Abstract
Study Design:
A retrospective case-controlled study.
Objectives:
To evaluate overall infection rate and adverse event after harvesting bone graft soaking and surgical wound irrigation by povidone iodine solution (PVI) in the minimally invasive instrumented spinal fusion surgery. In order to reduce the rate of surgical site infection in spinal surgery, surgical wound irrigation by povidone iodine solution has been well-established. However, the efficacy of autologous bone graft soaking by PVI has not been evaluated before.
Methods:
This is a retrospective cohort study. 120 patients were enrolled in the PVI group and compared with 124 patients in the historical cohort. In the PVI group, the harvesting autologous bone graft was soaking and the surgical wound was also irrigated by diluted PVI solution. The outcome measures were overall infection rate, superficial wound infection and deep infection. In addition, the delayed union of the fusion mass was also evaluated through the radiograph evaluation.
Results:
Both groups shared similar patient demographics instead of body mass index. The use of PVI solution had decreased the overall infection rate (0% versus 4.03%, p = 0.026) and deep infection rate (0% versus 3.23%, p = 0.047). In addition, there was no delayed bone healing in the PVI group after autologous bone graft soaking.
Conclusions:
In this study, we conclude that harvested autologous bone graft after PVI soaking in spinal fusion surgery can decrease the incidence of deep infection.
Keywords: povidone, lumbar interbody fusion, infection
Introduction
Surgical site infection (SSI) is a common postoperative complication in spinal surgeries, leading to both poor outcome and increased medical cost. The incidence of SSI in spinal surgery was reported diversely, ranging from 1% to 14% in open procedure and 0.6% to 4.6% in minimal invasive spine fusion procedure respectively.1-6 Specific risk factors were identified, increased blood loss, diabetes, obesity, prolonged operation time, posterior spinal approach, instrumentation and fusion have all been reported as risk factors in previous literatures.1-4
In order to reduce rate of SSI in spinal surgery, intrawound prophylaxis with antibiotics or antiseptics were proposed, including intrawound vancomycin powder and povidone-iodine (PVI) solution irrigation,7-10 result in reduced SSI rates without reported adverse events.11,12 Povidone-iodine is well known as a low-cost but effective antiseptic agent and has been widely used in wound disinfection, preoperative skin preparation for decades. Its broad antimicrobial spectrum, including methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecalis (VRE), had been reported in previous literature.13,14
In consideration of intraoperative contamination, both implants and autologous bone graft have been identified as potential sources of postoperative infection during spine fusion.15,16 A 328 cases retrospective study conducted by Lee at al. 16 revealed the 4.3% microbiological contamination rate of local autologous graft in posterior lumbar interbody fusion (PLIF) procedure, which account for 62.5% of all infection cases. However, although intraoperative autologous bone graft contamination was suspected to be a source of SSI, the efficacy of PVI soaking of autologous graft has not been evaluated.
In this study, a new protocol combining autologous bone graft soaking and surgical wound irrigation with PVI solution was performed. We aim to evaluate the efficacy and safety of disinfecting autologous graft with PVI in preventing surgical site infection in minimally invasive instrumented spine fusion procedure.
Material and Method
Study Design and Patient Selection
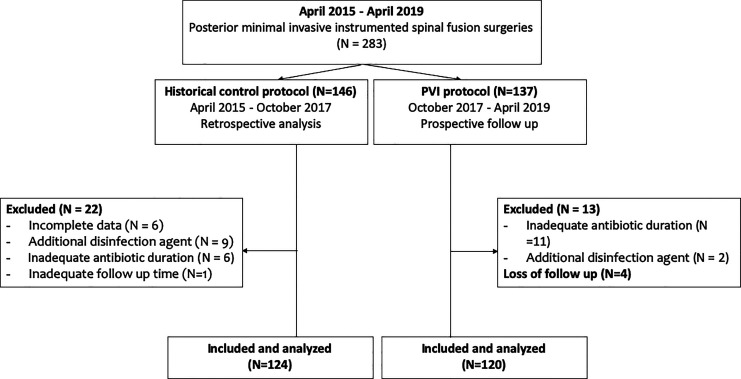
This retrospective cohort study was conducted by orthopedic department in the single center. The patient with persisted mechanical back pain or spinal stenosis symptoms without improvement after 3-month conservative treatment, were indicated for the surgery. The diagnosis consisted of degenerative spondylolisthesis, isthmic spondylolisthesis, pathological spondylolisthesis, traumatic spondylolisthesis, spinal stenosis and revision surgery. The PVI disinfection protocol, combining autologous bone graft povidone-iodine (PVI) solutions soaking and intrawound PVI irrigation was started on October, 2017. Patients underwent posterior minimal invasive spinal fusion surgeries under this new disinfection protocol were enrolled as the PVI cohort. Patients underwent spinal debridement surgery due to infection or with history of betadine allergy were excluded. Patient should keep postoperative follow up at outpatient department for at least 90 days, and those with inadequate follow up time would be excluded unless the SSI event occurred in 90 days. If patients receiving antibiotic or disinfection intervention other than standard disinfection protocol (as described in “surgical procedure and standard disinfection methods”), or received parenteral postoperative antibiotic for less than 48 hours, the patient would also be excluded. Until April, 2019, a total of 137 patients received spinal fusion surgeries under this new PVI disinfection protocol, while 13 patients were excluded (11 patients with inadequate postoperative antibiotics duration, 2 patient received additional antibiotics or disinfection intervention). During the 3-month follow up interval, there were 4 patients lost to follow-up. (Figure 1).
