± 12.33 | 11.25 ± 7.96 | 0.27 |
| Intraoperative SBPV (mmhg) | 14.23 ± 2.24 | 12.95 ± 2.89 | 0.07 |
| Intraoperative DBPV (mmhg) | 9.67 ± 3.62 | 8.40 ± 2.98 | 0.12 |
Values in Bold indicates p value < 0.05, which is considered as significance difference.
SBP systolic blood pressure; DBP diastolic blood pressure.
* By independent sample t test.
In terms of the radiographic parameters, there were significant differences in TK (SSEH 25.32 ± 9.94° vs control 15.79 ± 7.26°, p<0.01), the local kyphosis angle (SSEH 14.12 ± 6.61° vs control 6.54 ± 4.96°, p<0.01), the EFRT7 (SSEH 30.84 ± 6.34% vs control 24.71 ± 8.48%, p = 0.003), the occupying ratio of the cross-sectional area (SSEH 61.92 ± 14.86% vs control 44.41 ± 14.30%, p<0.01) and the SEVP grade (p<0.01) between the 2 groups. Lumbar lordosis (p = 0.24), EFRmax (p = 0.89), and the occupying ratio of the anterior-posterior diameter on axial (p = 0.099) and sagittal views (p = 0.40) showed no significant difference between the 2 groups (Table 4).
Table 4.
Radiographic Parameters.
| SSEH group (n = 22) | Control group (n = 53) | p Value* | |
|---|---|---|---|
| Thoracic Kyphosis (°) | 25.32 ± 9.94 | 15.79 ± 7.26 | <0.05 |
| Local kyphosis angle (°) | 14.12 ± 6.61 | 6.54 ± 4.96 | <0.05 |
| Lumbar Lordosis (°) | -39.00 ± 12.03 | -36.31 ± 7.27 | 0.24 |
| EFRmax (%) | 34.46 ± 7.21 | 34.89 ± 10.62 | 0.86 |
| EFRT7 (%) | 30.84 ± 6.34 | 24.71 ± 8.48 | 0.003 |
| Occupying ratio of anterior-posterior diameter at axial view (%) | 60.23 ± 17.44 | 53.16 ± 16.31 | 0.099 |
| Occupying ratio of anterior-posterior diameter at sagittal view (%) | 62.54 ± 18.10 | 59.52 ± 12.05 | 0.40 |
| Occupying ratio of cross-sectional area (%) | 61.92 ± 14.86 | 44.41 ± 14.30 | <0.05 |
| SEVP | |||
| Grade 0 (%) | 5 (22.73%) | 33 (62.26%) | <0.05 |
| Grade I (%) | 8 (36.36%) | 20 (37.74%) | |
| Grade II (%) | 9 (40.91%) | 0 (0) |
Values in Bold indicates p value < 0.05, which is considered as significance difference.
EFR epidural fat ratio; SEVP spinal epidural vein plexus.
* By independent sample t test or Chi-square test.
Multiple Logistic Regression Analysis (Table 5)
Table 5.
Multiple Logistic Regression Analysis.
| Adjusted OR | 95% CI | p Value | |
|---|---|---|---|
| Local kyphosis angle | 11.99 | 2.56-56.01 | 0.002 |
| occupying rate of cross-sectional area | 7.24 | 1.86-28.87 | 0.005 |
| cerebrospinal fluid leakage | 9.60 | 1.98-46.46 | 0.005 |
OR odds ratio; CI confidence interval.
Multiple logistic regression analysis was used to recognize the factors that were significantly related to SSEH and to adjust for potential confounding factors. Based on the results of the univariate analysis, factors with a p value less than 0.05 and clinical importance were selected for the multiple logistic regression. Multiple logistic regression analysis identified a large local kyphosis angle (adjusted odds ratio: 11.99, 95% confidence interval: 2.56-56.01, p = 0.002), a high occupying ratio of the cross-sectional area (adjusted odds ratio: 7.24, 95% confidence interval: 1.86-28.87, p = 0.005) and cerebrospinal fluid leakage (adjusted odds ratio: 9.60, 95% confidence interval: 1.98-46.46, p = 0.005) as independent risk factors for SSEH.