Figure 1.
Consort diagram.
The outcomes of the above PVI cohort was compared with historical control cohort, which applied the original disinfection protocol with intrawound normal saline irrigation in posterior minimal invasive spinal fusion procedure during April, 2015 to October, 2017. Through the electronic patient record system, 146 patients were retrospectively reviewed while a total of 124 illegible patients were finally included and analyzed (6 patients with incomplete data, 9 patients with inadequate postoperative antibiotics duration, 6 patients received addition antibiotics or disinfection intervention and 1 patient loss follow up) (Figure 1).
Surgical Procedure and Standard Disinfection Methods
All procedures were performed by 2 senior surgeons who participated in our study in standard operation rooms, without routine ultraviolet light, laminar flow, or other special air measures. Before surgical incision, aseptic technique was performed as usual, including skin preparing with PVI alcoholic solution, sterile adhesive drapes, sterile cloths and gloves. The PVI solution we used in this study were diluted from the commercially available Betadine solution with concentration of 10% PVI (0.1 g of PVI per 1 mL of Betadine solution). During preoperative preparation, 36 mL of Betadine was diluted with 1000 ml normal saline to achieve approximate 0.35% PVI solution 1036 mL and keep in the sterilized basin, covered by sterilized drapes. If extend wound size was expected, same concentration PVI solution would be prepared up to 2072 ml.
Under general anesthesia, patient was placed in prone position on the 4-poster frame. Intraoperative 3-dimensional C-arm (Vision FD Vario 3D, Ziehm Imaging, Nuremberg, Germany) and navigation system (Kick navigation system, Brainlab) were used for percutaneous pedicle screw insertions.
Intraoperative scanning was performed in both groups with CT data set automatically registered to the image guidance system. Before the skin incision was made, the spinal process was used as an anatomical landmark by navigation probe to confirm the accuracy of navigation. The ideal skin entry points were sought with the navigation probe tip in order to determine the precise skin incision based on the virtual elongated navigation trajectory on the intraoperative scanned images. Pedicle entry points were decided on navigation guidance, and pilot holes were made with a 3-mm awl. The direction of the pilot pedicle hole was rechecked with the navigation probe, and 4.5-mm tapping was done to a depth of 20 mm from the entry point. A guidewire was used for screw insertion. Rod was then placed and fixed with nuts after rechecking the position.
After microscopic decompression and preparation for transforaminal interbody fusion, the harvested autologous bone graft was collected and irrigated with 500 ml normal saline in original disinfection protocol then mixed with artificial bone material if needed. In PVI disinfection protocol, the harvested bone graft was collected and soaked in 100 ml PVI solution for 10 seconds then rinsed with 500 ml normal saline.
Transforminal lumbar interbody fusion was then performed with cage and autologous bone graft. All ischemic tissue was debrided before wound closure. The wound was then irrigated with 500 ml normal saline in original protocol while 500 ml PVI solution was irrigated before wound closure in PVI protocol. The irrigation volume can be titrated up to 1000 ml in extended wound. The wound was closed by layers and the skin was stapled and properly dressed with gauzes as usual. Postoperative cares were identical in both groups. We did not routinely insert drainage tube for postoperative wound drainage, if inserted, the tube would not be removed until daily amount of drainage was less than 100 ml.
Standard disinfection protocols applied in both groups were identical. Routine prophylaxis antibiotics were given. Single dose of parenteral cefazolin (1000 mg) were given 1 hour before surgery, and additional redosing of cefazolin would be given in prolonged surgery (more than 4 hours) to maintain antibiotics levels. Cefazolin 1000 mg was administered every 12 hours and lasted for at least 48 hours after surgery. Second antibiotic agent including aminoglycosides (gentamicin 80 mg every 12 hours, amikacin 500 mg every 12 hours or isepamicin 400 mg once daily), clindamycin (600 mg every 8 hours) or erythromycin (250 mg every 12 hours) was also administered as surgeon’s preference. The patient was kept bed rest with brace during the first 24 hours after surgery.
Postoperative Infection and Clinical Outcome
The postoperative outpatient department follow up occurred at 2 weeks, 1 month, 2 months and 3 months after surgery. The wound condition and symptoms of SSI were record. Lumbar spine x-ray with anterior-posterior view and lateral view were performed at every visit to observe if delay callus formation or instrument malposition occurred. The fusion status of the operated level would be accessed based on Bridwell grading system 17 during the last follow up. Visual analog scale (VAS) was accessed at the end of postoperative follow up.
When there were complains of symptoms and signs of suspected surgical site infection including unusual pain, induration, fever or wound discharge, C-reactive protein (CRP) level and white blood cell count with differential count would be checked. Pus-like discharge, if noted, would be collected and cultured. Advanced diagnosed tools including lumbar spine computed tomography (CT), magnetic resonance imaging (MRI) or CT-guide biopsy would be performed if needed. Once the SSI was highly suspected or diagnosed, the patient would be readmitted for intravenous empirical antibiotic therapy firstly then switched once the culture and antibiotic susceptibility test (AST) were confirmed. If the symptoms and signs of infection were not improved after antibiotic treatment after 1 week, further surgical debridement would be performed through the previous incision. Aerobic, anaerobic, fungal, and tubercular cultures would be collected if pus or discharge was noted during the debridement. After adequate debridement and normal saline irrigation, the wound was heavily sutured.
The classification and definition of superficial and deep surgical site infection was based on the Centers for Disease Control and Prevention (CDC) criteria for Surgical Site Infection (SSI), while both deep SSI and organ space SSI were classified as deep SSI in our study.
Data Extraction
All data was extracted from the electronic patient record system of our hospital by the assistant who did not involve the operation and patient care. Patient’s characteristic including age, gender, body mass index (BMI), underlying disease with diabetes mellitus (DM), indication of operation, preoperative visual analog scale (VAS), preoperative American society of anesthesiologists physical status (ASA) score and habits of smoking were recorded individually. The surgical outcomes including length of operative times, volume of intraoperative blood loss, use of artificial bone material, numbers of fusion levels, duration of postoperative antibiotic use and length of hospital stay were also recorded.
The primary endpoint of our study was comparing the rate of overall surgical site infection (SSI), superficial SSI, and deep SSI between 2 cohorts. The secondary endpoint of our study was rate of PVI related adverse effect, improvement of visual analog scale (VAS), rate of delay bone callus formation, and rate of instrument malposition or complication.
Statistical Analysis
Data was analyzed by SAS® 9.4 software and represented as the mean and standard deviation for continuous variables or percentages for discrete variables. The Fisher exact tests were used to compare difference between the 2 groups for each discrete variable, and the Student t test was used for each continuous variable. Univariate Logistic regression was used to evaluate the chosen individual factors 11 which may affect the outcome of SSI. If there were 2 or more factors with significant results (p-value < 0.05), these factors would be further analyzed in multivariate Logistic regression model to evaluate if the protective efficacy of intrawound intervention would be diminished. If there was only 1 or less factor with significant result, multivariate Logistic regression model would not be performed. The 95% confidence intervals (CI) and odds ratios of each variable were calculated. All tests were 2-tailed. Significance was set at P = 0.05 or less than 0.05 for each test.
Result
Characteristic of Patients
The characteristic of patients in both groups were compared in Table 1. The mean age of the patients was 67.75 years (range 27-91 years, SD = 12.20 years) included 76 (61.3%) patients elder than 65 years old in control cohort and 67.14 years (range 21-98 years, SD = 12.01 years) with 72 (60.0%) patients elder than 65 years old in PVI cohort. The mean BMI of the patients was 26.1 kg/m2 (SD = 4.27kg/m2) in control cohort and 27.1 kg/m2 (SD = 4.85 kg/m2) in PVI cohort respectively. In control cohort, 31 patients (25.0%) had underlying diabetes mellitus and 13 (10.5%) patients were smokers, while 36 patients (30.0%) with diabetes mellitus and 15 patients (12.5%) were smoker in PVI cohort. There were no significant differences in age, gender, BMI, ASA scores and underlying medical condition (diabetes mellitus and smoking) between 2 groups. The initial diagnosis was divided into 6 subgroups, consisted of degenerative spondylolisthesis, isthmic spondylolisthesis, pathological spondylolisthesis, traumatic spondylolisthesis, spinal stenosis and revision surgery. There was no difference of the proportion of diagnosis subgroups between 2 cohorts (Table 1).