Discussion
In the current study, based on the information collected, we revealed that the prevalence of SSEH in TSS after surgery was 1.49%, and the local kyphosis angle, the occupying ratio of the cross-sectional area and cerebrospinal fluid leakage were significant risk factors for SSEH. Other risk factors, such as advanced age (>60 years old), BMI, nonsteroidal drug use, high international standardized ratios (>1.2) and large increases in blood pressure after extubation (>50 mmHg), which have been considered predisposing factors for SSEH in previous studies, did not show any significant difference in our patients.2,5,7-9,15 Although the reason for the contradiction is unclear, different regions and surgical approaches are thought to explain this phenomenon. It is clear that the etiology of SSEH after single-level decompression differs from that of multilevel decompression.7,16,17 In addition, different spine regions have their own characteristics. Accordingly, we restricted the region of patients to the thoracic spine, and each SSEH patient was matched with 2 or 3 control patients who underwent the same procedures. To our knowledge, this is the first study to determine the incidence of and risk factors for SSEH in a cohort of patients undergoing posterior thoracic surgery in isolation.
In the current study, a large local kyphosis angle (>8.77°, adjusted odds ratio: 11.99, 95% confidence interval: 2.56-56.01) was considered a possible cause of SSEH. Nobuyuki Fujita demonstrated that in lumbar spinal canal stenosis, lumbar hypolordosis (LL less than 25°) was a significant risk factor for SSEH. 18 Similarly, a hypothesis has been proposed in the current study that when compared with patients in the control group, those with a large local kyphosis angle have more kyphotic alignment, creating a narrower space between the paravertebral muscles and spinal cord, especially in muscles with edema after surgery. 19 This is also the reason why SSEH occurs more frequently in the thoracic spine than in the cervical and lumbar spine.2,3,5 In multiple logistic regression, thoracic kyphosis failed to show a significant difference. We assumed that in our patients, the diseased segments in some cases were above T4, yet thoracic kyphosis was measured between T4 and T12. However, in clinical practice, the local kyphosis angle is an uncontrolled factor, and before surgery, surgeons need to inform these patients of their high risk of developing SSEH postoperatively.
Previous studies have reported that rupture of a pre-existing abnormal SEVP is the major bleeding source for epidural hematoma formation.17,20 Anatomically, the SEVP is a valveless network that, when compressed to any degree, very likely leads to dilatation.21,22 Dilated epidural veins bleed easily, and bleeding is difficult to stop when it starts owing to the thin vessel wall. Park et al visualized the increased incidence of epidural venous engorgement in patients with prominent epidural fat. 13 Likewise, another study revealed that SEVP dilatation was related to lumbar spinal stenosis.23,24 In the current study, the EFR and occupying ratio were calculated to evaluate the epidural fat and ossified ligaments. A high occupying ratio of the cross-sectional area (>49.58%, adjusted odds ratio: 7.24, 95% confidence interval: 1.86-28.87) was found in the SSEH group, indicating that severe compression by ossified ligaments occurred on the SEVP, leading to poor elasticity of the vessel wall and impedance of blood flow. As a result, dilatation of the SEVP was more common in the SSEH group (77.3%), contributing to the development of hematoma. Thus, for patients with a high occupying ratio (>49.58%), careful operation and control of bleeding on the SEVP are extremely important intraoperatively.
Several studies have also discussed the relationship between dural tears and SSEH.2,10,17 Only one study revealed a positive relationship in lumbar decompression and speculated that injured local vessels and a reduced use of drainage tubes following dural tears were the mechanisms. Our findings in the present study revealed that cerebrospinal fluid leakage (adjusted odds ratio: 9.6, 95% confidence interval: 1.98-46.46) greatly increased the incidence of SSEH. Anatomically, CSF provides internal pressure for the dura and its own contents, and a small hematoma does not easily induce symptoms. 24 Therefore, the incidence of asymptomatic SSEH is high, but symptomatic SSEH is very rare. It is the author’s hypothesis that if cerebrospinal fluid leakage occurs intraoperatively, internal pressure loss, even in a small hematoma, can cause compression. Previous studies have only reported the incidence of durotomy but have not described cerebrospinal fluid leakage and different regions, which may lead to inconsistency with our study. Therefore, if cerebrospinal fluid leakage occurs intraoperatively, surgeons should be conscious of not only intraoperative nerve injury and postoperative headache but also the increased risk of hematoma. Furthermore, our study revealed that the spinal surgery ratio, which has been reported as a risk factor, was higher in the SSEH group, but the difference was not significant in multiple logistic regression analysis. We believe that the elevated incidence of cerebrospinal fluid leakage in revision operations due to the formation of scar tissue and adhesions is the true cause in such patients.