Table 1.
Patient Demographic Data.
| Control cohort (n = 124) | PVI cohort (n = 120) | P-value | |
|---|---|---|---|
| Gender (Male) | 36 (29.03%) | 44 (36.67%) | 0.204 |
| Age (year) | 67.75 (SD = 12.20) | 67.14 (SD = 12.01) | 0.695 |
| ASA score | 0.354 | ||
| 1 | 9 (7.26%) | 4 (3.33%) | |
| 2 | 80 (64.52%) | 77 (64.17%) | |
| 3 | 34 (27.42%) | 39 (32.50%) | |
| 4 | 1 (0.81%) | 0 (0.00%) | |
| Diabetes mellitus | 31 (25.00%) | 36 (30.00%) | 0.382 |
| Habits of smoking | 13 (10.48%) | 15 (12.50%) | 0.621 |
| Body mass index | 26.07 (SD = 4.27) | 27.05 (SD = 4.85) | 0.095 |
| Fusion Levels | 0.236 | ||
| 1 | 68 (54.84%) | 76 (63.33%) | |
| 2 | 45 (36.29%) | 40 (33.33%) | |
| 3 | 10 (8.06%) | 4 (3.33%) | |
| 4 | 1 (0.81%) | 0 (0.00%) | |
| Diagnosis | |||
| Control cohort (n = 124) | PVI cohort (n = 120) | P-value | |
| Degenerative spondylolisthesis | |||
| 63 (50.81%) | 55 (45.83%) | 0.437 | |
| Traumatic spondylolisthesis | |||
| 6 (4.84%) | 2 (1.67%) | 0.164 | |
| Spinal stenosis | |||
| 36 (29.03%) | 49 (40.83%) | 0.053 | |
| Pathological spondylolisthesis | |||
| 1 (0.81%) | 1 (0.83%) | 0.981 | |
| Isthmic spondylolisthesis | |||
| 17 (13.71%) | 8 (6.67%) | 0.070 | |
| Revision of previous operation | |||
| 1 (0.81%) | 5 (4.17%) | 0.090 | |
| Surgical outcomes | |||
| Control cohort (n = 124) | PVI cohort (n = 120) | P-value | |
| Blood loss (ml) | 377.9 (SD = 201.9) | 373.3 (SD = 244.8) | 0.873 |
| Operation time (hour) | 4.27 (SD = 1.27) | 4.09 (SD = 1.12) | 0.224 |
| Length of hospitalization (day) | 8.40 (SD = 6.63) | 7.02 (SD = 4.30) | 0.055 |
| Drainage tube insertion | 15 (12.10%) | 9 (7.50%) | 0.228 |
| Artificial bone material use | 58 (46.77%) | 70 (58.33%) | 0.071 |
| Postoperative antibiotics duration (day) | 3.18 (SD = 1.13) | 3.42 (SD = 1.45) | 0.154 |
Abbreviation: ASA = American society of anesthesiologists physical status classification system, BMI = body mass index, DM = diabetes mellitus.
Disinfection Efficacy and Primary Endpoint
In terms of postoperative surgical site infection (SSI), there were totally 5 patients diagnosed with SSI (4.03%) in control cohort, consisted of 1 superficial infections (0.81%) and 4 deep infections (overall rate = 3.23%, including 2 deep incision SSI and 2 organ space SSI). The cases of SSI were listed (Table 2). Nevertheless, there was no infection incident in PVI cohort. Both overall infection rate (0% versus 4.03%, p = 0.026) and deep infection rate (0% versus 3.23%, p = 0.047) were significant lower under the intervention of PVI use while no similar significant disinfection efficacy was seen in superficial infection (0% versus 0.81%, p = 0.324) (Table 3).
Table 2.
Infection Cases of Control Cohort (N = 5).
| Patient | Indication | Date | SSI diagnosis criterion | Treatment |
|---|---|---|---|---|
| 59 year-old Female | L4-5 Degenerative spondylolisthesis | POD 14 | Superficial SSI (Criterion d) |
Parenteral antibiotics Teicoplanin (16 days) Daptomycin (13 days) |
| 60 year-old Female | L4-5 Degenerative spondylolisthesis | POD 46 | Organ space SSI (Criterion c) |
Parenteral antibiotics Vancomycin (19 days) |
| 62 year-old Female | L4 Pars fracture with L4-5 isthmic spondylolisthesis. |
POD 12 | Deep incision SSI (Criterion c) |
Parenteral antibiotics Vancomycin (7 days) Fosformycin(7 days) Linezolid (14 days) Wound debridement (POD 19) |
| 84 year-old Male | L2-5 spinal stenosis | POD 81 | Organ space SSI (Criterion c) |
Parenteral antibiotics Teicoplanin (14 days) Ceftazidime (14 days) Debridement and L3, L5 screws revision (POD 89) |
| 58 year-old Female | L4-5 Degenerative spondylolisthesis | POD 4 | Deep incision SSI (Criterion b) |
Parenteral antibiotics Teicoplanin (37 days) Ceftazidime (21 days) |
Abbreviation: POD: Postoperative date.
Table 3.
Postoperative Infection Rate of the 2 Groups.
| Control cohort (N = 124) | PVI cohort (N = 120) | P-value | |
|---|---|---|---|
| Overall Infection | 5 (4.03%) | 0 (0.00%) | 0.026* |
| Superficial wound infection | 1 (0.81%) | 0 (0.00%) | 0.324 |
| Deep wound infection | 4 (3.23%) | 0 (0.00%) | 0.047* |
* Significant results (p-value < 0.05).
Surgical Outcomes and Pain Improvement
There were no significant differences in intraoperative blood loss (p = 0.873), length of hospital stay (p = 0.055), drainage tube insertion rate (p = 0.228), artificial bone material use (p = 0.071), operation time (4.27 hours in control cohort versus 4.09 hours in PVI cohort, p = 0.224), or postoperative antibiotics duration (3.18 days in control cohort versus 3.42 days in PVI cohort, p = 0.154). There was no significant difference in preoperative visual analog scale (VAS) (p value = 0.293), postoperative VAS (p = 0.311), or the range of VAS improvement (p = 0.095). During the last follow up, through the X-ray evaluation by the surgeon, all patients were graded as grade 2 fusion status of Bridwell grading system and there was no event of delay bone callus formation noted in both groups (Table 4).
Table 4.
Postoperative Functional Outcomes and Perioperative Complications.
| Functional outcome | |||
|---|---|---|---|
| Control cohort (n = 124) | PVI cohort (n = 120) | P-value | |
| Preoperative VAS | 6.24 (SD = 1.45) | 6.00 (SD = 2.07) | 0.293 |
| Postoperative VAS | 2.25 (SD = 1.85) | 2.49 (SD = 1.71) | 0.311 |
| VAS Improvement range | 3.99 (SD = 2.14) | 3.51 (SD = 2.27) | 0.095 |
| Complication | |||
| Control cohort (n = 124) | PVI cohort (n = 120) | P-value | |
| Wound complication | 3 (2.42%) Poor healing (3) |
4 (3.33%) Prolonged discharge (3) Poor healing(1) |
0.669 |
| Surgical Complications | 10 (8.06%) Implant malposition, loosening or graft protrusion (4) Incidental durotomy (3) Residual symptom (1) Pseudoarthrosis (1) Iatrogenic fracture (1) |
6 (5.00%) Implant malposition or loosening (3) Incidental durotomy (3) |
0.334 |
Abbreviation: PVI = povidone iodine, VAS = visual analog scale.
Safety and Complication
There was no iodine related adverse event including iodine allergy or thyroid function abnormality reported during the 90-day interval of follow up. There was also no significant difference of wound complication (p = 0.669) or surgical complication (p = 0.334) (Table 4).
Efficacy or Risk Factor Analysis
The univariate Logistic regression was performed to evaluate the potential relation between overall infection events and different variates, revealing only the variate BMI as significant harmful factors (odds ratio = 1.152, p = 0.019) of overall SSI. Multivariate Logistic regression was not performed due to the variate BMI was the only significant harmful factors in univariate Logistic regression. (Table 5).
Table 5.