This study had some limitations. First, due to its retrospective nature, bias, data availability and confounders were difficult to control. Second, due to the low incidence of SSEH, the sample size was relatively small in the current study. Multicenter studies are needed to verify the results of the current study and fully investigate other potential factors. Even with this, because surgical region was restricted to the thoracic region, we were able to identify some significant risk factors for SSEH.
Conclusion
In conclusion, our study revealed an incidence of SSEH of 1.49% as well as 3 significant risk factors following posterior thoracic spinal surgeries. Consequently, patients with such characteristics need to be carefully observed, and once neurological deficits develop after surgery, spine surgeons should make a timely diagnosis of SSEH.
Footnotes
Author Contributions: Weishi Li contributed to the study conception and design. Data collection and analysis were performed by Longjie Wang and Hui Wang. The surgeries were performed by the Weishi Li, Zhongqiang Chen, Chuiguo Sun and Zhuoran Sun. The article was written by Longjie Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Availability of Data: The data used and analyzed during the current study was available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by Peking University Third hospital ethics committee (IRB00006761). For this type of study formal consent is not required. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Longjie Wang, MD  https://orcid.org/0000-0002-8179-0715
https://orcid.org/0000-0002-8179-0715
Weishi Li, MD  https://orcid.org/0000-0001-9512-5436
https://orcid.org/0000-0001-9512-5436
| Symptomatic spinal epidural hematoma | SSEH |
| Thoracic spinal stenosis | TSS |
| Prothrombin time | PT |
| Activated partial thromboplastin time | APTT |
| Thrombin time | TT |
| International Normalized ratio | INR |
| Blood pressure variability | BPV |
| Thoracic kyphosis | TK |
| Lumbar lordosis | LL |
| spinal epidural venous plexus | SEVP |
| cross-sectional area | CSA |
| Epidural fat | EF |
| Epidural fat ratio | EFR |
| Dural sac | DS |
References
- 1.Leonardi MA, Zanetti M, Saupe N, Min K. Early postoperative MRI in detecting hematoma and dural compression after lumbar spinal decompression: prospective study of asymptomatic patients in comparison to patients requiring surgical revision. Eur Spine J. 2010;19(12):2216–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri AR, Fouyas IP, Cro S, Casey AT. Postoperative spinal epidural hematoma (SEH): incidence, risk factors, onset, and management. Spine J. 2013;13(2):134–140. [DOI] [PubMed] [Google Scholar]
- 3.Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79(6):1165–1177. [DOI] [PubMed] [Google Scholar]
- 4.Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77(7):1042–1049. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Abe Y, Satoh S, Yanagibashi Y, Hyakumachi T, Masuda T. Large increase in blood pressure after extubation and high body mass index elevate the risk of spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2015;40(13):1046–1052. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara Y, Manabe H, Izumi B, et al. The impact of hypertension on the occurrence of postoperative spinal epidural hematoma following single level microscopic posterior lumbar decompression surgery in a single institute. Eur Spine J. 2017;26(10):2606–2615. [DOI] [PubMed] [Google Scholar]
- 7.Kao FC, Tsai TT, Chen LH, et al. Symptomatic epidural hematoma after lumbar decompression surgery. Eur Spine J. 2015;24(2):348–357. [DOI] [PubMed] [Google Scholar]