Univariate Logistic Regression of Overall Infection.
| Variate | Odds ratio | Confidence level | p-value |
|---|---|---|---|
| Age | 0.982 | 0.917-1.051 | 0.593 |
| ASA score = 3 or higher | 0.568 | 0.062-5.175 | 0.616 |
| Gender | 0.506 | 0.056-4.605 | 0.545 |
| DM | 1.785 | 0.292-10.924 | 0.530 |
| BMI | 1.152 | 1.024-1.296 | 0.019* |
| Fusion levels | 0.809 | 0.176-3.711 | 0.785 |
| Operation time | 1.844 | 0.974-3.491 | 0.601 |
| Drainage tube | 2.348 | 0.252-21.902 | 0.454 |
Abbreviation: ASA = American society of anesthesiologists physical status classification system, BMI = body mass index, DM = diabetes mellitus.
*Significant results (p-value < 0.05).
Discussion
In the present study, patients operated with PVI disinfection protocol showed significant decreased both overall and deep postoperative surgical site infection (SSI) rate during the 90 days follow up period comparing with the historical control group. The difference of infection rate was statistically significant in overall SSI rate (P = 0.026) and deep SSI rate (P = 0.047), while no same efficacy was seen in superficial SSI rate (P = 0.324).
The similar disinfection efficacy with same PVI concentration was also mention in previous literature. Cheng et al. 7 reported the disinfectant efficacy of betadine irrigation in spinal surgery in a 414 spinal surgery patients prospective study. The surgical wound irrigated by 3.5% Betadine solution (0.35% PVI solution) before wound closure, resulted in 0% of overall SSI in PVI-irrigated wounds, while still 3.4% of overall SSI in the saline-irrigated wounds. In instrumented spinal surgery, Chang et al. 12 also reported same disinfection efficacy of wound irrigation and soaking with 0.35% povidone iodine solution for 3 minutes. Similar efficacy was also seen in preventing deep periprosthetic joint infection. A retrospective study reviewed disinfection efficacy of dilute Betadine wound lavage (0.35%) for 3 minutes before wound closure in total hip (THA) and knee (TKA) arthroplasty, showing significant reduction in the infection rate (0.15% versus 0.97%, P = 0.04). 18
Previous literatures have identified some common strains of bacteria in spinal surgeries SSI4,19 or intraoperative bone autograft contamination. 16 The bactericidal sensitivity to povidone-iodine in some strains, including Staphylococcus epidermidis, Pseudomonas aeruginosa, Escherichia coli and methicillin sensitive Staphylococcus aureus (MSSA) and methicillin resistant Staphylococcus aureus (MRSA) have been identified.13,14,20 The remained bactericidal effect of PVI on Vancomycin-resistant Enterococcus faecalis (VRE), 14 and multi-resistant Gram-negative bacill have also been revealed on previous literature. 13 The broad spectrum antimicrobial efficacy of povidone-iodine made itself a reasonable agent not only in surgical wound but also bone autograft disinfection, which can be the explanation of the lower SSI rate of PVI protocol in the present study.
The overall infection rate in our control group (4.03%), which was comparable to previous literature. Mattehew et.al reported a 5170-patient retrospective comparative analysis of perioperative surgical site infection between minimally invasive versus open posterior or transforaminal lumbar interbody fusion in 2011, showing SSI rate of 4.5% in single level and 4.6% in 2 level minimally invasive fusion procedure respectively. 6 Another 298-case prospective comparative analysis between MI-TLIF and open TLIF also reported the rate of 4.2% in overall SSI among 144 MI-TLIF cases. 21
According to some in vitro studies, potential harmful effect on bone cell may be caused after irrigation with dilute PVI solution. Newton et al. reported the rapid and detrimental effect of PVI on human osteoblast cellular proliferation, metabolic function, and bone nodule mineralization after 0.35% PVI exposed for 3 minutes. 22 Another in vitro study conducted by van Meurs et al. 23 reported that only diluted PVI remained bactericidal at a cell-viable concentration, comparing with other antiseptics including hydrogen peroxide, and chlorhexidine digluconate. They concluded that 1.3 g/L (0.13%) diluted PVI could be the optimal antiseptic for intraoperative irrigation. In vivo study, however, no similar adverse effect has been reported. The safety of wound irrigation with o.35% PVI in spinal surgery was evaluated by Chang et al. 12 in a 244 patients prospective study, concluding that wound irrigation with diluted PVI solution followed by normal saline irrigation before the bone-grafting procedure exerted no adverse effects on spinal bone fusion, clinical outcome and wound healing, which revealed the same conclusion of the safety of PVI in related literature.7,8,10 The real wound environment contains certain factors including proteins and other organic compounds that may dilute and neutralize the PVI molecule, which may diminish both the cytotoxic and bactericidal effect of PVI, resulted in both higher cytotoxic and bactericidal concentration of PVI when clinically used.
According to another in vitro study, the bactericidal effect of PVI onset immediately on contact, suggesting that PVI exposure time is not the key factor of bactericidal efficacy. 20 In present study, under 0.35% diluted PVI solution, we shorten the PVI exposure time of bone graft soaking to 10 seconds, and the remained disinfection efficacy was revealed. There were no iodine allergies or other betadine related adverse effect noted during the 3-month follow-up. Moreover, based on VAS score improvement, clinical condition, length of hospital stay, reoperation and readmission rate, there was no difference of post-operative outcome between 2 cohorts.
There were several limitations of our study. First, the results of PVI cohort was compared with historical control group instead of concurrent control group. Consequently, the higher SSI rate in historical control group may be inevitably contributed to the learning curve of MIS fusion procedures for surgeons and operative team members. However, we also comparing other surgical outcomes including blood loss volume, surgical complication or length of hospital stay between the 2 groups, but no significant difference was found. Furthermore, all the data of the control group was retrospectively extracted from the electronic patient record system, which may lead to potential bias of data interpretation. Inadequate description of mild infection symptoms or signs of the outpatient department record may also lead to underestimate the actual rate of SSI event.
Besides, the follow up interval was set with only 90 days and the fusion process was accessed through the dynamic view of lumbar spine x-ray instead of lumbar CT. The longer follow up duration may be needed to evaluate the solid bone fusion condition through CT image, in order to monitor the long-term effects of PVI. Last but not least, the procedure in our study was limited to minimal invasive fusion spinal procedures, and further studies were needed to evaluate if the identical efficacy and safety can be seen in other spinal procedure.
To the best of our knowledge, this is the only study in the English language literature evaluating PVI-soaked autogenous bone graft in spine surgery. Our report is the first to propose the protective efficacy and safety of combining PVI soaked autogenous bone graft and PVI intrawound irrigation as a standard disinfectant protocol in spinal fusion procedures.
Conclusion
Harvested bone graft soaking and intrawound irrigation with diluted PVI solution showed significant efficacy in decreasing overall SSI rate and deep SSI rate. No PVI-related adverse effect or delayed bone callus formation was noted during the 90-day follow up duration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Division of Spine Surgery, Orthopaedic Department, Taipei Veterans General Hospital. We thank Hsin-Yi Huang from the Biostatistics Task Force, Taipei Veterans General Hospital, for the statistical assistance.
ORCID iD: Po-Hsin Chou, MD, PhD  https://orcid.org/0000-0001-5899-1124
https://orcid.org/0000-0001-5899-1124
Ming-Chau Chang, MD  https://orcid.org/0000-0002-4799-9339
https://orcid.org/0000-0002-4799-9339
References
- 1.Piper KF, Tomlinson SB, Santangelo G, et al. Risk factors for wound complications following spine surgery. Surg Neurol Int. 2017;8:269. doi:10.4103/sni.sni_306_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radcliff KE, Neusner AD, Millhouse PW, et al. What is new in the diagnosis and prevention of spine surgical site infections. Spine J. 2015;15(2):336–347. doi:10.1016/j.spinee.2014.09.022 [DOI] [PubMed] [Google Scholar]
- 3.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa 1976). 2009;34(13):1422–1428. doi:10.1097/BRS.0b013e3181a03013 [DOI] [PubMed] [Google Scholar]
- 4.Gerometta A, Rodriguez Olaverri JC, Bitan F. Infections in spinal instrumentation. Int Orthop. 2012;36(2):457–464. doi:10.1007/s00264-011-1426-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parker SL, Adogwa O, Witham TF, Aaronson OS, Cheng J, McGirt MJ. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg. 2011;54(1):33–37. doi:10.1055/s-0030-1269904 [DOI] [PubMed] [Google Scholar]
- 6.McGirt MJ, Parker SL, Lerner J, Engelhart L, Knight T, Wang MY. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14(6):771–778. doi:10.3171/2011.1.SPINE10571 [DOI] [PubMed] [Google Scholar]