- 8.Bovonratwet P, Fu MC, Tyagi V, et al. Incidence, risk factors, and clinical implications of postoperative hematoma requiring reoperation following anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2019;44(8):543–549. [DOI] [PubMed] [Google Scholar]
- 9.Liu JM, Deng HL, Zhou Y, et al. Incidence and risk factors for symptomatic spinal epidural haematoma following lumbar spinal surgery. Int Orthop. 2017;41(11):2297–2302. [DOI] [PubMed] [Google Scholar]
- 10.Sokolowski MJ, Garvey TA, Perl J, 2nd, et al. Prospective study of postoperative lumbar epidural hematoma: incidence and risk factors. Spine (Phila Pa 1976). 2008;33(1):108–113. [DOI] [PubMed] [Google Scholar]
- 11.Nakashima H, Tetreault L, Kato S, et al. Prediction of outcome following surgical treatment of cervical myelopathy based on features of ossification of the posterior longitudinal ligament: a systematic review. JBJS Rev. 2017;5(2):01874474–201702000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Li B, Yu Y, Li W, Qiu G, Zhao Y. The Relationship between dural ossification and spinal stenosis in thoracic ossification of the ligamentum flavum. J Bone Joint Surg Am. 2019;101(7):606–612. [DOI] [PubMed] [Google Scholar]
- 13.Park SK, Lee IS, Song YS, Moon JI, Song JW, Kang H. Dilatation of the spinal epidural venous plexus in patients with prominent epidural fat. Br J Radiol. 2016;89(1063):20160064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abul-Kasim K, Schlenzka D, Selariu E, Ohlin A. Spinal epidural lipomatosis: a common imaging feature in Scheuermann disease. J Spinal Disord Tech. 2012;25(7):356–361. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein CL, Bains I, Hurlbert RJ. Symptomatic spinal epidural hematoma after posterior cervical surgery: incidence and risk factors. Spine J. 2015;15(6):1179–1187. [DOI] [PubMed] [Google Scholar]
- 16.Awad JN, Kebaish KM, Donigan J, Cohen DB, Kostuik JP. Analysis of the risk factors for the development of post-operative spinal epidural haematoma. J Bone Joint Surg Br. 2005;87(9):1248–1252. [DOI] [PubMed] [Google Scholar]
- 17.Kou J, Fischgrund J, Biddinger A, Herkowitz H. Risk factors for spinal epidural hematoma after spinal surgery. Spine (Phila Pa 1976). 2002;27(15):1670–1673. [DOI] [PubMed] [Google Scholar]
- 18.Fujita N, Michikawa T, Yagi M, et al. Impact of lumbar hypolordosis on the incidence of symptomatic postoperative spinal epidural hematoma after decompression surgery for lumbar spinal canal stenosis. Eur Spine J. 2019;28(1):87–93. [DOI] [PubMed] [Google Scholar]
- 19.Aono H, Ohwada T, Hosono N, et al. Incidence of postoperative symptomatic epidural hematoma in spinal decompression surgery. J Neurosurg Spine. 2011;15(2):202–205. [DOI] [PubMed] [Google Scholar]
- 20.Groen RJ, Ponssen H.The spontaneous spinal epidural hematoma. A study of the etiology. J Neurol Sci. 1990;98(2-3):121–138. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa M, Sato S, Numaguchi Y, Mihara F, Rothman MI. Spinal epidural venous plexus: its MR enhancement patterns and their clinical significance. Radiat Med. 1996;14(5):221–227. [PubMed] [Google Scholar]
- 22.Paksoy Y, Gormus N. Epidural venous plexus enlargements presenting with radiculopathy and back pain in patients with inferior vena cava obstruction or occlusion. Spine (Phila Pa 1976). 2004;29(21):2419–2424. [DOI] [PubMed] [Google Scholar]
- 23.Slin’ko EI, Al Q, II. Surgical treatment of lumbar epidural varices. J Neurosurg Spine. 2006;5(5):414–423. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser MC, Capesius P, Roilgen A, Sandt G, Poos D, Gratia G.Epidural venous stasis in spinal stenosis. CT appearance. Neuroradiology. 1984;26(6):435–438. [DOI] [PubMed] [Google Scholar]