- 7.Cheng MT, Chang MC, Wang ST, Yu W-K, Liu C-L, Chen T-H. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689–1693. [DOI] [PubMed] [Google Scholar]
- 8.Tomov M, Mitsunaga L, Durbin-Johnson B, Nallur D, Roberto R. Reducing surgical site infection in spinal surgery with betadine irrigation and intrawound vancomycin powder. Spine (Phila Pa 1976). 2015;40(7):491–499. doi:10.1097/BRS.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011;36(24):2084–2088. doi:10.1097/BRS.0b013e3181ff2cb1 [DOI] [PubMed] [Google Scholar]
- 10.Lemans JVC, Oner FC, Wijdicks SPJ, et al. The efficacy of intrawound vancomycin powder and povidone-iodine irrigation to prevent surgical site infections in complex instrumented spine surgery. Spine J. 2019;19(10):1648–1656. doi:10.1016/j.spinee.2019.05.592 [DOI] [PubMed] [Google Scholar]
- 11.Lemans JVC, Wijdicks SPJ, Boot W, et al. Intrawound treatment for prevention of surgical site infections in instrumented spinal surgery: a systematic comparative effectiveness review and meta-analysis. Global Spine J. 2019;9(2):219–230. doi:10.1177/2192568218786252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang FY, Chang MC, Wang ST, Yu W-K, Liu C-L, Chen T-H. Can povidone-iodine solution be used safely in a spinal surgery? Eur Spine J. 2006;15(6):1005–1014. doi:10.1007/s00586-005-0975-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durani P, Leaper D. Povidone-iodine: use in hand disinfection, skin preparation and antiseptic irrigation. Int Wound J. 2008;5(3):376–387. doi:10.1111/j.1742-481X.2007.00405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Block C, Robenshtok E, Simhon A, Shapiro M. Evaluation of chlorhexidine and povidone iodine activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecalis using a surface test. J Hosp Infect. 2000;46(2):147–152. doi:10.1053/jhin.2000.0805 [DOI] [PubMed] [Google Scholar]
- 15.Bible JE, O’Neill KR, Crosby CG, Schoenecker JG, McGirt MJ, Devin CJ. Implant contamination during spine surgery. Spine J. 2013;13(6):637–640. doi:10.1016/j.spinee.2012.11.053 [DOI] [PubMed] [Google Scholar]
- 16.Lee CS, Kang KC, Chung SS, et al. Incidence of microbiological contamination of local bone autograft used in posterior lumbar interbody fusion and its association with postoperative spinal infection. J Neurosurg Spine. 2016;24(1):20–24. doi:10.3171/2015.3.SPINE14578 [DOI] [PubMed] [Google Scholar]
- 17.Bridwell KH, Lenke LG, McEnery KW, Baldus C, Blanke K.Anterior fresh frozen structural allografts in the thoracic and lumbar spine. Do they work if combined with posterior fusion and instrumentation in adult patients with kyphosis or anterior column defects? Spine (Phila Pa 1976). 1995;20(12):1410–1418. [PubMed] [Google Scholar]
- 18.Brown NM, Cipriano CA, Moric M, Sporer SM, Della Valle CJ. Dilute betadine lavage before closure for the prevention of acute postoperative deep periprosthetic joint infection. J Arthroplasty. 2012;27(1):27–30. doi:10.1016/j.arth.2011.03.034 [DOI] [PubMed] [Google Scholar]
- 19.Pawar AY, Biswas SK. Postoperative spine infections. Asian Spine J. 2016;10(1):176–183. doi:10.4184/asj.2016.10.1.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cichos KH, Andrews RM, Wolschendorf F, Narmore W, Mabry SE, Ghanem ES. Efficacy of intraoperative antiseptic techniques in the prevention of periprosthetic joint infection: superiority of betadine. J Arthroplasty. 2019;34(7S):S312–S318. doi:10.1016/j.arth.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 21.Wong AP, Smith ZA, Stadler JA, 3rd, et al. Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF): surgical technique, long-term 4-year prospective outcomes, and complications compared with an open TLIF cohort. Neurosurg Clin N Am. 2014;25(2):279–304. doi:10.1016/j.nec.2013.12.007 [DOI] [PubMed] [Google Scholar]
- 22.Newton Ede MP, Philp AM, Philp A, Richardson SM, Mohammad S, Jones SW. Povidone-Iodine has a profound effect on in vitro osteoblast proliferation and metabolic function and inhibits their ability to mineralize and form bone. Spine (Phila Pa 1976). 2016;41(9):729–734. doi:10.1097/BRS.0000000000001332 [DOI] [PubMed] [Google Scholar]
- 23.van Meurs SJ, Gawlitta D, Heemstra KA, Poolman RW, Vogely HC, Kruyt MC. Selection of an optimal antiseptic solution for intraoperative irrigation: an in vitro study. J Bone Joint Surg Am. 2014;96(4):285–291. doi:10.2106/JBJS.M.00313 [DOI] [PubMed] [Google Scholar]